Molecular, Physiological and Hematological Responses of Crossbred Dairy Cattle in a Tropical Savanna Climate
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location and Animal Management
2.2. Determination of the Temperature Humidity Index
2.3. Collection of Blood Samples and Hematological Analysis
2.4. Physiological Trait Recording
2.5. Total mRNA Extraction and Complementary DNA (cDNA) Synthesis
2.6. Quantitative PCR
2.7. Statistical Analysis
3. Results
3.1. Temperature Humidity Index
3.2. Physiological Response and Infrared Thermography
3.3. Hematological Profile
3.4. Milk Performance and Composition
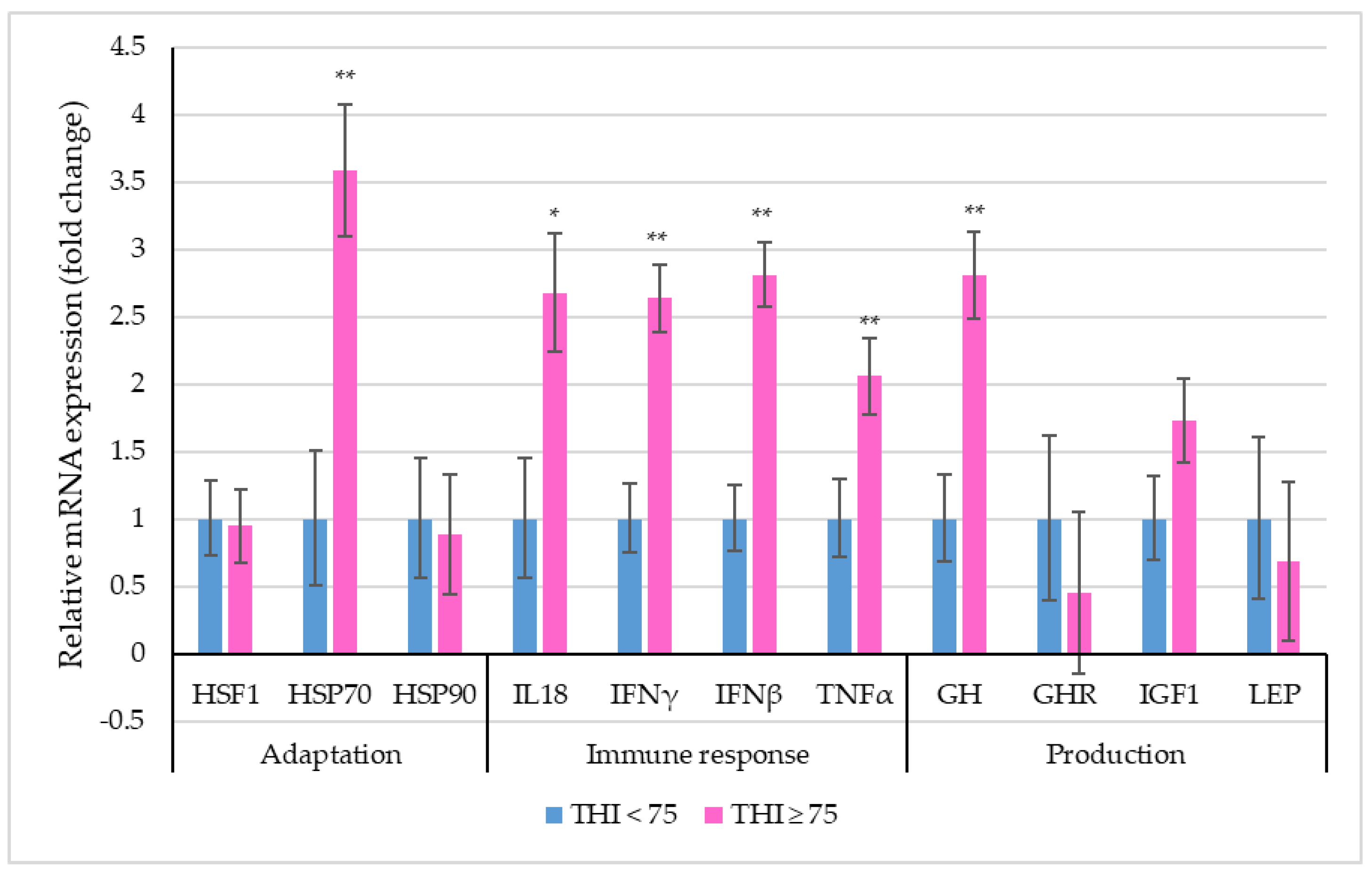
3.5. Selective Gene Expression Analysis
4. Discussion
4.1. Physiological Response and Infrared Thermography
4.2. Hematological Profile
4.3. Milk Performance and Composition
4.4. Selective Gene Expression Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wankar, A.K.; Rindhe, S.N.; Doijad, N.S. Heat Stress in Dairy Animals and Current Milk Production Trends, Economics, and Future Perspectives: The Global Scenario. Trop. Anim. Health Prod. 2021, 53, 70. [Google Scholar] [CrossRef] [PubMed]
- International Farm Comparison Network (IFCN). The IFCN Dairy Report 2019; IFCN: New Delhi, India, 2019. [Google Scholar]
- Devendra, C.; Swanepoel, F.J.C.; Stroebel, A.; van Rooyen, C.J. Implications and Innovative Strategies for Enhancing the Future Contribution of Livestock. In The Role of Livestock in Developing Communities: Enhancing Multifunctionality; Swanepoel, F., Stroebel, A., Moyo, S., Eds.; SunBonani Media: Free State, ZA, USA, 2010; ISBN 978-1-928424-81-9. [Google Scholar]
- Choudhary, B.B.; Sirohi, S. Economic Losses in Dairy Farms Due to Heat Stress in Sub-Tropics: Evidence from North Indian Plains. J. Dairy Res. 2022, 89, 141–147. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Effect of Environment on Nutrient Requirements of Domestic Animals; NRC: Singapore, 1981. [Google Scholar]
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.S.; Bernabucci, U. Effects of Climate Changes on Animal Production and Sustainability of Livestock Systems. Livest. Sci. 2010, 130, 57–69. [Google Scholar] [CrossRef]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of Animals to Heat Stress. Animal 2018, 12, s431–s444. [Google Scholar] [CrossRef] [Green Version]
- Collier, R.J.; Baumgard, L.H.; Zimbelman, R.B.; Xiao, Y. Heat Stress: Physiology of Acclimation and Adaptation. Anim. Front. 2019, 9, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Rashamol, V.P.; Sejian, V.; Bagath, M.; Krishnan, G.; Archana, P.R.; Bhatta, R. Physiological Adaptability of Livestock to Heat Stress: An Updated Review. JABB 2018, 6, 62–71. [Google Scholar] [CrossRef]
- Mishra, S.R. Behavioural, Physiological, Neuro-Endocrine and Molecular Responses of Cattle against Heat Stress: An Updated Review. Trop. Anim. Health Prod. 2021, 53, 400. [Google Scholar] [CrossRef]
- Collier, R.J.; Collier, J.L.; Rhoads, R.P.; Baumgard, L.H. Invited Review: Genes Involved in the Bovine Heat Stress Response. J. Dairy Sci. 2008, 91, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Basiricò, L.; Morera, P.; Primi, V.; Lacetera, N.; Nardone, A.; Bernabucci, U. Cellular Thermotolerance Is Associated with Heat Shock Protein 70.1 Genetic Polymorphisms in Holstein Lactating Cows. Cell Stress Chaperones 2011, 16, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Zhang, Y.; Zheng, N.; Cheng, J.; Wang, J. The Effect of Heat Stress on Gene Expression and Synthesis of Heat-Shock and Milk Proteins in Bovine Mammary Epithelial Cells: Heat Stress on Mammary Epithelial Cells. Anim. Sci. J. 2016, 87, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Ukita, H.; Kawahara, M.; Mitani, T.; Furukawa, E.; Yanagawa, Y.; Yabuuchi, N.; Kim, H.; Takahashi, M. Effect of Summer Heat Stress on Gene Expression in Bovine Uterine Endometrial Tissues. Anim. Sci. J. 2020, 91, e13474. [Google Scholar] [CrossRef]
- Corazzin, M.; Saccà, E.; Lippe, G.; Romanzin, A.; Foletto, V.; Da Borso, F.; Piasentier, E. Effect of Heat Stress on Dairy Cow Performance and on Expression of Protein Metabolism Genes in Mammary Cells. Animals 2020, 10, 2124. [Google Scholar] [CrossRef]
- Grewal, S.; Aggarwal, A.; Alhussien, M.N. Seasonal Alterations in the Expression of Inflammatory Cytokines and Cortisol Concentrations in Periparturient Sahiwal Cows. Biol. Rhythm. Res. 2021, 52, 1229–1239. [Google Scholar] [CrossRef]
- Wuebbles, D.J.; Fahey, D.W.; Hibbard, K.A. Climate Science Special Report: Fourth National Climate Assessment, Volume I; U.S. Global Change Research Program: Washington, DC, USA, 2017. [Google Scholar]
- National Research Council (NRC). A Guide to Environmental Research on Animals; NRC: Singapore, 1971. [Google Scholar]
- Mullakkalparambil Velayudhan, S.; Brügemann, K.; Pinto, A.; Yin, T.; Reichenbach, M.; Sejian, V.; Bhatta, R.; Schlecht, E.; König, S. Effects of Heat Stress across the Rural-Urban Interface on Phenotypic Trait Expressions of Dairy Cattle in a Tropical Savanna Region. Sustainability 2022, 14, 4590. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Collier, R.J.; Stiening, C.M.; Pollard, B.C.; VanBaale, M.J.; Baumgard, L.H.; Gentry, P.C.; Coussens, P.M. Use of Gene Expression Microarrays for Evaluating Environmental Stress Tolerance at the Cellular Level in Cattle1. J. Anim. Sci. 2006, 84, E1–E13. [Google Scholar] [CrossRef]
- Barwa, D.; Jain, A.; Jain, T.; Singh, M.; Mukherjee, K.; Ghosh, R. Haematological Profile in Calves of Kosali Breed of Cattle in Their Native Tract. Int. J. Livest. Res. 2018, 1. [Google Scholar] [CrossRef]
- Kumar, S.B.V.; Kumar, A.; Kataria, M. Effect of Heat Stress in Tropical Livestock and Different Strategies for Its Amelioration. J. Stress Physiol. Biochem. 2011, 7, 45–54. [Google Scholar]
- Aleena, J.; Sejian, V.; Bagath, M.; Krishnan, G.; Beena, V.; Bhatta, R. Resilience of Three Indigenous Goat Breeds to Heat Stress Based on Phenotypic Traits and PBMC HSP70 Expression. Int. J. Biometeorol. 2018, 62, 1995–2005. [Google Scholar] [CrossRef]
- Kumar, J.; Yadav, B.; Madan, A.K.; Kumar, M.; Sirohi, R.; Reddy, A.V. Dynamics of Heat-Shock Proteins, Metabolic and Endocrine Responses during Increasing Temperature Humidity Index (THI) in Lactating Hariana (Zebu) Cattle. Biol. Rhythm. Res. 2020, 51, 934–950. [Google Scholar] [CrossRef]
- Rhoads, M.L.; Rhoads, R.P.; VanBaale, M.J.; Collier, R.J.; Sanders, S.R.; Weber, W.J.; Crooker, B.A.; Baumgard, L.H. Effects of Heat Stress and Plane of Nutrition on Lactating Holstein Cows: I. Production, Metabolism, and Aspects of Circulating Somatotropin1. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutjens, M.F. Dairy Farm Management Systems | Dry Lot Dairy Cow Breeds. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 52–58. ISBN 978-0-12-374407-4. [Google Scholar]
- Yan, G.; Liu, K.; Hao, Z.; Shi, Z.; Li, H. The Effects of Cow-Related Factors on Rectal Temperature, Respiration Rate, and Temperature-Humidity Index Thresholds for Lactating Cows Exposed to Heat Stress. J. Therm. Biol. 2021, 100, 103041. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.G.; Maia, A.S.C. Evaporative Cooling and Cutaneous Surface Temperature of Holstein Cows in Tropical Conditions. R. Bras. Zootec. 2011, 40, 1143–1147. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, G.; Silpa, M.V.; Mylostyvyi, R.; Sejian, V. Non-Invasive Methods to Quantify the Heat Stress Response in Dairy Cattle. In Climate Change and Livestock Production: Recent Advances and Future Perspectives; Sejian, V., Chauhan, S.S., Devaraj, C., Malik, P.K., Bhatta, R., Eds.; Springer: Singapore, 2021; pp. 85–98. ISBN 9789811698361. [Google Scholar]
- Alsaaod, M.; Syring, C.; Dietrich, J.; Doherr, M.G.; Gujan, T.; Steiner, A. A Field Trial of Infrared Thermography as a Non-Invasive Diagnostic Tool for Early Detection of Digital Dermatitis in Dairy Cows. Vet. J. 2014, 199, 281–285. [Google Scholar] [CrossRef]
- Peng, D.; Chen, S.; Li, G.; Chen, J.; Wang, J.; Gu, X. Infrared Thermography Measured Body Surface Temperature and Its Relationship with Rectal Temperature in Dairy Cows under Different Temperature-Humidity Indexes. Int. J. Biometeorol. 2019, 63, 327–336. [Google Scholar] [CrossRef]
- Hoffmann, G.; Schmidt, M.; Ammon, C.; Rose-Meierhöfer, S.; Burfeind, O.; Heuwieser, W.; Berg, W. Monitoring the Body Temperature of Cows and Calves Using Video Recordings from an Infrared Thermography Camera. Vet. Res. Commun. 2013, 37, 91–99. [Google Scholar] [CrossRef]
- Yadav, B.; Singh, G.; Wankar, A. The Use of Infrared Skin Temperature Measurements for Monitoring Heat Stress and Welfare of Crossbred Cattle. Indian J. Dairy Sci. 2017, 70, 1–5. [Google Scholar]
- Kim, N.Y.; Moon, S.H.; Kim, S.J.; Kim, E.K.; Oh, M.; Tang, Y.; Jang, S.Y. Summer Season Temperature-Humidity Index Threshold for Infrared Thermography in Hanwoo (Bos Taurus Coreanae) Heifers. Asian-Australas J. Anim. Sci. 2020, 33, 1691–1698. [Google Scholar] [CrossRef] [Green Version]
- Collier, R.J.; Dahl, G.E.; VanBaale, M.J. Major Advances Associated with Environmental Effects on Dairy Cattle. J. Dairy Sci. 2006, 89, 1244–1253. [Google Scholar] [CrossRef] [Green Version]
- Martello, L.S.; Savastano Junior, H.; Silva, S.L.; Balieiro, J.C.C. Alternative Body Sites for Heat Stress Measurement in Milking Cows under Tropical Conditions and Their Relationship to the Thermal Discomfort of the Animals. Int. J. Biometeorol. 2010, 54, 647–652. [Google Scholar] [CrossRef]
- Fuquay, J.W. Heat Stress as It Affects Animal Production. J. Anim. Sci. 1981, 52, 164–174. [Google Scholar] [CrossRef]
- Kenilworth, N.J.; Sharp, M.; Corp, D. Normal Rectal Temperature Ranges; Merck Veterinary Manual: Rahway, NJ, USA, 2016. [Google Scholar]
- Kumar, B.; Pachauri, S.P. Haematological Profile of Crossbred Dairy Cattle to Monitor Herd Health Status at Medium Elevation in Central Himalayas. Res. Vet. Sci. 2000, 69, 141–145. [Google Scholar] [CrossRef]
- Casella, S.; Scianò, S.; Zumbo, A.; Monteverde, V.; Fazio, F.; Piccione, G. Effect of Seasonal Variations in Mediterranean Area on Haematological Profile in Dairy Cow. Comp. Clin. Pathol. 2013, 22, 691–695. [Google Scholar] [CrossRef]
- Giri, A.; Bharti, D.V.; Kalia, S.; Ravindran, V.; Ranjan, P.; Kundan, T.; Kumar, B. Seasonal Changes in Haematological and Biochemical Profile of Dairy Cows in High Altitude Cold Desert. Indian J. Anim. Sci. 2017, 87, 723–727. [Google Scholar]
- George, J.W.; Snipes, J.; Lane, V.M. Comparison of Bovine Hematology Reference Intervals from 1957 to 2006: Bovine Hematology Reference Intervals. Vet. Clin. Pathol. 2010, 39, 138–148. [Google Scholar] [CrossRef]
- Wood, D.; Quiroz-Rocha, G.F. Normal Hematology of Cattle. In Schalm’s Veterinary Hematology; Weiss, D.J., Wardrop, K.J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 829–835. ISBN 978-0-470-96183-4. [Google Scholar]
- Ferdous, F.; Scott, T.R. A Comparative Examination of Thrombocyte/Platelet Immunity. Immunol. Lett. 2015, 163, 32–39. [Google Scholar] [CrossRef]
- Habibu, B.; Dzenda, T.; Ayo, J.O.; Yaqub, L.S.; Kawu, M.U. Haematological Changes and Plasma Fluid Dynamics in Livestock during Thermal Stress, and Response to Mitigative Measures. Livest. Sci. 2018, 214, 189–201. [Google Scholar] [CrossRef]
- Mazzullo, G.; Rifici, C.; Caccamo, G.; Rizzo, M.; Piccione, G. Effect of Different Environmental Conditions on Some Haematological Parameters in Cow. Ann. Anim. Sci. 2014, 14, 947–954. [Google Scholar] [CrossRef] [Green Version]
- Roland, L.; Drillich, M.; Iwersen, M. Hematology as a Diagnostic Tool in Bovine Medicine. J. Vet. Diagn. Investig. 2014, 26, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Kocatürk, M.; Yeşilbağ, K.; Yilmaz, Z. Evaluation of Red Blood Cell and Platelet Indices in Cattle Naturally Infected with Bovine Viral Diarrhea Virus (BVDV). Uludağ Üniversitesi Vet. Fakültesi Derg. 2010, 29, 17–22. [Google Scholar]
- Jo, J.-H.; Ghassemi Nejad, J.; Peng, D.-Q.; Kim, H.-R.; Kim, S.-H.; Lee, H.-G. Characterization of Short-Term Heat Stress in Holstein Dairy Cows Using Altered Indicators of Metabolomics, Blood Parameters, Milk MicroRNA-216 and Characteristics. Animals 2021, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- Pragna, P.; Archana, P.R.; Aleena, J.; Sejian, V.; Krishnan, G.; Bagath, M.; Manimaran, A.; Beena, V.; Kurien, E.K.; Varma, G.; et al. Heat Stress and Dairy Cow: Impact on Both Milk Yield AndComposition. Int. J. Dairy Sci. 2016, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Parmar, P.; Lopez-Villalobos, N.; Tobin, J.T.; Murphy, E.; McDonagh, A.; Crowley, S.V.; Kelly, A.L.; Shalloo, L. The Effect of Compositional Changes Due to Seasonal Variation on Milk Density and the Determination of Season-Based Density Conversion Factors for Use in the Dairy Industry. Foods 2020, 9, 1004. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.B.; Kumar, M.; Pathak, V. Effect of Different Seasons on Cross-Bred Cow Milk Composition and Paneer Yield in Sub-Himalayan Region. Asian Australas. J. Anim. Sci 2002, 15, 528–530. [Google Scholar] [CrossRef]
- Veena, N.; Hundal, J.; Wadhwa, M.; Puniya, A. Factors Affecting the Milk Yield, Milk Composition and Physico-Chemical Parameters of Ghee in Lactating Crossbred Cows. IJDS 2021, 74, 68–73. [Google Scholar] [CrossRef]
- Gujar, G.; Choudhary, V.K.; Vivek, P.; Sodhi, M.; Choudhary, M.; Tiwari, M.; Masharing, N.; Mukesh, M. Characterization of Thermo-Physiological, Hematological, and Molecular Changes in Response to Seasonal Variations in Two Tropically Adapted Native Cattle Breeds of Bos Indicus Lineage in Hot Arid Ambience of Thar Desert. Int. J. Biometeorol. 2022, 66, 1515–1529. [Google Scholar] [CrossRef]
- Kim, W.S.; Nejad, J.G.; Peng, D.Q.; Jung, U.S.; Kim, M.J.; Jo, Y.H.; Jo, J.H.; Lee, J.S.; Lee, H.G. Identification of Heat Shock Protein Gene Expression in Hair Follicles as a Novel Indicator of Heat Stress in Beef Calves. Animal 2020, 14, 1502–1509. [Google Scholar] [CrossRef]
- Gill, J.K.; Arora, J.S.; Sunil Kumar, B.V.; Mukhopadhyay, C.S.; Kaur, S.; Kashyap, N. Cellular Thermotolerance Is Independent of HSF 1 Expression in Zebu and Crossbred Non-Lactating Cattle. Int. J. Biometeorol. 2017, 61, 1687–1693. [Google Scholar] [CrossRef]
- Wang, S.; Diller, K.R.; Aggarwal, S.J. Kinetics Study of Endogenous Heat Shock Protein 70 Expression. J. Biomech. Eng. 2003, 125, 794–797. [Google Scholar] [CrossRef]
- Kregel, K.C. Invited Review: Heat Shock Proteins: Modifying Factors in Physiological Stress Responses and Acquired Thermotolerance. J. Appl. Physiol. 2002, 92, 2177–2186. [Google Scholar] [CrossRef] [Green Version]
- Heled, Y.; Fleischmann, C.; Epstein, Y. Cytokines and Their Role in Hyperthermia and Heat Stroke. J. Basic Clin. Physiol. Pharmacol. 2013, 24, 85–96. [Google Scholar] [CrossRef]
- Alhussien, M.N.; Dang, A.K. Potential Roles of Neutrophils in Maintaining the Health and Productivity of Dairy Cows during Various Physiological and Physiopathological Conditions: A Review. Immunol. Res. 2019, 67, 21–38. [Google Scholar] [CrossRef]
- Tao, S.; Connor, E.E.; Bubolz, J.W.; Thompson, I.M.; do Amaral, B.C.; Hayen, M.J.; Dahl, G.E. Short Communication: Effect of Heat Stress during the Dry Period on Gene Expression in Mammary Tissue and Peripheral Blood Mononuclear Cells. J. Dairy Sci. 2013, 96, 378–383. [Google Scholar] [CrossRef]
- Kapila, N.; Sharma, A.; Kishore, A.; Sodhi, M.; Tripathi, P.K.; Mohanty, A.K.; Mukesh, M. Impact of Heat Stress on Cellular and Transcriptional Adaptation of Mammary Epithelial Cells in Riverine Buffalo (Bubalus Bubalis). PLoS ONE 2016, 11, e0157237. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wang, J.; Peng, D.; Li, G.; Chen, J.; Gu, X. Exposure to Heat-Stress Environment Affects the Physiology, Circulation Levels of Cytokines, and Microbiome in Dairy Cows. Sci. Rep. 2018, 8, 14606. [Google Scholar] [CrossRef] [Green Version]
- Rensis, F.D.; Scaramuzzi, R.J. Heat Stress and Seasonal Effects on Reproduction in the Dairy Cow—A Review. Theriogenology 2003, 60, 1139–1151. [Google Scholar] [CrossRef]
- Cordero, H.; Castro, M.A.S.; Algandar, Z.; Nevarez, L.; Rincon, G.; Medrano, J.F.; Speidel, S.E.; Enns, R.M.; Thomas, M.G. Research Article Genotypes within the Prolactin and Growth Hormone Insulin-like Growth Factor-I Pathways Associated with Milk Production in Heat Stressed Holstein Cattle: Genotypes and Milk Yield in Heat Stressed Holstein Cows. Genet. Mol. Res. 2017, 16. [Google Scholar] [CrossRef]
- Joudrey, E.M.; Lechniak, D.; Petrik, J.; King, W.A. Expression of Growth Hormone and Its Transcription Factor, Pit-1, in Early Bovine Development. Mol. Reprod. Dev. 2003, 64, 275–283. [Google Scholar] [CrossRef]
- Zhou, Y.; Akers, R.M.; Jiang, H. Growth Hormone Can Induce Expression of Four Major Milk Protein Genes in Transfected MAC-T Cells. J. Dairy Sci. 2008, 91, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Hull, K.L.; Harvey, S. Growth Hormone and Reproduction: A Review of Endocrine and Autocrine/Paracrine Interactions. Int. J. Endocrinol. 2014, 2014, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Pragna, P.; Sejian, V.; Soren, N.M.; Bagath, M.; Krishnan, G.; Beena, V.; Devi, P.I.; Bhatta, R. Summer Season Induced Rhythmic Alterations in Metabolic Activities to Adapt to Heat Stress in Three Indigenous (Osmanabadi, Malabari and Salem Black) Goat Breeds. Biol. Rhythm. Res. 2018, 49, 551–565. [Google Scholar] [CrossRef]
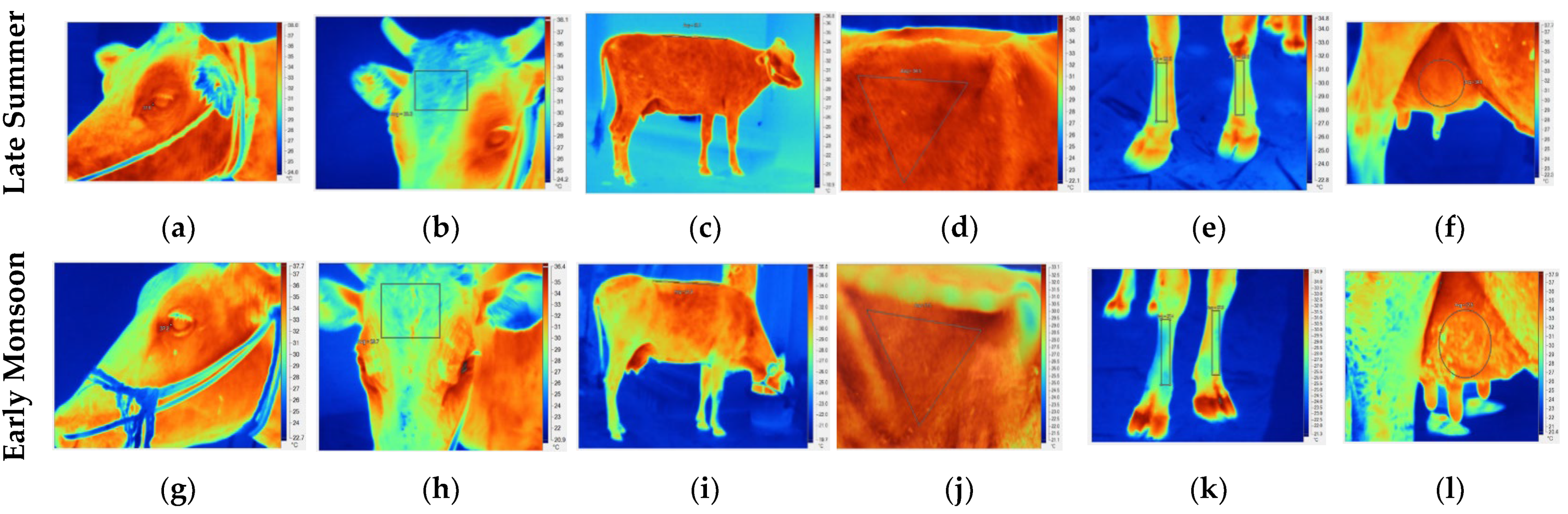

| Effects | PR (Beats/min) | RR (Breaths/min) | RT (°C) |
|---|---|---|---|
| FarmID | NS | * | NS |
| THI_1–7 | NS | NS | NS |
| 1 (THI < 75) | 69.19 ± 3.77 | 23.21 ± 2.38 | 37.14 ± 0.21 |
| 2 (THI ≥ 75) | 70.02 ± 2.39 | 29.90 ± 1.59 | 37.37 ± 0.14 |
| Season | NS | * | NS |
| Late summer | 72.94 ± 1.87 | 29.66 ± 1.17 | 37.40 ± 0.11 |
| Early monsoon | 66.27 ± 3.08 | 23.44 ± 1.93 | 37.11 ± 0.17 |
| Lactation stage | NS | NS | NS |
| Effects | Eye (°C) | Forehead (°C) | Back (°C) | Flank (°C) | Foreshank (°C) | Right Fore Quarter (°C) | Left Fore Quarter (°C) | Right Hind Quarter (°C) | Left Hind Quarter (°C) |
|---|---|---|---|---|---|---|---|---|---|
| FarmID | NS | NS | ** | ** | ** | ** | ** | ** | ** |
| THI_1–7 | NS | NS | ** | ** | ** | ** | ** | ** | ** |
| 1 (THI < 75) | 36.99 ± 0.12 | 29.63 ± 0.39 | 29.92 ± 0.42 | 32.56 ± 0.30 | 26.39 ± 0.38 | 33.12 ± 0.26 | 33.24 ± 0.29 | 32.95 ± 0.28 | 32.87 ± 0.31 |
| 2 (THI ≥ 75) | 37.24 ± 0.08 | 30.46 ± 0.25 | 32.21 ± 0.27 | 34.12 ± 0.19 | 28.74 ± 0.25 | 34.77 ± 0.17 | 34.68 ± 0.19 | 34.81 ± 0.18 | 34.90 ± 0.20 |
| Season | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| Late summer | 37.34 ± 0.05 | 30.69 ± 0.18 | 32.27 ± 0.20 | 34.53 ± 0.14 | 29.00 ± 0.18 | 34.85 ± 0.12 | 34.85 ± 0.14 | 34.76 ± 0.13 | 34.80 ± 0.14 |
| Early monsoon | 36.88 ± 0.09 | 29.41 ± 0.31 | 29.86 ± 0.34 | 32.15 ± 0.24 | 26.12 ± 0.31 | 33.04 ± 0.21 | 33.07 ± 0.24 | 33.00 ± 0.23 | 32.97 ± 0.25 |
| Lactation stage | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Effects | PLT (×10³/µL) | MPV (fL) | PDWc (%) | PCT (%) |
|---|---|---|---|---|
| Overall mean | 272.99 ± 137.30 | 5.70 ± 0.58 | 17.14 ± 0.69 | 0.15 ± 0.07 |
| THI_1–7 | ** | NS | ** | ** |
| 1 (THI < 75) | 440.10 ± 51.89 | 5.29 ± 0.24 | 16.51 ± 0.24 | 0.24 ± 0.03 |
| 2 (THI ≥ 75) | 132.12 ± 37.23 | 5.77 ± 0.16 | 17.51 ± 0.17 | 0.08 ± 0.02 |
| Season | NS | * | * | NS |
| Late summer | 240.17 ± 28.09 | 5.80 ± 0.11 | 17.26 ± 0.12 | 0.14 ± 0.01 |
| Early monsoon | 332.05 ± 45.46 | 5.25 ± 0.19 | 16.77 ± 0.19 | 0.17 ± 0.02 |
| Lactation stage | NS | NS | NS | NS |
| Reference range [23] | 151–359 | 5.3–7.2 | 15.6–18.4 | 0.12–0.25 |
| Effects | Milk Yield (L/Day) | Density (kg/m3) | Lactose (%) | SNF (%) | Protein (%) | Salts (%) |
|---|---|---|---|---|---|---|
| FarmID | ** | * | ** | ** | ** | * |
| THI_1–7 | NS | NS | NS | NS | NS | NS |
| 1 (THI < 75) | 11.93 ± 1.67 | 1022.85 ± 1.04 | 4.12 ± 0.11 | 7.50 ± 0.21 | 2.72 ± 0.09 | 0.62 ± 0.03 |
| 2 (THI ≥ 75) | 10.38 ± 1.07 | 1022.80 ± 0.77 | 3.94 ± 0.07 | 7.16 ± 0.13 | 2.62 ± 0.05 | 0.62 ± 0.02 |
| Season | * | * | * | * | NS | * |
| Late summer | 9.15 ± 0.76 | 1023.82 ± 0.66 | 4.14 ± 0.06 | 7.52 ± 0.11 | 2.74 ± 0.04 | 0.64 ± 0.02 |
| Early monsoon | 13.15 ± 1.34 | 1021.83 ± 0.77 | 3.91 ± 0.07 | 7.15 ± 0.14 | 2.61 ± 0.06 | 0.60 ± 0.02 |
| Lactation stage | ** | NS | NS | * | * | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velayudhan, S.M.; Brügemann, K.; Alam, S.; Yin, T.; Devaraj, C.; Sejian, V.; Schlecht, E.; König, S. Molecular, Physiological and Hematological Responses of Crossbred Dairy Cattle in a Tropical Savanna Climate. Biology 2023, 12, 26. https://doi.org/10.3390/biology12010026
Velayudhan SM, Brügemann K, Alam S, Yin T, Devaraj C, Sejian V, Schlecht E, König S. Molecular, Physiological and Hematological Responses of Crossbred Dairy Cattle in a Tropical Savanna Climate. Biology. 2023; 12(1):26. https://doi.org/10.3390/biology12010026
Chicago/Turabian StyleVelayudhan, Silpa Mullakkalparambil, Kerstin Brügemann, Shahin Alam, Tong Yin, Chinnasamy Devaraj, Veerasamy Sejian, Eva Schlecht, and Sven König. 2023. "Molecular, Physiological and Hematological Responses of Crossbred Dairy Cattle in a Tropical Savanna Climate" Biology 12, no. 1: 26. https://doi.org/10.3390/biology12010026
APA StyleVelayudhan, S. M., Brügemann, K., Alam, S., Yin, T., Devaraj, C., Sejian, V., Schlecht, E., & König, S. (2023). Molecular, Physiological and Hematological Responses of Crossbred Dairy Cattle in a Tropical Savanna Climate. Biology, 12(1), 26. https://doi.org/10.3390/biology12010026








