Exosomes: New Insights into the Pathogenesis of Metabolic Syndrome
Abstract
:Simple Summary
Abstract
1. Introduction
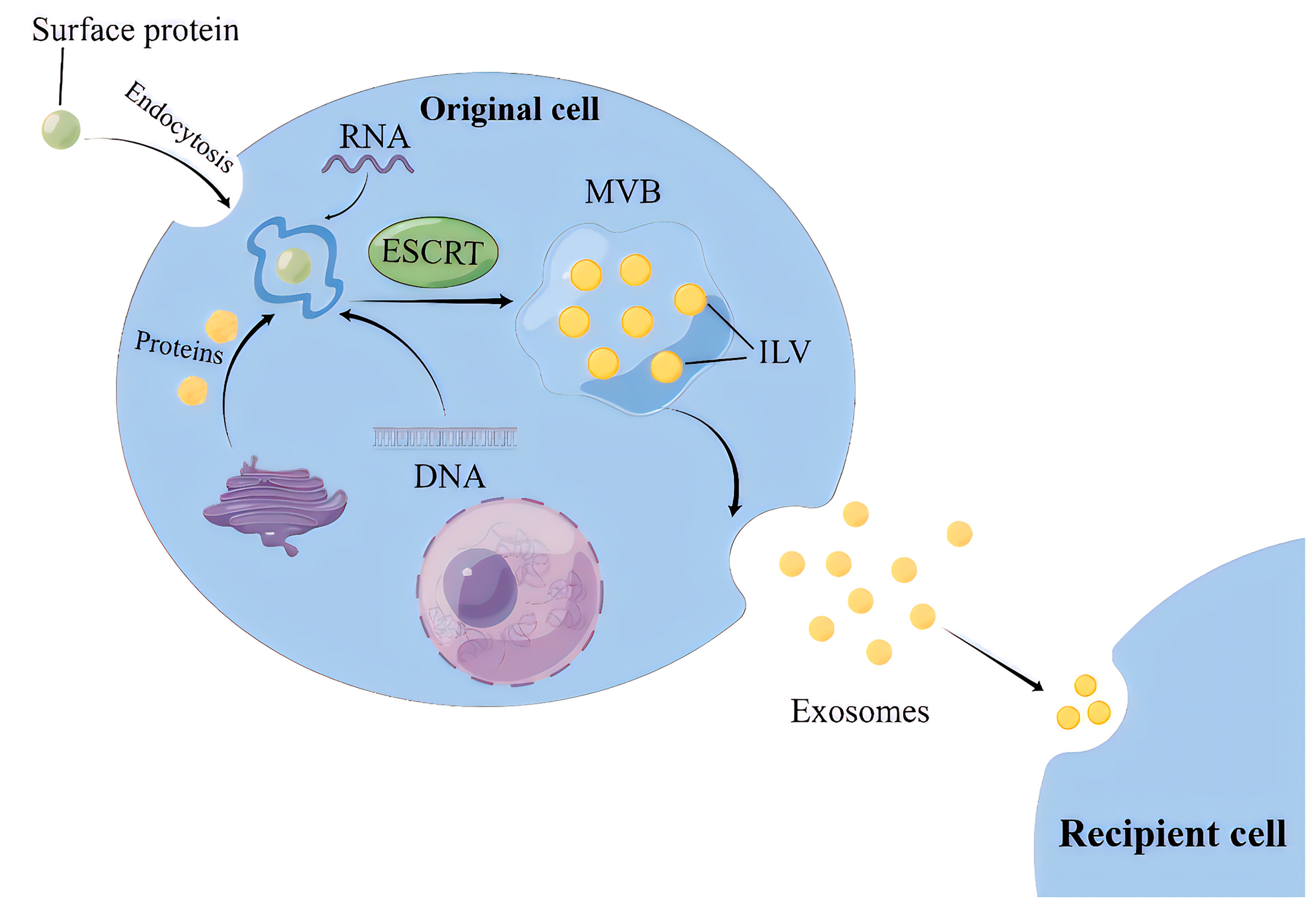
2. Biogenesis of Exosomes
2.1. ILVs Production Involves Two Mechanisms according to Their Reliance on Endosomal Sorting Complex Required for Transport (ESCRT), Hence Referred to as ESCRT-Dependent and -Independent Pathways
2.2. MVBs Transportation and Exosomes Release
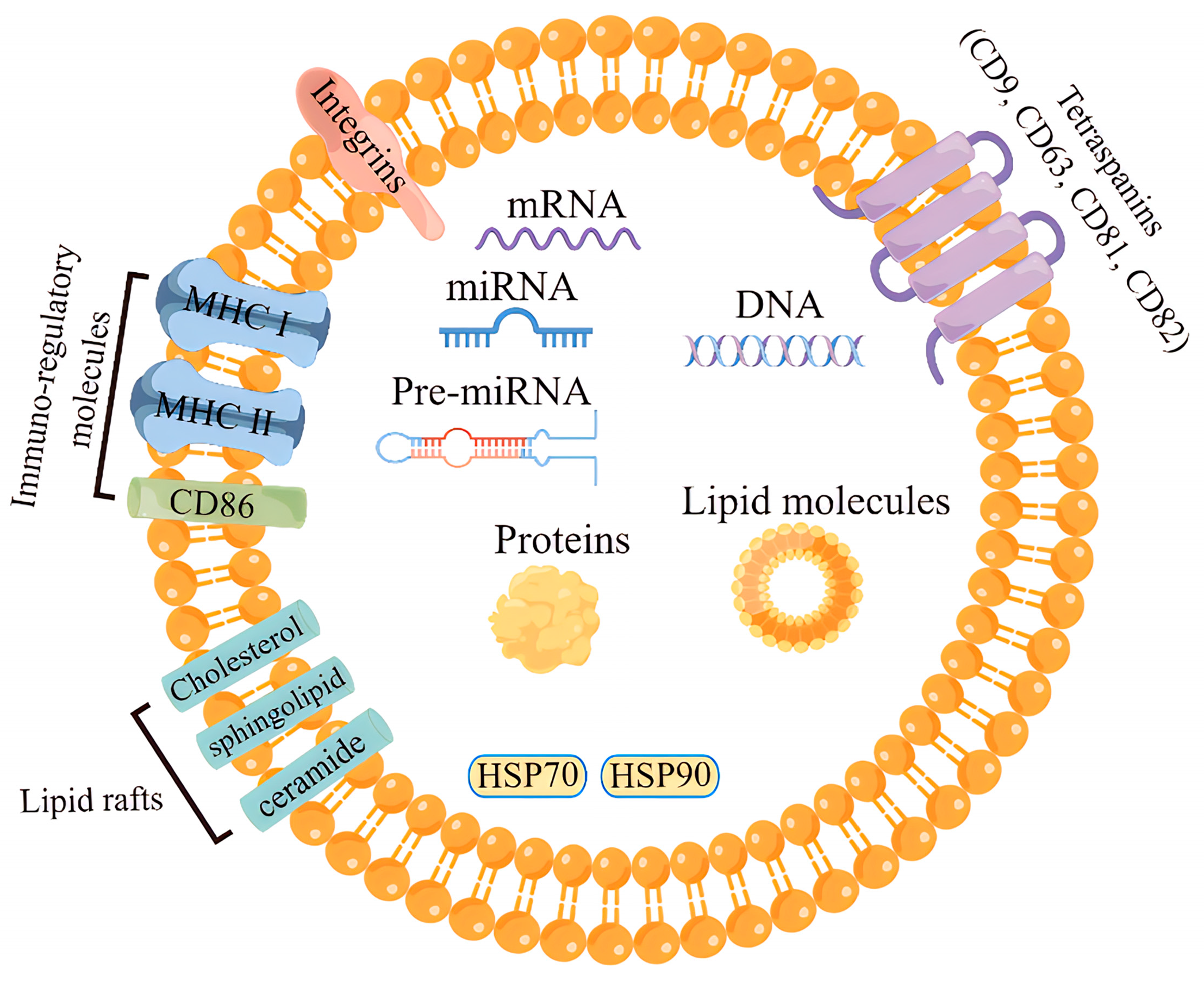
3. Biological Characteristics of Exosomes
4. Exosome Separation and Technical Challenges
5. Biological Functions of Exosomes
6. Application of Exosomes
7. Exosomes Involved in MS Progress
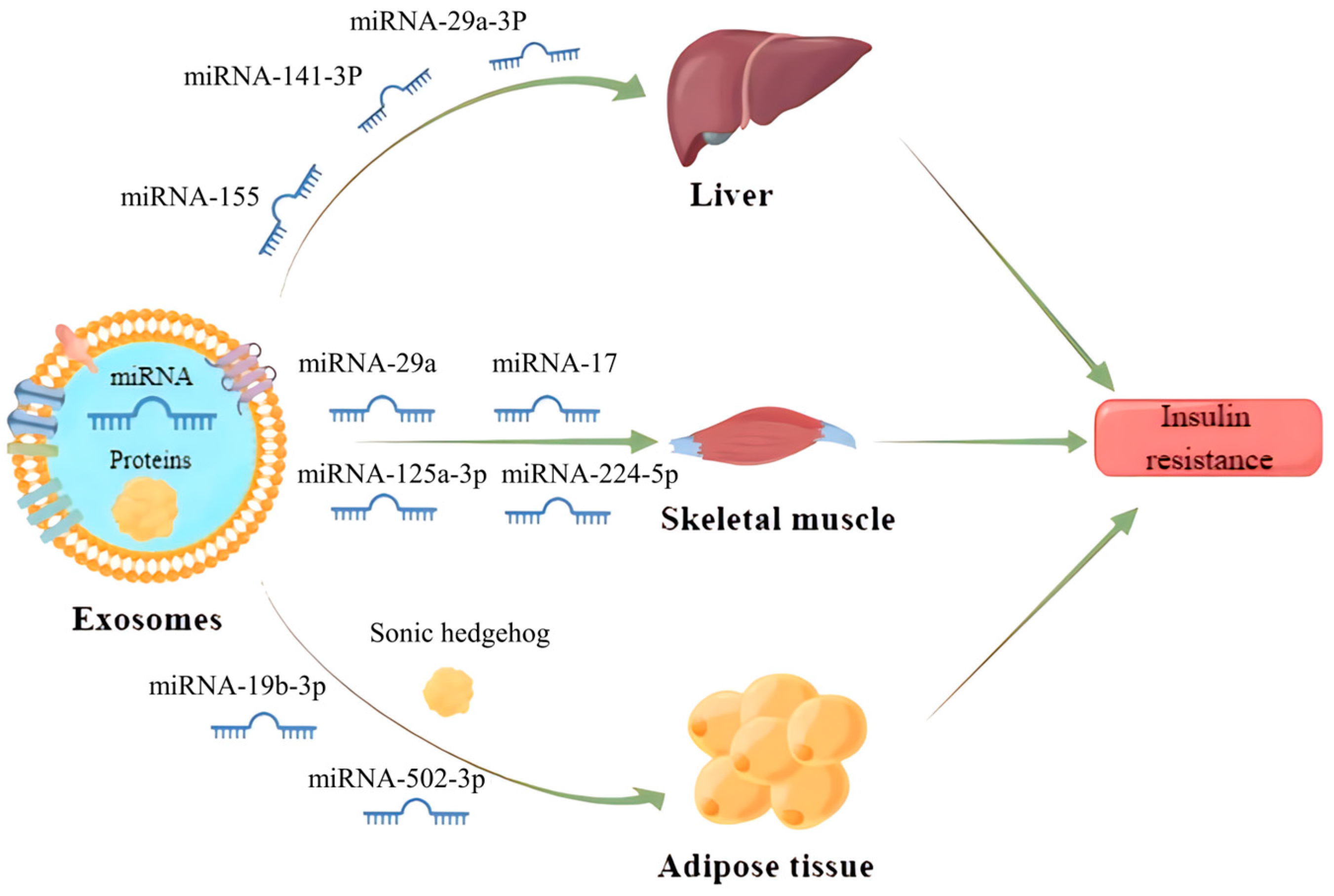
7.1. IR
7.2. DM and Its Related Complications
7.3. Obesity
7.4. NAFLD
7.5. Hyperlipidemia and AS
7.6. Hypertension
8. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome theranostics: Biology and translational medicine. Theranostics 2018, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-Y.; Chen, G.-H.; Yang, Y.-J. Exosomes: A rising star in failing hearts. Front. Physiol. 2017, 8, 494. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Golchin, A.; Hosseinzadeh, S.; Ardeshirylajimi, A. The exosomes released from different cell types and their effects in wound healing. J. Cell. Biochem. 2018, 119, 5043–5052. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Gopal, S.K.; Xu, R.; Simpson, R.J.; Chen, W. Exosomes and their roles in immune regulation and cancer. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2015; pp. 72–81. [Google Scholar]
- Gutierrez-Millan, C.; Calvo Díaz, C.; Lanao, J.M.; Colino, C.I. Advances in exosomes-based drug delivery systems. Macromol. Biosci. 2021, 21, 2000269. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Skowyra, M.L.; Schlesinger, P.H.; Naismith, T.V.; Hanson, P.I. Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science 2018, 360, eaar5078. [Google Scholar] [CrossRef]
- Nour, A.M.; Modis, Y. Endosomal vesicles as vehicles for viral genomes. Trends Cell Biol. 2014, 24, 449–454. [Google Scholar] [CrossRef]
- Mosesso, N.; Nagel, M.-K.; Isono, E. Ubiquitin recognition in endocytic trafficking–with or without ESCRT-0. J. Cell Sci. 2019, 132, jcs232868. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Feng, K.; Zhao, H.; Di, L.; Wang, L.; Wang, R. Tumor-derived extracellular vesicles as messengers of natural products in cancer treatment. Theranostics 2022, 12, 1683. [Google Scholar] [CrossRef] [PubMed]
- Zylbersztejn, K.; Galli, T. Vesicular traffic in cell navigation. FEBS J. 2011, 278, 4497–4505. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Hemler, M.E. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 2003, 19, 397–422. [Google Scholar] [CrossRef] [PubMed]
- Elsherbini, A.; Bieberich, E. Ceramide and exosomes: A novel target in cancer biology and therapy. Adv. Cancer Res. 2018, 140, 121–154. [Google Scholar] [PubMed]
- Soleimani, A.; Farshchi, H.K.; Mirzavi, F.; Zamani, P.; Ghaderi, A.; Amini, Y.; Khorrami, S.; Mashayekhi, K.; Jaafari, M.R. The therapeutic potential of targeting CD73 and CD73-derived adenosine in melanoma. Biochimie 2020, 176, 21–30. [Google Scholar] [CrossRef]
- Fei, X.; Li, Z.; Yang, D.; Kong, X.; Lu, X.; Shen, Y.; Li, X.; Xie, S.; Wang, J.; Zhao, Y.; et al. Neddylation of Coro1a determines the fate of multivesicular bodies and biogenesis of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12153. [Google Scholar] [CrossRef]
- Fader, C.M.; Sánchez, D.G.; Mestre, M.B.; Colombo, M.I. TI-VAMP/VAMP7 and VAMP3/cellubrevin: Two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim. Biophys. Acta 2009, 1793, 1901–1916. [Google Scholar] [CrossRef]
- Matsui, T.; Sakamaki, Y.; Hiragi, S.; Fukuda, M. VAMP5 and distinct sets of cognate Q-SNAREs mediate exosome release. Cell Struct. Funct. 2023, 48, 187–198. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Gangoda, L.; Liem, M.; Fonseka, P.; Atukorala, I.; Ozcitti, C.; Mechler, A.; Adda, C.G.; Ang, C.S.; Mathivanan, S. Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes. Oncotarget 2015, 6, 15375–15396. [Google Scholar] [CrossRef] [PubMed]
- De Gassart, A.; Geminard, C.; Fevrier, B.; Raposo, G.; Vidal, M. Lipid raft-associated protein sorting in exosomes. Blood 2003, 102, 4336–4344. [Google Scholar] [CrossRef] [PubMed]
- D’Souza-Schorey, C.; Clancy, J.W. Tumor-derived microvesicles: Shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012, 26, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Mardones, G.A.; Burgos, P.V.; Lin, Y.; Kloer, D.P.; Magadán, J.G.; Hurley, J.H.; Bonifacino, J.S. Structural basis for the recognition of tyrosine-based sorting signals by the μ3A subunit of the AP-3 adaptor complex. J. Biol. Chem. 2013, 288, 9563–9571. [Google Scholar] [CrossRef] [PubMed]
- Mesmin, B.; Maxfield, F.R. Intracellular sterol dynamics. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2009, 1791, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E.; Voelker, D.R. Cellular lipid transport processes and their role in human disease. Biochim. Biophys. Acta 2009, 1791, 561–562. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.-K.; Giebel, B. Exosomes: Small vesicles participating in intercellular communication. Int. J. Biochem. Cell Biol. 2012, 44, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, A.I.; Masyuk, T.V.; LaRusso, N.F. Exosomes in the pathogenesis, diagnostics and therapeutics of liver diseases. J. Hepatol. 2013, 59, 621–625. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R. Reassessment of exosome composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N. ExoCarta: A web-based compendium of exosomal cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Harmati, M.; Bukva, M.; Böröczky, T.; Buzás, K.; Gyukity-Sebestyén, E. The role of the metabolite cargo of extracellular vesicles in tumor progression. Cancer Metastasis Rev. 2021, 40, 1203–1221. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to extracellular vesicles: Biogenesis, RNA cargo selection, content, release, and uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Phuyal, S.; Brech, A.; Sandvig, K.; Llorente, A. Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochim. Biophys. Acta BBA Gene Regul. Mech. 2012, 1819, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Hvam, M.L.; Primdahl-Bengtson, B.; Boysen, A.T.; Whitehead, B.; Dyrskjøt, L.; Orntoft, T.F.; Howard, K.A.; Ostenfeld, M.S. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J. Extracell. Vesicles 2014, 3, 25011. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef]
- Niamtu, J., 3rd. Lip reduction surgery (reduction cheiloplasty). Facial Plast. Surg. Clin. N. Am. 2010, 18, 79–97. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Shehata Draz, M.; Zarghooni, M.; Sanati-Nezhad, A.; Ghavami, S.; Shafiee, H.; Akbari, M. Microfluidic approaches for isolation, detection, and characterization of extracellular vesicles: Current status and future directions. Biosens. Bioelectron. 2017, 91, 588–605. [Google Scholar] [CrossRef]
- Heinemann, M.L.; Vykoukal, J. Sequential Filtration: A Gentle Method for the Isolation of Functional Extracellular Vesicles. Methods Mol. Biol. 2017, 1660, 33–41. [Google Scholar] [PubMed]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.M.; Li, A.; Chen, J.J.; Sun, E.J. Research Development on Exosome Separation Technology. J. Membr. Biol. 2023, 256, 25–34. [Google Scholar] [CrossRef]
- Suharta, S.; Barlian, A.; Hidajah, A.C.; Notobroto, H.B.; Ana, I.D.; Indariani, S.; Wungu, T.D.K.; Wijaya, C.H. Plant-derived exosome-like nanoparticles: A concise review on its extraction methods, content, bioactivities, and potential as functional food ingredient. J. Food Sci. 2021, 86, 2838–2850. [Google Scholar] [CrossRef] [PubMed]
- Lihon, M.V.; Hadisurya, M.; Wu, X.; Iliuk, A.; Tao, W.A. Isolation and Identification of Plasma Extracellular Vesicles Protein Biomarkers. Methods Mol. Biol. 2023, 2660, 207–217. [Google Scholar] [PubMed]
- Jodo, S.; Xiao, S.; Hohlbaum, A.; Strehlow, D.; Marshak-Rothstein, A.; Ju, S.T. Apoptosis-inducing membrane vesicles. A novel agent with unique properties. J. Biol. Chem. 2001, 276, 39938–39944. [Google Scholar] [CrossRef] [PubMed]
- Montecalvo, A.; Larregina, A.T.; Shufesky, W.J.; Stolz, D.B.; Sullivan, M.L.; Karlsson, J.M.; Baty, C.J.; Gibson, G.A.; Erdos, G.; Wang, Z.; et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 2012, 119, 756–766. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Ocansey, D.K.W.; Xu, W.; Qian, H. Extracellular vesicles: A bright star of nanomedicine. Biomaterials 2021, 269, 120467. [Google Scholar] [CrossRef]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From biomarkers to mediators of physiology and disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Timmers, L.; Lim, S.K.; Hoefer, I.E.; Arslan, F.; Lai, R.C.; van Oorschot, A.A.; Goumans, M.J.; Strijder, C.; Sze, S.K.; Choo, A. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011, 6, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.A.; Andahur, E.I.; Valenzuela, R.; Castellón, E.A.; Fullá, J.A.; Ramos, C.G.; Triviño, J.C. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget 2016, 7, 3993. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—Latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 2020, 182, 1044–1061.e18. [Google Scholar] [CrossRef] [PubMed]
- Heiss, J.A.; Brennan, K.J.; Baccarelli, A.A.; Téllez-Rojo, M.M.; Estrada-Gutiérrez, G.; Wright, R.O.; Just, A.C. Battle of epigenetic proportions: Comparing Illumina’s EPIC methylation microarrays and TruSeq targeted bisulfite sequencing. Epigenetics 2020, 15, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Tutrone, R.; Donovan, M.J.; Torkler, P.; Tadigotla, V.; McLain, T.; Noerholm, M.; Skog, J.; McKiernan, J. Clinical utility of the exosome based ExoDx Prostate(IntelliScore) EPI test in men presenting for initial Biopsy with a PSA 2–10 ng/mL. Prostate Cancer Prostatic Dis. 2020, 23, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xia, T.; Fang, Y.; Zuo, H.; Dong, X.; Xu, P.; Ouyang, J. Overcoming the blood–brain barrier by using a multistage exosome delivery system to inhibit central nervous system lymphoma. Nanomed. Nanotechnol. Biol. Med. 2022, 41, 102523. [Google Scholar] [CrossRef]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell 2017, 171, 372–384.e12. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.-Y.; Leng, Y.; Wang, Z.-X.; Xiao, X.; Zhang, X.; Wen, T.; Gong, H.-Z.; Hong, A.; Ma, Y. Exosomal transfer of obesity adipose tissue for decreased miR-141-3p mediate insulin resistance of hepatocytes. Int. J. Biol. Sci. 2019, 15, 351. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.-J.; Weng, S.-Y.; Lin, M.; Chai, K.-F. Yunpi Heluo decoction attenuates insulin resistance by regulating liver miR-29a-3p in Zucker diabetic fatty rats. J. Ethnopharmacol. 2019, 243, 111966. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Jayabalan, N.; Guanzon, D.; Palma, C.; Scholz-Romero, K.; Elfeky, O.; Zuñiga, F.; Ormazabal, V.; Diaz, E.; Rice, G.E.; et al. Human placental exosomes in gestational diabetes mellitus carry a specific set of miRNAs associated with skeletal muscle insulin sensitivity. Clin. Sci. 2018, 132, 2451–2467. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Xiao, Y.; Xiao, Y.; Guo, Q.; Li, C.; Huang, Y.; Deng, Q.; Wen, J.; Zhou, F.; Luo, X.-H. Bone marrow mesenchymal stem cells-derived exosomal MiR-29b-3p regulates aging-associated insulin resistance. ACS Nano 2019, 13, 2450–2462. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Han, L.; Chen, F.-F.; Wang, D.; Wang, F.; Zhang, L.; Wang Z-h Zhong, M.; Tang, M.-X.; Zhang, W. Adipocyte-derived exosomes carrying sonic hedgehog mediate M1 macrophage polarization-induced insulin resistance via Ptch and PI3K pathways. Cell. Physiol. Biochem. 2018, 48, 1416–1432. [Google Scholar] [CrossRef] [PubMed]
- Al-Κafaji, G.; Al-Muhtaresh, H.A.; Salem, A.H. Expression and clinical significance of miR-1 and miR-133 in pre-diabetes. Biomed. Rep. 2021, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Yamada, T.; Takahashi, K.; Munakata, Y.; Hosaka, S.; Takahashi, H.; Gao, J.; Shirai, Y.; Kodama, S.; Asai, Y. MicroRNAs 106b and 222 improve hyperglycemia in a mouse model of insulin-deficient diabetes via pancreatic β-cell proliferation. eBioMedicine 2017, 15, 163–172. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Y.; Shi, Y.; Zhang, Y.; Liu, K.; Liang, R.; Sun, P.; Chang, X.; Tang, W.; Zhang, Y. Expression of miRNA-29 in pancreatic β cells promotes inflammation and diabetes via TRAF3. Cell Rep. 2021, 34, 108576. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Chen, L.; Bian, H.; Chen, X.; Zheng, H.; Yang, P.; Chen, Q.; Xu, H. Natural killer cell-derived exosomal miR-1249-3p attenuates insulin resistance and inflammation in mouse models of type 2 diabetes. Signal Transduct. Target. Ther. 2021, 6, 409. [Google Scholar] [CrossRef]
- Zhuang, M.; Du, D.; Pu, L.; Song, H.; Deng, M.; Long, Q.; Yin, X.; Wang, Y.; Rao, L. SPION-decorated exosome delivered BAY55-9837 targeting the pancreas through magnetism to improve the blood GLC response. Small 2019, 15, 1903135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-P.; Tian, M.-Y.; Yang, Y.-D.; Li, H.; Zhao, T.-T.; Zhu, J.; Mou, F.-F.; Cui, G.-H.; Guo, H.-D.; Shao, S.-J. Schwann cells-derived exosomal miR-21 participates in high glucose regulation of neurite outgrowth. iScience 2022, 25, 105141. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chen, J.; Zheng, Z.; Tao, Y.; Zhang, S.; Zou, M.; Yang, Y.; Xue, M.; Hu, F.; Li, Y. Tubular epithelial cell-derived extracellular vesicles induce macrophage glycolysis by stabilizing HIF-1α in diabetic kidney disease. Mol. Med. 2022, 28, 95. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.S.; Kim, S. Human adipose mesenchymal stem cells modulate inflammation and angiogenesis through exosomes. Sci. Rep. 2022, 12, 2776. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; An, Y.; Sun, Y.; Yang, F.; Xu, Q.; Wang, Z. Adipose Mesenchymal Stem Cell-Derived Exosomes Promote Wound Healing Through the WNT/β-catenin Signaling Pathway in Dermal Fibroblasts. Stem Cell Rev. Rep. 2022, 18, 2059–2073. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tao, R.; Chen, L.; Xiong, Y.; Xue, H.; Hu, L.; Yan, C.; Xie, X.; Lin, Z.; Panayi, A.C.; et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Theocharidis, G.; Vlachos, I.S.; Kounas, K.; Lobao, A.; Shu, B.; Wu, B.; Xie, J.; Hu, Z.; Qi, S. Exosomes derived from epidermal stem cells improve diabetic wound healing. J. Investig. Dermatol. 2022, 142, 2508–2517.e13. [Google Scholar] [CrossRef]
- Guo, B.; Shan, S.-K.; Xu, F.; Lin, X.; Li, F.-X.-Z.; Wang, Y.; Xu, Q.-S.; Zheng, M.-H.; Lei, L.-M.; Li, C.-C. Protective role of small extracellular vesicles derived from HUVECs treated with AGEs in diabetic vascular calcification. J. Nanobiotechnol. 2022, 20, 334. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, H.; Xu, H.; Tan, Y.; Zhang, C.; Zeng, Q.; Liu, L.; Qu, S. Targeting the microRNAs in exosome: A potential therapeutic strategy for alleviation of diabetes-related cardiovascular complication. Pharmacol. Res. 2021, 173, 105868. [Google Scholar] [CrossRef]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.Z.; Wang, Q.L.; Du, J.G.; Wang, B.B. Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placental exosomes in the maternal circulation across gestation. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3797. [Google Scholar] [PubMed]
- Rulkiewicz, A.; Pilchowska, I.; Lisik, W.; Pruszczyk, P.; Ciurzynski, M.; Domienik-Karlowicz, J. Prevalence of Obesity and Severe Obesity among Professionally Active Adult Population in Poland and Its Strong Relationship with Cardiovascular Co-Morbidities-POL-O-CARIA 2016–2020 Study. J. Clin. Med. 2022, 11, 3720. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Guo, X. The clinical potential of circulating microRNAs in obesity. Nat. Rev. Endocrinol. 2019, 15, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sundaram, K.; Mu, J.; Dryden, G.W.; Sriwastva, M.K.; Lei, C.; Zhang, L.; Qiu, X.; Xu, F.; Yan, J. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat. Commun. 2021, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Du, H.; Wei, S.; Feng, L.; Li, J.; Yao, F.; Zhang, M.; Hatch, G.M.; Chen, L. Adipocyte-derived exosomal MiR-27a induces insulin resistance in skeletal muscle through repression of PPARγ. Theranostics 2018, 8, 2171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, J.; Zhang, S.; Yan, S.; Wang, Z.; Wang, C.; Zhang, X. MiR-30e and miR-92a are related to atherosclerosis by targeting ABCA1. Mol. Med. Rep. 2019, 19, 3298–3304. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, N.; Yan, T.; Shi, Y.-N.; Chen, J.; Zhang, C.-J.; Xie, X.-J.; Liao, D.-F.; Qin, L. The crosstalk: Exosomes and lipid metabolism. Cell Commun. Signal. 2020, 18, 119. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Mark, H.E.; Anstee, Q.M.; Arab, J.P.; Batterham, R.L.; Castera, L.; Cortez-Pinto, H.; Crespo, J.; Cusi, K.; Dirac, M.A. Advancing the global public health agenda for NAFLD: A consensus statement. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 60–78. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, H.; Wang, H. Extracellular vesicles in non-alcoholic fatty liver disease and alcoholic liver disease. Front. Physiol. 2021, 12, 707429. [Google Scholar] [CrossRef]
- Liu, X.L.; Pan, Q.; Cao, H.X.; Xin, F.Z.; Zhao, Z.H.; Yang, R.X.; Zeng, J.; Zhou, H.; Fan, J.G. Lipotoxic hepatocyte-derived exosomal microRNA 192-5p activates macrophages through rictor/Akt/forkhead box transcription factor O1 signaling in nonalcoholic fatty liver disease. Hepatology 2020, 72, 454–469. [Google Scholar] [CrossRef]
- Hou, X.; Yin, S.; Ren, R.; Liu, S.; Yong, L.; Liu, Y.; Li, Y.; Zheng, M.H.; Kunos, G.; Gao, B. Myeloid-Cell–Specific IL-6 Signaling Promotes MicroRNA-223-Enriched Exosome Production to Attenuate NAFLD-Associated Fibrosis. Hepatology 2021, 74, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yu, P.; Li, F.; Jiang, X.; Jiao, X.; Shen, Y.; Lai, X. Human umbilical cord-derived mesenchymal stem cell-exosomal miR-627-5p ameliorates non-alcoholic fatty liver disease by repressing FTO expression. Hum. Cell 2021, 34, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Pasquino, C.; Sanchez, M.B.H.; Tapparo, M.; Figliolini, F.; Grange, C.; Chiabotto, G.; Cedrino, M.; Deregibus, M.C.; Tetta, C. HLSC-derived extracellular vesicles attenuate liver fibrosis and inflammation in a murine model of non-alcoholic steatohepatitis. Mol. Ther. 2020, 28, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Wang, Y.; Dakhlallah, D.; Moldovan, L.; Agarwal, K.; Batte, K.; Shah, P.; Wisler, J.; Eubank, T.D.; Tridandapani, S.; et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood 2013, 121, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, G.; Meng, C.; Guo, X.; Cheruku, P.S.; Shi, L.; Xu, H.; Li, H.; Wang, G.; Evans, A.R.; Safe, S.; et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation 2012, 125, 2892–2903. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, X.; Lin, C.; An, Z.; Yu, J.; Cao, H.; Fan, Y.; Liang, X. Exosomal MALAT1 derived from ox-LDL-treated endothelial cells induce neutrophil extracellular traps to aggravate atherosclerosis. Biol. Chem. 2020, 401, 367–376. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Liu, X.; Du, H.; Sun, C.; Shao, X.; Tian, J.; Gu, X.; Wang, H.; Tian, J. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J. Am. Heart Assoc. 2018, 7, e007442. [Google Scholar] [CrossRef]
- Bouchareychas, L.; Duong, P.; Covarrubias, S.; Alsop, E.; Phu, T.A.; Chung, A.; Gomes, M.; Wong, D.; Meechoovet, B.; Capili, A. Macrophage exosomes resolve atherosclerosis by regulating hematopoiesis and inflammation via MicroRNA cargo. Cell Rep. 2020, 32, 107881. [Google Scholar] [CrossRef]
- Gutmann, C.; Mayr, M. Circulating microRNAs as biomarkers and mediators of platelet activation. Platelets 2022, 33, 512–519. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Khanna, S.; Roy, S. Micromanaging vascular biology: Tiny microRNAs play big band. J. Vasc. Res. 2009, 46, 527–540. [Google Scholar] [CrossRef]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.F.; Wythe, J.D.; Ivey, K.N.; Bruneau, B.G.; Stainier, D.Y.; Srivastava, D. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, D.; Chen, X.; Li, J.; Li, L.; Bian, Z.; Sun, F.; Lu, J.; Yin, Y.; Cai, X. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol. Cell 2010, 39, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Ku, E.J.; Cho, K.-C.; Lim, C.; Kang, J.W.; Oh, J.W.; Choi, Y.R.; Park, J.-M.; Han, N.-Y.; Oh, J.J.; Oh, T.J. Discovery of plasma biomarkers for predicting the severity of coronary artery atherosclerosis by quantitative proteomics. BMJ Open Diabetes Res. Care 2020, 8, e001152. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.A. Hypertensive Crisis: A Review of Pathophysiology and Treatment. Crit. Care Nurs. Clin. 2015, 27, 439–447. [Google Scholar] [CrossRef]
- Jansen, F.; Yang, X.; Proebsting, S.; Hoelscher, M.; Przybilla, D.; Baumann, K.; Schmitz, T.; Dolf, A.; Endl, E.; Franklin, B.S.; et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J. Am. Heart Assoc. 2014, 3, e001249. [Google Scholar] [CrossRef]
- Ong, S.G.; Lee, W.H.; Huang, M.; Dey, D.; Kodo, K.; Sanchez-Freire, V.; Gold, J.D.; Wu, J.C. Cross talk of combined gene and cell therapy in ischemic heart disease: Role of exosomal microRNA transfer. Circulation 2014, 130, S60–S69. [Google Scholar] [CrossRef]
- Buntsma, N.; van der Pol, E.; Nieuwland, R.; Gąsecka, A. Extracellular Vesicles in Coronary Artery Disease. In Extracellular Vesicles in Cardiovascular and Metabolic Diseases; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2023; Volume 1418, pp. 81–103. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Li, J.; Hu, Z.; Ngowi, E.E.; Yan, B.; Qiao, A. Exosomes: New Insights into the Pathogenesis of Metabolic Syndrome. Biology 2023, 12, 1480. https://doi.org/10.3390/biology12121480
Wang N, Li J, Hu Z, Ngowi EE, Yan B, Qiao A. Exosomes: New Insights into the Pathogenesis of Metabolic Syndrome. Biology. 2023; 12(12):1480. https://doi.org/10.3390/biology12121480
Chicago/Turabian StyleWang, Ning, Jing Li, Zixuan Hu, Ebenezeri Erasto Ngowi, Baolong Yan, and Aijun Qiao. 2023. "Exosomes: New Insights into the Pathogenesis of Metabolic Syndrome" Biology 12, no. 12: 1480. https://doi.org/10.3390/biology12121480





