Prevalence of Cobalt in the Environment and Its Role in Biological Processes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Chemical and Physical Properties of Cobalt
3. Role of Cobalt in Physiological Processes
3.1. Vitamin B12 (Cobalamin): The Vitamin with Co
3.2. Cobalt Corrinoids
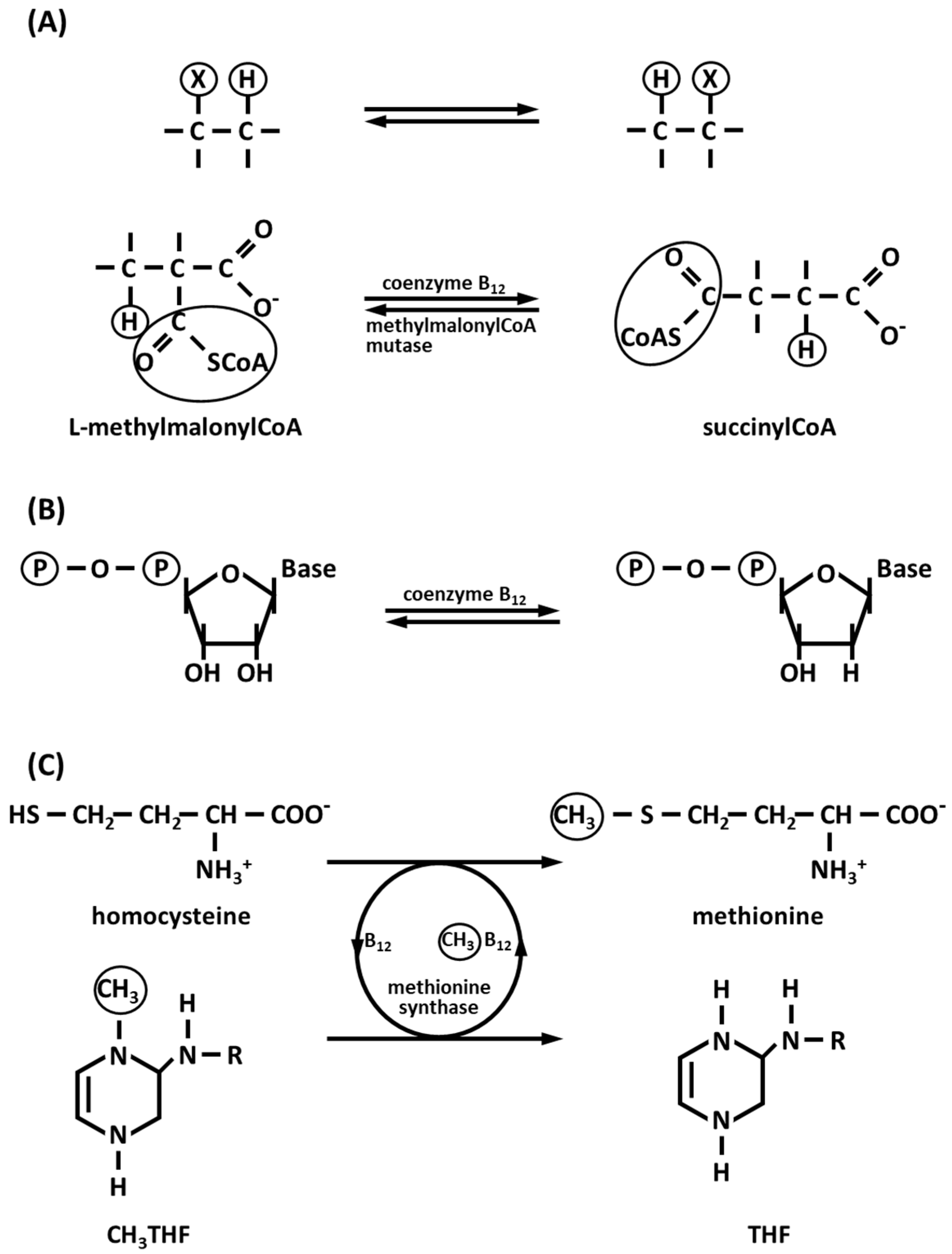
3.3. Co-Dependent Enzymes
3.3.1. Methylmalonyl-CoA Carboxytransferase
3.3.2. Prolidase
3.3.3. Methionine Aminopeptidase
3.3.4. Nitrile Hydratase (NHase)
3.3.5. Glucose Isomerase
3.3.6. Aldehyde Decarbonylase
3.3.7. Lysine-2,3-Aminomutase
3.3.8. Bromoperoxidase
4. Applications of Cobalt in Medicine
4.1. Cobalt in Prostheses
4.2. Cobalt in Radiotherapy and Teletherapy
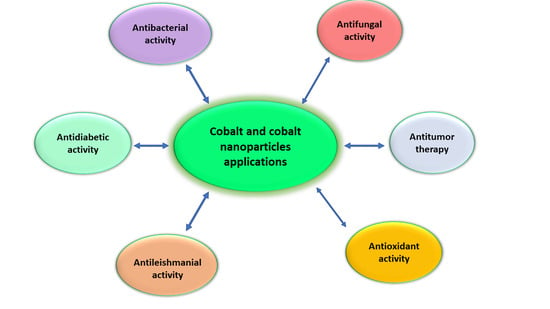
4.3. Cobalt Nanoparticles
5. Cobalt and Its Toxicological Implications
6. Chelation Therapy for Cobalt Intoxication
7. Environmentally Friendly Technology for the Reduction of Co Contamination
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gusenius, E.M. Beginnings of Greatness in Swedish Chemistry: Georg Brandt, (1694–1768). Source Trans. Kansas Acad. Sci. 1903, 70, 413–425. [Google Scholar] [CrossRef]
- Hu, X.; Wei, X.; Ling, J.; Chen, J. Cobalt: An essential micronutrient for plant growth? Front. Plant Sci. 2021, 12, 768523. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.J.M.; Khemiri, S.; Simões, S.; Prista, C.; Sousa, I.; Raymundo, A. The importance, prevalence and determination of vitamins B6 and B12 in food matrices: A review. Food Chem. 2023, 426, 136606. [Google Scholar] [CrossRef] [PubMed]
- Temova Rakuša, Ž.; Roškar, R.; Hickey, N.; Geremia, S. Vitamin B12 in foods, food supplements, and medicines—A review of its role and properties with a focus on its stability. Molecules 2022, 28, 240. [Google Scholar] [CrossRef]
- Guéant, J.L.; Guéant-Rodriguez, R.M.; Alpers, D.H. Vitamin B12 absorption and malabsorption. Vitam. Horm. 2022, 119, 241–274. [Google Scholar] [CrossRef]
- Sultana, S.; Bruns, S.; Wilkes, H.; Simon, M.; Wienhausen, G. Vitamin B12 is not shared by all marine prototrophic bacteria with their environment. ISME J. 2023, 17, 836–845. [Google Scholar] [CrossRef]
- Odaka, M.; Kobayashi, M. Cobalt proteins, overview. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 670–678. [Google Scholar]
- Ulhassan, Z.; Shah, A.M.; Khan, A.R.; Azhar, W.; Hamid, Y.; Zhou, W. Mechanisms of cobalt uptake, transport, and beneficial aspects in plants. In Beneficial Chemical Elements of Plants: Recent Developments and Future Prospects; Wiley: Hoboken, NJ, USA, 2023; pp. 169–181. [Google Scholar] [CrossRef]
- Watanabe, F.; Miyamoto, E. Hydrophilic Vitamins. In Handbook of Thin-Layer Chromatography, 3rd ed.; Sherma, J., Fried, B., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2003; pp. 589–605. [Google Scholar]
- Marques, H.M. The inorganic chemistry of the cobalt corrinoids–an update. J. Inorg. Biochem. 2023, 242, 112154. [Google Scholar] [CrossRef]
- Watanabe, F.; Yabuta, Y.; Bito, T.; Teng, F. Vitamin B12-containing plant food sources for vegetarians. Nutrients 2014, 6, 1861–1873. [Google Scholar] [CrossRef]
- Cracan, V.; Banerjee, R. Cobalt and corrinoid transport and biochemistry. Met. Ions Life Sci. 2013, 1212, 333–374. [Google Scholar] [CrossRef]
- Bilandžić, N.; Čalopek, B.; Sedak, M.; Đokić, M.; Murati, T.; Kmetič, I.; Gajger, I.T. The content of cobalt, silver and vanadium in raw cow’s milk in Croatia and risk assessment for consumers. Bull. Environ. Contam. Toxicol. 2022, 108, 936–942. [Google Scholar] [CrossRef]
- Haddad, R.N.; Hascoet, S.; Karsenty, C.; Houeijeh, A.; Baruteau, A.E.; Ovaert, C.; Valdeolmillos, E.; Jalal, Z.; Bonnet, D.; Malekzadeh-Milani, S. Multicentre experience with Optimus balloon-expandable cobalt–chromium stents in congenital heart disease interventions. Open Heart 2023, 10, e002157. [Google Scholar] [CrossRef]
- Choi, S.; Shin, D.; Kim, S.E.; Yun, C.; Tan, Y.Y.; Lee, C.S. Nano-capsuled thermal interface materials filler using defective multilayered graphene-coated silver nanoparticles. Microelectron. Eng. 2023, 281, 112082. [Google Scholar] [CrossRef]
- Król, G.; Fortunka, K.; Majchrzak, M.; Piktel, E.; Paprocka, P.; Mańkowska, A.; Lesiak, A.; Karasiński, M.; Strelecka, A.; Durnaś, B.; et al. Metallic nanoparticles and core-shell nanosystems in the treatment, diagnosis, and prevention of parasitic diseases. Pathogens 2023, 12, 838. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Mariconda, A.; Rosano, C.; Scumaci, D.; Saturnino, C.; Longo, C.; Sinicropi, M.S. New achievements for the treatment of triple-negative breast cancer. Appl. Sci. 2022, 12, 5554. [Google Scholar] [CrossRef]
- Vinayagam, R.; Hebbar, A.; Kumar, P.S.; Rangasamy, G.; Varadavenkatesan, T.; Murugesan, G.; Srivastava, S.; Goveas, L.C.; Kumar, N.M.; Selvaraj, R. Green synthesized cobalt oxide nanoparticles with photocatalytic activity towards dye removal from water environment. Environ. Res. 2023, 216, 114766. [Google Scholar] [CrossRef] [PubMed]
- Maron, G.K.; Masteghin, M.G.; Gehrke, V.; Rodrigues, L.S.; Alano, J.H.; Rossato, J.H.; Mastelaro, V.R.; Dupont, J.; Escote, M.Y.; Silva, S.R.P.; et al. Enhanced pseudocapacitive behaviour in laser-written graphene micro-supercapacitors decorated with nickel cobalt sulphide nanoparticles. Mat. Res. Bull. 2023, 168, 112490. [Google Scholar] [CrossRef]
- Anupong, W.; Onuma, R.; Jutamas, K.; Joshi, D.; Salmen, S.H.; Alahmadi, T.A.; Jhanani, G.K. Cobalt nanoparticles synthesizing potential of orange peel aqueous extract and their antimicrobial and antioxidant activity. Environ. Res. 2023, 216, 114594. [Google Scholar] [CrossRef]
- Pillai, A.S.; Alexander, A.; Manikantan, V.; Varalakshmi, G.S.; Akash, B.A.; Enoch, I.V. Camptothecin-carrying cobalt-doped copper sulfide nanoparticles. J. Clust. Sci. 2023. [Google Scholar] [CrossRef]
- Ma, Y.; Lin, W.; Ruan, Y.; Lu, H.; Fan, S.; Chen, D.; Huang, Y.; Zhang, T.; Pi, J.; Xu, J.F. Advances of cobalt nanomaterials as anti-infection agents, drug carriers, and immunomodulators for potential infectious disease treatment. Pharmaceutics 2022, 14, 2351. [Google Scholar] [CrossRef]
- Vodyashkin, A.A.; Kezimana, P.; Prokonov, F.Y.; Vasilenko, I.A.; Stanishevskiy, Y.M. Current methods for synthesis and potential applications of cobalt nanoparticles: A review. Crystals 2022, 12, 272. [Google Scholar] [CrossRef]
- Di Bella, C.; Traina, A.; Giosuè, C.; Carpintieri, D.; Lo Dico, G.M.; Bellante, A.; Del Core, M.; Falco, F.; Gherardi, S.; Uccello, M.M.; et al. Heavy metals and PAHs in meat, milk, and seafood from Augusta Area (Southern Italy): Contamination levels, dietary intake, and human exposure assessment. Front. Public Health 2020, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Preisser, A.M.; Scheit, L.; Kraft, A.; Thieme, O.; Harth, V. Long-term clinical and toxicological follow-up of severe cobalt and chromium intoxication—A case report. SN Comprehens. Clin. Med. 2023, 5, 58. [Google Scholar] [CrossRef]
- Rahman, A.; Haque, M.A.; Ghosh, S.; Shinu, P.; Attimarad, M.; Kobayashi, G. Modified shrimp-based chitosan as an emerging adsorbent removing heavy metals (chromium, nickel, arsenic, and cobalt) from polluted water. Sustainability 2023, 15, 2431. [Google Scholar] [CrossRef]
- Carocci, A.; Catalano, A.; Sinicropi, M.S.; Genchi, G. Oxidative stress and neurodegeneration: The involvement of iron. Biometals 2018, 31, 715–735. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Betancourt-Cantera, J.; Jesús, F.S.-D.; Bolarín-Miró, A.; Torres-Villaseñor, G.; Betancourt-Cantera, L. Magnetic properties and crystal structure of elemental cobalt powder modified by high-energy ball milling. J. Mater. Res. Technol. 2019, 8, 4995–5003. [Google Scholar] [CrossRef]
- Ahmad, Z.; Choudhary, M.A.; Mehmood, A.; Wakeeh, R.; Akhtar, T.; Rafiq, M.A. Synthesis of polypyrrole nano/microspheres using cobalt(III) as an oxidizing agent and its ammonia sensing behavior. Macromol. Res. 2016, 24, 596–601. [Google Scholar] [CrossRef]
- Wong, Y.J.; Petersen, J.D.; Geldard, J.F. Racemization, reduction, and substitution reactions of tris (chelating ligand) metal complexes. 1. Thermolysis of (-)-tris (2,2’-dipyr.idylamine) cobalt (III) perchlorate in water and DMF. Inorg. Chem. 1985, 24, 3352–3357. [Google Scholar] [CrossRef]
- Scott, E.R. Iron meteorites: Composition, age, and origin. In Oxford Research Encyclopedia of Planetary Science; Oxford University Press: Oxford, UK, 2020. [Google Scholar] [CrossRef]
- Yilanci, V.; Turkmen, N.C.; Shah, M.I. An empirical investigation of resource curse hypothesis for cobalt. Res. Policy 2022, 78, 102843. [Google Scholar] [CrossRef]
- Gulley, A.L. One hundred years of cobalt production in the Democratic Republic of the Congo. Res. Policy 2022, 79, 103007. [Google Scholar] [CrossRef]
- Crider, B.P.; Prokop, C.J.; Liddick, S.N.; Albers, H.M.; Alshudifat, M.; Ayangeakaa, A.D.; Carpenter, M.P.; Carroll, J.J.; Chen, J.; Chiara, C.J.; et al. New method for level-lifetime measurements with thick scintillators. Nucl. Instrum. Methods Phys. Res. A 2023, 1055, 168525. [Google Scholar] [CrossRef]
- Rickes, E.L.; Brink, N.G.; Koniuszy, F.R.; Wood, T.R.; Folkers, K. Crystalline Vitamin B12. Science 1948, 107, 396. [Google Scholar] [CrossRef] [PubMed]
- Calvillo, Á.; Pellicer, T.; Carnicer, M.; Planas, A. Bioprocess strategies for vitamin B12 production by microbial fermentation and its market applications. Bioengineering 2022, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, R.; Gouda, H.; Ruetz, M.; Banerjee, R. Human B12-dependent enzymes: Methionine synthase and Methylmalonyl-CoA mutase. Methods Enzym. 2022, 668, 309–326. [Google Scholar] [CrossRef]
- Calderón-Ospina, C.A.; Nava-Mesa, M.O. B vitamins in the Nervous System: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci. 2019, 26, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Geller, M.; Oliveira, L.; Nigri, R.; Mezitis, S.G.E.; Ribeiro, M.G.; de Souza da Fonseca, A.; Guimarães, O.R.; Kaufman, R.; Wajnsztajn, F. B Vitamins for neuropathy and neuropathic pain. Vitam. Miner. 2017, 6, 1000161. [Google Scholar] [CrossRef]
- Moore, S.J.; Lawrence, A.D.; Biedendieck, R.; Deery, E.; Frank, S.; Howard, M.J.; Rigby, S.E.J.; Warren, M.J. Elucidation of the Anaerobic Pathway for the Corrin Component of Cobalamin (Vitamin B12). Proc. Natl. Acad. Sci. USA 2013, 110, 14906–14911. [Google Scholar] [CrossRef]
- Osman, D.; Cooke, A.; Young, T.R.; Deery, E.; Robinson, N.J.; Warren, M.J. The requirement for cobalt in vitamin B-12: A paradigm for protein metalation. Biochim. Biophys. Acta-Mol. Cell Res. 2021, 1868, 118896. [Google Scholar] [CrossRef]
- Wendolowicz, A.; Stefanska, E.; Ostrowska, L. Influence of Selected Dietary Components on the Functioning of the Human Nervous System. Rocz. Państw. Zakładu Hig. 2018, 69, 15–21. Available online: http://wydawnictwa.pzh.gov.pl/roczniki_pzh/ (accessed on 15 October 2023).
- Mikkelsen, K.; Apostolopoulos, V. Vitamin B12, folic acid, and the immune system. In Nutrition and Immunity; Springer: Cham, Switzerland, 2019; pp. 103–114. [Google Scholar] [CrossRef]
- Rizzo, G.; Marino, A. Cognitive impairment and micronutrients: Vitamin B12, folate, and homocysteine and implications for dementia. In Vitamins and Minerals in Neurological Disorders; Academic Press: Cambridge, MA, USA, 2023; pp. 29–46. [Google Scholar] [CrossRef]
- Ma, F.; Wu, T.; Zhao, J.; Ji, L.; Song, A.; Zhang, M.; Huang, G. Plasma homocysteine and serum folate and vitamin B12 levels in mild cognitive impairment and Alzheimer’s Disease: A case-control study. Nutrients 2017, 9, 725. [Google Scholar] [CrossRef]
- Lauer, A.A.; Grimm, H.S.; Apel, B.; Golobrodska, N.; Kruse, L.; Ratanski, E.; Schulten, N.; Schwarze, L.; Slawik, T.; Sperlich, S.; et al. Mechanistic link between vitamin B12 and Alzheimer’s Disease. Biomolecules 2022, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Ge, B.; Zhou, D.; Li, M.; Li, W.; Ma, F.; Liu, Z.; Ji, Y.; Huang, G. Effects of folic acid and vitamin B12 supplementation on cognitive impairment and inflammation in patients with Alzheimer’s disease: A randomized, single-blinded, placebo-controlled trial. J. Prev. Alzheimers Dis. 2021, 8, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Batista, K.S.; Cintra, V.M.; Lucena, P.A.F.; Manhães-De-Castro, R.; Toscano, A.E.; Costa, L.P.; Queiroz, M.E.B.S.; De Andrade, S.M.; Guzman-Quevedo, O.; Aquino, J.D.S. The role of vitamin B12 in viral infections: A comprehensive review of its relationship with the muscle-gut-brain axis and implications for SARS-CoV-2 infection. Nutr. Rev. 2022, 80, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Pellegrino, M.; Mariconda, A.; Longo, P.; Sinicropi, M.S.; Aquaro, S. COVID-19 at a glance: An up-to-date overview on variants, drug design and therapies. Viruses 2022, 14, 573. [Google Scholar] [CrossRef] [PubMed]
- Yahn, G.B.; Abato, J.E.; Jadavji, N.M. Role of vitamin B12 deficiency in ischemic stroke risk and outcome. Neural Regen. Res. 2021, 16, 470–474. [Google Scholar] [CrossRef]
- Hodgkin, D.C.; Kamper, J.; Mackay, M.; Pickworth, J.; Trueblood, K.N.; White, J.G. Structure of vitamin B12. Nature 1956, 178, 64–66. [Google Scholar] [CrossRef]
- Krautler, B. Biochemistry of B12-cofactors in human metabolism. Subcell. Biochem. 2012, 56, 323–346. [Google Scholar] [CrossRef]
- Kozyraki, R.; Cases, O. Vitamin B12 absorption: Mammalian physiology and acquired and inherited disorders. Biochimie 2013, 95, 1002–1007. [Google Scholar] [CrossRef]
- Smith, D.M.; Golding, B.T.; Radom, L. Understanding the mechanism of B12-dependent methylmalonyl-CoA mutase: Partial proton transfer in action. J. Am. Chem. Soc. 1999, 121, 9388–9399. [Google Scholar] [CrossRef]
- Fang, H.; Kang, J.; Zhang, D. Microbial production of vitamin B12: A review and future perspectives. Microb. Cell Factories 2017, 16, 15. [Google Scholar] [CrossRef]
- McCorvie, T.J.; Ferreira, D.; Yue, W.W.; Froese, D.S. The complex machinery of human cobalamin metabolism. J. Inher. Metab. Dis. 2023, 46, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, T.; Harita, Y.; Miura, K.; Mitsui, J.; Ode, K.L.; Morishita, S.; Urae, S.; Kanda, S.; Kajiho, Y.; Tsurumi, H.; et al. Amnionless-mediated glycosylation is crucial for cell surface targeting of cubilin in renal and intestinal cells. Sci. Rep. 2018, 8, 2351. [Google Scholar] [CrossRef] [PubMed]
- Hariz, A.; Bhattacharya, P.T. Megaloblastic Anemia. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Vaqar, S.; Shackelford, K. Pernicious Anemia. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Rashid, S.; Meier, V.; Patrick, H. Review of vitamin B12 deficiency in pregnancy: A Diagnosis not to miss as veganism and vegetarianism become more prevalent. Eur. J. Haematol. 2021, 106, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Allen, L.H.; Bjorke-Monsen, A.L.; Brito, A.; Gueant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef]
- Ljungblad, U.W.; Lindberg, M.; Eklund, E.A.; Saeves, I.; Bjørke-Monsen, A.-L.; Tangeraas, T. Nitrous oxide in labour pre-dicted newborn screening total homocysteine and is a potential risk factor for infant vitamin B12 deficiency. Acta Paediatr. 2022, 111, 2315–2321. [Google Scholar] [CrossRef]
- Pratama, S.; Lauren, B.C.; Wisnu, W. The efficacy of vitamin B12 supplementation for treating vitamin B12 deficiency and peripheral neuropathy in metformin-treated type 2 diabetes mellitus patients: A systematic review. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102634. [Google Scholar] [CrossRef]
- Greibe, E. Nutritional and biochemical aspects of cobalamin throughout life. In Vitamin B12: Advances and Insights; CRC Press: Boca Raton, FL, USA, 2017; pp. 30–45. [Google Scholar]
- Govender, P.; Opoku, F.; Wahab, O.; Kiarii, E. Molecular Modelling of Vitamin B12 and Its Analogues; Jenny Stanford Publishing: Singapore, 2021; 176p. [Google Scholar] [CrossRef]
- Randaccio, L.; Geremia, S.; Demitri, N.; Wuerges, J. Vitamin B12: Unique Metalorganic Compounds and the Most Complex Vitamins. Molecules 2010, 15, 3228–3259. [Google Scholar] [CrossRef]
- Giedyk, M.; Goliszewska, K.; Gryko, D. Vitamin B12 catalysed reactions. Chem. Soc. Rev. 2015, 44, 3391–3404. [Google Scholar] [CrossRef]
- Jones, A.R. The photochemistry and photobiology of vitamin B12. Photochem. Photobiol. Sci. 2017, 16, 820–834. [Google Scholar] [CrossRef]
- Toda, M.J.; Lodowski, P.; Al Mamun, A.; Jaworska, M.; Kozlowski, P.M. Photolytic properties of the biologically active forms of vitamin B12. Coord. Chem. Rev. 2019, 385, 20–43. [Google Scholar] [CrossRef]
- Lennon, S.R.; Wierzba, A.J.; Siwik, S.H.; Gryko, D.; Palmer, A.E.; Batey, R.T. Targeting Riboswitches with Beta-Axial-Substituted Cobalamins. ACS Chem. Biol. 2023, 18, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Bito, T. Determination of cobalamin and related compounds in foods. J. AOAC Int. 2018, 101, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Yabuta, Y.; Tanioka, Y.; Bito, T. Biologically Active Vitamin B12 Compounds in Foods for Preventing Deficiency among Vegetarians and Elderly Subjects. J. Agric. Food Chem. 2013, 61, 6769–6775. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.; Jost, M.; Drennan, C.L.; Elias-Arnanz, M. A new facet of vitamin B12: Gene regulation by cobalamin-based phoreceptors. Annu. Rev. Biochem. 2017, 86, 485–514. [Google Scholar] [CrossRef]
- Hall, P.R.; Zheng, R.; Antony, L.; Pusztai-Carey, M.; Carey, P.R.; Yee, V.C. Transcarboxylase 5S structures: Assembly and catalytic mechanism of a multienzyme complex subunit. EMBO J. 2004, 23, 3621–3631. [Google Scholar] [CrossRef]
- Ghosh, M.; Grunden, A.M.; Dunn, D.M.; Weiss, R.; Adams, M.W. Characterization of native and recombinant forms of an unusual cobalt-dependent proline dipeptidase (prolidase) from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 1998, 180, 4781–4789. [Google Scholar] [CrossRef]
- Eni-Aganga, I.; Lanaghan, Z.M.; Balasubramaniam, M.; Dash, C.; Pandhare, J. PROLIDASE: A review from discovery to its role in health and disease. Front. Mol. Biosci. 2021, 8, 723003. [Google Scholar] [CrossRef]
- Arfin, S.M.; Kendall, R.L.; Hall, L.; Weaver, L.H.; Stewart, A.E.; Matthews, B.W.; Bradshaw, R.A. Eukaryotic methionyl aminopeptidases: Two classes of cobalt-dependent enzymes. Proc. Natl. Acad. Sci. USA 1995, 92, 7714–7718. [Google Scholar] [CrossRef]
- Roderick, S.L.; Matthews, B.W. Structure of the cobalt-dependent methionine aminopeptidase from Escherichia coli a new type of proteolytic enzyme. Biochemistry 1993, 32, 3907–3912. [Google Scholar] [CrossRef]
- Yamada, H.; Kobayashi, M. Nitrile hydratase and its application to industrial production of acrylamide. Biosci. Biotechnol. Biochem. 1996, 60, 1391–1400. [Google Scholar] [CrossRef]
- Nagasawa, T.; Mathew, C.D.; Mauger, J.; Yamada, H. Nitrile hydratase-catalyzed production of nicotinamide from 3-cyanopyridine in Rhodococcus rhodochrous J1. Appl. Environ. Microbiol. 1988, 54, 1766–1769. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.H.; Rao, M.B.; Deshpande, V.V. Molecular and industrial aspects of glucose isomerase. Microbiol. Rev. 1996, 60, 280–300. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.P.; Gowtham, Y.K.; Henson, J.M.; Harcum, S.W. Xylose isomerase improves growth and ethanol production rates from biomass sugars for both Saccharomyces pastorianus and Saccharomyces cerevisiae. Biotechnol. Prog. 2012, 28, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.; Kolattukudy, P.E. A cobalt-porphyrin enzyme convers a fatty aldehyde to a hydrocarbon and CO. Proc. Natl. Acad. Sci. USA 1992, 89, 5306–5310. [Google Scholar] [CrossRef] [PubMed]
- Marsh, E.N.; Waugh, M.W. Aldehyde decarbonylases: Enigmatic enzymes of hydrocarbon biosynthesis. ACS Catal. 2013, 3, 2515–2521. [Google Scholar] [CrossRef] [PubMed]
- Frey, P.A. Lysine 2,3-aminomutase: Is adenosylmethionine a poor man’s adenosylcobalamin? FASEB J. 1993, 7, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Szymański, W.; Heberling, M.M.; Feringa, B.L.; Janssen, D.B. Aminomutases: Mechanistic diversity, biotechnological applications and future perspectives. Trends Biotechnol. 2011, 29, 352–362. [Google Scholar] [CrossRef]
- Itoh, N.; Morinaga, N.; Kouzai, T. Purification and characterization of a novel metal-containing nonheme bromoperoxidase from Pseudomonas putida. Biochim. Biophys. Acta 1994, 1207, 208–216. [Google Scholar] [CrossRef]
- Itoh, N.; Kawanami, T.; Liu, J.Q.; Dairi, T.; Miyakoshi, M.; Nitta, C.; Kimoto, Y. Cloning and biochemical characterization of Co2+-activated bromoperoxidase-esterase (perhydrolase) from Pseudomonas putida IF-3 strain. Biochim. Biophys. Acta 2001, 1545, 53–66. [Google Scholar] [CrossRef]
- Rangrazi, A.; Mirmortazavi, A.; Imani, R.; Nodehi, D. Effect of ozone on corrosion behavior of a cobalt–chromium alloy used in removable partial denture framework: An in vitro study J. Adv. Oral Res. 2021, 12, 304–309. [Google Scholar] [CrossRef]
- Vaicelyte, A.; Janssen, C.; Le Borgne, M.; Grosgogeat, B. Cobalt–chromium dental alloys: Metal exposures, toxicological risks, CMR Classification, and EU regulatory framework. Crystals 2020, 10, 1151. [Google Scholar] [CrossRef]
- ASTM F75-18; Standard Specification for Co-Balt-28 Chromium-6 Molybdenum Alloy Castings and Casting Alloy for Surgical Implants-UNS R30075. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2018.
- Pontes, J.R.; Alves, A.C.; Toptan, F.; Galo, R.; Ariza, E. Effect of commercial mouthwashes on the corrosion and tribocorrosion behaviour of a Co–Cr dental casting alloy. Mat. Corros. 2016, 67, 305–311. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Dionisopoulou, N.K. Irradiation of Food Commodities. Techniques, Applications, Detection, Legislation, Safety and Consumer Opinion, 1st ed.; Arvanitoyannis, I.S., Ed.; Academic Press: London, UK, 2010; pp. 609–634. ISBN 13 978-0128101919. [Google Scholar]
- Wooten, H.O.; Green, O.; Yang, M.; DeWees, T.; Kashani, R.; Olsen, J.; Michalski, J.; Yang, D.; Tanderup, K.; Hu, Y.; et al. Quality of intensity modulated radiation therapy treatment plans using a 60Co magnetic resonance image guidance radiation therapy system. Int. J. Radiat. Oncol. Biol. Physics 2015, 92, 771–778. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine fight against antibacterial resistance: An overview of the recent pharmaceutical innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, T.; Zaib, M.; Riaz, T.; Shehzadi, S.; Abbasi, M.; Shahid, M. Synthesis of eco-friendly cobalt nanoparticles using Celosia argentea plant extract and their efficacy studies as antioxidant, antibacterial, hemolytic and catalytical agent. Arab. J. Sci. Eng. 2019, 44, 6435–6444. [Google Scholar] [CrossRef]
- Omran, B.A.; Nassar, H.N.; Younis, S.A.; El-Salamony, R.A.; Fatthallah, N.A.; Hamdy, A.; El-Shatoury, E.H.; El-Gendy, N.S. Novel mycosynthesis of cobalt oxide nanoparticles using Aspergillus brasiliensis ATCC 16404—Optimization, characterization and antimicrobial activity. J. Appl. Microbiol. 2020, 128, 438–457. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, C.T.; Raji, P. Effect of annealing temperature on antibacterial, antifungal and structural properties of bio-synthesized Co3O4 nanoparticles using Hibiscus rosa-sinensis. Mater. Res. Express 2019, 6, 095063. [Google Scholar] [CrossRef]
- Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; Taher, M.M.; Khalaf, M. Benign synthesis of cobalt oxide nanoparticles containing red algae extract: Antioxidant, antimicrobial, anticancer, and anticoagulant activity. J. Clust. Sci. 2022, 33, 717–728. [Google Scholar] [CrossRef]
- Kgosiemang, I.K.; Lefojane, R.; Direko, P.; Madlanga, Z.; Mashele, S.; Sekhoacha, M. Green synthesis of magnesium and cobalt oxide nanoparticles using Euphorbia tirucalli: Characterization and potential application for breast cancer inhibition. Inorg. Nano-Metal Chem. 2020, 50, 1070–1080. [Google Scholar] [CrossRef]
- Gao, Z.; Ding, C.; Wang, J.; Ding, G.; Xue, Y.; Zhang, Y.; Zhang, K.; Liu, P.; Gao, X. Cobalt nanoparticles packaged into nitrogen-doped porous carbon derived from metal-organic framework nanocrystals for hydrogen production by hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2019, 44, 8365–8375. [Google Scholar] [CrossRef]
- Li, J.H.; Hong, X.Y.; Wang, Y.L.; Luo, Y.M.; Huang, P.R.; Li, B.; Zhang, K.X.; Zou, Y.J.; Sun, L.X.; Xu, F.; et al. Encapsulated cobalt nanoparticles as a recoverable catalyst for the hydrolysis of sodium borohydride. Energy Storage Mater. 2020, 27, 187–197. [Google Scholar] [CrossRef]
- Wu, D.; Ye, P.; Wang, M.; Wei, Y.; Li, X.; Xu, A. Cobalt nanoparticles encapsulated in nitrogen-rich carbon nanotubes as efficient catalysts for organic pollutants degradation via sulfite activation. J. Hazard. Mater. 2018, 352, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Nabeel, F.; Bilal, M.; Iqbal, H.M. Biogenic synthesis and characterization of cobalt oxide nanoparticles for catalytic reduction of direct yellow-142 and methyl orange dyes. Biocatal. Agric. Biotechnol. 2019, 19, 101154. [Google Scholar] [CrossRef]
- El-Sayed, M.M.; Elsayed, R.E.; Attia, A.; Farghal, H.H.; Azzam, R.A.; Madkour, T.M. Novel nanoporous membranes of bio-based cellulose acetate, poly(lactic acid) and biodegradable polyurethane in-situ impregnated with catalytic cobalt nanoparticles for the removal of Methylene Blue and Congo Red dyes from wastewater. Carbohydr. Polym. Technol. Appl. 2021, 2, 100123. [Google Scholar] [CrossRef]
- Jia, Y.; Hu, Y.; Li, Y.; Zeng, Q.; Jiang, X.; Cheng, Z. Boron doped carbon dots as a multifunctional fluorescent probe for sorbate and vitamin B12. Microchim. Acta 2019, 186, 84. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.; Schmid, A. Effect of fermentation on vitamin content in food. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 131–157. [Google Scholar] [CrossRef]
- (Elci, S.G. Determination of cobalt in food by magnetic solid-phase extraction (MSPE) preconcentration by polyaniline (PANI) and polythiophene (PTH) coated magnetic nanoparticles (MNPs) and microsample injection system–flame atomic absorption spectrometry (MIS-FAAS). Instrum. Sci. Technol. 2021, 49, 258–275. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Postnikov, P.V.; Ordzhonikidze, Z.G.; Badtieva, V.A.; Turin, I.A.; Pavlov, V.I. Determination of cobalt in plasma blood samples by the ICP-MS method after oral intake of dietary supplements containing low doses of cobalt. Vopr. Pitan. 2022, 91, 92–101. [Google Scholar] [CrossRef]
- Gheorghe, D.C.; Stefan-van Staden, R.I.; van Staden, J.K.F. Mini-review: Electrochemical sensors used for the determination of water-and fat-soluble vitamins: B, D, K. Crit. Rev. Anal. Chem. 2022, 1–10. [Google Scholar] [CrossRef]
- Karastogianni, S.; Girousi, S. Square Wave Voltammetric (SWV) Determination of cyanocobalamin (Vitamin B12) in pharmaceuticals and supplements on a Carbon Paste Electrode (CPE) modified by a manganese(II) polymeric film. Anal. Lett. 2021, 55, 399–410. [Google Scholar] [CrossRef]
- Sharma, A.; Arya, S.; Chauhan, D.; Solanki, P.R.; Khajuria, S.; Khosla, A. Synthesis of Au-SnO2 nanoparticles for electrochemical determination of vitamin B12. J. Mater. Res. Technol. 2020, 9, 14321–14337. [Google Scholar] [CrossRef]
- Lison, D. Chapter 25—Cobalt. In Handbook on the Toxicology of Metals; Academic Press: Cambridge, MA, USA, 2007; pp. 511–528. [Google Scholar] [CrossRef]
- Committee for Human Medicinal Products. ICH Guideline Q3D (R1) on Elemental Impurities; European Medicines Agency: Amsterdam, The Netherlands, 2019; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-32.pdf (accessed on 2 December 2022).
- Viegas, V.; Burzlaff, A.; Brock, T.O., III; Danzeisen, R. A tiered approach to investigate the inhalation toxicity of cobalt substances. Tier 3: Inflammatory response following acute inhalation exposure correlates with lower tier data. Reg. Toxicol. Pharmacol. 2022, 130, 105127. [Google Scholar] [CrossRef] [PubMed]
- Danzeisen, R.; Weight, D.; Blakeney, M.; Boyle, D. A tiered approach to investigate the inhalation toxicity of cobalt substances. Introduction: Cobalt’s essential role in nature and technology. Reg. Toxicol. Pharmacol. 2022, 130, 105125. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Lee, V.R. Cobalt Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- International Agency for Research on Cancer. Cobalt in Hard Metals and Cobalt Sulfate, Gallium Arsenide, Indium Phosphide and Vanadium Pentoxide Monographs on the Evaluation of Carcinogenic Risks to Humans IARC; International Agency for Research on Cancer: Lyon, France, 2006.
- Crutsen, J.R.W.; Koper, M.C.; Jelsma, J.; Heymans, M.; Heyligers, I.C.; Grimm, B.; Schotanus, M.G.M. Prosthetic hip-associated cobalt toxicity: A systematic review of case series and case reports. EFORT Open Rev. 2022, 7, 188–199. [Google Scholar] [CrossRef]
- Szedlak, P.; Virdi, A.; Cacciottolo, P.; Shepherd, S.; Pettit, S.; Falter, F. Cardiac transplantation following cobalt cardiomyopathy from bilateral metal-on-metal hip replacements. Case Rep. Anesthesiol. 2022, 2022, 3373363. [Google Scholar] [CrossRef]
- García, A.D.; Meseguer, M.J.; Pérez, J.M.S.; Martínez, S.G.; Correas, F.H.; Díaz, B.G. [Translated article] Cobalt poisoning secondary to hip prosthesis: A case report. Farm. Hosp. 2023, 47, T139–T140. [Google Scholar] [CrossRef]
- Świątkowska, I.; Akinfosile, O.J.; Badhe, R.V.; Barba, M.; Mathew, M.T.; Bijukumar, D. Hip implants and systemic cobalt toxicity: A comprehensive review with case studies. In Biomarkers of Hip Implant Function; Academic Press: Cambridge, MA, USA, 2023; pp. 205–247. [Google Scholar] [CrossRef]
- Iavicoli, I.; Falcone, G.; Alessandrelli, M.; Cresti, R.; De Santis, V.; Salvatori, S.; Alimonti, A.; Carelli, G. The release of metals from metal-on-metal surface arthroplasty of the hip. J. Trace Elem. Med. Biol. 2006, 20, 25–31. [Google Scholar] [CrossRef]
- Sargeant, A.; Goswami, T. Hip implants: Paper, V. Physiological effects. Mater. Des 2006, 27, 287–307. [Google Scholar] [CrossRef]
- Rizzetti, M.C.; Liberini, P.; Zarattini, G.; Catalani, S.; Pazzaglia, U.; Apostoli, P.; Padovani, A. Loss of sight and sound. Could it be the hip? Lancet 2009, 373, 1052. [Google Scholar] [CrossRef]
- Bradberry, S.M.; Wilkinson, J.M.; Ferner, R.E. Systemic toxicity related to metal hip prostheses. Clin. Toxicol. 2014, 52, 837–847. [Google Scholar] [CrossRef]
- Sadler, P.J.; Tucker, A.; Viles, J.H. Involvement of a lysine residue in the N-terminal Ni2+ and Cu2+ binding site of serum albumins. Comparison with Co2+, Cd2+ and Al3+. Eur. J. Biochem. 1994, 220, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Mothes, E.; Faller, P. Evidence that the principal CoII-binding site in human serum albumin is not at the N-terminus: Implication on the albumin cobalt binding test for detecting myocardial ischemia. Biochemistry 2007, 46, 2267–2274. [Google Scholar] [CrossRef]
- Bar-Or, D.; Lau, E.; Winkler, J.V. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia—A preliminary report. J. Emerg. Med. 2000, 19, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Eom, J.E.; Jeon, K.H.; Kim, T.H.; Kim, E.; Jhon, G.J.; Kwon, Y. Evaluation of albumin structural modifications through cobalt-albumin binding (CAB) assay. J. Pharm. Biomed. Anal. 2014, 91, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Henretig, F.; Shannon, M. An 11-year-old boy develops vomiting, weakness, weight loss and a neck mass. Int. J. Med. Toxicol. 1988, 1, 13–15. [Google Scholar]
- Pazzaglia, U.E.; Apostoli, P.; Congiu, T.; Catalani, S.; Marchese, M.; Zarattini, G. Cobalt, chromium and molybdenum ions kinetics in the human body: Data gained from a total hip replacement with massive third body wear of the head and neuropathy by cobalt intoxication. Arch. Orthop. Trauma Surg. 2011, 131, 1299–1308. [Google Scholar] [CrossRef]
- Pelclova, D.; Sklensky, M.; Janicek, P.; Lach, K. Severe cobalt intoxication following hip replacement revision: Clinical features and outcome. Clin. Toxicol. 2012, 50, 262–265. [Google Scholar] [CrossRef]
- Giampreti, A.; Lonati, D.; Ragghianti, B.; Ronchi, A.; Petrolini, V.M.; Vecchio, S.; Locatelli, C.A. N-Acetyl-cysteine as effective and safe chelating agent in metal-on-metal hip-implanted patients: Two cases. Case Rep. Orthop. 2016, 2016, 8682737. [Google Scholar] [CrossRef]
- D’Ambrosi, R.; Ursino, N. N-Acetyl-cysteine reduces blood chromium and cobalt levels in metal-on-metal hip arthroplasty. Arthroplast. Today 2020, 6, 149–152. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. The double face of metals: The intriguing case of chromium. Appl. Sci. 2021, 11, 638. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Arsenic: A review on a great health issue worldwide. Appl. Sci. 2022, 12, 6184. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological activity of selenium and its impact on human health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Catalano, A.; Sinicropi, M.S. Thallium use, toxicity, and detoxification therapy: An overview. Appl. Sci. 2021, 11, 8322. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef]
- Gopal, R.; Dube, B.K.; Sinha, P.; Chatterjee, C. Cobalt toxicity effects on growth and metabolism of tomato. Commun. Soil Sci. Plant Anal. 2003, 34, 619–628. [Google Scholar] [CrossRef]
- Wendling, L.A.; Kirbz, J.K.; Kosiorek, M.; Wyszkowski, M.; Mclaughlin, M.J. Aging effects on cobalt availability in soils. Environ. Toxicol. Chem. 2009, 28, 1609–1617. [Google Scholar] [CrossRef]
- Malik, M.; Chaney, R.L.; Brewer, E.P.; Li, Y.; Angle, J.S. Phytoextraction of soil cobalt using hyperaccumulator plants. Int. J. Phytoremediat. 2000, 2, 319–329. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Meravi, N.; Singh, S. Phytoremediation of chromium and cobalt using Pistia stratiotes: A sustainable approach. Proc. Int. Acad. Ecol. Environ. Sci. 2012, 2, 136. [Google Scholar] [CrossRef]
- Lotfy, S.M.; Mostafa, A.Z. Phytoremediation of contaminated soil with cobalt and chromium. J. Geochem. Explor. 2014, 144, 367–373. [Google Scholar] [CrossRef]
- van der Ent, A.; Mak, R.; de Jonge, M.D.; Harris, H.H. Simultaneous hyperaccumulation of nickel and cobalt in the tree Glochidion cf. sericeum (Phyllanthaceae): Elemental distribution and chemical speciation. Sci. Rep. 2018, 8, 9683. [Google Scholar] [CrossRef]
- Khalid, A.; Farid, M.; Zubair, M.; Rizwan, M.; Iftikhar, U.; Ishaq, H.K.; Farid, S.; Latif, U.; Hina, K.; Ali, S. Efficacy of Alternanthera bettzickiana to remediate copper and cobalt contaminated soil physiological and biochemical alterations. Int. J. Environ. Res. 2020, 14, 243–255. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Prevalence of Cobalt in the Environment and Its Role in Biological Processes. Biology 2023, 12, 1335. https://doi.org/10.3390/biology12101335
Genchi G, Lauria G, Catalano A, Carocci A, Sinicropi MS. Prevalence of Cobalt in the Environment and Its Role in Biological Processes. Biology. 2023; 12(10):1335. https://doi.org/10.3390/biology12101335
Chicago/Turabian StyleGenchi, Giuseppe, Graziantonio Lauria, Alessia Catalano, Alessia Carocci, and Maria Stefania Sinicropi. 2023. "Prevalence of Cobalt in the Environment and Its Role in Biological Processes" Biology 12, no. 10: 1335. https://doi.org/10.3390/biology12101335
APA StyleGenchi, G., Lauria, G., Catalano, A., Carocci, A., & Sinicropi, M. S. (2023). Prevalence of Cobalt in the Environment and Its Role in Biological Processes. Biology, 12(10), 1335. https://doi.org/10.3390/biology12101335