Ventral Morphology of the Non-Trilobite Artiopod Retifacies abnormalis Hou, Chen & Lu, 1989, from the Early Cambrian Chengjiang Biota, China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Systematic Palaeontology
3.1.1. Emended Diagnosis
3.1.2. Remarks
3.2. Description
3.2.1. Preservation and Overall Morphology
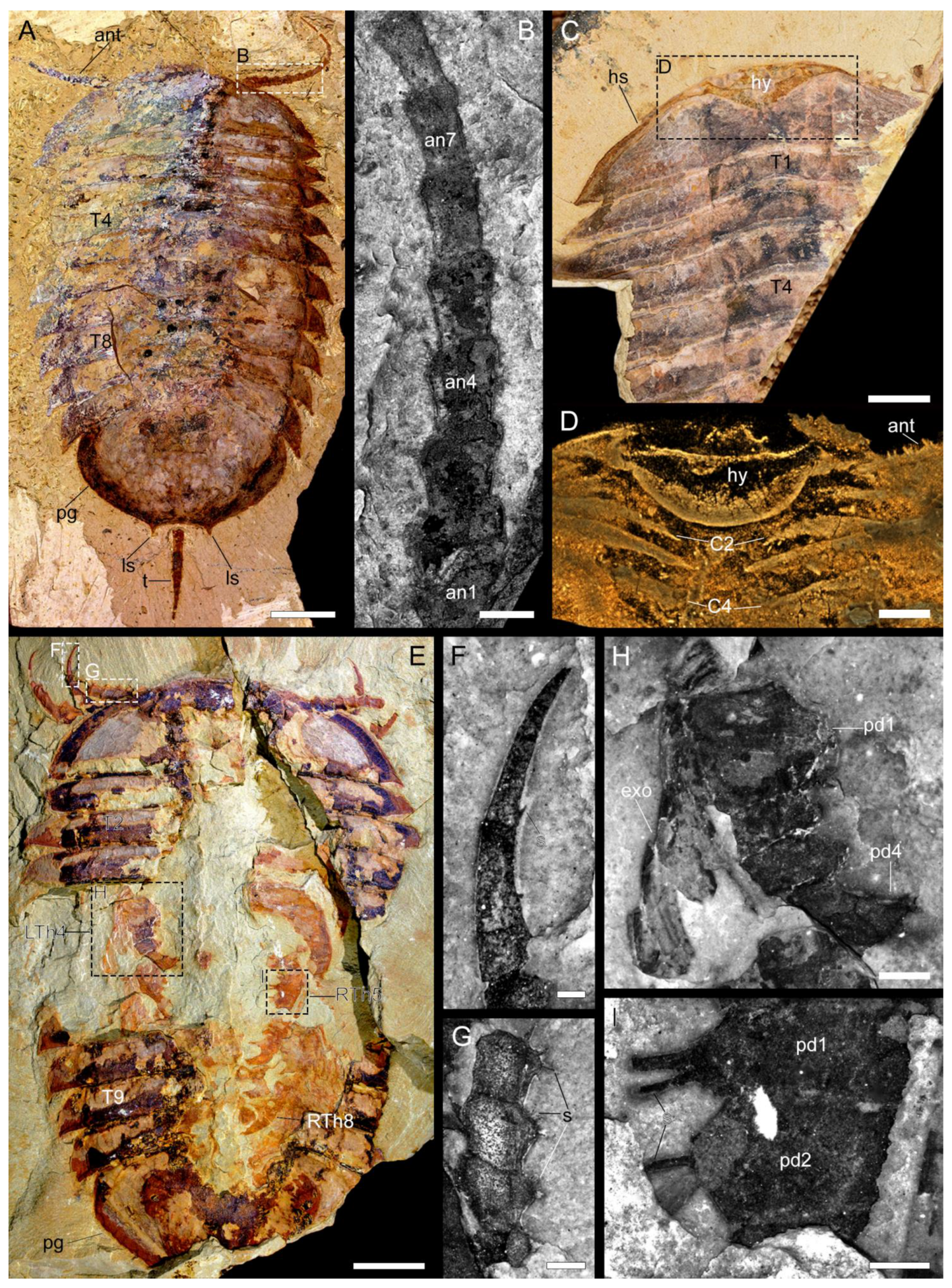
3.2.2. Cephalon


3.2.3. Thoracic tergites and Appendages
3.2.4. Pygidium and Pygidial Appendages



3.3. Phylogenetic Analyses

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


Appendix B
| Specimen Number | Body Length | Cephalon | Thorax | Pygidium | |||
|---|---|---|---|---|---|---|---|
| L0 | L1 | R(L1/L0) | L2 | R(L2/L0) | L3 | R(L3/L0) | |
| YKLP 11434 | 94.88 | 14.88 | 16% | 51.46 | 54% | 28.55 | 30% |
| YKLP 11432 | 51.06 | 4.26 | 8% | 32.55 | 64% | 14.26 | 28% |
| YKLP 11430 | 41.32 | 6.77 | 16% | 25.27 | 61% | 9.27 | 22% |
| YKLP 11446 | 35.61 | 5.00 | 14% | 18.39 | 52% | 12.22 | 34% |
| YKLP 11447 | 59.81 | 5.06 | 8% | 38.07 | 64% | 16.68 | 28% |
| YKLP 11448 | 45.40 | 7.49 | 16% | 24.18 | 53% | 13.74 | 30% |
| YKLP 11449 | 36.88 | 5.84 | 16% | 21.22 | 58% | 9.82 | 27% |
| YKLP 11450 | 29.86 | 4.64 | 16% | 17.42 | 58% | 7.81 | 26% |
References
- Hou, X.; Jan, B. Arthropods of the Lower Cambrian Chengjiang Fauna, Southwest China; Scandinavian University Press: Oslo, Norway, 1997; pp. 44–57. ISBN 82-00-37693-1. [Google Scholar]
- Edgecombe, G.; Ramskold, L. Relationships of Cambrian Arachnata and the Systematic Position of Trilobita. J. Paleontol. 1999, 73, 263–287. [Google Scholar] [CrossRef]
- Paterson, J.R.; Edgecombe, G.D.; Garcia-Bellido, D.C.; Jago, J.B.; Gehling, J.G. Nektaspid Arthropods from the Lower Cambrian Emu Bay Shale Lagerstatte, South Australia, with a Reassessment of Lamellipedian Relationships. Palaeontology 2010, 53, 377–402. [Google Scholar] [CrossRef]
- Stein, M.; Selden, P.A. A Restudy of the Burgess Shale (Cambrian) Arthropod Emeraldella Brocki and Reassessment of Its Affinities. J. Syst. Palaeontol. 2012, 10, 361–383. [Google Scholar] [CrossRef]
- Ortega-Hernández, J.; Legg, D.A.; Braddy, S.J. The Phylogeny of Aglaspidid Arthropods and the Internal Relationships within Artiopoda. Cladistics 2013, 29, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Budd, G.E.; Peel, J.S.; Harper, D.A. Arthroaspis n. Gen., a Common Element of the Sirius Passet Lagerstätte (Cambrian, North Greenland), Sheds Light on Trilobite Ancestry. BMC Evol. Biol. 2013, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.D.; Paterson, J.R.; García-Bellido, D.C. The Trilobite Redlichia from the Lower Cambrian Emu Bay Shale Konservat-Lagerstätte of South Australia: Systematics, Ontogeny and Soft-Part Anatomy. J. Syst. Palaeontol. 2020, 18, 295–334. [Google Scholar] [CrossRef]
- Bicknell, R.D.C.; Holmes, J.D.; Edgecombe, G.D.; Losso, S.R.; Ortega-Hernández, J.; Wroe, S.; Paterson, J.R. Biomechanical Analyses of Cambrian Euarthropod Limbs Reveal Their Effectiveness in Mastication and Durophagy. Proc. R. Soc. B Biol. Sci. 2021, 288, 20202075. [Google Scholar] [CrossRef]
- Losso, S.R.; Ortega-Hernandez, J. Claspers in the Mid-Cambrian Olenoides Serratus Indicate Horseshoe Crab-like Mating in Trilobites. Geology 2022, 50, 897–901. [Google Scholar] [CrossRef]
- Chen, X.; Ortega-Hernández, J.; Wolfe, J.M.; Zhai, D.; Hou, X.; Chen, A.; Mai, H.; Liu, Y. The Appendicular Morphology of Sinoburius Lunaris and the Evolution of the Artiopodan Clade Xandarellida (Euarthropoda, Early Cambrian) from South China. BMC Evol. Biol. 2019, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Hou, X.; Zhai, D.; Mai, H.; Belojevic, J.; Chen, X.; Melzer, R.R.; Ortega-Hernandez, J.; Liu, Y. Before Trilobite Legs: Pygmaclypeatus Daziensis Reconsidered and the Ancestral Appendicular Organization of Cambrian Artiopods. Philos. Trans. R. Soc. B-Biol. Sci. 2022, 377, 20210030. [Google Scholar] [CrossRef]
- Liu, Y.; Edgecombe, G.; Schmidt, M.; Bond, A.; Melzer, R.; Zhai, D.; Huijuan, M.; Zhang, M.; Hou, X. Exites in Cambrian Arthropods and Homology of Arthropod Limb Branches. Nat. Commun. 2021, 12, 4619. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Siveter, D.J.; Siveter, D.J.; Aldridge, R.J.; Cong, P.; Gabbott, S.E.; Ma, X.; Purnell, M.A.; Williams, M. The Cambrian Fossils of Chengjiang, China the Flowering of Early Animal Life, 2nd ed.; Wiley-Blackwell: Oxford, MS, USA, 2017; pp. 162–222. ISBN 978-1-118-89638-9. [Google Scholar]
- Hou, X.; Chen, J.; Lu, H. Early Cambrian new arthropods from Chengjiang, Yunnan. Acta Palaeontol. Sin. 1989, 28, 42–57+131–136. [Google Scholar] [CrossRef]
- Delle Cave, L.; Simonetta, A.M. (Eds.) Early Palaeozoic Arthropods and Problems of Arthropod Phylogeny; With Some Notes on Taxa of Doubtful Affinities. In The Early Evolution of Metazoa and the Significance of Problematic Taxa; Proc. symposium, Camerino, 1989; Cambridge University Press: Cambridge, UK, 1991; pp. 189–244. [Google Scholar]
- Liu, Y.; Melzer, R.R.; Haug, J.T.; Haug, C.; Briggs, D.E.G.; Hoernig, M.K.; He, Y.; Hou, X. Three-Dimensionally Preserved Minute Larva of a Great-Appendage Arthropod from the Early Cambrian Chengjiang Biota. Proc. Natl. Acad. Sci. USA. 2016, 113, 5542–5546. [Google Scholar] [CrossRef]
- Paterson, J.R.; Garcia-Bellido, D.C.; Edgecombe, G.D. New Artiopodan Arthropods from the Early Cambrian Emu Bay Shale Konservat-Lagerstatte of South Australia. J. Paleontol. 2012, 86, 340–357. [Google Scholar] [CrossRef]
- Limaye, A. Drishti: A volume exploration and presentation tool. In Developments in X-ray Tomography VIII; SPIE: Bellingham, WA, USA, 2012; Volume 8506, pp. 191–199. [Google Scholar]
- Lankester, E.R. The Structure and Classification of Arthropoda. Q. J Microsc. Sci. 1904, 47, 523–582. [Google Scholar]
- Luo, H.; Hu, S.; Zhang, S.; Tao, Y. New Occurrence of the Early Cambrian Chengjiang Fauna in Haikou, Kunming, Yunnan Province, and Study on Trilobitoidea. Acta Geol. Sin. Ed. 1997, 71, 122–132+231–232. [Google Scholar]
- Luo, H.; Hu, S.; Chen, L.; Zhang, S.; Tao, Y. Early Cambrian Chengjiang Fauna from Kunming Region, China; Yunnan Science and Technology Press: Kunming, China, 1999. [Google Scholar]
- Du, K.; Ortega-Hernandez, J.; Yang, J.; Zhang, X. A Soft-Bodied Euarthropod from the Early Cambrian Xiaoshiba Lagerstatte of China Supports a New Clade of Basal Artiopodans with Dorsal Ecdysial Sutures. Cladistics 2019, 35, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Goloboff, P.A.; Catalano, S.A. TNT Version 1.5, Including a Full Implementation of Phylogenetic Morphometrics. Cladistics 2016, 32, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.O. Phylogenetic Systematics Turns over a New Leaf. Trends Ecol. Evol. 2001, 16, 30–37. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Waloszek, D.; Chen, J.-Y.; Maas, A.; Wang, X. Early Cambrian Arthropods—New Insights into Arthropod Head and Structural Evolution. Arthropod Struct. Dev. 2005, 34, 189–205. [Google Scholar] [CrossRef]
- Lu, Y. On the Ontogeny and Phylogeny of Redlichia Intermedia Lu (Sp. Nov.). Bull. Geol. Soc. China 1940, 20, 333–342+393. [Google Scholar]
- Fu, D.; Ortega-Hernandez, J.; Daley, A.C.; Zhang, X.; Shu, D. Anamorphic Development and Extended Parental Care in a 520 Million-Year-Old Stem-Group Euarthropod from China. Bmc Evol. Biol. 2018, 18, 147. [Google Scholar] [CrossRef] [PubMed]
- Zacaï, A.; Vannier, J.; Lerosey-Aubril, R. Reconstructing the Diet of a 505-Million-Year-Old Arthropod: Sidneyia Inexpectans from the Burgess Shale Fauna. Arthropod Struct. Dev. 2016, 45, 200–220. [Google Scholar] [CrossRef] [PubMed]
| Specimen | Panel | Pixel Size (um) | Detector | Voltage (kV) | Power (W) | Filter | Rotation | Projection | Average Step Rotation |
|---|---|---|---|---|---|---|---|---|---|
| YKLP 11436 | Figure 1D | 18.03 | FP | 60 | 5 | no | −120°–+120° | 1001 × 7 | 0.2398° |
| YKLP 11430 | Figure 2A,E | 35.92 | 0.4X | 70 | 6 | LE4 | −103°–+103° | 801 × 2 | 0.2572° |
| Figure 2C,D | 17.385 | 0.4X | 70 | 6 | LE4 | −103°–+103° | 1601 | 0.1287° | |
| Figure 2B | 9.71 | FP | 60 | 5 | no | −120°–+120° | 1401 | 0.1713° | |
| Figure A2, Figure 5B | 8.59 | 0.4X | 90 | 8 | LE4 | −103°–+103° | 1401 × 3 | 0.1470° | |
| Figure 5A | 7.39 | 0.4X | 90 | 8 | LE4 | −103°–+103° | 1401 × 2 | 0.1470° | |
| YKLP 11426 | Figure 3D | 27.45 | FP | 60 | 5 | LE4 | −120°–+120° | 801 × 5 | 0.2996° |
| Figure 3A,B,C | 17.01 | 0.4X | 60 | 5 | no | −103°–+103° | 1001 × 5 | 0.2058° | |
| YKLP 11432 | Figure 4D | 19.83 | FP | 60 | 5 | no | −120°–+120° | 1001 × 3 | 0.2398° |
| Figure 5C,D,E | 13.31 | 0.4X | 60 | 5 | no | −103°–+103° | 1201 × 3 | 0.1715° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Liu, Y.; Hou, X.; Ortega-Hernández, J.; Mai, H.; Schmidt, M.; Melzer, R.R.; Guo, J. Ventral Morphology of the Non-Trilobite Artiopod Retifacies abnormalis Hou, Chen & Lu, 1989, from the Early Cambrian Chengjiang Biota, China. Biology 2022, 11, 1235. https://doi.org/10.3390/biology11081235
Zhang M, Liu Y, Hou X, Ortega-Hernández J, Mai H, Schmidt M, Melzer RR, Guo J. Ventral Morphology of the Non-Trilobite Artiopod Retifacies abnormalis Hou, Chen & Lu, 1989, from the Early Cambrian Chengjiang Biota, China. Biology. 2022; 11(8):1235. https://doi.org/10.3390/biology11081235
Chicago/Turabian StyleZhang, Maoyin, Yu Liu, Xianguang Hou, Javier Ortega-Hernández, Huijuan Mai, Michel Schmidt, Roland R. Melzer, and Jin Guo. 2022. "Ventral Morphology of the Non-Trilobite Artiopod Retifacies abnormalis Hou, Chen & Lu, 1989, from the Early Cambrian Chengjiang Biota, China" Biology 11, no. 8: 1235. https://doi.org/10.3390/biology11081235
APA StyleZhang, M., Liu, Y., Hou, X., Ortega-Hernández, J., Mai, H., Schmidt, M., Melzer, R. R., & Guo, J. (2022). Ventral Morphology of the Non-Trilobite Artiopod Retifacies abnormalis Hou, Chen & Lu, 1989, from the Early Cambrian Chengjiang Biota, China. Biology, 11(8), 1235. https://doi.org/10.3390/biology11081235






