Autologous Human Mesenchymal Stem Cell-Based Therapy in Infertility: New Strategies and Future Perspectives
Abstract
:Simple Summary
Abstract
1. Introduction
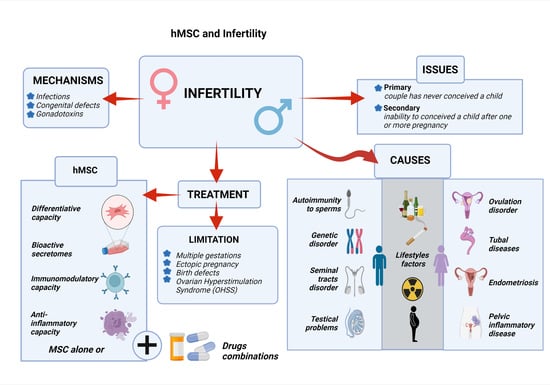
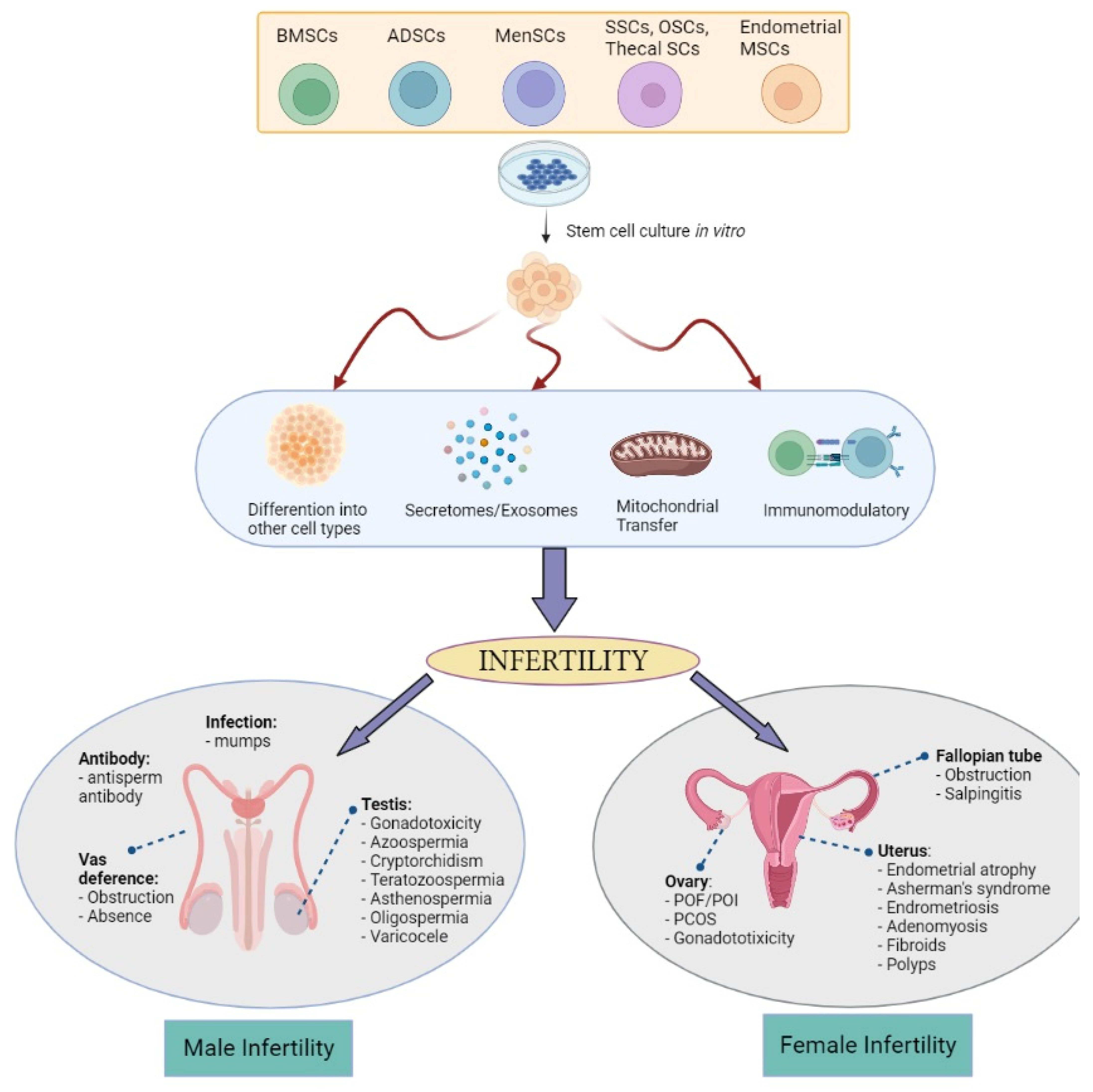
2. Causes and Mechanisms Leading to Infertility
3. Limitations in The Current Conception Treatment
3.1. Multifetal Gestations and The Effects
3.2. Ectopic Pregnancy
3.3. Ovarian Hyperstimulation Syndrome (OHSS)
3.4. Birth Defects
3.5. Cryopreservation of Testes and Ovary
4. MSCs and Their Mechanism in The Treatment of Infertility
5. Potential Usage of MSCs in Infertility
5.1. Preclinical Studies
5.2. Clinical Studies
| Cell Source | Disease | Mode of Treatment | Outcome | Reference/ NCT ID |
|---|---|---|---|---|
| BMSCs | IUA (Asherman’s syndrome) | Transplantation in the endometrial cavity | Restoration of the menstrual cycle | [132] |
| Increased endometrium thickness and good vascularity | [130] | |||
| IUA (Asherman’s syndrome) | Intra-arterial to the uterus |
| [131] | |
| IUA (Asherman’s syndrome) | BMSCs-loaded collagen scaffold |
| [133] | |
| IUA (Asherman’s syndrome) | Transplantation to the uterine cavity | Recovered endometrium | [148] | |
| POI | Laparoscopic instillation into ovaries |
| [149] | |
| Injection into the ovary |
| [128] | ||
| [129] | |||
| NCT03069209 | |||
| NCT02043743 | |||
| NCT02062931 | |||
| Injection via peripheral vein | No results posted (unknown status) | NCT02779374 | ||
| Azoospermia | Injection into rete testis |
| [137] | |
| Azoospermia | Intra-testicular transplantation | No results posted (recruiting) | NCT02641769 | |
| Azoospermia (Klinefelter Syndrome) | Injection into testicular tubules and artery | No results posted (recruiting) | NCT02414295 | |
| Non-obstructive azoospermia | Injection into testis | No results posted (recruiting) | NCT02041910 | |
| Non-obstructive azoospermia | Injection into testis | No results posted (recruiting) | NCT02008799 | |
| Ovarian reserve | Intra-ovarian artery injection |
| [135] | |
| ADSCs | Thin endometrium syndrome | Subendometrial injection |
| [140] |
| IUA (Asherman’s syndrome) | Transcervical instillation |
| [139] | |
| Azoospermia and oligozoospermia | Injection into testis | No results posted (Enrolling by invitation) | NCT03762967 | |
| POI | Intra ovarian transplantation |
| [138] | |
| POI and Ovarian Ageing | Injection into ovary |
| [150] | |
| Post-cancer surgical removal of erectile dysfunction | Single intracavernous injection | Improved erectile function | [141] | |
| MenSCs | IUA (Asherman’s syndrome) | Transplanted into uterus | Regenerating the endometrium, prolonging menstrual duration, and increasing the rate of pregnancy | [142] |
| Transplantation |
| [143] | ||
| Endometrial-MSCs | Thin endometrium syndrome | Submucosal injection |
| [146] |
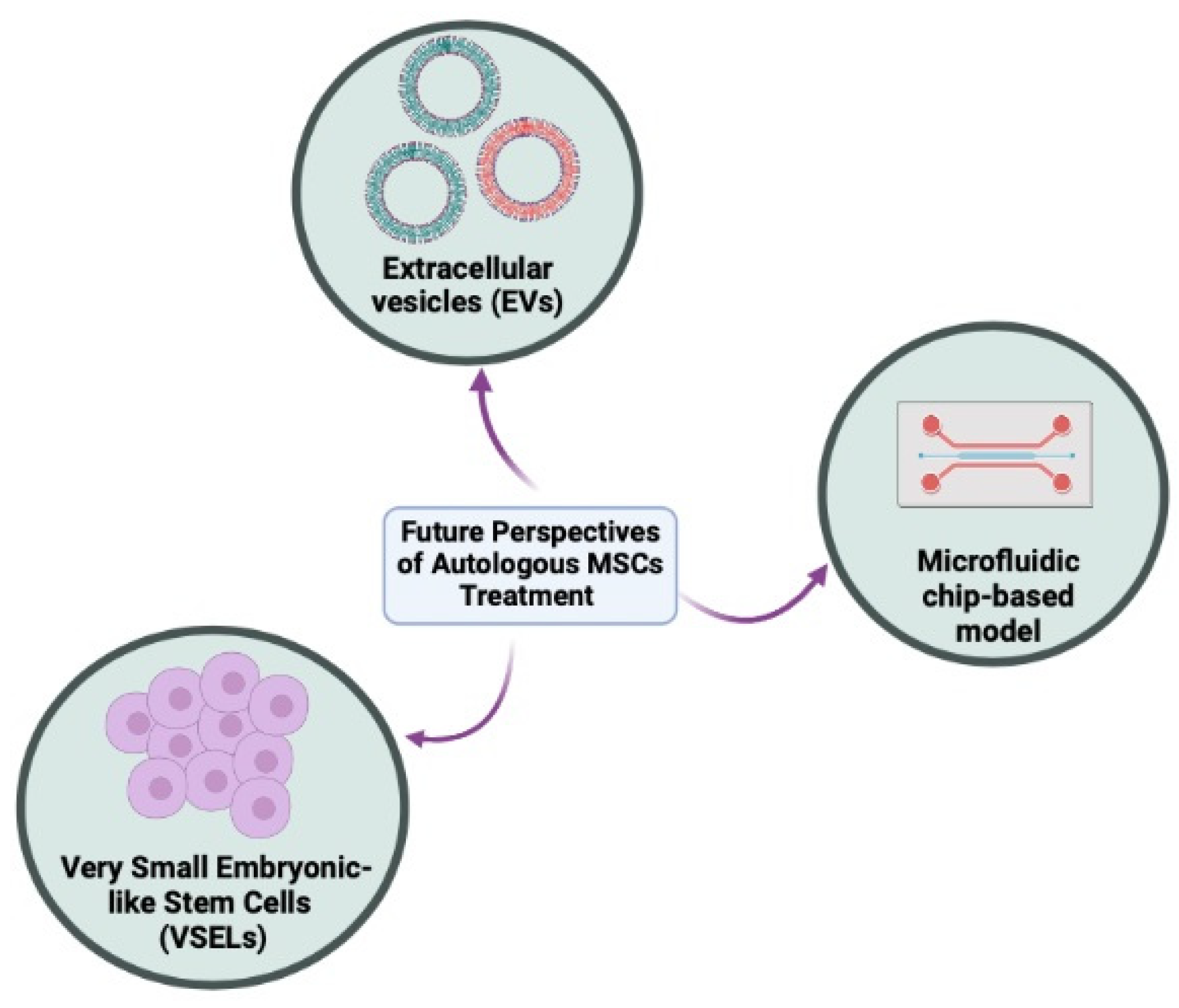
6. New Strategies and Future Perspectives
6.1. Cell-Free Therapy
6.1.1. MSCs-Derived Exosomes/miRNA
6.1.2. ADSCs-Derived Exosomes/miRNA
6.1.3. Menstrual SCs-Derived Exosomes/miRNA
6.2. Very Small Embryonic-like Stem Cells (VSELs)
6.3. Regenerative Therapy
7. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2020, 113, 533–535. [Google Scholar] [CrossRef]
- World Health Organization. Infertility. Available online: https://www.who.int/health-topics/infertility#tab=tab_1 (accessed on 14 April 2022).
- Stevenson, E.L.; Hershberger, P.E.; Bergh, P.A. Evidence-Based Care for Couples with Infertility. J. Obstet. Gynecol. Neonatal. Nurs. 2016, 45, 100–110; quiz e101–e102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayernia, K.; Lee, J.H.; Drusenheimer, N.; Nolte, J.; Wulf, G.; Dressel, R.; Gromoll, J.; Engel, W. Derivation of male germ cells from bone marrow stem cells. Lab. Investig. 2006, 86, 654–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemzadeh-Hasankolaei, M.; Sedighi-Gilani, M.A.; Eslaminejad, M.B. Induction of ram bone marrow mesenchymal stem cells into germ cell lineage using transforming growth factor-beta superfamily growth factors. Reprod. Domest. Anim. 2014, 49, 588–598. [Google Scholar] [CrossRef]
- Cakici, C.; Buyrukcu, B.; Duruksu, G.; Haliloglu, A.H.; Aksoy, A.; Isik, A.; Uludag, O.; Ustun, H.; Subasi, C.; Karaoz, E. Recovery of fertility in azoospermia rats after injection of adipose-tissue-derived mesenchymal stem cells: The sperm generation. Biomed. Res. Int. 2013, 2013, 529589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamadon, A.; Mehrabani, D.; Rahmanifar, F.; Jahromi, A.R.; Panahi, M.; Zare, S.; Khodabandeh, Z.; Jahromi, I.R.; Tanideh, N.; Dianatpour, M.; et al. Induction of Spermatogenesis by Bone Marrow-derived Mesenchymal Stem Cells in Busulfan-induced Azoospermia in Hamster. Int. J. Stem Cells 2015, 8, 134–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, Y.; Liu, X. Mesenchymal stem cells to treat female infertility; future perspective and challenges: A review. Int. J. Reprod. Biomed. 2022, 20, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Wood, A. Ethics and embryonic stem cell research. Stem Cell Rev. 2005, 1, 317–324. [Google Scholar] [CrossRef]
- Hyun, I. The bioethics of stem cell research and therapy. J. Clin. Investig. 2010, 120, 71–75. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transpl. 2014, 23, 1045–1059. [Google Scholar] [CrossRef]
- Fazeli, Z.; Abedindo, A.; Omrani, M.D.; Ghaderian, S.M.H. Mesenchymal Stem Cells (MSCs) Therapy for Recovery of Fertility: A Systematic Review. Stem Cell Rev. Rep. 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Saha, S.; Roy, P.; Corbitt, C.; Kakar, S.S. Application of Stem Cell Therapy for Infertility. Cells 2021, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Pizzol, D.; Carosso, A.R.; Borini, A.; Ubaldi, F.M.; Calogero, A.E.; Ferlin, A.; Lanzone, A.; Tomei, F.; Engl, B.; et al. Practical Clinical and Diagnostic Pathway for the Investigation of the Infertile Couple. Front. Endocrinol. 2020, 11, 591837. [Google Scholar] [CrossRef] [PubMed]
- Gelbaya, T.A.; Potdar, N.; Jeve, Y.B.; Nardo, L.G. Definition and epidemiology of unexplained infertility. Obstet. Gynecol. Surv. 2014, 69, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, M.; Juul, A.; Feldt-Rasmussen, U.; Jorgensen, N. Semen quality in patients with pituitary disease and adult-onset hypogonadotropic hypogonadism. Endocr. Connect. 2018, 7, 523–533. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Zhao, F.; Wang, Q.; Liu, C.; Lan, Y.; Wang, S.; Xin, Z.; Yang, X. Salpingectomy may decrease antral follicle count but not live birth rate for IVF-ET patients aged 35–39 years: A retrospective study. J. Ovarian Res. 2020, 13, 80. [Google Scholar] [CrossRef]
- Dreisler, E.; Kjer, J.J. Asherman’s syndrome: Current perspectives on diagnosis and management. Int. J. Womens Health 2019, 11, 191–198. [Google Scholar] [CrossRef]
- Egbe, T.O.; Nana-Njamen, T.; Elong, F.; Tchounzou, R.; Simo, A.G.; Nzeuga, G.P.; Njamen Nana, C.; Manka’a, E.; Tchente Nguefack, C.; Halle-Ekane, G.E. Risk factors of tubal infertility in a tertiary hospital in a low-resource setting: A case-control study. Fertil. Res. Pract. 2020, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Tao, X.; Ge, S.Q.; Chen, L.; Cai, L.S.; Hwang, M.F.; Wang, C.L. Relationships between female infertility and female genital infections and pelvic inflammatory disease: A population-based nested controlled study. Clinics 2018, 73, e364. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Sun, X.; Liu, J.; Zheng, H.; Yang, B.; Tang, W.; Wang, C. Chlamydia infection, PID, and infertility: Further evidence from a case-control study in China. BMC Womens Health 2022, 22, 294. [Google Scholar] [CrossRef]
- Aitken, R.J. COVID-19 and human spermatozoa-Potential risks for infertility and sexual transmission? Andrology 2021, 9, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Dukhovny, S.; Wilkins-Haug, L. Genetic Basis of Female Infertility. In Clinical Genomics: Practical Applications in Adult Patient Care; Murray, M.F., Babyatsky, M.W., Giovanni, M.A., Alkuraya, F.S., Stewart, D.R., Eds.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Wikstrom, A.M.; Hoei-Hansen, C.E.; Dunkel, L.; Rajpert-De Meyts, E. Immunoexpression of androgen receptor and nine markers of maturation in the testes of adolescent boys with Klinefelter syndrome: Evidence for degeneration of germ cells at the onset of meiosis. J. Clin. Endocrinol. Metab. 2007, 92, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Poganitsch-Korhonen, M.; Masliukaite, I.; Nurmio, M.; Lahteenmaki, P.; van Wely, M.; van Pelt, A.M.M.; Jahnukainen, K.; Stukenborg, J.B. Decreased spermatogonial quantity in prepubertal boys with leukaemia treated with alkylating agents. Leukemia 2017, 31, 1460–1463. [Google Scholar] [CrossRef]
- Bar-Shira Maymon, B.; Yogev, L.; Marks, A.; Hauser, R.; Botchan, A.; Yavetz, H. Sertoli cell inactivation by cytotoxic damage to the human testis after cancer chemotherapy. Fertil. Steril. 2004, 81, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.; Bath, L.E.; Wallace, W.H. Radiation damage to the uterus -- review of the effects of treatment of childhood cancer. Hum. Fertil. 2002, 5, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Sklar, C.A.; Mertens, A.C.; Mitby, P.; Whitton, J.; Stovall, M.; Kasper, C.; Mulder, J.; Green, D.; Nicholson, H.S.; Yasui, Y.; et al. Premature menopause in survivors of childhood cancer: A report from the childhood cancer survivor study. J. Natl. Cancer Inst. 2006, 98, 890–896. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Balasundaram, P. Ovulation Induction Techniques. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kroese, A.C.; de Lange, N.M.; Collins, J.; Evers, J.L. Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst. Rev. 2012, 10, CD000479. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.F.; Chen, B.; Liu, W.; Huang, Y.P.; Wang, H.X.; Huang, Y.R.; Ping, P. Microsurgical vasoepididymostomy for patients with infectious obstructive azoospermia: Cause, outcome, and associated factors. Asian J. Androl. 2016, 18, 759–762. [Google Scholar] [CrossRef]
- Rives, N.; Courbiere, B.; Almont, T.; Kassab, D.; Berger, C.; Grynberg, M.; Papaxanthos, A.; Decanter, C.; Elefant, E.; Dhedin, N.; et al. What should be done in terms of fertility preservation for patients with cancer? The French 2021 guidelines. Eur. J. Cancer 2022, 173, 146–166. [Google Scholar] [CrossRef]
- Sunderam, S.; Kissin, D.M.; Zhang, Y.; Folger, S.G.; Boulet, S.L.; Warner, L.; Callaghan, W.M.; Barfield, W.D. Assisted Reproductive Technology Surveillance—United States, 2016. MMWR Surveill. Summ. 2019, 68, 1–23. [Google Scholar] [CrossRef]
- McClamrock, H.D.; Jones, H.W., Jr.; Adashi, E.Y. Ovarian stimulation and intrauterine insemination at the quarter centennial: Implications for the multiple births epidemic. Fertil. Steril. 2012, 97, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Kurinczuk, J.J.; Bower, C.; Webb, S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N. Engl. J. Med. 2002, 346, 725–730. [Google Scholar] [CrossRef] [Green Version]
- Wisborg, K.; Ingerslev, H.J.; Henriksen, T.B. IVF and stillbirth: A prospective follow-up study. Hum. Reprod. 2010, 25, 1312–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahu, K.; Allvee, K.; Karro, H.; Rahu, M. Singleton pregnancies after in vitro fertilization in Estonia: A register-based study of complications and adverse outcomes in relation to the maternal socio-demographic background. BMC Pregnancy Childbirth 2019, 19, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE); Wyns, C.; Bergh, C.; Calhaz-Jorge, C.; De Geyter, C.; Kupka, M.S.; Motrenko, T.; Rugescu, I.; Smeenk, J. ART in Europe, 2016: Results generated from European registries by ESHRE. Hum. Reprod. Open 2020, 2020, hoaa032. [Google Scholar] [CrossRef]
- von Wolff, M.; Haaf, T. In Vitro Fertilization Technology and Child Health. Dtsch. Arztebl. Int. 2020, 117, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.D.; Neri, Q.V.; Takeuchi, T.; Rosenwaks, Z. ICSI: Where we have been and where we are going. Semin. Reprod. Med. 2009, 27, 191–201. [Google Scholar] [CrossRef]
- Assisted reproductive technology in the United States: 1996 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil. Steril. 1999, 71, 798–807. [CrossRef]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gulmezoglu, A.M.; Van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Clayton, H.B.; Schieve, L.A.; Peterson, H.B.; Jamieson, D.J.; Reynolds, M.A.; Wright, V.C. Ectopic pregnancy risk with assisted reproductive technology procedures. Obstet Gynecol. 2006, 107, 595–604. [Google Scholar] [CrossRef]
- Li, C.; Zhao, W.H.; Zhu, Q.; Cao, S.J.; Ping, H.; Xi, X.; Qin, G.J.; Yan, M.X.; Zhang, D.; Qiu, J.; et al. Risk factors for ectopic pregnancy: A multi-center case-control study. BMC Pregnancy Childbirth 2015, 15, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubuisson, J.B.; Aubriot, F.X.; Mathieu, L.; Foulot, H.; Mandelbrot, L.; de Joliere, J.B. Risk factors for ectopic pregnancy in 556 pregnancies after in vitro fertilization: Implications for preventive management. Fertil. Steril. 1991, 56, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Audebert, A.; Pouly, J.L.; Bonifacie, B.; Yazbeck, C. Laparoscopic surgery for distal tubal occlusions: Lessons learned from a historical series of 434 cases. Fertil. Steril. 2014, 102, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Jia-Rong, Z.; Shuang-Di, L.; Xiao-Ping, W. Eutopic or ectopic pregnancy: A competition between signals derived from the endometrium and the fallopian tube for blastocyst implantation. Placenta 2009, 30, 835–839. [Google Scholar] [CrossRef]
- Shao, R.; Nutu, M.; Weijdegard, B.; Egecioglu, E.; Fernandez-Rodriguez, J.; Karlsson-Lindahl, L.; Gemzell-Danielsson, K.; Bergh, C.; Billig, H. Clomiphene citrate causes aberrant tubal apoptosis and estrogen receptor activation in rat fallopian tube: Implications for tubal ectopic pregnancy. Biol. Reprod. 2009, 80, 1262–1271. [Google Scholar] [CrossRef]
- Lekovich, J.; Witkin, S.S.; Doulaveris, G.; Orfanelli, T.; Shulman, B.; Pereira, N.; Rosenwaks, Z.; Spandorfer, S.D. Elevated serum interleukin-1beta levels and interleukin-1beta-to-interleukin-1 receptor antagonist ratio 1 week after embryo transfer are associated with ectopic pregnancy. Fertil. Steril. 2015, 104, 1190–1194. [Google Scholar] [CrossRef]
- Papanikolaou, E.G.; Pozzobon, C.; Kolibianakis, E.M.; Camus, M.; Tournaye, H.; Fatemi, H.M.; Van Steirteghem, A.; Devroey, P. Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil. Steril. 2006, 85, 112–120. [Google Scholar] [CrossRef]
- Griesinger, G.; Diedrich, K.; Devroey, P.; Kolibianakis, E.M. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: A systematic review and meta-analysis. Hum. Reprod. Update 2006, 12, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Palermo, G.D.; Schlegel, P.N.; Hariprashad, J.J.; Ergun, B.; Mielnik, A.; Zaninovic, N.; Veeck, L.L.; Rosenwaks, Z. Fertilization and pregnancy outcome with intracytoplasmic sperm injection for azoospermic men. Hum. Reprod. 1999, 14, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Ponjaert-Kristoffersen, I.; Bonduelle, M.; Barnes, J.; Nekkebroeck, J.; Loft, A.; Wennerholm, U.B.; Tarlatzis, B.C.; Peters, C.; Hagberg, B.S.; Berner, A.; et al. International collaborative study of intracytoplasmic sperm injection-conceived, in vitro fertilization-conceived, and naturally conceived 5-year-old child outcomes: Cognitive and motor assessments. Pediatrics 2005, 115, e283–e289. [Google Scholar] [CrossRef]
- Woldringh, G.H.; Horvers, M.; Janssen, A.J.; Reuser, J.J.; de Groot, S.A.; Steiner, K.; D’Hauwers, K.W.; Wetzels, A.M.; Kremer, J.A. Follow-up of children born after ICSI with epididymal spermatozoa. Hum. Reprod. 2011, 26, 1759–1767. [Google Scholar] [CrossRef] [Green Version]
- Abel, K.; Healey, M.; Finch, S.; Osianlis, T.; Vollenhoven, B. Associations between embryo grading and congenital malformations in IVF/ICSI pregnancies. Reprod. Biomed. Online 2019, 39, 981–989. [Google Scholar] [CrossRef]
- Lv, H.; Diao, F.; Du, J.; Chen, T.; Meng, Q.; Ling, X.; Li, H.; Song, C.; Xi, Q.; Jiang, Y.; et al. Assisted reproductive technology and birth defects in a Chinese birth cohort study. Lancet Reg. Health West Pac. 2021, 7, 100090. [Google Scholar] [CrossRef]
- El-Chaar, D.; Yang, Q.; Gao, J.; Bottomley, J.; Leader, A.; Wen, S.W.; Walker, M. Risk of birth defects increased in pregnancies conceived by assisted human reproduction. Fertil. Steril. 2009, 92, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Bonduelle, M.; Van Assche, E.; Joris, H.; Keymolen, K.; Devroey, P.; Van Steirteghem, A.; Liebaers, I. Prenatal testing in ICSI pregnancies: Incidence of chromosomal anomalies in 1586 karyotypes and relation to sperm parameters. Hum. Reprod. 2002, 17, 2600–2614. [Google Scholar] [CrossRef] [Green Version]
- Braye, A.; Tournaye, H.; Goossens, E. Setting Up a Cryopreservation Programme for Immature Testicular Tissue: Lessons Learned After More Than 15 Years of Experience. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119886342. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Lee, S.; Kim, T. Ovarian tissue cryopreservation and transplantation in patients with cancer. Obstet Gynecol. Sci. 2018, 61, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Nottola, S.A.; Camboni, A.; Van Langendonckt, A.; Demylle, D.; Macchiarelli, G.; Dolmans, M.M.; Martinez-Madrid, B.; Correr, S.; Donnez, J. Cryopreservation and xenotransplantation of human ovarian tissue: An ultrastructural study. Fertil. Steril. 2008, 90, 23–32. [Google Scholar] [CrossRef]
- Cheng, H.; Ye, X.; Zhu, H.; Li, Y.; Xudong, l.; Chang, X.; Cui, H. An experimental study on autologous transplantation of fresh ovarian tissue in sheep. Gynecol. Obstet. Clin. Med. 2021, 1, 87–93. [Google Scholar] [CrossRef]
- Blum, B.; Benvenisty, N. The tumorigenicity of human embryonic stem cells. Adv. Cancer Res. 2008, 100, 133–158. [Google Scholar] [CrossRef]
- Dezawa, M.; Ishikawa, H.; Itokazu, Y.; Yoshihara, T.; Hoshino, M.; Takeda, S.; Ide, C.; Nabeshima, Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 2005, 309, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.K.; Thiemermann, C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells 2010, 28, 585–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orciani, M.; Caffarini, M.; Lazzarini, R.; Delli Carpini, G.; Tsiroglou, D.; Di Primio, R.; Ciavattini, A. Mesenchymal Stem Cells from Cervix and Age: New Insights into CIN Regression Rate. Oxid. Med. Cell. Longev. 2018, 2018, 1545784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Qu, J.; Xiang, C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res. Ther. 2019, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, P.; Lombardi, F.; Siragusa, G.; Cifone, M.G.; Cinque, B.; Giuliani, M. Methods of Isolation, Characterization and Expansion of Human Adipose-Derived Stem Cells (ASCs): An Overview. Int. J. Mol. Sci. 2018, 19, 1897. [Google Scholar] [CrossRef] [Green Version]
- Lee, O.K.; Kuo, T.K.; Chen, W.M.; Lee, K.D.; Hsieh, S.L.; Chen, T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004, 103, 1669–1675. [Google Scholar] [CrossRef] [Green Version]
- Amati, E.; Sella, S.; Perbellini, O.; Alghisi, A.; Bernardi, M.; Chieregato, K.; Lievore, C.; Peserico, D.; Rigno, M.; Zilio, A.; et al. Generation of mesenchymal stromal cells from cord blood: Evaluation of in vitro quality parameters prior to clinical use. Stem Cell Res. Ther. 2017, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Roberts, E.G.; Piekarski, B.L.; Huang, K.; Emani, S.; Wong, J.Y.; Emani, S.M. Evaluation of Placental Mesenchymal Stem Cell Sheets for Myocardial Repair and Regeneration. Tissue Eng. Part A 2019, 25, 867–877. [Google Scholar] [CrossRef] [Green Version]
- In‘t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.; Claas, F.H.; Fibbe, W.E.; Kanhai, H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- O’Donoghue, K.; Fisk, N.M. Fetal stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 853–875. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Brinster, R.L. Spermatogonial stem cells. Biol. Reprod. 2018, 99, 52–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhartiya, D.; Sharma, D. Ovary does harbor stem cells—Size of the cells matter! J. Ovarian Res. 2020, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Rungsiwiwut, R.; Virutamasen, P.; Pruksananonda, K. Mesenchymal stem cells for restoring endometrial function: An infertility perspective. Reprod. Med. Biol. 2021, 20, 13–19. [Google Scholar] [CrossRef]
- Xin, L.; Lin, X.; Pan, Y.; Zheng, X.; Shi, L.; Zhang, Y.; Ma, L.; Gao, C.; Zhang, S. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater. 2019, 92, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ding, L.; Wang, L.; Cao, Y.; Zhu, H.; Lu, J.; Li, X.; Song, T.; Hu, Y.; Dai, J. Umbilical cord-derived mesenchymal stem cells on scaffolds facilitate collagen degradation via upregulation of MMP-9 in rat uterine scars. Stem Cell Res. Ther. 2017, 8, 84. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.X.; Chen, S.R.; Su, P.P.; Huang, F.H.; Shi, Y.C.; Shi, Q.Y.; Lin, S. Using Mesenchymal Stem Cells to Treat Female Infertility: An Update on Female Reproductive Diseases. Stem Cells Int. 2019, 2019, 9071720. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.; Zou, Q.; Wang, F.; Wu, H.; Wang, W.; Li, H.; Huang, B. HGF and BFGF Secretion by Human Adipose-Derived Stem Cells Improves Ovarian Function During Natural Aging via Activation of the SIRT1/FOXO1 Signaling Pathway. Cell. Physiol. Biochem. 2018, 45, 1316–1332. [Google Scholar] [CrossRef] [Green Version]
- Sagaradze, G.D.; Basalova, N.A.; Efimenko, A.Y.; Tkachuk, V.A. Mesenchymal Stromal Cells as Critical Contributors to Tissue Regeneration. Front. Cell. Dev. Biol. 2020, 8, 576176. [Google Scholar] [CrossRef]
- Feng, X.; Ling, L.; Zhang, W.; Liu, X.; Wang, Y.; Luo, Y.; Xiong, Z. Effects of Human Amnion-Derived Mesenchymal Stem Cell (hAD-MSC) Transplantation In Situ on Primary Ovarian Insufficiency in SD Rats. Reprod. Sci. 2020, 27, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Feng, X.; Wei, T.; Wang, Y.; Wang, Y.; Wang, Z.; Tang, D.; Luo, Y.; Xiong, Z. Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism. Stem Cell Res. Ther. 2019, 10, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervello, I.; Gil-Sanchis, C.; Santamaria, X.; Cabanillas, S.; Diaz, A.; Faus, A.; Pellicer, A.; Simon, C. Human CD133(+) bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil. Steril. 2015, 104, 1552–1560.e1551–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilic, S.; Yuksel, B.; Pinarli, F.; Albayrak, A.; Boztok, B.; Delibasi, T. Effect of stem cell application on Asherman syndrome, an experimental rat model. J. Assist. Reprod. Genet. 2014, 31, 975–982. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, R.C.; Xiao, E.; Husami, N.; Sauer, M.V.; Lobo, R.; Kitajewski, J.; Ferin, M. Short-term administration of antivascular endothelial growth factor antibody in the late follicular phase delays follicular development in the rhesus monkey. J. Clin. Endocrinol. Metab. 2001, 86, 768–772. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.; Kim, T.H.; Seok, J.; Jun, J.H.; Park, H.; Kweon, M.; Lim, J.Y.; Kim, G.J. Vascular remodeling by placenta-derived mesenchymal stem cells restores ovarian function in ovariectomized rat model via the VEGF pathway. Lab. Investig. 2021, 101, 304–317. [Google Scholar] [CrossRef]
- Sun, B.; Ma, Y.; Wang, F.; Hu, L.; Sun, Y. miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res. Ther. 2019, 10, 360. [Google Scholar] [CrossRef]
- Yang, M.; Lin, L.; Sha, C.; Li, T.; Zhao, D.; Wei, H.; Chen, Q.; Liu, Y.; Chen, X.; Xu, W.; et al. Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab. Investig. 2020, 100, 342–352. [Google Scholar] [CrossRef]
- Deng, C.; Xie, Y.; Zhang, C.; Ouyang, B.; Chen, H.; Lv, L.; Yao, J.; Liang, X.; Zhang, Y.; Sun, X.; et al. Urine-Derived Stem Cells Facilitate Endogenous Spermatogenesis Restoration of Busulfan-Induced Nonobstructive Azoospermic Mice by Paracrine Exosomes. Stem Cells Dev. 2019, 28, 1322–1333. [Google Scholar] [CrossRef]
- Gangaraju, V.K.; Lin, H. MicroRNAs: Key regulators of stem cells. Nat. Rev. Mol. Cell Biol. 2009, 10, 116–125. [Google Scholar] [CrossRef]
- Kim, K.; Kenigsberg, S.; Jurisicova, A.; Bentov, Y. The Role of Mitochondria in Oocyte and Early Embryo Health. OBM Genet. 2019, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Varela, C.; Labarta, E. Role of Mitochondria Transfer in Infertility: A Commentary. Cells 2022, 11, 1867. [Google Scholar] [CrossRef] [PubMed]
- Costa-Borges, N.; Nikitos, E.; Spath, K.; Rink, K.; Kostaras, K.; Zervomanolakis, I.; Kontopoulos, G.; Polyzos, P.; Grigorakis, S.; Prokopakis, T.; et al. First registered pilot trial to validate the safety and effectiveness of maternal spindle transfer to overcome infertility associated with poor oocyte quality. Fertil. Steril. 2020, 114, E71–E72. [Google Scholar] [CrossRef]
- Zhang, H.; Panula, S.; Petropoulos, S.; Edsgard, D.; Busayavalasa, K.; Liu, L.; Li, X.; Risal, S.; Shen, Y.; Shao, J.; et al. Adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat. Med. 2015, 21, 1116–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.B.; Hao, J.X.; Meng, T.G.; Guo, L.; Dong, M.Z.; Fan, L.H.; Ouyang, Y.C.; Wang, G.; Sun, Q.Y.; Ou, X.H.; et al. Transfer of autologous mitochondria from adipose tissue-derived stem cells rescues oocyte quality and infertility in aged mice. Aging 2017, 9, 2480–2488. [Google Scholar] [CrossRef] [Green Version]
- Sheng, X.; Yang, Y.; Zhou, J.; Yan, G.; Liu, M.; Xu, L.; Li, Z.; Jiang, R.; Diao, Z.; Zhen, X.; et al. Mitochondrial transfer from aged adipose-derived stem cells does not improve the quality of aged oocytes in C57BL/6 mice. Mol. Reprod. Dev. 2019, 86, 516–529. [Google Scholar] [CrossRef]
- Gong, D.; Zhang, C.; Li, T.; Zhang, J.; Zhang, N.; Tao, Z.; Zhu, W.; Sun, X. Are Sertoli cells a kind of mesenchymal stem cells? Am. J. Transl. Res. 2017, 9, 1067–1074. [Google Scholar]
- Porubska, B.; Vasek, D.; Somova, V.; Hajkova, M.; Hlaviznova, M.; Tlapakova, T.; Holan, V.; Krulova, M. Sertoli Cells Possess Immunomodulatory Properties and the Ability of Mitochondrial Transfer Similar to Mesenchymal Stromal Cells. Stem Cell Rev. Rep. 2021, 17, 1905–1916. [Google Scholar] [CrossRef]
- Li, C.; Cheung, M.K.H.; Han, S.; Zhang, Z.; Chen, L.; Chen, J.; Zeng, H.; Qiu, J. Mesenchymal stem cells and their mitochondrial transfer: A double-edged sword. Biosci. Rep. 2019, 39, BSR20182417. [Google Scholar] [CrossRef] [Green Version]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef]
- Xie, M.; Xiong, W.; She, Z.; Wen, Z.; Abdirahman, A.S.; Wan, W.; Wen, C. Immunoregulatory Effects of Stem Cell-Derived Extracellular Vesicles on Immune Cells. Front. Immunol. 2020, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, L.; Xu, W.; Li, X.; Meng, W.; Hu, L.; Luo, Z.; Wang, Y.; Luo, S.; Li, S. Differential Expression Profile of Immunological Cytokines in Local Ovary in Patients with Polycystic Ovarian Syndrome: Analysis by Flow Cytometry. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 197, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Su, N.J.; Ma, J.; Feng, D.F.; Zhou, S.; Li, Z.T.; Zhou, W.P.; Deng, H.; Liang, J.Y.; Yang, X.H.; Zhang, Y.M.; et al. The peripheral blood transcriptome identifies dysregulation of inflammatory response genes in polycystic ovary syndrome. Gynecol. Endocrinol. 2018, 34, 584–588. [Google Scholar] [CrossRef]
- Xie, Q.; Xiong, X.; Xiao, N.; He, K.; Chen, M.; Peng, J.; Su, X.; Mei, H.; Dai, Y.; Wei, D.; et al. Mesenchymal Stem Cells Alleviate DHEA-Induced Polycystic Ovary Syndrome (PCOS) by Inhibiting Inflammation in Mice. Stem Cells Int. 2019, 2019, 9782373. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, Z.; Chen, X.; Zhou, J.; Xiao, X.M. Treatment evaluation of Wharton’s jelly-derived mesenchymal stem cells using a chronic salpingitis model: An animal experiment. Stem Cell Res. Ther. 2017, 8, 232. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Tang, X.; Li, X.; Li, T. Therapeutic effect of human umbilical cord mesenchymal stem cells on tubal factor infertility using a chronic salpingitis murine model. Arch. Gynecol. Obstet. 2019, 300, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Volkova, N.; Yukhta, M.; Goltsev, A. Mesenchymal Stem Cells in Restoration of Fertility at Experimental Pelvic Inflammatory Disease. Stem Cells Int. 2017, 2017, 2014132. [Google Scholar] [CrossRef] [Green Version]
- Sreenivasa, G.; Kavitha, P.; Chaithra, P.; Vineeth, V.; Kumar, C.S.; Malini, S.S. Clinical Significance of Antisperm Antibody Analysis in Evaluating Male Infertility of South Karnataka. Bioscan 2011, 6, 125–128. [Google Scholar]
- Aghamir, S.M.; Salavati, A.; Yousefie, R.; Tootian, Z.; Ghazaleh, N.; Jamali, M.; Azimi, P. Does bone marrow-derived mesenchymal stem cell transfusion prevent antisperm antibody production after traumatic testis rupture? Urology 2014, 84, 82–86. [Google Scholar] [CrossRef]
- Hajizadeh Maleki, B.; Tartibian, B. COVID-19 and male reproductive function: A prospective, longitudinal cohort study. Reproduction 2021, 161, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Ozveri, H.; Eren, M.T.; Kirisoglu, C.E.; Sariguzel, N. Atypical presentation of SARS-CoV-2 infection in male genitalia. Urol. Case Rep. 2020, 33, 101349. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention What is Assisted Reproductive Technology? Available online: https://www.cdc.gov/art/whatis.html (accessed on 3 September 2022).
- Hwang, J.W.; Lee, N.K.; Yang, J.H.; Son, H.J.; Bang, S.I.; Chang, J.W.; Na, D.L. A Comparison of Immune Responses Exerted Following Syngeneic, Allogeneic, and Xenogeneic Transplantation of Mesenchymal Stem Cells into the Mouse Brain. Int. J. Mol. Sci. 2020, 21, 3052. [Google Scholar] [CrossRef] [PubMed]
- Joswig, A.J.; Mitchell, A.; Cummings, K.J.; Levine, G.J.; Gregory, C.A.; Smith, R., 3rd; Watts, A.E. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res. Ther. 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berglund, A.K.; Fortier, L.A.; Antczak, D.F.; Schnabel, L.V. Immunoprivileged no more: Measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 288. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.P.; Sun, Z.; Miyagi, Y.; McDonald Kinkaid, H.; Zhang, L.; Weisel, R.D.; Li, R.K. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation 2010, 122, 2419–2429. [Google Scholar] [CrossRef] [Green Version]
- Lohan, P.; Treacy, O.; Griffin, M.D.; Ritter, T.; Ryan, A.E. Anti-Donor Immune Responses Elicited by Allogeneic Mesenchymal Stem Cells and Their Extracellular Vesicles: Are We Still Learning? Front. Immunol. 2017, 8, 1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderi, R.; Makdissy, N.; Azar, A.; Rizk, F.; Hamade, A. Cellular therapy with human autologous adipose-derived adult cells of stromal vascular fraction for alopecia areata. Stem Cell Res. Ther. 2018, 9, 141. [Google Scholar] [CrossRef]
- Izadyar, F.; Den Ouden, K.; Stout, T.A.; Stout, J.; Coret, J.; Lankveld, D.P.; Spoormakers, T.J.; Colenbrander, B.; Oldenbroek, J.K.; Van der Ploeg, K.D.; et al. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction 2003, 126, 765–774. [Google Scholar] [CrossRef]
- Jahnukainen, K.; Ehmcke, J.; Quader, M.A.; Saiful Huq, M.; Epperly, M.W.; Hergenrother, S.; Nurmio, M.; Schlatt, S. Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation. Hum. Reprod. 2011, 26, 1945–1954. [Google Scholar] [CrossRef]
- Hermann, B.P.; Sukhwani, M.; Winkler, F.; Pascarella, J.N.; Peters, K.A.; Sheng, Y.; Valli, H.; Rodriguez, M.; Ezzelarab, M.; Dargo, G.; et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell 2012, 11, 715–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.Y.; Chen, S.R.; Zhao, Y.X.; Chen, J.M.; Chen, W.H.; Lin, S.; Shi, Q.Y. Melatonin enhances autologous adipose-derived stem cells to improve mouse ovarian function in relation to the SIRT6/NF-kappaB pathway. Stem Cell Res. Ther. 2022, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh-Hasankolaei, M.; Batavani, R.; Eslaminejad, M.B.; Sayahpour, F. Transplantation of Autologous Bone Marrow Mesenchymal Stem Cells into the Testes of Infertile Male Rats and New Germ Cell Formation. Int. J. Stem Cells 2016, 9, 250–263. [Google Scholar] [CrossRef] [Green Version]
- Edessy, M.; Hosni, H.; Shady, Y.; Waf, Y.; Bakr, S.; Kamel, M. Autologous stem cells therapy, The first baby of idiopathic premature ovarian failure. Acta Med. Int. 2016, 3, 19–23. [Google Scholar] [CrossRef]
- Igboeli, P.; El Andaloussi, A.; Sheikh, U.; Takala, H.; ElSharoud, A.; McHugh, A.; Gavrilova-Jordan, L.; Levy, S.; Al-Hendy, A. Intraovarian injection of autologous human mesenchymal stem cells increases estrogen production and reduces menopausal symptoms in women with premature ovarian failure: Two case reports and a review of the literature. J. Med. Case Rep. 2020, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Nagori, C.B.; Panchal, S.Y.; Patel, H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman’s syndrome. J. Hum. Reprod. Sci. 2011, 4, 43–48. [Google Scholar] [CrossRef]
- Santamaria, X.; Cabanillas, S.; Cervello, I.; Arbona, C.; Raga, F.; Ferro, J.; Palmero, J.; Remohi, J.; Pellicer, A.; Simon, C. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: A pilot cohort study. Hum. Reprod. 2016, 31, 1087–1096. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Mohanty, S.; Seth, T.; Shankar, M.; Bhaskaran, S.; Dharmendra, S. Autologous stem cell transplantation in refractory Asherman’s syndrome: A novel cell based therapy. J. Hum. Reprod. Sci. 2014, 7, 93–98. [Google Scholar] [CrossRef]
- Zhao, G.; Cao, Y.; Zhu, X.; Tang, X.; Ding, L.; Sun, H.; Li, J.; Li, X.; Dai, C.; Ru, T.; et al. Transplantation of collagen scaffold with autologous bone marrow mononuclear cells promotes functional endometrium reconstruction via downregulating DeltaNp63 expression in Asherman’s syndrome. Sci. China Life Sci. 2017, 60, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Tao, M.; Wei, M.; Du, S.; Wang, H.; Wang, X. Mesenchymal stem cells derived exosomal miR-323-3p promotes proliferation and inhibits apoptosis of cumulus cells in polycystic ovary syndrome (PCOS). Artif. Cells Nanomed. Biotechnol. 2019, 47, 3804–3813. [Google Scholar] [CrossRef]
- Herraiz, S.; Romeu, M.; Buigues, A.; Martinez, S.; Diaz-Garcia, C.; Gomez-Segui, I.; Martinez, J.; Pellicer, N.; Pellicer, A. Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil. Steril. 2018, 110, 496–505.e491. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, D.; Yang, L.; Hou, Q.; Ma, H.; Xu, X. The therapeutic potential of bone marrow mesenchymal stem cells in premature ovarian failure. Stem Cell Res. Ther. 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Gabr, H.; Elkheir, W.A. Autologous MSC therapy for azoospermia: A pilot clinical. Cytotherapy 2015, 17, S51. [Google Scholar] [CrossRef]
- Mashayekhi, M.; Mirzadeh, E.; Chekini, Z.; Ahmadi, F.; Eftekhari-Yazdi, P.; Vesali, S.; Madani, T.; Aghdami, N. Evaluation of safety, feasibility and efficacy of intra-ovarian transplantation of autologous adipose derived mesenchymal stromal cells in idiopathic premature ovarian failure patients: Non-randomized clinical trial, phase I, first in human. J. Ovarian Res. 2021, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Shin, J.E.; Kwon, H.; Choi, D.H.; Kim, J.H. Effect of Autologous Adipose-Derived Stromal Vascular Fraction Transplantation on Endometrial Regeneration in Patients of Asherman’s Syndrome: A Pilot Study. Reprod. Sci. 2020, 27, 561–568. [Google Scholar] [CrossRef]
- Sudoma, I.; Pylyp, L.; Kremenska, Y.; Goncharova, Y. Application of autologous adipose-derived stem cells for thin endometrium treatment in patients with failed ART programs. J. Stem Cell Ther. Transpl. 2019, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Haahr, M.K.; Jensen, C.H.; Toyserkani, N.M.; Andersen, D.C.; Damkier, P.; Sorensen, J.A.; Lund, L.; Sheikh, S.P. Safety and Potential Effect of a Single Intracavernous Injection of Autologous Adipose-Derived Regenerative Cells in Patients with Erectile Dysfunction Following Radical Prostatectomy: An Open-Label Phase I Clinical Trial. EBioMedicine 2016, 5, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Liu, M.; Li, Y.; Wang, W.; Yang, K.; Lu, L.; He, M.; Deng, T.; Li, M.; Wu, D. Intrauterine transplantation of autologous menstrual blood stem cells increases endometrial thickness and pregnancy potential in patients with refractory intrauterine adhesion. J. Obstet. Gynaecol. Res. 2020, 46, 2347–2355. [Google Scholar] [CrossRef]
- Tan, J.; Li, P.; Wang, Q.; Li, Y.; Li, X.; Zhao, D.; Xu, X.; Kong, L. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum. Reprod. 2016, 31, 2723–2729. [Google Scholar] [CrossRef] [Green Version]
- Gellert, S.E.; Pors, S.E.; Kristensen, S.G.; Bay-Bjorn, A.M.; Ernst, E.; Yding Andersen, C. Transplantation of frozen-thawed ovarian tissue: An update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J. Assist. Reprod. Genet 2018, 35, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, F.; Marin, L.; Oktay, K.H. Autologous cryopreserved ovarian tissue transplantation (acott): An update from our previous meta-analytical dataset as of march 2021. Fertil. Steril. 2021, 116, e217. [Google Scholar] [CrossRef]
- Sapozhak, I.M.; Gubar, S.; Rodnichenko, A.E.; Zlatska, A.V. Application of autologous endometrial mesenchymal stromal/stem cells increases thin endometrium receptivity: A case report. J. Med. Case Rep. 2020, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Zlatska, A.V.; Rodnichenko, A.E.; Gubar, O.S.; Zubov, D.O.; Novikova, S.N.; Vasyliev, R.G. Endometrial stromal cells: Isolation, expansion, morphological and functional properties. Exp. Oncol. 2017, 39, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, A.; Tang, X.; Li, M.; Yan, L.; Shang, W.; Gao, M. Intrauterine transplantation of autologous bone marrow derived mesenchymal stem cells followed by conception in a patient of severe intrauterine adhesions. Open J. Obstet. Gynecol. 2013, 3, 31293. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Lodha, P.; Karthick, M.S.; Tandulwadkar, S.R. Role of Autologous Bone Marrow-Derived Stem Cell Therapy for Follicular Recruitment in Premature Ovarian Insufficiency: Review of Literature and a Case Report of World’s First Baby with Ovarian Autologous Stem Cell Therapy in a Perimenopausal Woman of Age 45 Year. J. Hum. Reprod. Sci. 2018, 11, 125–130. [Google Scholar] [CrossRef]
- Estuardo, L.I.J.; Carlos, D.M.; Roberto, H.R.; Jesús, Á.P.F.d.; Juan, G.V.J.; Alejandro, K.B.; Daniela, Á.R.; Maruxa, P.F.; Angélica, P.N.M.; Ruiz, G.d.l.R.; et al. Therapeutic Potential of Autologous Adipose Derived Mesenchymal Stem Cells in Human POI and Ovarian Aging. J. Evol. Stem Cell Res. 2021, 1, 5–18. [Google Scholar] [CrossRef]
- Gao, M.; Yu, Z.; Yao, D.; Qian, Y.; Wang, Q.; Jia, R. Mesenchymal stem cells therapy: A promising method for the treatment of uterine scars and premature ovarian failure. Tissue Cell 2022, 74, 101676. [Google Scholar] [CrossRef]
- Fu, X.; He, Y.; Wang, X.; Peng, D.; Chen, X.; Li, X.; Wang, Q. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res. Ther. 2017, 8, 187. [Google Scholar] [CrossRef] [Green Version]
- Tan, Q.; Xia, D.; Ying, X. miR-29a in Exosomes from Bone Marrow Mesenchymal Stem Cells Inhibit Fibrosis during Endometrial Repair of Intrauterine Adhesion. Int. J. Stem Cells 2020, 13, 414–423. [Google Scholar] [CrossRef]
- Xiao, B.; Zhu, Y.; Huang, J.; Wang, T.; Wang, F.; Sun, S. Exosomal transfer of bone marrow mesenchymal stem cell-derived miR-340 attenuates endometrial fibrosis. Biol. Open 2019, 8, bio039958. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Chen, R.; Wang, G.; Zhang, Y.; Liu, F. Exosomes derived from mesenchymal stem cells reverse EMT via TGF-beta1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res. Ther. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, Z.; Sun, J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-beta/Smad signaling pathway. Stem Cell Res. Ther. 2020, 11, 198. [Google Scholar] [CrossRef]
- Qamar, A.Y.; Fang, X.; Kim, M.J.; Cho, J. Improved Post-Thaw Quality of Canine Semen after Treatment with Exosomes from Conditioned Medium of Adipose-Derived Mesenchymal Stem Cells. Animals 2019, 9, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Qi, W.; Zheng, J.; Tian, Y.; Qi, X.; Kong, D.; Zhang, J.; Huang, X. Exosomes Derived from Adipose Mesenchymal Stem Cells Restore Functional Endometrium in a Rat Model of Intrauterine Adhesions. Reprod. Sci. 2020, 27, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lu, J.; Ding, C.; Zou, Q.; Wang, W.; Li, H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res. Ther. 2018, 9, 216. [Google Scholar] [CrossRef]
- Zhu, L.L.; Huang, X.; Yu, W.; Chen, H.; Chen, Y.; Dai, Y.T. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia 2018, 50, e12871. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, B.; Su, P.; Chang, Q.; Li, P.; Song, A.; Zhao, X.; Yuan, Z.; Tan, J. Concentrated exosomes from menstrual blood-derived stromal cells improves ovarian activity in a rat model of premature ovarian insufficiency. Stem Cell Res. Ther. 2021, 12, 178. [Google Scholar] [CrossRef]
- Claridge, B.; Lozano, J.; Poh, Q.H.; Greening, D.W. Development of Extracellular Vesicle Therapeutics: Challenges, Considerations, and Opportunities. Front. Cell Dev. Biol. 2021, 9, 734720. [Google Scholar] [CrossRef]
- Kucia, M.; Reca, R.; Campbell, F.R.; Zuba-Surma, E.; Majka, M.; Ratajczak, J.; Ratajczak, M.Z. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia 2006, 20, 857–869. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.M.; Liu, R.; Wu, W.; Waigel, S.J.; Zacharias, W.; Ratajczak, M.Z.; Kucia, M. Global gene expression analysis of very small embryonic-like stem cells reveals that the Ezh2-dependent bivalent domain mechanism contributes to their pluripotent state. Stem Cells Dev. 2012, 21, 1639–1652. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.M.; Liu, R.; Klich, I.; Ratajczak, J.; Kucia, M.; Ratajczak, M.Z. Molecular characterization of isolated from murine adult tissues very small embryonic/epiblast like stem cells (VSELs). Mol. Cells 2010, 29, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Ratajczak, J.; Suszynska, M.; Miller, D.M.; Kucia, M.; Shin, D.M. A Novel View of the Adult Stem Cell Compartment From the Perspective of a Quiescent Population of Very Small Embryonic-Like Stem Cells. Circ. Res. 2017, 120, 166–178. [Google Scholar] [CrossRef]
- Bhartiya, D.; Unni, S.; Parte, S.; Anand, S. Very small embryonic-like stem cells: Implications in reproductive biology. Biomed. Res. Int. 2013, 2013, 682326. [Google Scholar] [CrossRef] [PubMed]
- Lahlil, R.; Scrofani, M.; Barbet, R.; Tancredi, C.; Aries, A.; Henon, P. VSELs Maintain their Pluripotency and Competence to Differentiate after Enhanced Ex Vivo Expansion. Stem Cell Rev. Rep. 2018, 14, 510–524. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, D.; Kasiviswanathan, S.; Unni, S.K.; Pethe, P.; Dhabalia, J.V.; Patwardhan, S.; Tongaonkar, H.B. Newer insights into premeiotic development of germ cells in adult human testis using Oct-4 as a stem cell marker. J. Histochem. Cytochem. 2010, 58, 1093–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parte, S.; Bhartiya, D.; Telang, J.; Daithankar, V.; Salvi, V.; Zaveri, K.; Hinduja, I. Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells Dev. 2011, 20, 1451–1464. [Google Scholar] [CrossRef]
- Virant-Klun, I. Functional Testing of Primitive Oocyte-like Cells Developed in Ovarian Surface Epithelium Cell Culture from Small VSEL-like Stem Cells: Can They Be Fertilized One Day? Stem Cell Rev. Rep. 2018, 14, 715–721. [Google Scholar] [CrossRef]
- Kurkure, P.; Prasad, M.; Dhamankar, V.; Bakshi, G. Very small embryonic-like stem cells (VSELs) detected in azoospermic testicular biopsies of adult survivors of childhood cancer. Reprod. Biol. Endocrinol. 2015, 13, 122. [Google Scholar] [CrossRef] [Green Version]
- Sriraman, K.; Bhartiya, D.; Anand, S.; Bhutda, S. Mouse Ovarian Very Small Embryonic-Like Stem Cells Resist Chemotherapy and Retain Ability to Initiate Oocyte-Specific Differentiation. Reprod. Sci. 2015, 22, 884–903. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Sim, W.Y.; Min, B.H.; Yang, S.S.; Khademhosseini, A.; Kaplan, D.L. Chip-based comparison of the osteogenesis of human bone marrow- and adipose tissue-derived mesenchymal stem cells under mechanical stimulation. PLoS ONE 2012, 7, e46689. [Google Scholar] [CrossRef] [Green Version]
- Tahmasbpour Marzouni, E.; Sinclair, A.H.; Stern, C.; Tucker, E.J. Stem cells and organs-on-chips: New promising technologies for human infertility treatment. Endocr. Rev. 2021, 43, 878–906. [Google Scholar] [CrossRef] [PubMed]
- Dossena, M.; Piras, R.; Cherubini, A.; Barilani, M.; Dugnani, E.; Salanitro, F.; Moreth, T.; Pampaloni, F.; Piemonti, L.; Lazzari, L. Standardized GMP-compliant scalable production of human pancreas organoids. Stem Cell Res. Ther. 2020, 11, 94. [Google Scholar] [CrossRef] [PubMed]
| Common Causes of Male Infertility | Common Causes of Female Infertility |
|---|---|
| Hypothalamic hypophyseal causes | Ovulation Disorders Causes |
| Pituitary insufficiency | Ageing (diminished ovarian reserve) |
| Hyperprolactinemia | Premature ovarian failure/insufficiency (POF/POI) |
| Kallmann’s syndrome (anosmia) | Endocrine disorders (such as PCOS) |
| Testicular Disorders | Tubal Causes |
| Klinefelter syndrome | Pelvic inflammatory disease |
| Chromosome anomalies (AZF microdeletions) | Tubal surgery |
| Testicular atrophy | Previous ectopic pregnancy |
| Varicocele (excessive heats) | Salpingectomy |
| Cryptorchidism | Uterine/Cervical Causes |
| Infections (such as mumps) | Congenital uterine anomaly |
| Disorders of the Seminal Tract | Fibroids (Asherman’s syndrome) |
| Retrograde Ejaculation | Endometriosis |
| Obstructive azoospermia | Poor cervical mucus quality/quantity |
| Immunological causes | Infection (salpingitis) |
| Autoimmunity to sperm | Others |
| Tumours/treatment | |
| Obesity | |
| Environment |
| Cell Source | Disease | Mode of Treatment | Model | Outcome | Reference |
|---|---|---|---|---|---|
| ADSCs | POI (aging mice) | Microinjection into oocytes | Mice |
| [98] |
| POI (aging mice) | Microinjection into oocytes | Mice | Did not mitigate the poor fertilization and embryonic development rates of aged oocytes | [99] | |
| POI | Melatonin-pretreated intraovarian injection | Mouse |
| [126] | |
| BMSCs | Testicular damage due to chemotherapy | Injection into the testes | Rats |
| [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed Rasheed, Z.B.; Nordin, F.; Wan Kamarul Zaman, W.S.; Tan, Y.-F.; Abd Aziz, N.H. Autologous Human Mesenchymal Stem Cell-Based Therapy in Infertility: New Strategies and Future Perspectives. Biology 2023, 12, 108. https://doi.org/10.3390/biology12010108
Mohamed Rasheed ZB, Nordin F, Wan Kamarul Zaman WS, Tan Y-F, Abd Aziz NH. Autologous Human Mesenchymal Stem Cell-Based Therapy in Infertility: New Strategies and Future Perspectives. Biology. 2023; 12(1):108. https://doi.org/10.3390/biology12010108
Chicago/Turabian StyleMohamed Rasheed, Zahirrah Begam, Fazlina Nordin, Wan Safwani Wan Kamarul Zaman, Yuen-Fen Tan, and Nor Haslinda Abd Aziz. 2023. "Autologous Human Mesenchymal Stem Cell-Based Therapy in Infertility: New Strategies and Future Perspectives" Biology 12, no. 1: 108. https://doi.org/10.3390/biology12010108
APA StyleMohamed Rasheed, Z. B., Nordin, F., Wan Kamarul Zaman, W. S., Tan, Y.-F., & Abd Aziz, N. H. (2023). Autologous Human Mesenchymal Stem Cell-Based Therapy in Infertility: New Strategies and Future Perspectives. Biology, 12(1), 108. https://doi.org/10.3390/biology12010108