Analysis of Vibration Frequency and Direction for Facilitating Upper-Limb Muscle Activity
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
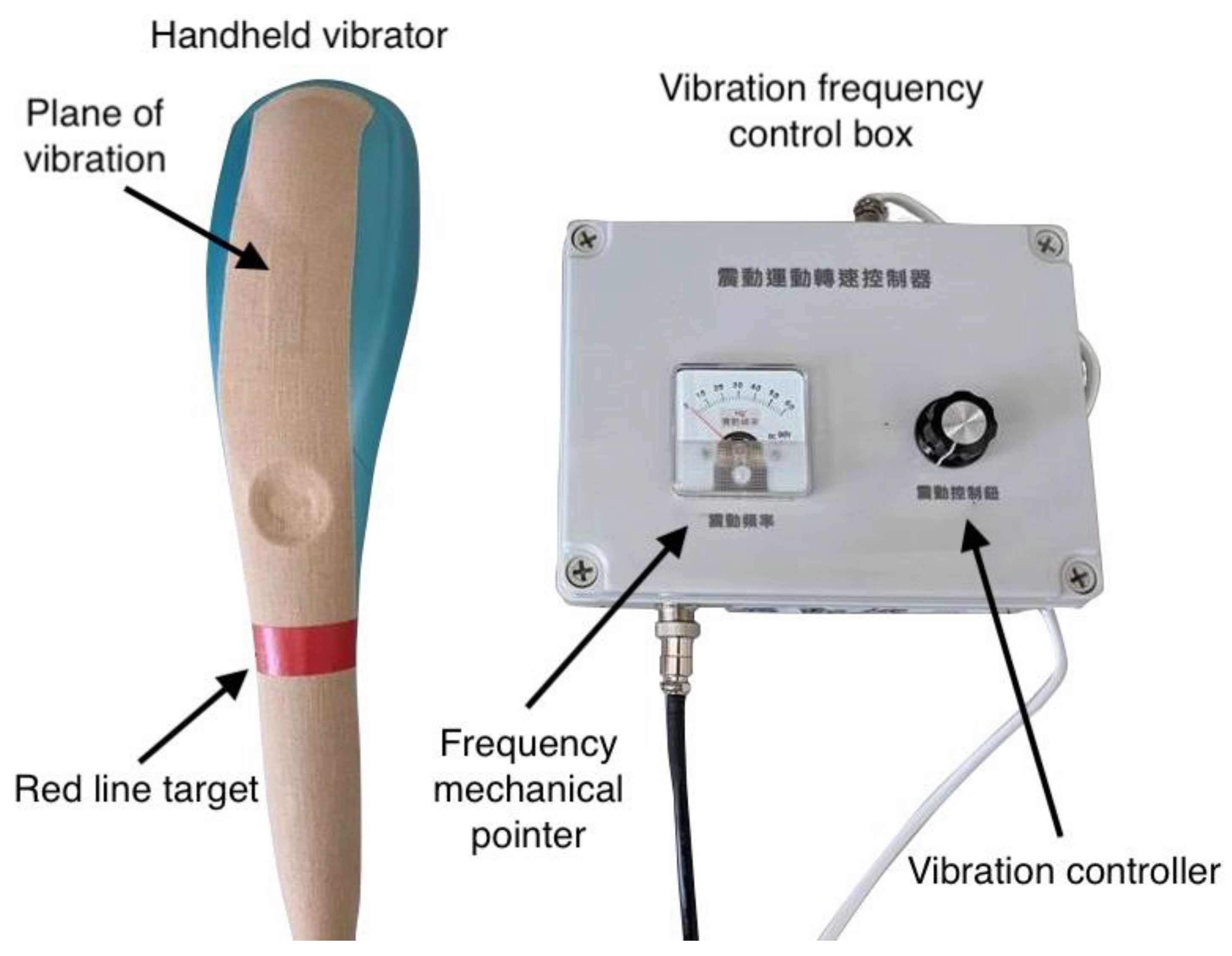
2.2. Research Device and Data Processing
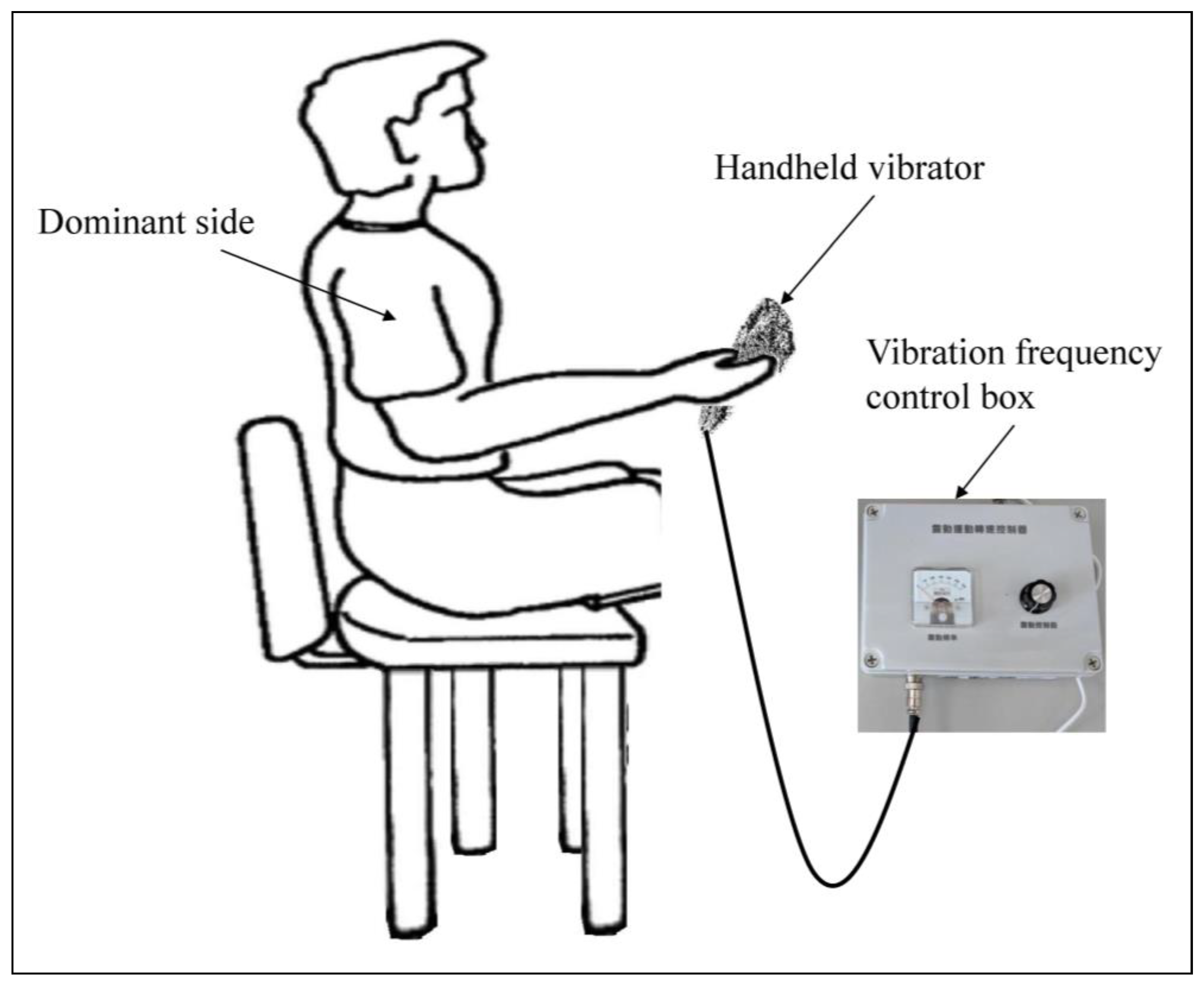
2.3. Experimental Procedures and Positioning of Participants
2.4. EMG Analysis
2.5. Statistical Analysis
3. Results
3.1. Participants
3.2. EMG Analysis
4. Discussion
4.1. Vibrator Types and Applications in Upper-Limb Muscle Activity Induction
4.2. Influence of Vibration Frequency on Upper-Limb Muscle Activation
4.3. Vibration Direction Affects Upper-Limb Muscle Activation
4.4. Study Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shumway-Cook, A.; Woollacott, M.H. Motor Control: Theory and Practical Applications; Lippincqtt Williams & Wilkins: Philadelphia, PA, USA, 1995. [Google Scholar]
- Roos, M.R.; Rice, C.L.; Vandervoort, A.A. Age-related changes in motor unit function. Muscle Nerve 1997, 20, 679–690. [Google Scholar] [CrossRef]
- Desrosiers, J.; Hebert, R.; Bravo, G.; Rochette, A. Age-related changes in upper extremity performance of elderly people: A longitudinal study. Exp. Gerontol. 1999, 34, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Yu, R.; Wong, M.; Yeung, F.; Wong, M.; Lum, C. Frailty Screening in the Community Using the FRAIL Scale. J. Am. Med. Dir. Assoc. 2015, 16, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Mühlberg, W.; Sieber, C. Sarcopenia and frailty in geriatric patients: Implications for training and prevention. Z. Gerontol. Geriatr. 2004, 37, 2–8. [Google Scholar] [CrossRef]
- Seene, T.; Kaasik, P. Muscle weakness in the elderly: Role of sarcopenia, dynapenia, and possibilities for rehabilitation. Eur. Rev. Aging Phys. Act. 2012, 9, 109–117. [Google Scholar] [CrossRef]
- Teixeira-Santos, L.; Bobrowicz-Campos, E.; Parola, V.; Coelho, A.; Gil, I.; Almeida, M.d.L.; Apóstolo, J.L. What Is the Relationship between Lifestyle and Frailty Status? Data from the Portuguese Multicentre Descriptive Study. Nurs. Rep. 2022, 12, 39–49. [Google Scholar] [CrossRef]
- Shechtman, O.; Mann, W.C.; Justiss, M.D.; Tomita, M. Grip strength in the frail elderly. Am. J. Phys. Med. Rehabil. 2004, 83, 819–826. [Google Scholar] [CrossRef]
- Lin, C.H.; Chou, L.W.; Wei, S.H.; Lieu, F.K.; Chiang, S.L.; Sung, W.H. Influence of aging on bimanual coordination control. Exp. Gerontol. 2014, 53, 40–47. [Google Scholar] [CrossRef]
- Lee, S.C.; Wu, L.C.; Chiang, S.L.; Lu, L.H.; Chen, C.Y.; Lin, C.H.; Ni, C.H.; Lin, C.H. Validating the Capability for Measuring Age-Related Changes in Grip-Force Strength Using a Digital Hand-Held Dynamometer in Healthy Young and Elderly Adults. BioMed Res. Int. 2020, 2020, 6936879. [Google Scholar] [CrossRef]
- Lin, C.H.; Sung, W.H.; Chiang, S.L.; Lee, S.C.; Lu, L.H.; Wang, P.C.; Wang, X.M. Influence of aging and visual feedback on the stability of hand grip control in elderly adults. Exp. Gerontol. 2019, 119, 74–81. [Google Scholar] [CrossRef]
- Kao, C.-H.; Chiang, S.-L.; Chou, L.-W.; Lin, C.-H.; Lu, Y.-H.; Lu, L.-H.; Wang, X.-M.; Lin, C.-H. Validation of Vibration Exercises on Enhancing Muscle Strength and Upper Limb Functionality among Pre-Frail Community-Dwelling Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 14509. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-S.; Kuo, S.-F.; Lee, I.; Lu, L.-H.; Chen, P.-Y.; Wang, P.-C.; Lai, C.-H.; Wang, X.-M.; Lin, C.-H. The impact of aging and reaching movements on grip stability control during manual precision tasks. BMC Geriatr. 2021, 21, 703. [Google Scholar] [CrossRef] [PubMed]
- Greenlund, K.J.; Keenan, N.L.; Clayton, P.F.; Pandey, D.K.; Hong, Y. Public Health Options for Improving Cardiovascular Health Among Older Americans. Am. J. Public Health 2012, 102, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Hewett, T.E.; Ford, K.R.; Levine, P.; Page, S.J. Reaching kinematics to measure motor changes after mental practice in stroke. Top. Stroke Rehabil. 2007, 14, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Page, S.J.; Levine, P.; Leonard, A.; Szaflarski, J.P.; Kissela, B.M. Modified constraint-induced therapy in chronic stroke: Results of a single-blinded randomized controlled trial. Phys. Ther. 2008, 88, 333–340. [Google Scholar] [CrossRef]
- Cirstea, M.C.; Levin, M.F. Improvement of arm movement patterns and endpoint control depends on type of feedback during practice in stroke survivors. Neurorehabil. Neural Repair 2007, 21, 398–411. [Google Scholar] [CrossRef]
- Lai, C.H.; Sung, W.H.; Chiang, S.L.; Lu, L.H.; Lin, C.H.; Tung, Y.C.; Lin, C.H. Bimanual coordination deficits in hands following stroke and their relationship with motor and functional performance. J. Neuroeng. Rehabil. 2019, 16, 101. [Google Scholar] [CrossRef]
- Hendricks, H.T.; van Limbeek, J.; Geurts, A.C.; Zwarts, M.J. Motor recovery after stroke: A systematic review of the literature. Arch. Phys. Med. Rehabil. 2002, 83, 1629–1637. [Google Scholar] [CrossRef]
- Bogaerts, A.C.; Delecluse, C.; Claessens, A.L.; Troosters, T.; Boonen, S.; Verschueren, S.M. Effects of whole body vibration training on cardiorespiratory fitness and muscle strength in older individuals (a 1-year randomised controlled trial). Age Ageing 2009, 38, 448–454. [Google Scholar] [CrossRef]
- Pang, M.Y.; Lau, R.W.; Yip, S.P. The effects of whole-body vibration therapy on bone turnover, muscle strength, motor function, and spasticity in chronic stroke: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2013, 49, 439–450. [Google Scholar]
- Morel, D.S.; Marín, P.J.; Moreira-Marconi, E.; Dionello, C.F.; Bernardo-Filho, M. Can Whole-Body Vibration Exercises in Different Positions Change Muscular Activity of Upper Limbs? A Randomized Trial. Dose-Response 2018, 16, 1559325818804361. [Google Scholar] [CrossRef] [PubMed]
- Stania, M.; Juras, G.; Słomka, K.; Chmielewska, D.; Król, P. The application of whole-body vibration in physiotherapy—A narrative review. Acta Physiol. Hung. 2016, 103, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.J. Vibration exercise: The potential benefits. Int. J. Sports Med. 2011, 32, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Khan, A.A.; Farooq, M. Effect of whole-body vibration on neuromuscular performance: A literature review. Work 2018, 59, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, U.; Villagra, H.A.; Oliva, L.L.; Marconi, N.F. EMG activity of upper limb on spinal cord injury individuals during whole-body vibration. Physiol. Int. 2016, 103, 361–367. [Google Scholar] [CrossRef]
- Cristino de Souza, A.L.; Mendonca, V.A.; Coelho de Oliveira, A.C.; Ferreira da Fonseca, S.; Mello Santos, L.M.; Cunha Fernandes, J.S.; Leite, H.R.; Luiz de Mendonca Martins, F.; Marcia Dos Santos, J.; de Fatima Silva, A.; et al. Whole body vibration in the static modified push-up position in untrained healthy women stimulates neuromuscular system potentiating increased handgrip myogenic response. J. Bodyw. Mov. Ther. 2020, 24, 233–238. [Google Scholar] [CrossRef]
- Jones, M.T.; Martin, J.R.; Jagim, A.R.; Oliver, J.M. Effect of Direct Whole-Body Vibration on Upper-Body Muscular Power in Recreational, Resistance-Trained Men. J. Strength Cond. Res. 2017, 31, 1371–1377. [Google Scholar] [CrossRef]
- Ashnagar, Z.; Shadmehr, A.; Hadian, M.; Talebian, S.; Jalaei, S. The effects of whole body vibration on EMG activity of the upper extremity muscles in static modified push up position. J. Back Musculoskelet. Rehabil. 2016, 29, 557–563. [Google Scholar] [CrossRef]
- Costa, V.; da Silva, F.F.; de Lima, R.M.; Mezêncio, B.; Ferreira, J.C. Mechanical Vibration Increases EMG Activity But Does Not Affect Strength Resistance Performance. J. Prof. Exerc. Physiol. 2019, 16, 120–129. [Google Scholar]
- Lee, J.S.; Kim, C.Y.; Kim, H.D. Short-Term Effects of Whole-Body Vibration Combined with Task-Related Training on Upper Extremity Function, Spasticity, and Grip Strength in Subjects with Poststroke Hemiplegia: A Pilot Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2016, 95, 608–617. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Kim, H.; Park, C.B. Effects of Whole-Body Vibration on Upper Extremity Function and Grip Strength in Patients with Subacute Stroke: A Randomised Single-Blind Controlled Trial. Occup. Ther. Int. 2019, 2019, 5820952. [Google Scholar] [CrossRef] [PubMed]
- Boo, J.A.; Moon, S.H.; Lee, S.M.; Choi, J.H.; Park, S.E. Effect of whole-body vibration exercise in a sitting position prior to therapy on muscle tone and upper extremity function in stroke patients. J. Phys. Ther. Sci. 2016, 28, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Pollock, R.D.; Woledge, R.C.; Mills, K.R.; Martin, F.C.; Newham, D.J. Muscle activity and acceleration during whole body vibration: Effect of frequency and amplitude. Clin. Biomech. 2010, 25, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Wakeling, J. Whole body vibration exercise: Are vibrations good for you? Br. J. Sports Med. 2005, 39, 585–589. [Google Scholar] [CrossRef]
- Lee, G. Does whole-body vibration training in the horizontal direction have effects on motor function and balance of chronic stroke survivors? A preliminary study. J. Phys. Ther. Sci. 2015, 27, 1133–1136. [Google Scholar] [CrossRef]
- Morel, D.S.; Moreira-Marconi, E.; Neto, S.B.S.; Domingos, L.L.P.; de Souza, P.L.; Caputo, D.; Costa, G.D.; de Figueiredo, C.F.; Carmo, R.C.R.; de Paiva, P.C.; et al. Effects of Whole Body Vibration Intervention on Handgrip Strength of Brazilian Healthy Soldiers. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 28–32. [Google Scholar] [CrossRef]
- Kiiski, J.; Heinonen, A.; Jarvinen, T.L.; Kannus, P.; Sievanen, H. Transmission of vertical whole body vibration to the human body. J. Bone Miner. Res. 2008, 23, 1318–1325. [Google Scholar] [CrossRef]
- Hazell, T.J.; Jakobi, J.M.; Kenno, K.A. The effects of whole-body vibration on upper- and lower-body EMG during static and dynamic contractions. Appl. Physiol. Nutr. Metab. 2007, 32, 1156–1163. [Google Scholar] [CrossRef]
- Kurt, C.; Pekunlu, E. Acute effect of whole body vibration on isometric strength, squat jump, and flexibility in well-trained combat athletes. Biol. Sport 2015, 32, 115–122. [Google Scholar] [CrossRef]
- Cochrane, D.J.; Hawke, E.J. Effects of acute upper-body vibration on strength and power variables in climbers. J. Strength Cond. Res. 2007, 21, 527–531. [Google Scholar] [CrossRef]
- Sanudo, B.; Taiar, R.; Furness, T.; Bernardo-Filho, M. Clinical Approaches of Whole-Body Vibration Exercises in Individuals with Stroke: A Narrative Revision. Rehabil. Res. Pract. 2018, 2018, 8180901. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Pang, M.Y.C. Muscle activity and vibration transmissibility during whole-body vibration in chronic stroke. Scand. J. Med. Sci. Sports 2019, 29, 816–825. [Google Scholar] [CrossRef]
- Lu, J.; Xu, G.; Wang, Y. Effects of whole body vibration training on people with chronic stroke: A systematic review and meta-analysis. Top. Stroke Rehabil. 2015, 22, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liao, L.R.; Pang, M.Y. Effects of whole body vibration on muscle spasticity for people with central nervous system disorders: A systematic review. Clin. Rehabil. 2017, 31, 23–33. [Google Scholar] [CrossRef]
- Bogaerts, A.; Delecluse, C.; Claessens, A.L.; Coudyzer, W.; Boonen, S.; Verschueren, S.M. Impact of whole-body vibration training versus fitness training on muscle strength and muscle mass in older men: A 1-year randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, D.R.; Weir, P.L.; Leigh, L.; Kenno, K. The Effects of a Whole-Body Advanced Vibration Exercise Program on Flexibility, Balance, and Strength in Seniors. Phys. Occup. Ther. Geriatr. 2010, 28, 225–234. [Google Scholar] [CrossRef]
- Marin, P.J.; Ferrero, C.M.; Menendez, H.; Martin, J.; Herrero, A.J. Effects of whole-body vibration on muscle architecture, muscle strength, and balance in stroke patients: A randomized controlled trial. Am. J. Phys. Med. Rehabil. 2013, 92, 881–888. [Google Scholar] [CrossRef]
- Tihanyi, T.K.; Horvath, M.; Fazekas, G.; Hortobagyi, T.; Tihanyi, J. One session of whole body vibration increases voluntary muscle strength transiently in patients with stroke. Clin. Rehabil. 2007, 21, 782–793. [Google Scholar] [CrossRef]
- Armstrong, W.J.; Nestle, H.N.; Grinnell, D.C.; Cole, L.D.; Van Gilder, E.L.; Warren, G.S.; Capizzi, E.A. The acute effect of whole-body vibration on the hoffmann reflex. J. Strength Cond. Res. 2008, 22, 471–476. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Masani, K.; Alizadeh-Meghrazi, M.; Popovic, M.R.; Craven, B.C. Acute effects of whole body vibration during passive standing on soleus H-reflex in subjects with and without spinal cord injury. Neurosci. Lett. 2010, 482, 66–70. [Google Scholar] [CrossRef]
- Dias, J.A.; Ovando, A.C.; Külkamp, W.; Borges Junior, N.G. Força de preensão palmar: Métodos de avaliação e fatores que influenciam a medida. Rev. Bras. Cineantropometria Desempenho Hum. 2010, 12, 209–216. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Pujari, A.N.; Neilson, R.D.; Aphale, S.S.; Cardinale, M. Upper limb vibration prototype with sports and rehabilitation applications: Development, evaluation and preliminary study. Healthc. Technol. Lett. 2017, 4, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Ju, Y.Y.; Chen, C.L.; Chuang, L.L.; Cheng, C.H. Effects of whole body vibration on spasticity and lower extremity function in children with cerebral palsy. Hum. Mov. Sci. 2015, 39, 65–72. [Google Scholar] [CrossRef]
- Lau, R.W.; Yip, S.P.; Pang, M.Y. Whole-body vibration has no effect on neuromotor function and falls in chronic stroke. Med. Sci. Sports Exerc. 2012, 44, 1409–1418. [Google Scholar] [CrossRef]
- Lee, D.K.; Han, J.W. Effects of active vibration exercise using a Flexi-Bar on balance and gait in patients with chronic stroke. J. Phys. Ther. Sci. 2018, 30, 832–834. [Google Scholar] [CrossRef][Green Version]
- Liao, L.R.; Ng, G.Y.; Jones, A.Y.; Chung, R.C.; Pang, M.Y. Effects of Vibration Intensity, Exercise, and Motor Impairment on Leg Muscle Activity Induced by Whole-Body Vibration in People With Stroke. Phys Ther. 2015, 95, 1617–1627. [Google Scholar] [CrossRef]
- Bovenzi, M. Metrics of whole-body vibration and exposure-response relationship for low back pain in professional drivers: A prospective cohort study. Int. Arch. Occup. Environ. Health 2009, 82, 893–917. [Google Scholar] [CrossRef]
- Cardinale, M.; Lim, J. The acute effects of two different whole body vibration frequencies on vertical jump performance. Med. Dello Sport 2003, 56, 287–292. [Google Scholar]
- Hwang, K.J.; Ryu, Y.U. Whole body vibration may have immediate adverse effects on the postural sway of stroke patients. J. Phys. Ther. Sci. 2016, 28, 473–477. [Google Scholar] [CrossRef]
- Liao, L.R.; Ng, G.Y.; Jones, A.Y.; Huang, M.Z.; Pang, M.Y. Whole-Body Vibration Intensities in Chronic Stroke: A Randomized Controlled Trial. Med. Sci. Sports Exerc. 2016, 48, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Nawayseh, N.; Sinan, H.A.; Alteneiji, S.; Hamdan, S. Effect of gender on the biodynamic responses to vibration induced by a whole-body vibration training machine. Proc. Inst. Mech. Eng. H—J. Eng. Med. 2019, 233, 383–392. [Google Scholar] [CrossRef] [PubMed]



| Participants (N = 19) | |
|---|---|
| Age (years) | 38.2 ± 14.0 |
| Body weight (kg) | 64.3 ± 13.4 |
| Body height (cm) | 163.8 ± 9.9 |
| Sex (F/M) | 14/5 |
| Dominant side (R/L) | 17/2 |
| Vertical Vibration b | Horizontal Vibration b | ||||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Wilcoxon Signed-Rank Test c | Friedman Test b | Mean (SD) | Wilcoxon Signed-Rank Test c | Friedman Test b | Mann–Whitney U Test a | |
| Z-Value (p Value) | X2-Value (p Value) | Z-Value (p Value) | X2-Value (p Value) | Z-Value (p Value) | |||
| Flexor digitorum superficialis | 35.07 (0.000 **b) | 46.82 (0.000 **b) | |||||
| 0 Hz | 10.92 (6.58) | - | 9.44 (5.69) | - | 158.50 (0.521 a) | ||
| 15 Hz | 29.26 (17.91) | −3.783 (0.000 **c) | 26.95 (17.16) | −3.662 (0.000 **c) | 166.00 (0.672 a) | ||
| 30 Hz | 25.61 (14.00) | −3.743 (0.000 **c) | 32.28 (18.62) | −3.783 (0.000 **c) | 217.00 (0.287 a) | ||
| 45 Hz | 25.01 (13.73) | −3.421 (0.000 **c) | 38.01 (18.54) | −3.823 (0.000 **c) | 261.00 (0.019 *a) | ||
| 60 Hz | 27.76 (17.79) | −3.421 (0.000 **c) | 35.17 (18.23) | −3.783 (0.000 **c) | 223.00 (0.215 a) | ||
| Flexor carpi radialis | 43.41 (0.000 **b) | 34.31 (0.000 **b) | |||||
| 0 Hz | 11.32 (4.83) | - | 13.07 (8.70) | - | 188.00 (0.827 a) | ||
| 15 Hz | 33.21 (17.15) | −3.823 (0.000 **c) | 35.92 (19.38) | −3.461 (0.000 **c) | 190.00 (0.782 a) | ||
| 30 Hz | 31.69 (17.81) | −3.783 (0.000 **c) | 44.23 (26.75) | −3.501 (0.000 **c) | 234.00 (0.118 a) | ||
| 45 Hz | 31.52 (17.20) | −3.823 (0.000 **c) | 49.40 (29.40) | −3.622 (0.000 **c) | 253.00 (0.034 *a) | ||
| 60 Hz | 37.66 (20.64) | −3.823 (0.000 **c) | 49.20 (32.62) | −3.541 (0.000 **c) | 222.00 (0.226 a) | ||
| Extensor carpi radialis | 39.70 (0.000 **b) | 59.95 (0.000 **b) | |||||
| 0 Hz | 14.76 (7.40) | - | 15.74 (12.68) | - | 175.00 (0.872 a) | ||
| 15 Hz | 53.20 (34.15) | −3.823 (0.000 **c) | 38.83 (21.39) | −3.823 (0.000 **c) | 136.00 (0.194 a) | ||
| 30 Hz | 48.80 (28.18) | −3.783 (0.000 **c) | 48.03 (23.24) | −3.823 (0.000 **c) | 182.00 (0.965 a) | ||
| 45 Hz | 49.5 6 (24.13) | −3.823 (0.000 **c) | 60.47 (28.83) | −3.823 (0.000 **c) | 222.00 (0.226 a) | ||
| 60 Hz | 55.50 (29.73) | −3.823 (0.000 **c) | 59.85 (30.65) | −3.823 (0.000 **c) | 194.00 (0.693 a) | ||
| Extensor carpi ulnaris | 38.82 (0.000 **b) | 49.72 (0.000 **b) | |||||
| 0 Hz | 11.83 (5.64) | - | 9.83 (5.05) | - | 145.50 (0.307 a) | ||
| 15 Hz | 38.97 (22.46) | −3.823 (0.000 **c) | 22.95 (11.22) | −3.823 (0.000 **c) | 101.00 (0.020 *a) | ||
| 30 Hz | 34.21 (18.26) | −3.823 (0.000 **c) | 26.64 (14.94) | −3.823 (0.000**c) | 133.00 (0.166 a) | ||
| 45 Hz | 34.91 (23.07) | −3.823 (0.000 **c) | 34.25 (22.56) | −3.823(0.000**c) | 183.00 (0.942 a) | ||
| 60 Hz | 35.90 (29.90) | −3.702 (0.000 **c) | 31.39 (20.81) | −3.823 (0.000**c) | 173.00 (0.827 a) | ||
| Biceps | 54.61 (0.000 **b) | 53.72 (0.000 **b) | |||||
| 0 Hz | 7.22 (3.92) | - | 7.37 (3.57) | - | 191.50 (0.748 a) | ||
| 15 Hz | 21.89 (10.69) | −3.823 (0.000 **c) | 21.56 (7.64) | −3.823(0.000 **c) | 185.00 (0.895 a) | ||
| 30 Hz | 27.12 (13.23) | −3.823 (0.000 **c) | 29.24 (12.58) | −3.823(0.000 **c) | 202.00 (0.530 a) | ||
| 45 Hz | 32.21 (15.63) | −3.823 (0.000 **c) | 41.18 (29.81) | −3.823(0.000 **c) | 202.00 (0.530 a) | ||
| 60 Hz | 33.38 (16.27) | −3.823 (0.000 **c) | 34.64 (17.89) | −3.823(0.000 **c) | 189.00 (0.804 a) | ||
| Triceps | 35.74 (0.000 **b) | 48.46 (0.000 **b) | |||||
| 0 Hz | 3.19 (2.02) | - | 3.74 (3.00) | - | 191.50 (0.748 a) | ||
| 15 Hz | 9.94 (6.11) | −3.823 (0.000 **c) | 8.89 (7.30) | −3.823 (0.000 **c) | 157.00 (0.493 a) | ||
| 30 Hz | 9.35 (5.39) | −3.743 (0.000 **c) | 9.27 (6.34) | −3.823 (0.000 **c) | 168.00 (0.715 a) | ||
| 45 Hz | 11.17 (9.14) | −3.662 (0.000 **c) | 11.64 (7.88) | −3.823 (0.000 **c) | 192.00 (0.737 a) | ||
| 60 Hz | 13.27 (11.54) | −3.823 (0.000 **c) | 12.62 (10.05) | −3.823 (0.000 **c) | 176.00 (0.895 a) | ||
| Deltoid anterior | 39.11 (0.000 **b) | 43.91 (0.000 **b) | |||||
| 0 Hz | 8.01 (8.14) | - | 12.72 (11.66) | - | 246.50 (0.054 a) | ||
| 15 Hz | 31.36 (32.06) | −3.823 (0.000 **c) | 43.04 (34.16) | −3.823 (0.000 **c) | 241.00 (0.077 a) | ||
| 30 Hz | 30.46 (23.82) | −3.823 (0.000 **c) | 43.79 (27.79) | −3.823 (0.000 **c) | 240.00 (0.082 a) | ||
| 45 Hz | 28.91 (19.48) | −3.823 (0.000 **c) | 43.55 (28.83) | −3.823 (0.000 **c) | 237.00 (0.099 a) | ||
| 60 Hz | 32.99 (25.55) | −3.823 (0.000 **c) | 40.16 (32.46) | −3.702 (0.000 **c) | 205.00 (0.474 a) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, C.-H.; Lu, Y.-H.; Chou, L.-W.; Kuo, S.-F.; Lin, C.-H.; Chiang, S.-L.; Lu, L.-H.; Wang, X.-M.; Chang, J.-L.; Lin, C.-H. Analysis of Vibration Frequency and Direction for Facilitating Upper-Limb Muscle Activity. Biology 2023, 12, 48. https://doi.org/10.3390/biology12010048
Ni C-H, Lu Y-H, Chou L-W, Kuo S-F, Lin C-H, Chiang S-L, Lu L-H, Wang X-M, Chang J-L, Lin C-H. Analysis of Vibration Frequency and Direction for Facilitating Upper-Limb Muscle Activity. Biology. 2023; 12(1):48. https://doi.org/10.3390/biology12010048
Chicago/Turabian StyleNi, Cheng-Hua, Yueh-Hsun Lu, Li-Wei Chou, Shu-Fen Kuo, Chia-Huei Lin, Shang-Lin Chiang, Liang-Hsuan Lu, Xin-Miao Wang, Jia-Lan Chang, and Chueh-Ho Lin. 2023. "Analysis of Vibration Frequency and Direction for Facilitating Upper-Limb Muscle Activity" Biology 12, no. 1: 48. https://doi.org/10.3390/biology12010048
APA StyleNi, C.-H., Lu, Y.-H., Chou, L.-W., Kuo, S.-F., Lin, C.-H., Chiang, S.-L., Lu, L.-H., Wang, X.-M., Chang, J.-L., & Lin, C.-H. (2023). Analysis of Vibration Frequency and Direction for Facilitating Upper-Limb Muscle Activity. Biology, 12(1), 48. https://doi.org/10.3390/biology12010048







