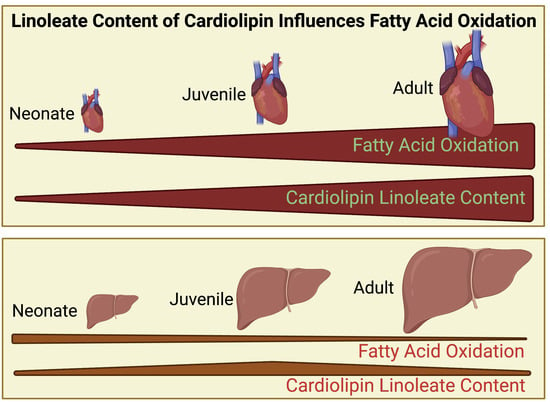
Linoleate-Enrichment of Mitochondrial Cardiolipin Molecular Species Is Developmentally Regulated and a Determinant of Metabolic Phenotype
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Studies
2.3. Cardiolipin Quantitation
2.4. Real-Time quantitative PCR (RT-qPCR)
2.5. Carnitine Palmitoyl Transferase I and II activity
2.6. Rat Cardiac primary Cell Isolation
2.7. Alteration of CL Fatty Acyl Side Chains in NRVMs
2.8. Agilent Seahorse
2.9. Statistical Analysis
3. Results
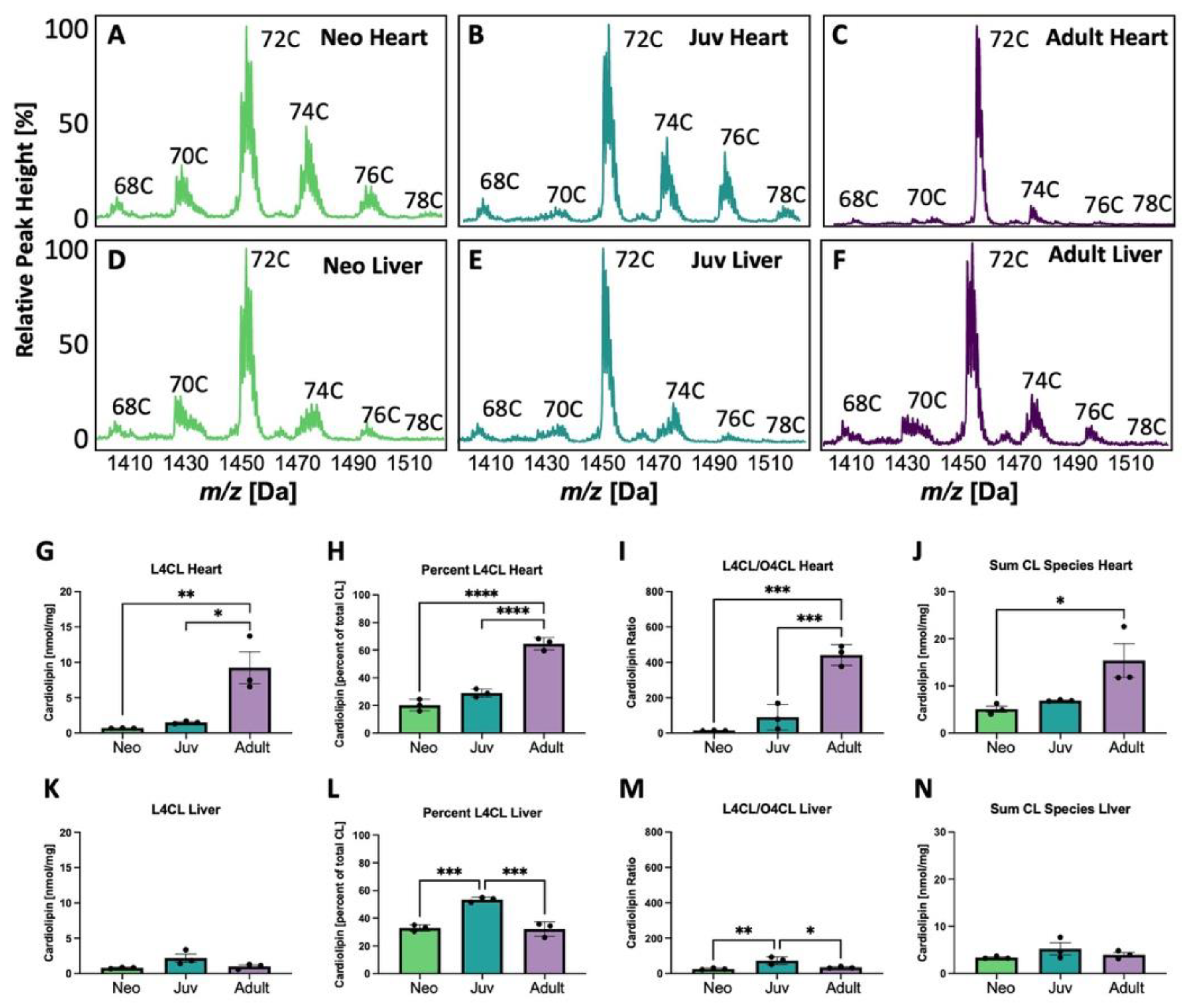
3.1. Cardiolipin Is Altered Proportionally with Age in Heart but Not Liver
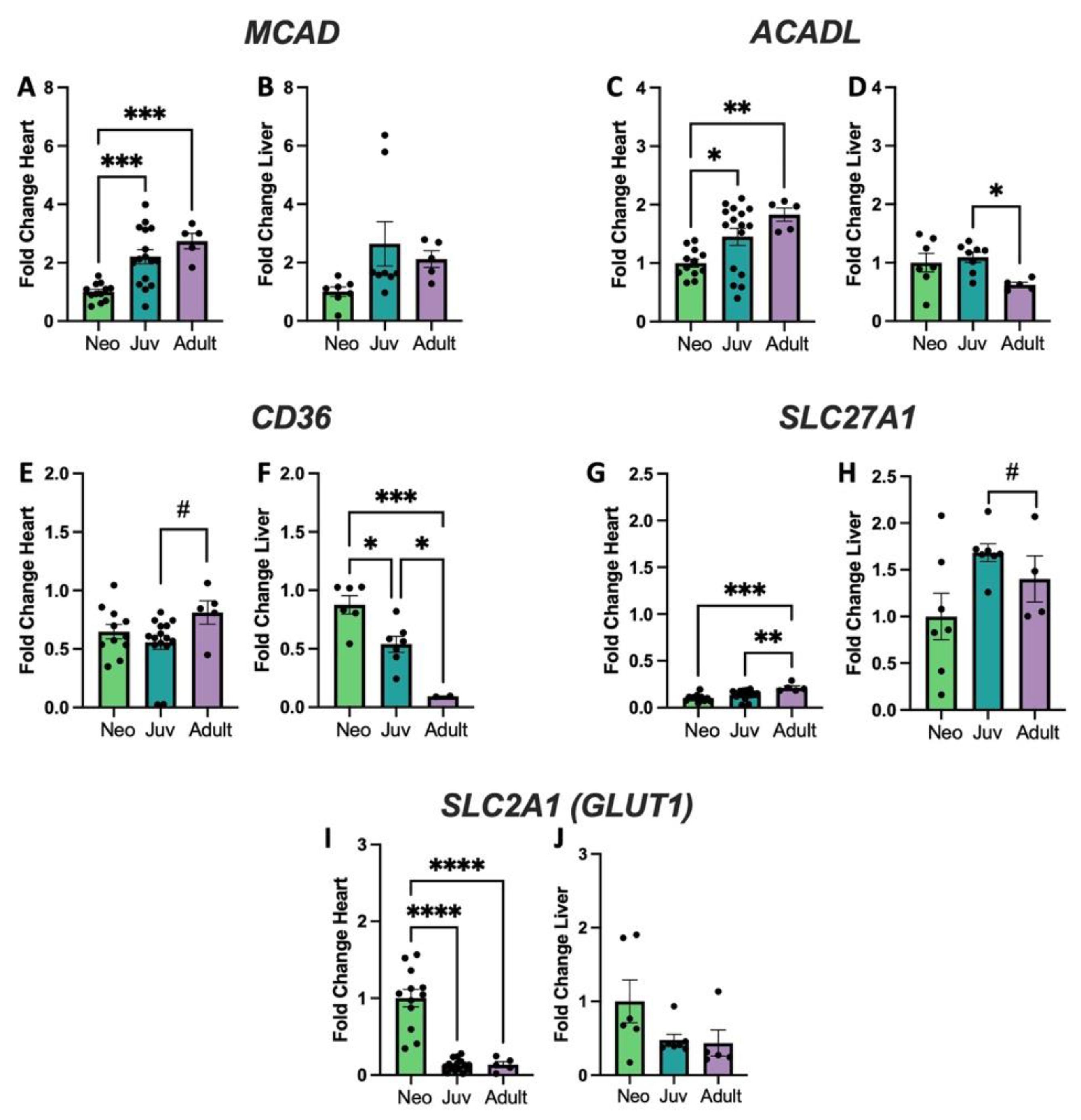
3.2. Genes Encoding Fatty Acid Metabolism and Glycolysis Show Changes with Age and Organ Type That Correlate with Cl Alterations
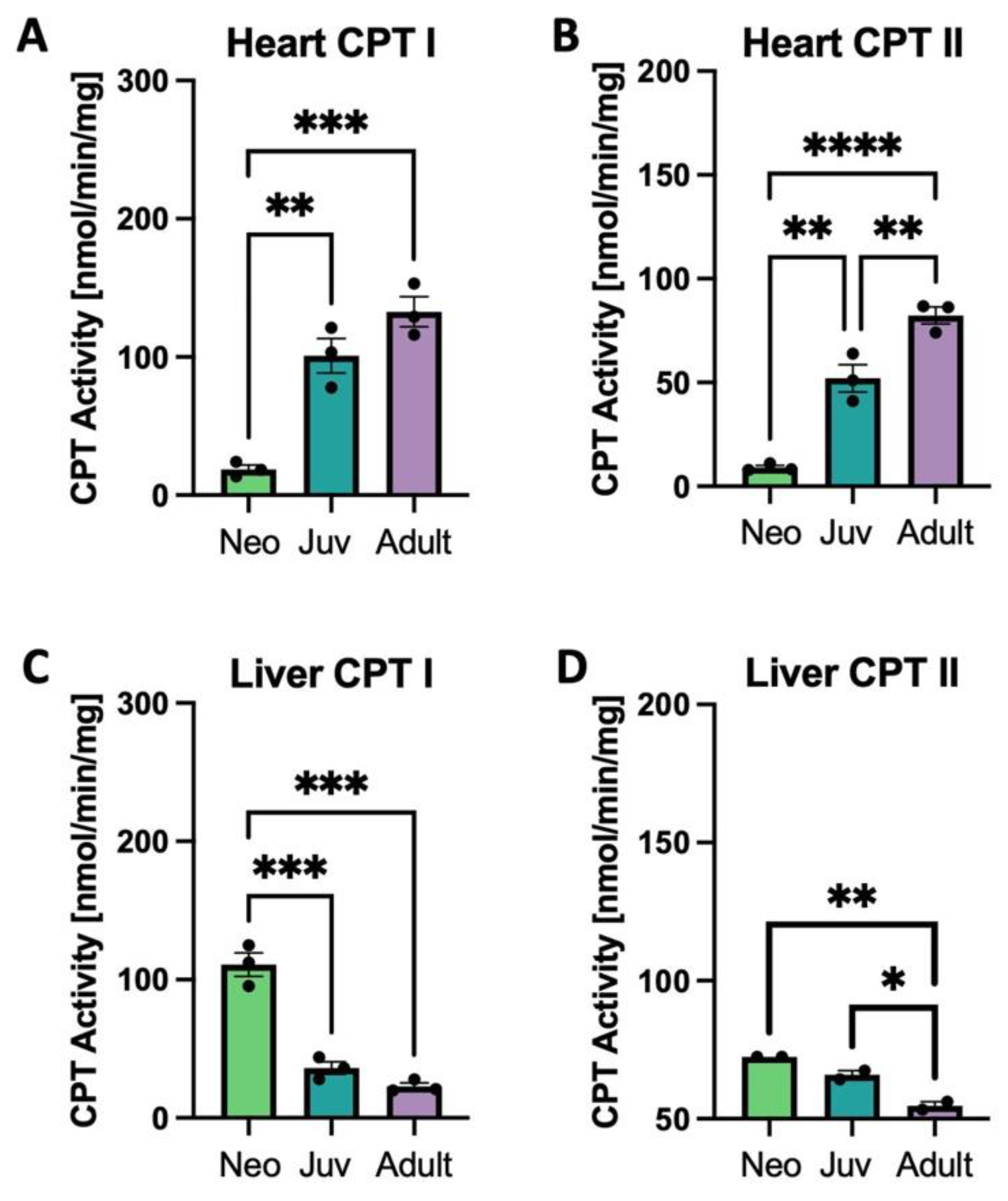
3.3. Carnitine Palmitoyltransferase Activity and Expression in the Heart and Liver
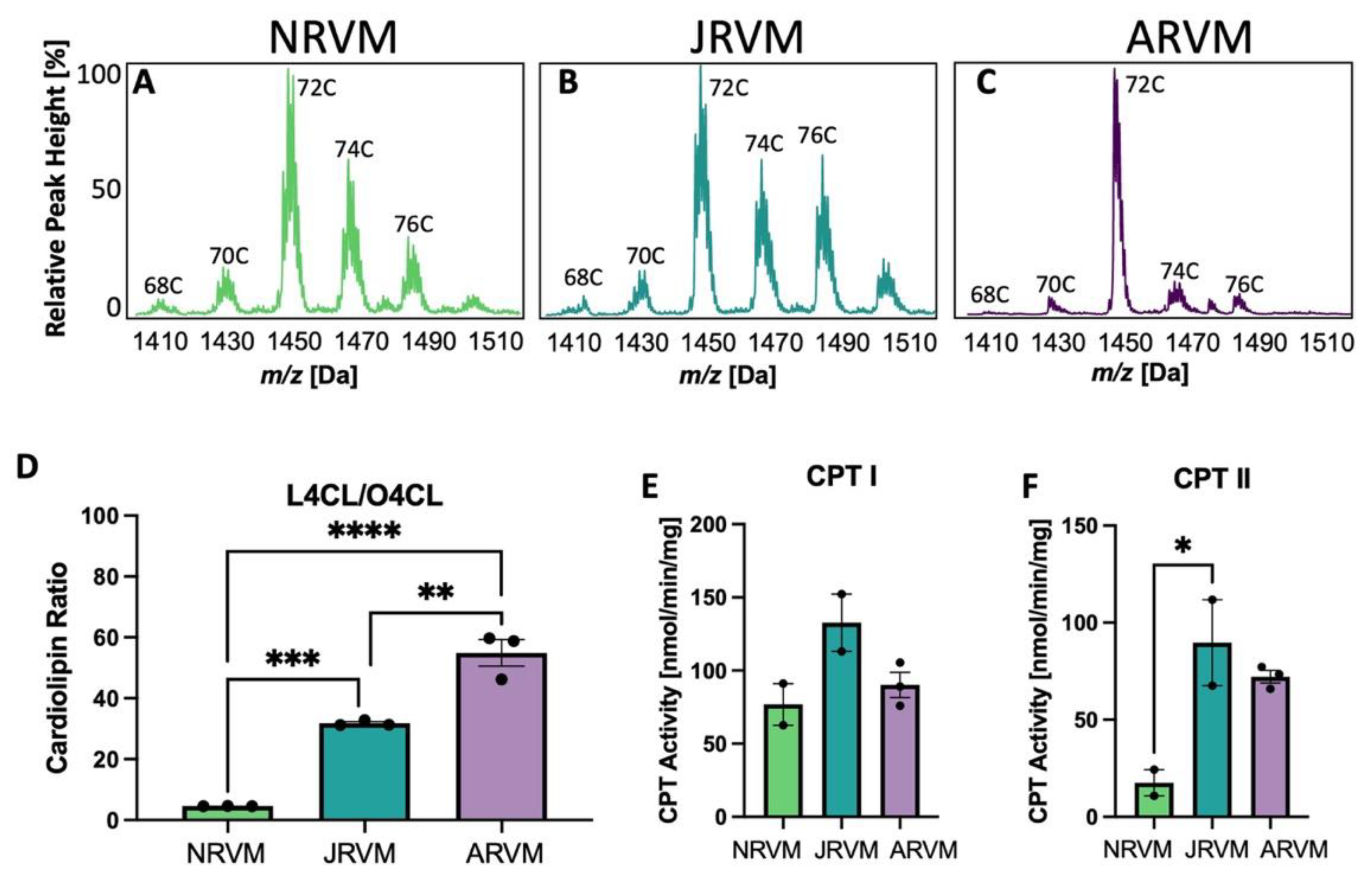
3.4. Cardiolipin and CPT Patterns Are Recapitulated in Primary Cultures of Neonatal, Juvenile, and Adult Cardiac Myocytes
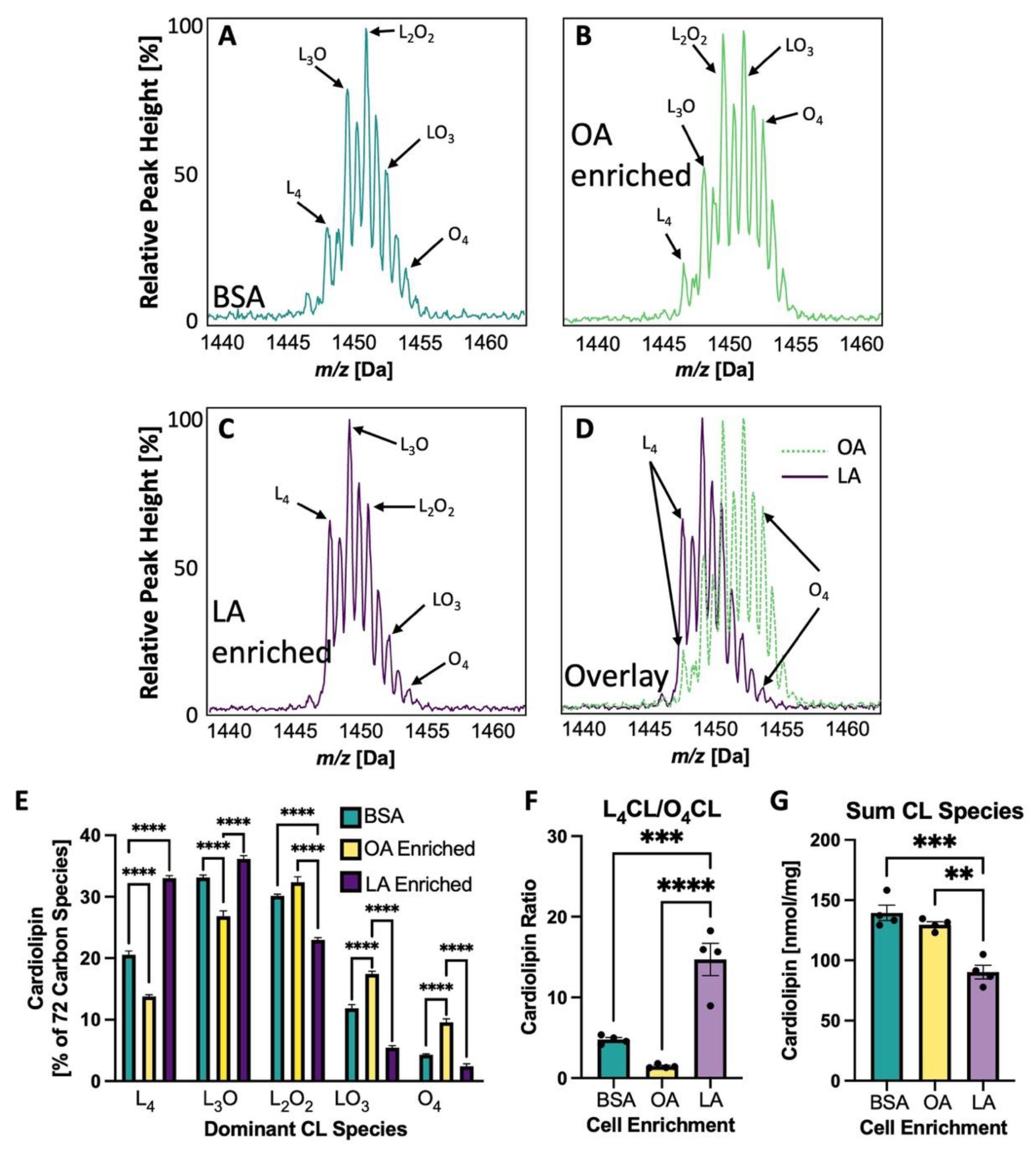
3.5. Cardiolipin Side Chains Are Experimentally Altered in NRVMs to Vary LA and OA Content
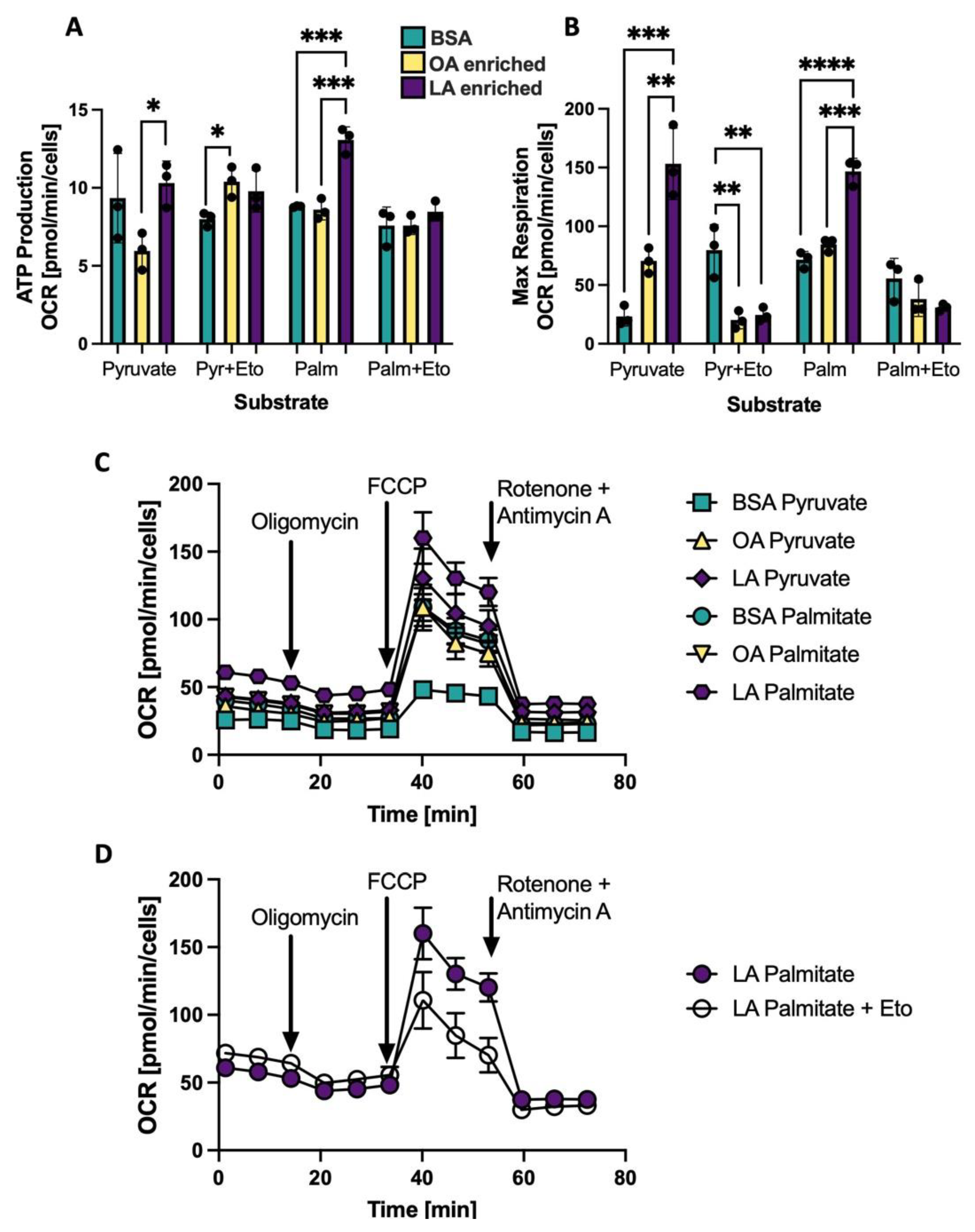
3.6. Changing the CL Profile Alters Oxygen Consumption Using Palmitate as a Substrate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Mohsen, A.W.; Mihalik, S.J.; Goetzman, E.S.; Vockley, J. Evidence for physical association of mitochondrial fatty acid oxidation and oxidative phosphorylation complexes. J. Biol. Chem. 2010, 285, 29834–29841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chicco, A.J.; Sparagna, G. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Physiol. Cell Physiol. 2007, 292, C33–C44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.; Mancuso, D.J.; Jiang, X.; Guan, S.; Yang, J.; Yang, K.; Han, X. Shotgun lipidomics reveals the temporally dependent, highly diversified cardiolipin profile in the mammalian brain: Temporally coordinated postnatal diversification of cardiolipin molecular species with neuronal remodeling. Biochemistry 2008, 47, 5869–5880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, S. The failing heart—An engine out of fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef] [Green Version]
- Allard, M.F.; Schonekess, B.O.; Henning, S.L.; English, D.R.; Lopaschuk, G.D. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am. J. Physiol. 1994, 267 Pt 2, H742–H750. [Google Scholar] [CrossRef]
- Lai, L.; Leone, T.C.; Keller, M.P.; Martin, O.J.; Broman, A.T.; Nigro, J.; Kelly, D.P. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: A multisystems approach. Circ. Heart Fail. 2014, 7, 1022–1031. [Google Scholar] [CrossRef] [Green Version]
- Chatfield, K.C.; Sparagna, G.C.; Sucharov, C.C.; Miyamoto, S.D.; Grudis, J.E.; Sobus, R.D.; Stauffer, B.L. Dysregulation of cardiolipin biosynthesis in pediatric heart failure. J. Mol. Cell Cardiol. 2014, 74, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Sparagna, G.C.; Chicco, A.J.; Murphy, R.C.; Bristow, M.R.; Johnson, C.A.; Rees, M.L.; Moore, R.L. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J. Lipid Res. 2007, 48, 1559–1570. [Google Scholar] [CrossRef] [Green Version]
- Sparagna, G.C.; Johnson, C.A.; McCune, S.A.; Moore, R.L.; Murphy, R.C. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J. Lipid Res. 2005, 46, 1196–1204. [Google Scholar] [CrossRef]
- Chicco, A.J.; Sparagna, G.C.; McCune, S.A.; Johnson, C.A.; Murphy, R.C.; Bolden, D.A.; Moore, R.L. Linoleate-rich high-fat diet decreases mortality in hypertensive heart failure rats compared with lard and low-fat diets. Hypertension 2008, 52, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cade, W.T.; Bohnert, K.L.; Peterson, L.R.; Patterson, B.W.; Bittel, A.J.; Okunade, A.L.; Reeds, D.N. Blunted fat oxidation upon submaximal exercise is partially compensated by enhanced glucose metabolism in children, adolescents, and young adults with Barth syndrome. J. Inherit. Metab. Dis. 2019, 42, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Le, C.H.; Benage, L.G.; Specht, K.S.; Puma, L.C.L.; Mulligan, C.M.; Heuberger, A.L.; Chicco, A.J. Tafazzin deficiency impairs CoA-dependent oxidative metabolism in cardiac mitochondria. J. Biol. Chem. 2020, 295, 12485–12497. [Google Scholar] [CrossRef] [PubMed]
- Chatfield, K.C.; Sparagna, G.C.; Specht, K.S.; Whitcomb, L.A.; Omar, A.K.; Miyamoto, S.D.; Chicco, A.J. Long-chain fatty acid oxidation and respiratory complex I deficiencies distinguish Barth Syndrome from idiopathic pediatric cardiomyopathy. J. Inherit. Metab. Dis. 2022, 45, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.R.; Hong, Y.M.; Boriack, R.L.; Bennett, M.J. Effect of L-carnitine supplementation on cardiac carnitine palmitoyltransferase activities and plasma carnitine concentrations in adriamycin-treated rats. Pediatr. Res. 2003, 53, 788–792. [Google Scholar] [CrossRef] [Green Version]
- Sucharov, C.C.; Mariner, P.D.; Nunley, K.R.; Long, C.; Leinwand, L.; Bristow, M.R. A β1-adrenergic receptor CaM kinase II-dependent pathway mediates cardiac myocyte fetal gene induction. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H1299–H1308. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.H.; Schmidt, W.; Fritz, K.S.; Jeong, M.Y.; Cammarato, A.; Foster, D.B.; Woulfe, K.C. Site-specific acetyl-mimetic modification of cardiac troponin I modulates myofilament relaxation and calcium sensitivity. J. Mol. Cell. Cardiol. 2020, 139, 135–147. [Google Scholar] [CrossRef] [Green Version]
- Woulfe, K.C.; Ferrara, C.; Pioner, J.M.; Mahaffey, J.H.; Coppini, R.; Scellini, B.; Jeong, M. A novel method of isolating myofibrils from primary cardiomyocyte culture suitable for myofibril mechanical study. Front. Cardiovasc. Med. 2019, 6, 12. [Google Scholar] [CrossRef]
- Corrado, M.; Edwards-Hicks, J.; Villa, M.; Flachsmann, L.J.; Sanin, D.E.; Jacobs, M.; Pearce, E.L. Dynamic Cardiolipin Synthesis Is Required for CD8(+) T Cell Immunity. Cell Metab. 2020, 32, 981–995.e7. [Google Scholar] [CrossRef]
- Mulligan, C.M.; Sparagna, G.C.; Le, C.H.; De Mooy, A.B.; Routh, M.A.; Holmes, M.G.; Chicco, A.J. Dietary linoleate preserves cardiolipin and attenuates mitochondrial dysfunction in the failing rat heart. Cardiovasc. Res. 2012, 94, 460–468. [Google Scholar] [CrossRef]
- Porter, G.A., Jr.; Hom, J.R.; Hoffman, D.L.; Quintanilla, R.A.; de Mesy Bentley, K.L.; Sheu, S.S. Bioenergetics, mitochondria, and cardiac myocyte differentiation. Prog. Pediatr. Cardiol. 2011, 31, 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [PubMed] [Green Version]
- Prola, A.; Blondelle, J.; Vandestienne, A.; Piquereau, J.; Denis, R.G.; Guyot, S.; Pilot-Storck, F. Cardiolipin content controls mitochondrial coupling and energetic efficiency in muscle. Sci. Adv. 2021, 7, eabd6322. [Google Scholar] [CrossRef] [PubMed]
- Claypool, S.M.; Koehler, C. The complexity of cardiolipin in health and disease. Trends Biochem. Sci. 2012, 37, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Chabowski, A.; Coort, S.L.; Calles-Escandon, J.; Tandon, N.N.; Glatz, J.F.; Luiken, J.J.; Bonen, A. Insulin stimulates fatty acid transport by regulating expression of FAT/CD36 but not FABPpm. Am. J. Physiol.-Endocrinol. Metab. 2004, 287, E781–E789. [Google Scholar] [CrossRef] [Green Version]
- Young, M.E.; Yan, J.; Razeghi, P.; Cooksey, R.C.; Guthrie, P.H.; Stepkowski, S.M.; Taegtmeyer, H. Proposed regulation of gene expression by glucose in rodent heart. Gene Regul. Syst. Biol. 2007, 1, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Cole, R.M.; Angelotti, A.; Sparagna, G.C.; Ni, A.; Belury, M.A. Linoleic Acid-Rich Oil Alters Circulating Cardiolipin Species and Fatty Acid Composition in Adults: A Randomized Controlled Trial. Mol. Nutr. Food Res. 2022, 66, e2101132. [Google Scholar] [CrossRef]
- Schlame, M.; Ren, M.; Xu, Y.; Greenberg, M.L.; Haller, I. Molecular symmetry in mitochondrial cardiolipins. Chem. Phys. Lipids 2005, 138, 38–49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparagna, G.C.; Jonscher, R.L.; Shuff, S.R.; Phillips, E.K.; Wilson, C.E.; Woulfe, K.C.; Garcia, A.M.; Stauffer, B.L.; Chatfield, K.C. Linoleate-Enrichment of Mitochondrial Cardiolipin Molecular Species Is Developmentally Regulated and a Determinant of Metabolic Phenotype. Biology 2023, 12, 32. https://doi.org/10.3390/biology12010032
Sparagna GC, Jonscher RL, Shuff SR, Phillips EK, Wilson CE, Woulfe KC, Garcia AM, Stauffer BL, Chatfield KC. Linoleate-Enrichment of Mitochondrial Cardiolipin Molecular Species Is Developmentally Regulated and a Determinant of Metabolic Phenotype. Biology. 2023; 12(1):32. https://doi.org/10.3390/biology12010032
Chicago/Turabian StyleSparagna, Genevieve C., Raleigh L. Jonscher, Sydney R. Shuff, Elisabeth K. Phillips, Cortney E. Wilson, Kathleen C. Woulfe, Anastacia M. Garcia, Brian L. Stauffer, and Kathryn C. Chatfield. 2023. "Linoleate-Enrichment of Mitochondrial Cardiolipin Molecular Species Is Developmentally Regulated and a Determinant of Metabolic Phenotype" Biology 12, no. 1: 32. https://doi.org/10.3390/biology12010032
APA StyleSparagna, G. C., Jonscher, R. L., Shuff, S. R., Phillips, E. K., Wilson, C. E., Woulfe, K. C., Garcia, A. M., Stauffer, B. L., & Chatfield, K. C. (2023). Linoleate-Enrichment of Mitochondrial Cardiolipin Molecular Species Is Developmentally Regulated and a Determinant of Metabolic Phenotype. Biology, 12(1), 32. https://doi.org/10.3390/biology12010032