Colour Variation in the Crocodile Lizard (Shinisaurus crocodilurus) and Its Relationship to Individual Quality
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Colour Measurements of Lizards and Their Habitat
2.3. Colour Variation
2.4. Conspicuousness of Colour to the Lizard Visual System
2.5. Morphology and Bite Force Measurements
2.6. Age, Parentage and Reproductive Output
2.7. Relationship between Colour, Morphology and Functional Performance Traits
2.8. Colour Variation and Reproductive Output
3. Results
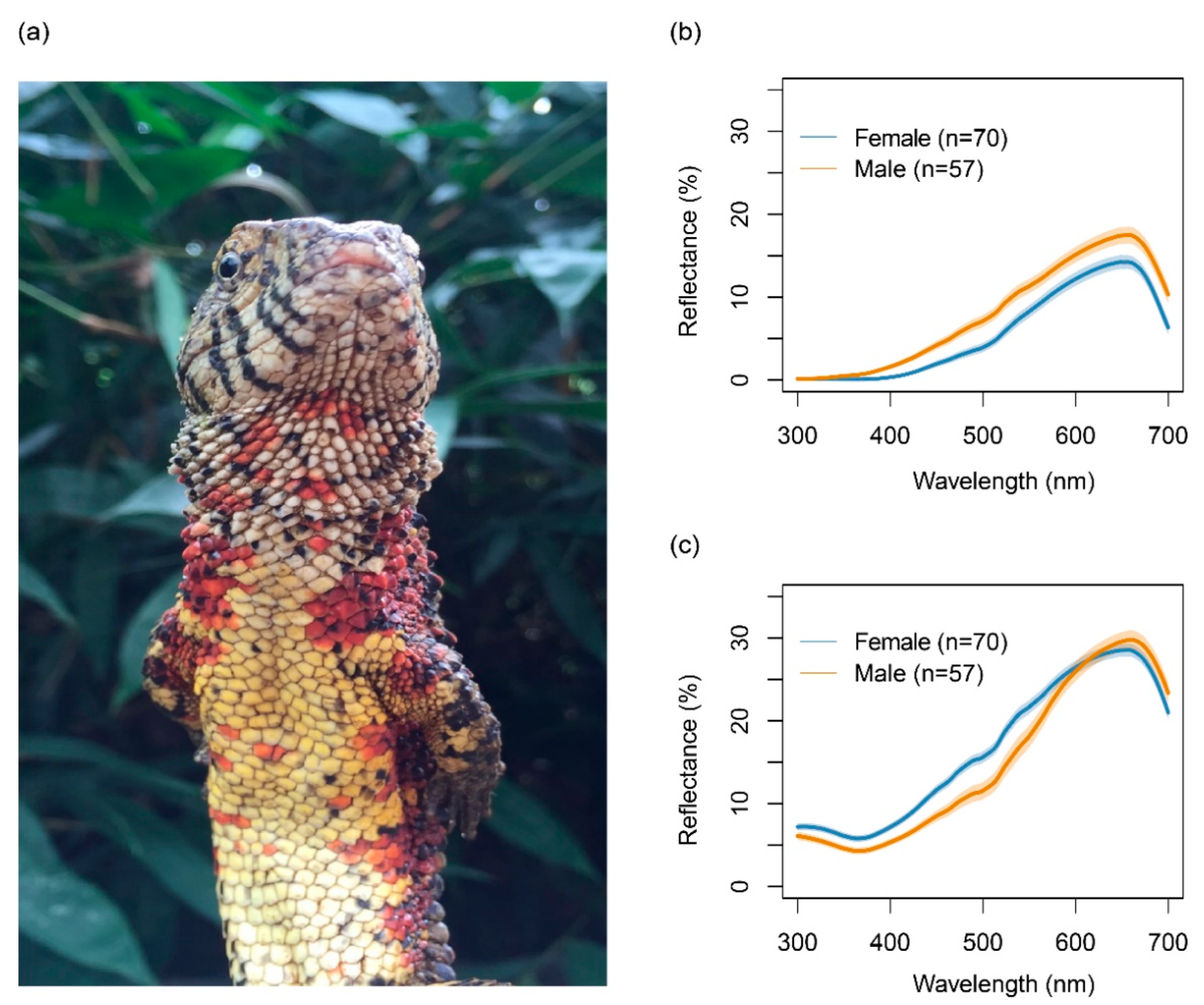
3.1. Colour Variation
3.2. Conspicuousness of Colour Patch to a Lizard Receiver
3.3. Relationships between Colour Variation and Phenotypic Traits
3.4. Relationship between Colour Variation and Reproductive Output
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kemp, D.J.; Herberstein, M.E.; Fleishman, L.J.; Endler, J.A.; Bennett, A.T.D.; Dyer, A.G.; Hart, N.S.; Marshall, J.; Whiting, M.J. An Integrative Framework for the Appraisal of Coloration in Nature. Am. Nat. 2015, 185, 705–724. [Google Scholar] [CrossRef] [PubMed]
- Stuart-Fox, D.; Aulsebrook, A.; Rankin, K.J.; Dong, C.M.; McLean, C.A. Convergence and Divergence in Lizard Colour Polymorphisms. Biol. Rev. 2020, 96, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.; Fleishman, L.J. Differences in Visual Signal Design and Detectability between Allopatric Populations of Anolis Lizards. Am. Nat. 2004, 163, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Pérez i de Lanuza, G.; Ábalos, J.; Bartolomé, A.; Font, E. Through the Eye of a Lizard: Hue Discrimination in a Lizard with Ventral Polymorphic Coloration. J. Exp. Biol. 2018, 221, jeb169565. [Google Scholar] [CrossRef]
- Tibbetts, E.A.; Mullen, S.P.; Dale, J. Signal Function Drives Phenotypic and Genetic Diversity: The Effects of Signalling Individual Identity, Quality or Behavioural Strategy. Phil. Trans. R. Soc. B 2017, 372, 20160347. [Google Scholar] [CrossRef]
- Lind, O.; Henze, M.J.; Kelber, A.; Osorio, D. Coevolution of Coloration and Colour Vision? Phil. Trans. R. Soc. B 2017, 372, 20160338. [Google Scholar] [CrossRef]
- Tibbetts, E.A.; Dale, J. Individual Recognition: It Is Good to Be Different. Trends Ecol. Evol. 2007, 22, 529–537. [Google Scholar] [CrossRef]
- Lai, Y.T.; Keka, J. Male Ornamentation in the European Minnow (Phoxinus Phoxinus) Signals Swimming Performance. Ethology 2013, 119, 1077–1085. [Google Scholar] [CrossRef]
- Whiting, M.J.; Stuart-Fox, D.M.; O’Connor, D.; Firth, D.; Bennett, N.C.; Blomberg, S.P. Ultraviolet Signals Ultra-Aggression in a Lizard. Anim. Behav. 2006, 72, 353–363. [Google Scholar] [CrossRef]
- Sinervo, B.; Lively, C.M. The Rock-Paper-Scissors Game and the Evolution of Alternative Male Strategies. Nature 1996, 380, 240–243. [Google Scholar] [CrossRef]
- Vercken, E.; Massot, M.; Sinervo, B.; Clobert, J. Colour Variation and Alternative Reproductive Strategies in Females of the Common Lizard Lacerta Vivipara. J. Evol. Biol. 2007, 20, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Earl, A.D.; Simpson, R.K.; Yorzinski, J.L. Dominant Females Have Brighter Ornamentation in a Sexually Dimorphic Lekking Species. Ethology 2022, 128, 85–93. [Google Scholar] [CrossRef]
- Belliure, J.; Fresnillo, B.; Cuervo, J.J. Male Mate Choice Based on Female Coloration in a Lizard: The Role of a Juvenile Trait. Behav. Ecol. 2018, 29, 543–552. [Google Scholar] [CrossRef]
- He, N.; Wu, Z.; Cai, F.; Wang, Z.; Yu, H.; Huang, C. The Sexual Dimorphism of Shinisaurus Crocodilurus. Chin. J. Ecol. 2011, 30, 7–11. [Google Scholar]
- Van Schingen, M.; Duc Le, M.; Thi Ngo, H.; The Pham, C.; Quy Ha, Q.; Quang Nguyen, T.; Ziegler, T. Is There More than One Crocodile Lizard? An Integrative Taxonomic Approach Reveals Vietnamese and Chinese Shinisaurus Crocodilurus Represent Separate Conservation and Taxonomic Units. Der Zool. Gart. 2016, 85, 240–260. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Hamilton, P.; Ziegler, T. Shinisaurus Crocodilurus. IUCN Red List. Threat. Species 2014, e.T57287221A57287235. [Google Scholar] [CrossRef]
- Le, K.Q.; Ziegler, T. First Record of the Chinese Crocodile Lizard from Outside of China: Report on a Population of Shinisaurus Crocodilurus Ahl, 1930 from North-Eastern Vietnam. Hamadryad 2003, 27, 193–199. [Google Scholar]
- Huang, C.M.; Yu, H.; Wu, Z.J.; Li, Y.B.; Wei, F.W.; Gong, M.H. Population and Conservation Srategies for the Chinese Crocodile Lizard (Shinisaurus Crocodilurus) in China. Anim. Biodivers. Conserv. 2008, 32, 63–70. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Syakur, M.A.; Khotimah, B.K.; Rochman, E.M.S.; Satoto, B.D. Integration K-Means Clustering Method and Elbow Method For Identification of The Best Customer Profile Cluster. IOP Conf. Ser. Mater. Sci. Eng. 2018, 336, 012017. [Google Scholar] [CrossRef]
- Maia, R.; Eliason, C.M.; Bitton, P.-P.; Doucet, S.M.; Shawkey, M.D. Pavo: An R Package for the Analysis, Visualization and Organization of Spectral Data. Methods Ecol. Evol. 2013, 4, 906–913. [Google Scholar] [CrossRef]
- Maia, R.; Gruson, H.; Endler, J.A.; White, T.E. Pavo 2: New Tools for the Spectral and Spatial Analysis of Colour in r. Methods Ecol. Evol. 2019, 10, 1097–1107. [Google Scholar] [CrossRef]
- Chan, R.; Stuart-Fox, D.; Jessop, T.S. Why Are Females Ornamented? A Test of the Courtship Stimulation and Courtship Rejection Hypotheses. Behav. Ecol. 2009, 20, 1334–1342. [Google Scholar] [CrossRef]
- Fleishman, L.J.; Loew, E.R.; Whiting, M.J. High Sensitivity to Short Wavelengths in a Lizard and Implications for Understanding the Evolution of Visual Systems in Lizards. Proc. Biol. Sci. 2011, 278, 2891–2899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqi, A.; Cronin, T.W.; Loew, E.R.; Vorobyev, M.; Summers, K. Interspecific and Intraspecific Views of Color Signals in the Strawberry Poison Frog Dendrobates Pumilio. J. Exp. Biol. 2004, 207, 2471–2485. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Peig, J.; Green, A.J. New Perspectives for Estimating Body Condition from Mass/Length Data: The Scaled Mass Index as an Alternative Method. Oikos 2009, 118, 1883–1891. [Google Scholar] [CrossRef]
- Vanhooydonck, B.; Herrel, A.Y.; Van Damme, R.; Irschick, D.J. Does Dewlap Size Predict Male Bite Performance in Jamaican Anolis Lizards? Funct. Ecol. 2005, 19, 38–42. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, Y.; Li, K.; Qi, L.; Zhang, Q.; Wang, M.; Xiao, J. A Novel Three-Round Multiplex PCR for SNP Genotyping with next Generation Sequencing. Anal. Bioanal. Chem. 2016, 408, 4371–4377. [Google Scholar] [CrossRef]
- Jones, A.G.; Small, C.M.; Paczolt, K.A.; Ratterman, N.L. A Practical Guide to Methods of Parentage Analysis. Mol. Ecol. Resour. 2010, 10, 6–30. [Google Scholar] [CrossRef]
- Forslund, P.; Pärt, T. Age and Reproduction in Birds - Hypotheses and Tests. Trends Ecol. Evol. 1995, 10, 374–378. [Google Scholar] [CrossRef]
- Whiting, M.J.; Nagy, K.A.; Bateman, P.W. Evolution and maintenance of social status-signaling badges: Experimental Manipulations in Lizards. In Lizard Social Behavior; Johns Hopkins University Press: Baltimore, MD, USA, 2003; pp. 47–82. [Google Scholar]
- Yu, S.; Wu, Z.; Wang, Z.; Chen, L.; Huang, C.; Yu, H. Courtship and Mating Behavior of Shinisaurus Crocodilurus Bred in Luokeng Nature Reserve, Guangdong. Chin. J. Zool. 2009, 44, 38–44. [Google Scholar]
- Meyers, J.J.; Irschick, D.J.; Vanhooydonck, B.; Herrel, A. Divergent Roles for Multiple Sexual Signals in a Polygynous Lizard. Funct. Ecol. 2006, 20, 709–716. [Google Scholar] [CrossRef]
- Robinson, C.D.; Gifford, M.E. Covariation between Thermally Mediated Color and Performance Traits in a Lizard. Physiol. Biochem. Zool. 2018, 91, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Stuart-Fox, D.; Rankin, K.J.; Lutz, A.; Elliott, A.; Hugall, A.F.; McLean, C.A.; Medina, I. Environmental Gradients Predict the Ratio of Environmentally Acquired Carotenoids to Self-Synthesised Pteridine Pigments. Ecol. Lett. 2021, 24, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- McLean, C.A.; Lutz, A.; Rankin, K.J.; Elliott, A.; Moussalli, A.; Stuart-Fox, D. Red Carotenoids and Associated Gene Expression Explain Colour Variation in Frillneck Lizards. Proc. R. Soc. B 2019, 286, 20191172. [Google Scholar] [CrossRef] [PubMed]
- Zajitschek, S.R.K.; Zajitschek, F.; Miles, D.B.; Clobert, J. The Effect of Coloration and Temperature on Sprint Performance in Male and Female Wall Lizards. Biol. J. Linn. Soc. 2012, 107, 573–582. [Google Scholar] [CrossRef] [Green Version]





| JNDs | Female (n = 70) | Male (n = 57) | ||
|---|---|---|---|---|
| Throat (n = 127) | Non-red (n = 25) | Red (n = 45) | Non-red (n = 21) | Red (n = 36) |
| Chromatic | 30.77 ± 20.20 | 36.34 ± 17.05 | 15.02 ± 11.90 | 20.41 ± 17.88 |
| Luminance | 9.62 ± 5.75 | 10.23 ± 5.25 | 7.04 ± 5.17 | 6.37 ± 5.75 |
| Venter (n = 127) | Non-red (n = 29) | Red (n = 41) | Non-red (n = 10) | Red (n = 47) |
| Chromatic | 3.29 ± 1.71 | 3.21 ± 1.55 | 4.53 ± 1.31 | 4.62 ± 1.74 |
| Luminance | 6.40 ± 2.47 | 3.87 ± 2.33 | 7.55 ± 3.32 | 3.65 ± 3.10 |
| Throat (n = 59) | Venter (n = 59) | ||||
|---|---|---|---|---|---|
| Female (n = 35) | Combined (n = 35) | Non-Red (n = 12) | Red (n = 23) | Non-Red (n = 16) | Red (n =19) |
| Age (year) | 2.83 ± 1.44 | 2.75 ± 1.71 | 2.87 ± 1.32 | 2.50 ± 1.32 | 3.11 ± 1.52 |
| SMI | 82.57 ± 12.12 | 90.25 ± 7.20 | 78.56 ± 12.33 | 85.29 ± 9.62 | 80.28 ± 13.71 |
| Head size (PC1) | 0.07 ± 4.86 | 0.89 ± 5.49 | −0.36 ± 4.56 | 2.40 ± 4.90 | −1.89 ± 3.96 |
| Head length (mm) | 29.63 ± 3.21 | 29.51 ± 3.79 | 29.69 ± 2.96 | 28.03 ± 3.22 | 30.97 ± 2.58 |
| Head width (mm) | 23.62 ± 2.82 | 23.07 ± 3.23 | 23.90 ± 2.62 | 22.28 ± 2.92 | 24.74 ± 2.24 |
| Head height (mm) | 18.30 ± 3.67 | 17.38 ± 2.59 | 18.79 ± 4.09 | 17.26 ± 3.85 | 19.18 ± 3.36 |
| Tail length (mm) | 192.54 ± 26.28 | 184.33 ± 31.76 | 196.83 ± 22.52 | 182.19 ± 19.96 | 201.26 ± 28.22 |
| Bite force (n) | 39.52 ± 12.40 | 34.18 ± 11.15 | 42.31 ± 12.32 | 36.61 ± 13.28 | 41.97 ± 11.39 |
| Male (n = 24) | Combined (n = 24) | Non-red (n = 7) | Red (n = 17) | Non-red (n = 5) | Red (n = 19) |
| Age (year) | 3.04 ± 1.37 | 2.86 ± 0.38 | 3.12 ± 1.62 | 3.40 ± 0.55 | 2.95 ± 1.51 |
| SMI | 81.47 ± 7.81 | 80.62 ± 12.77 | 81.83 ± 5.11 | 82.65 ± 10.30 | 81.17 ± 7.34 |
| Head size (PC1) | −2.35 ± 5.13 | −3.06 ± 1.87 | −2.06 ± 6.01 | −3.43 ± 1.42 | −2.06 ± 5.72 |
| Head length (mm) | 31.13 ± 3.83 | 31.86 ± 1.31 | 30.84 ± 4.49 | 32.21 ± 0.67 | 30.85 ± 4.27 |
| Head width (mm) | 25.54 ± 2.92 | 25.87 ± 1.86 | 25.40 ± 3.29 | 25.66 ± 1.50 | 25.50 ± 3.22 |
| Head height (mm) | 19.01 ± 2.55 | 19.07 ± 1.08 | 18.99 ± 2.98 | 19.57 ± 0.64 | 18.86 ± 2.84 |
| Tail length (mm) | 202.21 ± 20.23 | 209.00 ± 16.05 | 199.41 ± 21.52 | 209.80 ± 13.76 | 200.21 ± 21.46 |
| Bite force (n) | 41.33 ± 10.40 | 44.23 ± 4.10 | 40.14 ± 12.00 | 47.44 ± 4.19 | 39.72 ± 11.01 |
| Female (N = 16) | Male (N = 13) | ||||||
|---|---|---|---|---|---|---|---|
| Estimate | t Value | p Value | Estimate | t Value | p Value | ||
| Throat | |||||||
| Chromatic contrast | Age | −0.01 | −2.41 | 0.03 | −0.01 | −1.73 | 0.11 |
| SMI | −0.002 | −1.14 | 0.27 | 0.001 | 0.31 | 0.76 | |
| Head size (PC1) | 0.01 | 0.50 | 0.62 | 0.10 | 2.08 | 0.05 | |
| Tail length | −0.002 | −1.77 | 0.09 | −0.002 | −1.45 | 0.16 | |
| Bite force | −0.003 | −1.27 | 0.21 | −0.01 | −2.32 | 0.03 | |
| Litter size | 0.001 | 0.17 | 0.86 | 0.01 | 0.54 | 0.60 | |
| Litter mass | 0.01 | 1.19 | 0.26 | 0.001 | 0.06 | 0.96 | |
| Total live mass | 0.02 | 1.54 | 0.15 | −0.02 | −1.40 | 0.25 | |
| Total live number | 0.01 | 1.48 | 0.16 | 0.01 | 0.28 | 0.79 | |
| Luminance contrast | Age | −0.02 | −2.02 | 0.05 | −0.02 | −1.82 | 0.10 |
| SMI | −0.005 | −0.95 | 0.35 | 0.001 | 0.25 | 0.81 | |
| Head size (PC1) | 0.10 | 1.03 | 0.31 | 0.13 | 1.65 | 0.13 | |
| Tail length | −0.01 | −2.37 | 0.02 | −0.002 | −1.03 | 0.33 | |
| Bite force | −0.01 | −1.34 | 0.19 | −0.01 | −1.03 | 0.32 | |
| Litter size | −0.01 | −0.95 | 0.36 | −0.04 | −0.90 | 0.39 | |
| Litter mass | 0.001 | 0.05 | 0.96 | −0.02 | −1.06 | 0.35 | |
| Total live mass | 0.03 | 0.84 | 0.42 | 0.04 | 1.00 | 0.34 | |
| Total live number | 0.03 | 0.87 | 0.40 | 0.02 | 0.50 | 0.63 | |
| Venter | |||||||
| Chromatic contrast | Age | −0.05 | −1.45 | 0.16 | −0.03 | -0.95 | 0.37 |
| SMI | −0.01 | −0.58 | 0.57 | −0.02 | −1.05 | 0.31 | |
| Head size (PC1) | 0.42 | 1.23 | 0.23 | 0.21 | 0.64 | 0.54 | |
| Tail length | 0.0 | −0.21 | 0.83 | −0.01 | −0.66 | 0.52 | |
| Bite force | 0.01 | 0.28 | 0.78 | −0.04 | −1.24 | 0.24 | |
| Litter size | −0.06 | −1.32 | 0.21 | 0.03 | 0.45 | 0.68 | |
| Litter mass | −0.02 | −0.30 | 0.77 | −0.01 | −0.58 | 0.61 | |
| Total live mass | 0.11 | 0.82 | 0.43 | 0.08 | 23.71 | 0.002 | |
| Total live number | 0.08 | 0.71 | 0.49 | 0.16 | 3.02 | 0.04 | |
| Luminance contrast | Age | −0.02 | −1.19 | 0.25 | 0.002 | 0.15 | 0.88 |
| SMI | 0.003 | 0.28 | 0.78 | −0.003 | −0.46 | 0.65 | |
| Head size (PC1) | 0.07 | 0.41 | 0.69 | 0.09 | 0.65 | 0.53 | |
| Tail length | 0.004 | 0.60 | 0.56 | −0.001 | −0.36 | 0.73 | |
| Bite force | −0.002 | −0.15 | 0.88 | 0.02 | 1.36 | 0.19 | |
| Litter size | 0.02 | 1.05 | 0.31 | 0.01 | 0.43 | 0.70 | |
| Litter mass | −0.02 | −0.63 | 0.54 | 0.01 | 6.63 | 0.02 | |
| Total live mass | −0.09 | −1.60 | 0.14 | −0.01 | −0.55 | 0.64 | |
| Total live number | −0.06 | −1.09 | 0.29 | −0.03 | −0.74 | 0.51 | |
| Throat (n = 29) | Venter (n = 29) | ||||
|---|---|---|---|---|---|
| Female (n = 16) | Combined (n = 16) | Non-red (n = 7) | Red (n = 9) | Non-red (n = 10) | Red (n = 6) |
| Litter size | 7.31 ± 2.18 | 7.57 ± 0.98 | 7.11 ± 2.85 | 6.80 ± 1.32 | 8.17 ± 3.13 |
| Litter mass | 21.60 ± 7.29 | 21.91 ± 6.91 | 21.36 ± 7.98 | 19.60 ± 6.08 | 24.93 ± 8.47 |
| Total live mass | 20.04 ± 8.68 | 20.37 ± 9.72 | 19.78 ± 8.38 | 18.14 ± 8.04 | 23.20 ± 9.51 |
| Total live number | 6.31 ± 2.91 | 6.43 ± 2.70 | 6.22 ± 3.23 | 5.50 ± 2.32 | 7.67 ± 3.50 |
| Male (n = 13) | Combined (n = 13) | Non-red(n = 5) | Red (n = 8) | Non-red(n = 1) | Red (n = 12) |
| Litter size | 7.46 ± 4.96 | 7.20 ± 4.82 | 7.63 ± 5.37 | 14.00 | 6.92 ± 4.76 |
| Litter mass | 21.75 ± 13.60 | 21.48 ± 13.59 | 21.93 ± 14.55 | 35.60 | 20.60 ± 13.53 |
| Total live mass | 19.28 ± 12.63 | 18.42 ± 12.11 | 19.83 ± 13.74 | 28.10 | 18.55 ± 12.90 |
| Total live number | 5.85 ± 3.69 | 5.40 ± 3.21 | 6.13 ± 4.16 | 8.00 | 5.67 ± 3.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, X.; Whiting, M.J.; Du, W.; Wu, Z.; Luo, S.; Yue, B.; Fu, J.; Qi, Y. Colour Variation in the Crocodile Lizard (Shinisaurus crocodilurus) and Its Relationship to Individual Quality. Biology 2022, 11, 1314. https://doi.org/10.3390/biology11091314
Qiu X, Whiting MJ, Du W, Wu Z, Luo S, Yue B, Fu J, Qi Y. Colour Variation in the Crocodile Lizard (Shinisaurus crocodilurus) and Its Relationship to Individual Quality. Biology. 2022; 11(9):1314. https://doi.org/10.3390/biology11091314
Chicago/Turabian StyleQiu, Xia, Martin J. Whiting, Weiguo Du, Zhengjun Wu, Shuyi Luo, Bisong Yue, Jinzhong Fu, and Yin Qi. 2022. "Colour Variation in the Crocodile Lizard (Shinisaurus crocodilurus) and Its Relationship to Individual Quality" Biology 11, no. 9: 1314. https://doi.org/10.3390/biology11091314
APA StyleQiu, X., Whiting, M. J., Du, W., Wu, Z., Luo, S., Yue, B., Fu, J., & Qi, Y. (2022). Colour Variation in the Crocodile Lizard (Shinisaurus crocodilurus) and Its Relationship to Individual Quality. Biology, 11(9), 1314. https://doi.org/10.3390/biology11091314





