Further Insights into Invasion: Field Observations of Behavioural Interactions between an Invasive and Critically Endangered Freshwater Crayfish Using Baited Remote Underwater Video (BRUV)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Behavioural Observations
2.3. Statistical Analysis
3. Results
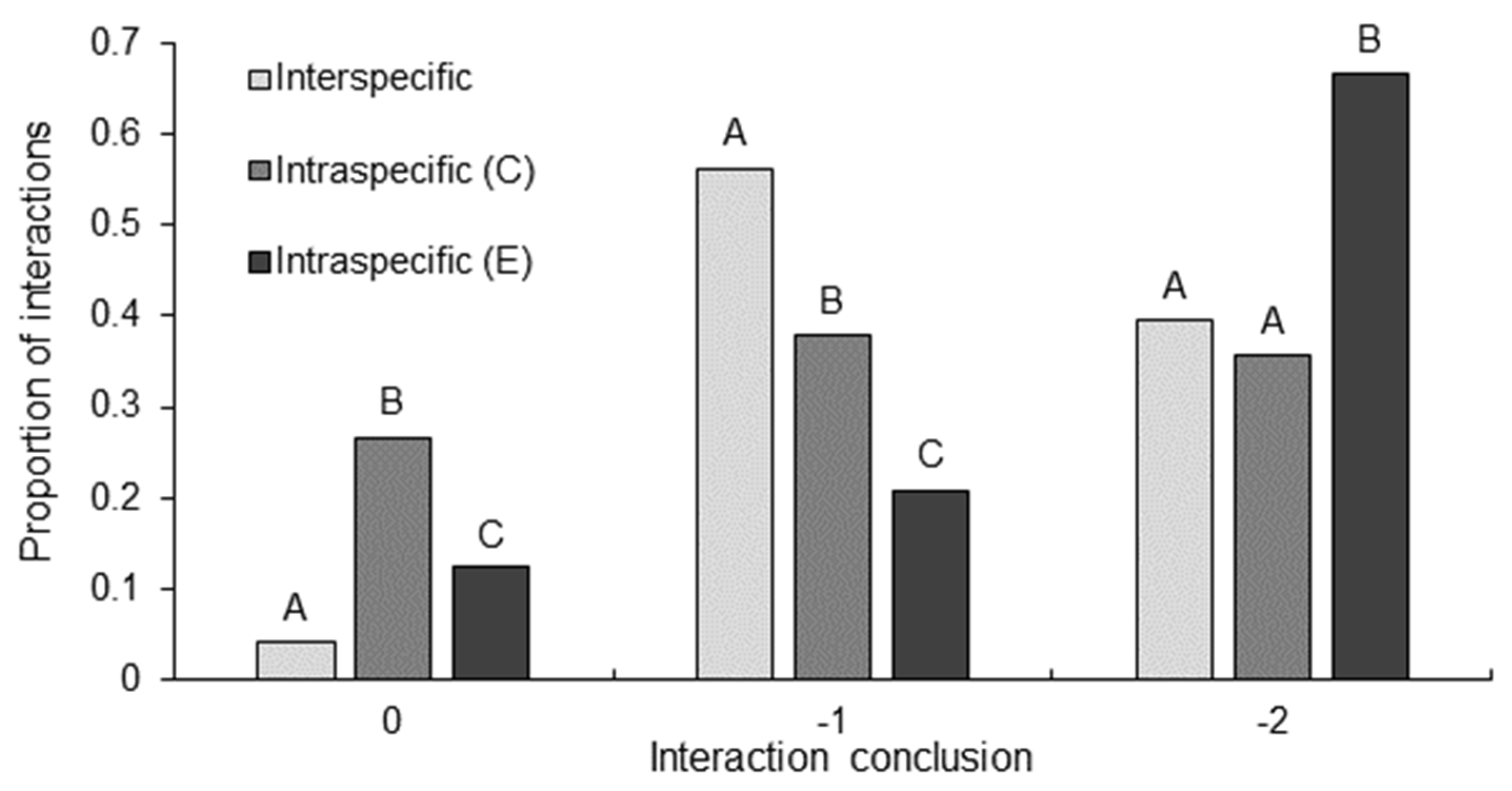
3.1. Contest Dynamics: Duration, Intensity, and Conclusion
3.2. Interspecific Interaction Outcome and Initiation
3.3. Effect of Size on Interaction Outcome and Initiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardy, I.C.; Briffa, M. Animal Contests, 1st ed.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Peiman, K.S.; Robinson, B.W. Ecology and evolution of resource-related heterospecific aggression. Q. Rev. Biol. 2010, 85, 133–158. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.A. Assessment strategy and evolution of fighting behaviour. J. Theor. Biol. 1974, 47, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.B.; Halliday, T.R. Deep croaks and fighting assessment in toads Bufo bufo. Nature 1978, 274, 683–685. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Mayer, M.K. The effects of size and reproductive quality on the outcomes of duels between honey bee queens (Apis mellifera L.). Ethol. Ecol. Evol. 2009, 21, 146–153. [Google Scholar] [CrossRef]
- Colella, D.J.; Paijmans, K.C.; Wong, Y.L.W. Size, sex and social experience: Experimental tests of multiple factors mediating contest behaviour in a rockpool fish. Ethology 2018, 125, 369–379. [Google Scholar] [CrossRef]
- Figler, M.H.; Blank, G.S.; Peeke, H.V.S. Maternal aggression and post-hatch care in red swamp crayfish, Procambarus clarkii (Girard): The influences of presence of offspring, fostering and maternal moulting. Mar. Freshw. Behav. Physiol. 1997, 30, 173–194. [Google Scholar] [CrossRef]
- Chamorro-Florescano, I.A.; Favila, M.E.; Macías-Ordóñezm, R. Ownership, size and reproductive status affect the outcome of food ball contests in a dung roller beetle: When do enemies share? Evol. Ecol. 2011, 25, 277–289. [Google Scholar] [CrossRef]
- Snell-Rood, E.C.; Cristol, D.A. Prior residence influences contest outcome in flocks of non-breeding birds. Ethology 2005, 111, 441–454. [Google Scholar] [CrossRef]
- Tsai, Y.J.J.; Barrows, E.M.; Weiss, M.R. Why do larger and older males win contests in the parasitoid wasp Nasonia vitripennis? Anim. Behav. 2014, 91, 151–159. [Google Scholar] [CrossRef]
- Yang, S.; Premel, V.; Richards-Zawacki, C.L. Prior residence effect determines success of male-male territorial competition in a color polymorphic poison frog. Ethology 2020, 126, 1131–1140. [Google Scholar] [CrossRef]
- Petren, K.; Case, T.J. An experimental demonstration of exploitation competition in an ongoing invasion. Ecology 1996, 77, 118–132. [Google Scholar] [CrossRef]
- Chock, R.Y.; Shier, D.M.; Grether, G.F. Body size, not phylogenetic relationship or residency, drives interspecific dominance in a little pocket mouse community. Anim. Behav. 2018, 137, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Graham, Z.A.; Angilletta, M.J. Claw size predicts dominance within and between invasive species of crayfish. Anim. Behav. 2020, 166, 153–161. [Google Scholar] [CrossRef]
- Robinson, S.K.; Terborgh, J. Interspecific aggression and habitat selection by Amazonian birds. J. Anim. Ecol. 1995, 64, 1–11. [Google Scholar] [CrossRef]
- Pearce, D.; Pryke, S.R.; Griffith, S.C. Interspecific aggression for nest sites: Model experiments with long-tailed finches (Poephila acuticauda) and endangered Gouldian finches (Erythrura gouldiae). Auk 2011, 128, 497–505. [Google Scholar] [CrossRef]
- Ferretti, F. Interspecific aggression between fallow and roe deer. Ethol. Ecol. Evol. 2011, 23, 179–186. [Google Scholar] [CrossRef]
- Mills, M.D.; Rader, R.B.; Belk, M.C. Complex interactions between native and invasive fish: The simultaneous effects of multiple negative interactions. Oecologia 2004, 141, 713–721. [Google Scholar] [CrossRef]
- Polo-Cavia, N.; López, P.; Martín, J. Aggressive interactions during feeding between invasive and native freshwater turtles. Biol. Invasions 2011, 13, 1387–1396. [Google Scholar] [CrossRef]
- Kakareko, T.; Kobak, J.; Grabowska, J.; Jermacz, L.; Przybylski, M.; Poznańska, M.; Pietraszewski, D.; Copp, G.H. Competitive interactions for food resources between invasive racer goby Babka gymnotrachelus and native European bullhead Cottus gobio. Biol. Invasions 2013, 15, 2519–2530. [Google Scholar] [CrossRef] [Green Version]
- Culbertson, K.A.; Herrmann, N.C. Asymmetric interference competition and niche partitioning between native and invasive Anolis lizards. Oecologia 2019, 190, 811–820. [Google Scholar] [CrossRef]
- Holway, D.A. Competitive mechanisms underlying the displacement of native ants by the invasive Argentine ant. Ecology 1999, 80, 238–251. [Google Scholar] [CrossRef]
- Gherardi, F.; Cioni, A. Agonism and interference competition in freshwater decapods. Behaviour 2004, 141, 1297–1324. [Google Scholar] [CrossRef] [Green Version]
- Hajek, A.E.; Henry, J.C.; Standley, C.R.; Foelker, C.J. Comparing functional traits and abundance of invasive versus native woodwasps. Neobiota 2017, 36, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Sanches, F.H.C.; Miyai, C.A.; Costa, T.M.; Christofoletti, R.A.; Volpato, G.L.; Barreto, R.E. Aggressiveness overcomes body-size effects in fights staged between invasive and native fish species with overlapping niches. PLoS ONE 2012, 7, e29746. [Google Scholar] [CrossRef] [Green Version]
- Blank, G.S.; Figler, M.H. Interspecific shelter competition between the sympatric crayfish species Procambarus clarkii (Girard) and Procambarus zonangulus (Hobbs and Hobbs). J. Crustac. Biol. 1996, 16, 300–309. [Google Scholar] [CrossRef]
- Nakata, K.; Goshima, S. Competition for shelter of preferred sizes between the native crayfish species Cambaroides japonicus and the alien crayfish species Pacifastacus leniusculus in Japan in relation to prior residence, sex difference, and body size. J. Crustac. Biol. 2003, 23, 897–907. [Google Scholar] [CrossRef] [Green Version]
- Hudina, S.; Hock, K.; Radović, A.; Klobučar, G.; Petković, J.; Jelić, M.; Maquire, I. Species-specific differences in dynamics of agonistic interactions may contribute to the competitive advantage of the invasive signal crayfish (Pacifastacus leniusculus) over the native narrow-clawed crayfish (Astacus leptodactylus). Mar. Freshw. Behav. Physiol. 2016, 49, 147–157. [Google Scholar] [CrossRef]
- Klocker, C.A.; Strayer, D.L. Interactions among an invasive crayfish (Orconectes rusticus), a native crayfish (Orconectes limosus), and native bivalves (Sphaeriidae and Unionidae). Northeast. Nat. 2004, 11, 167–178. [Google Scholar] [CrossRef]
- O’Hea Miller, S.B.; Davis, A.R.; Wong, M.Y.L. Does habitat complexity and prior residency influence aggression between invasive and native freshwater crayfish? Ethology 2022, 128, 443–452. [Google Scholar] [CrossRef]
- Pârvulescu, L.; Pîrvu, M.; Moroşan, L.G.; Zaharia, C. Plasticity in fecundity highlights the females’ importance in the spiny-cheek crayfish invasion mechanism. Zoology 2015, 118, 424–432. [Google Scholar] [CrossRef]
- Beatty, S.; Morgan, D.; Gill, H. Role of life history strategy in the colonisation of Western Australian aquatic systems by the introduced crayfish Cherax destructor Clark, 1936. Hydrobiologia 2005, 549, 219–237. [Google Scholar] [CrossRef]
- Larson, E.R.; Magoulick, D.D.; Turner, C.; Laycock, K.H. Disturbance and species displacement: Different tolerances to stream drying and desiccation in a native and an invasive crayfish. Freshw. Biol. 2009, 54, 1899–1908. [Google Scholar] [CrossRef]
- Smith, K.R.; Roth, B.M.; Jones, M.L.; Hayes, D.B.; Hervst, S.J.; Popoff, N. Changes in the distribution of Michigan crayfishes and the influence of invasive rusty crayfish (Faxonius rusticus) on native crayfish substrate associations. Biol. Invasions 2019, 21, 637–656. [Google Scholar] [CrossRef]
- Söderback, B. Replacement of the native crayfish Astacus astacus by the introduced species Pacifastacus leniusculus in a Swedish lake: Possible causes and mechanisms. Freshw. Biol. 1995, 33, 291–304. [Google Scholar] [CrossRef]
- Gherardi, F. Crayfish invading Europe: The case study of Procambarus clarkii. Mar. Freshw. Behav. Physiol. 2006, 39, 175–191. [Google Scholar] [CrossRef]
- Rabalais, M.R.; Magoulick, D.D. Is competition with the invasive crayfish Orconectes neglectus chaenodactylus responsible for the displacement of the native crayfish Orconectes eupunctus? Biol. Invasions 2006, 8, 1039–1048. [Google Scholar] [CrossRef]
- Westhoff, J.T.; Rabeni, C.F. Resource selection and space use of a native and an invasive crayfish: Evidence for competitive exclusion? Freshw. Sci. 2013, 32, 1383–1397. [Google Scholar] [CrossRef]
- Vorburger, C.; Ribi, G. Aggression and competition for shelter between a native and an introduced crayfish in Europe. Freshw. Biol. 1999, 41, 111–119. [Google Scholar] [CrossRef]
- Lopez, L.K.; Hendry, K.; Wong, M.Y.; Davis, A.R. Insight into invasion: Interactions between a critically endangered and invasive crayfish. Austral Ecol. 2019, 44, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Hazlett, B.A.; Lawler, S.; Geoffery, E. Agonistic behaviour of the crayfish Euastacus armatus and Cherax destructor. Mar. Freshw. Behav. Physiol. 2007, 40, 257–266. [Google Scholar] [CrossRef]
- Roessink, I.; van der Zon, K.A.E.; de Reus, S.R.M.M.; Peeters, E.R.H.M. Native European crayfish Astacus astacus competitive in staged confrontation with the invasive crayfish Faxonius limosus and Procambarus acutus. PLoS ONE 2022, 17, e0263133. [Google Scholar] [CrossRef] [PubMed]
- Cerato, S.; Davis, A.R.; Coleman, D.; Wong, M.L.Y. Reversal of competitive dominance between invasive and native freshwater crayfish species under near-future elevated water temperature. Aquat. Invasions 2019, 14, 518–530. [Google Scholar] [CrossRef]
- Bergman, D.A.; Moore, P.A. Field observations of intraspecific agonistic behaviour of two crayfish species, Orconectes rusticus and Orconectes virilis, in different habitats. Biol. Bull. 2003, 205, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Bubb, D.H.; Thom, T.J.; Lucas, M.C. Movement, dispersal and refuge use of co-occurring introduced and native crayfish. Freshw. Biol. 2006, 51, 1359–1368. [Google Scholar] [CrossRef]
- Olsson, K.; Stenroth, P.; Nyström, P.; Granéli, W. Invasions and niche width: Does niche width of an introduced crayfish differ from a native crayfish? Freshw. Biol. 2009, 54, 1731–1740. [Google Scholar] [CrossRef]
- Dresser, C.M.; Kuhlmann, M.L.; Swanson, B.J. Variation in native crayfish agonistic response to the invasion of the rusty crayfish Orconectes rusticus (Girard, 1852). J. Crustac. Biol. 2016, 36, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Coughran, J.; McCormack, R.B.; Daly, G. Translocation of the yabby Cherax destructor into eastern drainages of New South Wales, Australia. Aust. Zool. 2009, 35, 100–103. [Google Scholar] [CrossRef]
- Coughran, J.; Daly, G. Potential threats posed by a translocation crayfish: The case of Cherax destructor in coastal drainages of New South Wales, Australia. Crust. Res. 2012, 7, 5–13. [Google Scholar] [CrossRef] [Green Version]
- McCormack, R.B. A Guide to Australia’s Spiny Freshwater Crayfish, 1st ed.; CSIRO Publishing: Collingwood, VIC, Australia, 2012. [Google Scholar]
- Lynas, J.; Storey, A.W.; Knott, B. Aggressive interactions between three species of freshwater crayfish of the genus Cherax (Decapoda: Parastacidae). Mar. Freshw. Behav. Physiol. 2007, 40, 105–116. [Google Scholar] [CrossRef]
- Williamson, J. Final Determination, the Fitzroy Falls Spiny Crayfish, Euastacus dharawalus, as a Critically Endangered Species; Ref. No. FD 49. File No. FSC 11/01; NSW Fisheries Scientific Committee: Wollstonecraft, Australia, 2011. [Google Scholar]
- McCormack, R.B. Conservation of imperiled crayfish, Euastacus dharawalus (Decapoda: Astacidae: Parastacidae), from the Southern highlands of New South Wales, Australia. J. Crustac. Biol. 2013, 33, 432–439. [Google Scholar] [CrossRef]
- McCormack, R.B. Euastacus dharawalus. The IUCN Red List of Threatened Species, 2016: e.T153704A188805771. Available online: https://www.iucnredlist.org/species/153704/188805771 (accessed on 13 September 2022).
- Van Der Meulen, D.; (Department of Primary Industries, Batemans Bay, NSW, Australia). Personal communication, 2021.
- O’Hea Miller, S.B.; Davis, A.R.; Broadhurst, B.; Wong, M.Y.L. Shifts in the Movement and Habitat Use of a Critically Endangered Native Crayfish in the Presence of an Invader; University of Wollongong: Wollongong, NSW, Australia, 2022; to be submitted. [Google Scholar]
- Wraith, J.; Lynch, T.; Minchinton, T.E.; Broad, A.; Davis, A.R. Bait type affects fish assemblages and feeding guilds observed at baited remote underwater video stations. Mar. Ecol. Prog. Ser. 2013, 477, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.A.; Moore, P.A. Field observation of agonism in the crayfish, Orconectes rusticus: Shelter use in a natural environment. Ethology 2007, 113, 1192–1201. [Google Scholar] [CrossRef]
- Daws, A.G.; Grills, J.; Konzen, K.; Moore, P.A. Previous experiences alter the outcome of aggressive interactions between males in the crayfish, Procambarus Clarkii. Mar. Freshw. Behav. Physiol. 2002, 35, 139–148. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects model using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Marascuilo, L.A.; McSweeney, M. Nonparametric post hoc comparisons for trend. Psychol. Bull. 1967, 67, 401–412. [Google Scholar] [CrossRef]
- Pavey, C.R.; Fielder, D.R. The influence of size difference on agonistic behaviour in freshwater crayfish, Cherax cuspidatus (Decapoda: Parastacidae). J. Zool. 1996, 238, 445–457. [Google Scholar] [CrossRef]
- Just, W.; Morris, M.R. The Napoleon Complex: Why smaller males pick fights. Evol. Ecol. 2003, 17, 509–522. [Google Scholar] [CrossRef]
- Sih, A.; Cote, J.; Evans, M.; Forgarty, S.; Pruitt, J. Ecological implications of behavioural syndromes. Ecol. Lett. 2012, 15, 278–289. [Google Scholar] [CrossRef]
- Bruce, M.; Doherty, T.; Kaplan, J.; Sutherland, C.; Atema, J. American lobsters, Homarus americanus, use vision for initial opponent evaluation and subsequent memory. Bull. Mar. Sci. 2018, 94, 517–532. [Google Scholar] [CrossRef]
- Percival, D.T.; Moore, P.A. Shelter size influences self-assessment of size in crayfish, Orconectes rusticus: Consequences for agonistic fights. Behaviour 2010, 147, 103–119. [Google Scholar] [CrossRef]
- Wofford, S.J.; Earley, R.L.; Moore, P.A. Evidence for assessment disappears in mixed-sex contests of the crayfish, Orconectes virilis. Behaviour 2015, 152, 995–1018. [Google Scholar] [CrossRef]
- Lane, S.M.; Briffa, M. The price of attack: Rethinking damage costs in animal contests. Anim. Behav. 2017, 126, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Arnott, G.; Elwood, R.W. Information gathering and decision making about resource value in animal contests. Anim. Behav. 2008, 76, 529–542. [Google Scholar] [CrossRef]




| Intensity Level | Behaviour | Description |
|---|---|---|
| −2 | Tailflip away | A rapid beating of the tail to quickly retreat from an opponent. |
| −1 | Slowly back away | A slow retreat from an opponent usually in a submissive posture. |
| 0 | Ignore | Individuals come within proximity (≤1 body length away), but no response or threat display is exhibited. |
| 1 | Approach without threat display | One or both individuals approach each other with no threat display. |
| 2 | Approach with threat display | An approach with threat display is made, this includes some or all of the following: raised posture, meral spread and antennal whip. |
| 3 | Closed claw contact | Use of closed claws to stab, punch, push or touch opponent. |
| 4 | Open claw contact | Use of open claws to snip, grab or hold opponent. |
| 5 | Unrestrained fighting | Unrestrained fighting by grasping and pulling opponents claws or appendages. |
| Interaction Type | Number Observed |
|---|---|
| Intraspecific (C) | 51 |
| Intraspecific (E) | 26 |
| Interspecific (E vs. C) | 54 |
| Size-matched (E vs. C) | 17 |
| Small Cherax vs. large Euastacus | 29 |
| Small Euastacus vs. large Cherax | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Hea Miller, S.B.; Davis, A.R.; Wong, M.Y.L. Further Insights into Invasion: Field Observations of Behavioural Interactions between an Invasive and Critically Endangered Freshwater Crayfish Using Baited Remote Underwater Video (BRUV). Biology 2023, 12, 18. https://doi.org/10.3390/biology12010018
O’Hea Miller SB, Davis AR, Wong MYL. Further Insights into Invasion: Field Observations of Behavioural Interactions between an Invasive and Critically Endangered Freshwater Crayfish Using Baited Remote Underwater Video (BRUV). Biology. 2023; 12(1):18. https://doi.org/10.3390/biology12010018
Chicago/Turabian StyleO’Hea Miller, Sarah B., Andrew R. Davis, and Marian Y. L. Wong. 2023. "Further Insights into Invasion: Field Observations of Behavioural Interactions between an Invasive and Critically Endangered Freshwater Crayfish Using Baited Remote Underwater Video (BRUV)" Biology 12, no. 1: 18. https://doi.org/10.3390/biology12010018
APA StyleO’Hea Miller, S. B., Davis, A. R., & Wong, M. Y. L. (2023). Further Insights into Invasion: Field Observations of Behavioural Interactions between an Invasive and Critically Endangered Freshwater Crayfish Using Baited Remote Underwater Video (BRUV). Biology, 12(1), 18. https://doi.org/10.3390/biology12010018







