Intestinal Barrier Dysfunction and Gut Microbiota in Non-Alcoholic Fatty Liver Disease: Assessment, Mechanisms, and Therapeutic Considerations
Abstract
:Simple Summary
Abstract
1. Introduction
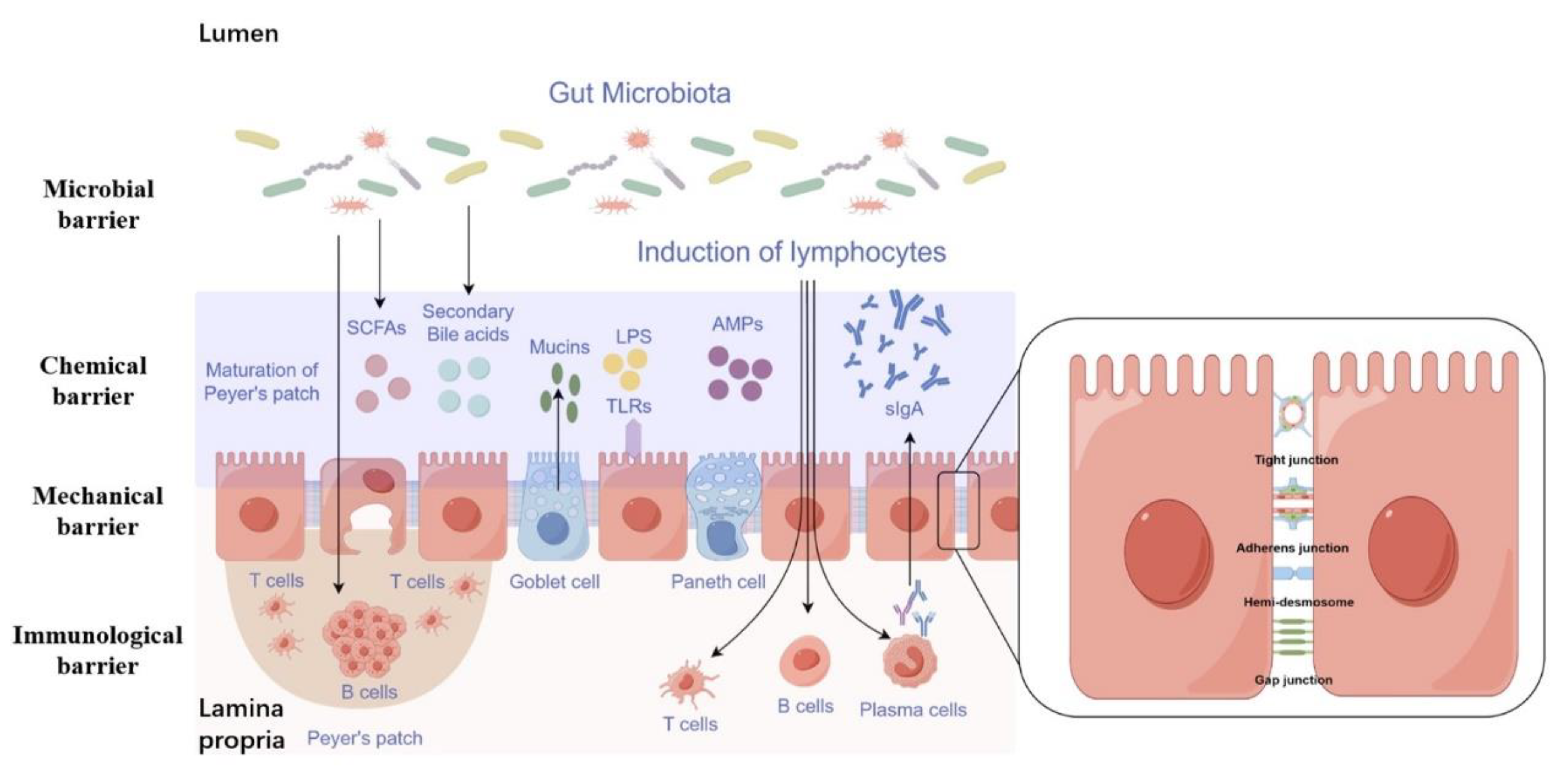
2. Function and Composition of the Intestinal Barrier
2.1. Mechanical Barrier
2.2. Chemical Barrier
2.3. Immunological Barrier
2.4. Microbial Barrier
3. Assessment of Intestinal Barrier
3.1. Evaluation of the Gut Barrier in a Living Organism
3.1.1. Exogenous Inert Probes
3.1.2. Exogenous Inert Probes
3.2. Evaluation of the Intestinal Barrier in a Laboratory Setting
3.2.1. Monoculture Models
3.2.2. Co-Culture Models and Intestinal Organoids
4. Gut Dysbiosis and Gut–Liver Axis
5. Inflammation and TLR Signaling
6. Therapeutic Strategies Targeting Gut Permeability and Gut Microbiota
6.1. Probiotics
6.1.1. Conventional Probiotics
6.1.2. Next-Generation Probiotics
- Strain selection: NGPs mostly derived from commensals and that belong to diverse genera, which are selected based on advanced screening techniques, including genomics, metagenomics, and functional assays, that allow for identifying and isolating strains with specific beneficial properties, such as enhanced colonization, improved survival in the gastrointestinal tract, and targeted health benefits.
- Precision and personalized approach: NGPs are designed to be more precise and personalized in their application. They can be tailored to address specific health conditions or individual needs, considering factors like microbiome composition, genetic background, and lifestyle factors.
- Mechanistic understanding: NGPs are characterized by a better understanding of their mechanisms of action. Advances in molecular biology and omics technologies have enabled researchers to uncover the molecular interactions between NGPs and the host, providing insights into the underlying mechanisms through which they exert their beneficial effects.
- Therapeutic potential: NGPs have the potential to be used as therapeutic agents for various diseases beyond gut health. By manipulating the gut microbiota and engaging with the host’s immune system, they can address ailments like metabolic disorders, inflammatory conditions, and neurological disorders.
- Combination therapies: NGPs can be combined with other therapies, such as pharmaceutical drugs or dietary interventions, to enhance their efficacy. This approach, known as synbiotics, involves the synergistic interaction between NGPs and other therapeutic agents to achieve improved health outcomes.
6.2. Fecal Microbiota Transplantation
6.3. Lifestyle Changes
6.3.1. Exercise
6.3.2. Dietary Carbohydrates
6.3.3. Dietary Proteins
6.3.4. Dietary Fats
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metab. Clin. Exp. 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Juanola, O.; Martínez-López, S.; Francés, R.; Gómez-Hurtado, I. Non-Alcoholic Fatty Liver Disease: Metabolic, Genetic, Epigenetic and Environmental Risk Factors. Int. J. Environ. Res. Public Health 2021, 18, 5227. [Google Scholar] [CrossRef] [PubMed]
- Gariani, K.; Jornayvaz, F.R. Pathophysiology of NASH in endocrine diseases. Endocr. Connect. 2021, 10, R52–R65. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.T.; Kleiner, D.E. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metab. Clin. Exp. 2016, 65, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- Guo, Z.; Fan, X.; Yao, J.; Tomlinson, S.; Yuan, G.; He, S. The role of complement in nonalcoholic fatty liver disease. Front. Immunol. 2022, 13, 1017467. [Google Scholar] [CrossRef] [PubMed]
- Lemoinne, S.; Friedman, S.L. New and emerging anti-fibrotic therapeutics entering or already in clinical trials in chronic liver diseases. Curr. Opin. Pharmacol. 2019, 49, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Forlano, R.; Mullish, B.H.; Nathwani, R.; Dhar, A.; Thursz, M.R.; Manousou, P. Non-Alcoholic Fatty Liver Disease and Vascular Disease. Curr. Vasc. Pharmacol. 2021, 19, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62 (Suppl. S1), S47–S64. [Google Scholar] [CrossRef]
- Moretti, R.; Caruso, P.; Gazzin, S. Non-alcoholic fatty liver disease and neurological defects. Ann. Hepatol. 2019, 18, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; N.AFLD Nomenclature Consensus Group; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Peverill, W.; Powell, L.W.; Skoien, R. Evolving concepts in the pathogenesis of NASH: Beyond steatosis and inflammation. Int. J. Mol. Sci. 2014, 15, 8591–8638. [Google Scholar] [CrossRef] [PubMed]
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell. Mol. Life Sci. CMLS 2019, 76, 99–128. [Google Scholar] [CrossRef] [PubMed]
- Amorim, R.; Magalhães, C.C.; Borges, F.; Oliveira, P.J.; Teixeira, J. From Non-Alcoholic Fatty Liver to Hepatocellular Carcinoma: A Story of (Mal)Adapted Mitochondria. Biology 2023, 12, 595. [Google Scholar] [CrossRef] [PubMed]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Farré, R.; Vicario, M. Abnormal Barrier Function in Gastrointestinal Disorders. Handb. Exp. Pharmacol. 2017, 239, 193–217. [Google Scholar] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Forlano, R.; Mullish, B.H.; Roberts, L.A.; Thursz, M.R.; Manousou, P. The Intestinal Barrier and Its Dysfunction in Patients with Metabolic Diseases and Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 662. [Google Scholar] [CrossRef]
- Liu, L.; Yin, M.; Gao, J.; Yu, C.; Lin, J.; Wu, A.; Zhu, J.; Xu, C.; Liu, X. Intestinal Barrier Function in the Pathogenesis of Nonalcoholic Fatty Liver Disease. J. Clin. Transl. Hepatol. 2023, 11, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, X.F.; Su, Y.S.; He, W.; Zhang, J.B.; Zhang, Q.; Zhan, L.B.; Jing, X.H. Mechanism of Acupuncture and Moxibustion on Promoting Mucosal Healing in Ulcerative Colitis. Chin. J. Integr. Med. 2023, 29, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Raya-Sandino, A.; Luissint, A.C.; Kusters, D.H.M.; Narayanan, V.; Flemming, S.; Garcia-Hernandez, V.; Godsel, L.M.; Green, K.J.; Hagen, S.J.; Conway, D.E.; et al. Regulation of intestinal epithelial intercellular adhesion and barrier function by desmosomal cadherin desmocollin-2. Mol. Biol. Cell 2021, 32, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Rusu, A.D.; Georgiou, M. The multifarious regulation of the apical junctional complex. Open Biol. 2020, 10, 190278. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar] [CrossRef] [PubMed]
- Douhara, A.; Moriya, K.; Yoshiji, H.; Noguchi, R.; Namisaki, T.; Kitade, M.; Kaji, K.; Aihara, Y.; Nishimura, N.; Takeda, K.; et al. Reduction of endotoxin attenuates liver fibrosis through suppression of hepatic stellate cell activation and remission of intestinal permeability in a rat non-alcoholic steatohepatitis model. Mol. Med. Rep. 2015, 11, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Obert, E.; Strauss, R.; Brandon, C.; Grek, C.; Ghatnekar, G.; Gourdie, R.; Rohrer, B. Targeting the tight junction protein, zonula occludens-1, with the connexin43 mimetic peptide, αCT1, reduces VEGF-dependent RPE pathophysiology. J. Mol. Med. 2017, 95, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Peglion, F.; Llense, F.; Etienne-Manneville, S. Adherens junction treadmilling during collective migration. Nat. Cell Biol. 2014, 16, 639–651. [Google Scholar] [CrossRef] [PubMed]
- le Duc, Q.; Shi, Q.; Blonk, I.; Sonnenberg, A.; Wang, N.; Leckband, D.; de Rooij, J. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J. Cell Biol. 2010, 189, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, W.; Qiu, Y.; Yu, M.; Yin, J.; Yang, H.; Mei, J. KGF inhibits hypoxia-induced intestinal epithelial cell apoptosis by upregulating AKT/ERK pathway-dependent E-cadherin expression. Biomed. Pharmacother. 2018, 105, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.C.; Hwang, J.M.; Bang, S.J.; Kim, B.T.; Kim, D.H.; Chae, M.; Lee, S.A.; Choi, G.J.; Kim, D.H.; Lee, J.C. Chloroform extract of alfalfa (Medicago sativa) inhibits lipopolysaccharide-induced inflammation by downregulating ERK/NF-κB signaling and cytokine production. J. Med. Food 2013, 16, 410–420. [Google Scholar] [CrossRef]
- Campbell, H.K.; Maiers, J.L.; DeMali, K.A. Interplay between tight junctions & adherens junctions. Exp. Cell Res. 2017, 358, 39–44. [Google Scholar] [PubMed]
- Cui, Y.; Wang, Q.; Chang, R.; Zhou, X.; Xu, C. Intestinal Barrier Function-Non-alcoholic Fatty Liver Disease Interactions and Possible Role of Gut Microbiota. J. Agric. Food Chem. 2019, 67, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, K.F.; Herzberg, M.C. Antimicrobial peptides: Defending the mucosal epithelial barrier. Front. Oral Health. 2022, 1, 958480. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, A.; Ponziani, F.R.; Biolato, M.; Valenza, V.; Marrone, G.; Sganga, G.; Gasbarrini, A.; Miele, L.; Grieco, A. Intestinal permeability in the pathogenesis of liver damage: From non-alcoholic fatty liver disease to liver transplantation. World J. Gastroenterol. 2019, 25, 4814–4834. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Baj, J.; Garruti, G.; Celano, G.; De Angelis, M.; Wang, H.H.; Di Palo, D.M.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk. J. Clin. Med. 2020, 9, 2648. [Google Scholar] [PubMed]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, L.; Chen, T. The Effects of Secretory IgA in the Mucosal Immune System. BioMed Res. Int. 2020, 2020, 2032057. [Google Scholar] [CrossRef] [PubMed]
- Xichun, W.; Jinjie, W.; Liang, C.; Xiaojia, H. Effect of high-level zinc diets on intestinal mucosal immunity and morphology of weanling piglets. Chin. J. Vet. Sci. 2010, 30, 1371–1376. [Google Scholar]
- Mahdally, S.M.; Izquierdo, M.; Viscardi, R.M.; Magder, L.S.; Crowley, H.M.; Bafford, A.C.; Drachenberg, C.B.; Farfan, M.J.; Fasano, A.; Sztein, M.B.; et al. Secretory-IgA binding to intestinal microbiota attenuates inflammatory reactions as the intestinal barrier of preterm infants matures. Clin. Exp. Immunol. 2023, 213, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Philips, C.A.; Augustine, P. Gut Barrier and Microbiota in Cirrhosis. J. Clin. Exp. Hepatol. 2022, 12, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Gillingham, T.; Guo, Y.; Meng, D.; Zhu, W.; Walker, W.A.; Ganguli, K. Secretions of Bifidobacterium infantis and Lactobacillus acidophilus Protect Intestinal Epithelial Barrier Function. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.J.; Colgan, S.P. Breathless in the Gut: Implications of Luminal O2 for Microbial Pathogenicity. Cell Host Microbe 2016, 19, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.S.; Chamberlain, R.S. Probiotics are effective at preventing Clostridium difficile-associated diarrhea: A systematic review and meta-analysis. Int. J. Gen. Med. 2016, 9, 27–37. [Google Scholar] [PubMed]
- De Munck, T.J.I.; Xu, P.; Verwijs, H.J.A.; Masclee, A.A.M.; Jonkers, D.; Verbeek, J.; Koek, G.H. Intestinal permeability in human nonalcoholic fatty liver disease: A systematic review and meta-analysis. Liver Int. Off. J. Int. Assoc. Study Liver 2020, 40, 2906–2916. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.P.; Zhang, Y.T.; Zhang, R.X.; Zhong, H.J.; He, X.X. The Gut-Liver Axis in Nonalcoholic Fatty Liver Disease: Association of Intestinal Permeability with Disease Severity and Treatment Outcomes. Int. J. Clin. Pract. 2022, 2022, 4797453. [Google Scholar] [CrossRef] [PubMed]
- Seethaler, B.; Basrai, M.; Neyrinck, A.M.; Nazare, J.A.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Biomarkers for assessment of intestinal permeability in clinical practice. Am. J. Physiol.-Gastrointest. Liver Physiology. 2021, 321, G11–G17. [Google Scholar] [CrossRef] [PubMed]
- Volynets, V.; Reichold, A.; Bárdos, G.; Rings, A.; Bleich, A.; Bischoff, S.C. Assessment of the Intestinal Barrier with Five Different Permeability Tests in Healthy C57BL/6J and BALB/cJ Mice. Dig. Dis. Sci. 2016, 61, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Khoshbin, K.; Khanna, L.; Maselli, D.; Atieh, J.; Breen-Lyles, M.; Arndt, K.; Rhoten, D.; Dyer, R.B.; Singh, R.J.; Nayar, S.; et al. Development and Validation of Test for “Leaky Gut” Small Intestinal and Colonic Permeability Using Sugars in Healthy Adults. Gastroenterology 2021, 161, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Larkey, N.E.; Fatica, E.M.; Singh, R.J. Detection of 13C-Mannitol and Other Saccharides Using Tandem Mass Spectrometry for Evaluation of Intestinal Permeability or Leaky Gut. Methods Mol. Biol. 2022, 2546, 285–294. [Google Scholar]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, D.; Olander, T.; Sjöblom, M.; Hedeland, M.; Lennernäs, H. Effect of paracellular permeation enhancers on intestinal permeability of two peptide drugs, enalaprilat and hexarelin, in rats. Acta Pharm. Sinica. B 2021, 11, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, J.; Liu, J.; Zhu, H.; Li, R.; Wan, X.; Lei, J.; Li, Y.; You, C.; Hu, F.; et al. Programmed cell death 10 increased blood-brain barrier permeability through HMGB1/TLR4 mediated downregulation of endothelial ZO-1 in glioblastoma. Cell. Signal. 2023, 107, 110683. [Google Scholar] [CrossRef] [PubMed]
- Melichar, B.; Hyspler, R.; Kalábová, H.; Dvorák, J.; Tichá, A.; Zadák, Z. Gastroduodenal, intestinal and colonic permeability during anticancer therapy. Hepatogastroenterology 2011, 58, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- van Wijck, K.; Verlinden, T.J.; van Eijk, H.M.; Dekker, J.; Buurman, W.A.; Dejong, C.H.; Lenaerts, K. Novel multi-sugar assay for site-specific gastrointestinal permeability analysis: A randomized controlled crossover trial. Clin. Nutr. 2013, 32, 245–251. [Google Scholar] [CrossRef] [PubMed]
- van Wijck, K.; van Eijk, H.M.; Buurman, W.A.; Dejong, C.H.; Lenaerts, K. Novel analytical approach to a multi-sugar whole gut permeability assay. J. Chromatography. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2794–2801. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Intestinal permeability and its regulation by zonulin: Diagnostic and therapeutic implications. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2012, 10, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef] [PubMed]
- Maget, A.; Dalkner, N.; Hamm, C.; Bengesser, S.A.; Fellendorf, F.T.; Platzer, M.; Queissner, R.; Birner, A.; Lenger, M.; Mörkl, S.; et al. Sex differences in zonulin in affective disorders and associations with current mood symptoms. J. Affect. Disord. 2021, 294, 441–446. [Google Scholar] [CrossRef]
- Dumoulin, E.N.; Van Biervliet, S.; Langlois, M.R.; Delanghe, J.R. Proteolysis is a confounding factor in the interpretation of faecal calprotectin. Clin. Chem. Lab. Med. 2015, 53, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Shabani, F.; Farasat, A.; Mahdavi, M.; Gheibi, N. Calprotectin (S100A8/S100A9): A key protein between inflammation and cancer. Inflamm. Res. Off. J. Eur. Histamine Res. Society. 2018, 67, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From biomarker to biological function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Khaki-Khatibi, F.; Qujeq, D.; Kashifard, M.; Moein, S.; Maniati, M.; Vaghari-Tabari, M. Calprotectin in inflammatory bowel disease. Clin. Chim. Acta; Int. J. Clin. Chem. 2020, 510, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Grad, C.; David, L.; Portincasa, P.; Dumitraşcu, D.L. Diagnostic value of calprotectin in irritable bowel syndrome and in inflammatory bowel disease. Rom. J. Intern. Med. 2012, 50, 3–6. [Google Scholar] [PubMed]
- Demirbaş, F.; Çaltepe, G.; Comba, A.; Abbasguliyev, H.; Yurttan Uyar, N.; Kalaycı, A.G. Association of obesity and non-alcoholic fatty liver disease with the fecal calprotectin level in children. Arab J. Gastroenterol. Off. Publ. Pan-Arab Assoc. Gastroenterol. 2020, 21, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; van den Berg, E.H.; Kieneker, L.M.; Nilsen, T.; Hidden, C.; Bakker, S.J.L.; Blokzijl, H.; Dullaart, R.P.F.; van Goor, H.; Abdulle, A.E. Plasma Calprotectin Levels Associate with Suspected Metabolic-Associated Fatty Liver Disease and All-Cause Mortality in the General Population. Int. J. Mol. Sci. 2022, 23, 15708. [Google Scholar] [CrossRef]
- Bıçakçı, E.; Demirtaş, C.; Çelikel, Ç.; Haklar, G.; Duman, D.G. Myeloperoxidase and calprotectin; Any role as non-invasive markers for the prediction of ınflammation and fibrosis in non-alcoholic steatohepatitis? Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2020, 31, 681–687. [Google Scholar] [CrossRef]
- Gajda, A.M.; Tawfeeq, H.R.; Lackey, A.I.; Zhou, Y.X.; Kanaan, H.; Pappas, A.; Xu, H.; Kodukula, S.; Storch, J. The proximal intestinal Fatty Acid-Binding Proteins liver FABP (LFABP) and intestinal FABP (IFABP) differentially modulate whole body energy homeostasis but are not centrally involved in net dietary lipid absorption: Studies of the LFABP/IFABP double knockout mouse. Biochim. Et Biophys. Acta. Mol. Cell Biol. Lipids 2023, 1868, 159238. [Google Scholar]
- Wu, G.; Tawfeeq, H.R.; Lackey, A.I.; Zhou, Y.; Sifnakis, Z.; Zacharisen, S.M.; Xu, H.; Doran, J.M.; Sampath, H.; Zhao, L.; et al. Gut Microbiota and Phenotypic Changes Induced by Ablation of Liver- and Intestinal-Type Fatty Acid-Binding Proteins. Nutrients 2022, 14, 1762. [Google Scholar] [CrossRef] [PubMed]
- van de Poll, M.C.; Derikx, J.P.; Buurman, W.A.; Peters, W.H.; Roelofs, H.M.; Wigmore, S.J.; Dejong, C.H. Liver manipulation causes hepatocyte injury and precedes systemic inflammation in patients undergoing liver resection. World J. Surg. 2007, 31, 2033–2038. [Google Scholar] [CrossRef]
- Ho, S.S.C.; Keenan, J.I.; Day, A.S. The Role of Gastrointestinal-Related Fatty Acid-Binding Proteins as Biomarkers in Gastrointestinal Diseases. Dig. Dis. Sci. 2020, 65, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Funaoka, H.; Kanda, T.; Fujii, H. Intestinal fatty acid-binding protein (I-FABP) as a new biomarker for intestinal diseases. Rinsho Byori. 2010, 58, 162–168. [Google Scholar] [PubMed]
- Evennett, N.J.; Petrov, M.S.; Mittal, A.; Windsor, J.A. Systematic review and pooled estimates for the diagnostic accuracy of serological markers for intestinal ischemia. World J. Surg. 2009, 33, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Marques, C.; Pestana, D.; Santoalha, M.; Carvalho, D.; Freitas, P.; Calhau, C. The role of I-FABP as a biomarker of intestinal barrier dysfunction driven by gut microbiota changes in obesity. Nutr. Metab. 2016, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Crenn, P.; Hanachi, M.; Neveux, N.; Cynober, L. Circulating citrulline levels: A biomarker for intestinal functionality assessment. Ann. De Biol. Clin. 2011, 69, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Sellmann, C.; Jin, C.J.; Engstler, A.J.; De Bandt, J.P.; Bergheim, I. Oral citrulline supplementation protects female mice from the development of non-alcoholic fatty liver disease (NAFLD). Eur. J. Nutr. 2017, 56, 2519–2527. [Google Scholar] [CrossRef] [PubMed]
- Jegatheesan, P.; Beutheu, S.; Freese, K.; Waligora-Dupriet, A.J.; Nubret, E.; Butel, M.J.; Bergheim, I.; De Bandt, J.P. Preventive effects of citrulline on Western diet-induced non-alcoholic fatty liver disease in rats. Br. J. Nutr. 2016, 116, 191–203. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Freeman, M.; Zhang, X.; Sandhu, A.; Edirisinghe, I. Watermelon and L-Citrulline in Cardio-Metabolic Health: Review of the Evidence 2000–2020. Curr. Atheroscler. Rep. 2021, 23, 81. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, O.; Biesiekierska, M.; Panthu, B.; Soszynski, M.; Pirola, L.; Balcerczyk, A. Citrullination in the pathology of inflammatory and autoimmune disorders: Recent advances and future perspectives. Cell. Mol. Life Sci. CMLS 2022, 79, 94. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Chai, Z.; Chen, S.; Zhang, H.; Zhang, X.; Zhou, Y. Intestinal mucus components and secretion mechanisms: What we do and do not know. Exp. Mol. Medicine. 2023, 55, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Xu, L.F.; Sun, M. The protective effect of trefoil factor 3 on the intestinal tight junction barrier is mediated by toll-like receptor 2 via a PI3K/Akt dependent mechanism. Biochem. Biophys. Res. Commun. 2013, 440, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, Z.; Lin, Q.; Bei, W.; Guo, J. Pathological and therapeutic roles of bioactive peptide trefoil factor 3 in diverse diseases: Recent progress and perspective. Cell Death Dis. 2022, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, K.; Kong, W. Intestinal Trefoil Factor 3 Alleviates the Intestinal Barrier Function Through Reducing the Expression of TLR4 in Rats with Nonalcoholic Steatohepatitis. Arch. Med. Res. 2019, 50, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Pinton, P.; Olleik, H.; Pujol, A.; Nicoletti, C.; Sicre, M.; Quinson, N.; Ajandouz, E.H.; Perrier, J.; Pasquale, E.D.; et al. Deoxynivalenol inhibits the expression of trefoil factors (TFF) by intestinal human and porcine goblet cells. Arch. Toxicol. 2019, 93, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Q.; Chen, Z.; Lv, S.; Tang, J.; Xing, Z.; Shi, M.; Lei, A.; Xiao, G.; He, Y. TFF3 mediates the NF-κB/COX2 pathway to regulate PMN-MDSCs activation and protect against necrotizing enterocolitis. Eur. J. Immunol. 2021, 51, 1110–1125. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, H.; Yang, Z.; Shao, D.; Zhang, W.; Ren, Y.; Sun, B.; Lin, J.; Xu, M.; Nie, S. Intestinal trefoil factor activates the PI3K/Akt signaling pathway to protect gastric mucosal epithelium from damage. Int. J. Oncol. 2014, 45, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.X.; Zhong, X.Y.; Cui, Y.F.; Liu, W.; Tai, S.; Wang, Z.D.; Shi, Y.G.; Zhao, S.Y.; Li, C.L. IL-6/STAT3/TFF3 signaling regulates human biliary epithelial cell migration and wound healing in vitro. Mol. Biol. Rep. 2010, 37, 3813–3818. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.X.; Han, H.; Shao, J.; Gao, Y.; Yin, X.; Zhu, W.L.; Han, Y.; Shi, H.S. mTOR signalling in the nucleus accumbens shell is critical for augmented effect of TFF3 on behavioural response to cocaine. Sci. Rep. 2016, 6, 27895. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Santamatilde, E.; McCreath, K.J.; Cervera, A.M.; Díez, I.; Ortiz-Masiá, D.; Martínez, N.; Calatayud, S.; Esplugues, J.V.; Barrachina, M.D. Induction of trefoil factor (TFF)1, TFF2 and TFF3 by hypoxia is mediated by hypoxia inducible factor-1: Implications for gastric mucosal healing. Br. J. Pharmacol. 2009, 156, 262–272. [Google Scholar] [CrossRef]
- Ding, X.; Hu, X.; Chen, Y.; Xie, J.; Ying, M.; Wang, Y.; Yu, Q. Differentiated Caco-2 cell models in food-intestine interaction study: Current applications and future trends. Trends Food Sci. Technol. 2021, 107, 455–465. [Google Scholar] [CrossRef]
- Panse, N.; Gerk, P.M. The Caco-2 Model: Modifications and enhancements to improve efficiency and predictive performance. Int. J. Pharm. 2022, 624, 122004. [Google Scholar] [CrossRef] [PubMed]
- Valdez, J.C.; Cho, J.; Bolling, B.W. Aronia berry inhibits disruption of Caco-2 intestinal barrier function. Arch. Biochem. Biophys. 2020, 688, 108409. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Li, Y.; Deng, L.; Ren, R.; Ge, B.; Liao, Z.; Xiang, S.; Zhou, B. Alisol B 23-Acetate Ameliorates Lipopolysaccharide-Induced Intestinal Barrier Dysfunction by Inhibiting TLR4-NOX1/ROS Signaling Pathway in Caco-2 Cells. Front. Pharmacol. 2022, 13, 911196. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, J.; Gui, W.; Sun, D.; Dai, H.; Xiao, L.; Chu, H.; Du, F.; Zhu, Q.; Schnabl, B.; et al. Tauroursodeoxycholic acid inhibits intestinal inflammation and barrier disruption in mice with non-alcoholic fatty liver disease. Br. J. Pharmacol. 2018, 175, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Vendrell-Pacheco, M.; Heskitt, B.; Chitchumroonchokchai, C.; Failla, M.; Sastry, S.K.; Francis, D.M.; Martin-Belloso, O.; Elez-Martínez, P.; Kopec, R.E. Novel Processing Technologies as Compared to Thermal Treatment on the Bioaccessibility and Caco-2 Cell Uptake of Carotenoids from Tomato and Kale-Based Juices. J. Agric. Food Chem. 2019, 67, 10185–10194. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Burmester, M.; Langeheine, M.; Brehm, R.; Empl, M.T.; Seeger, B.; Breves, G. Caco-2/HT29-MTX co-cultured cells as a model for studying physiological properties and toxin-induced effects on intestinal cells. PLoS ONE 2021, 16, e0257824. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Geloen, A. Adipocytes as lipid sensors of oleic acid transport through a functional Caco-2/HT29-MTX intestinal barrier. Adipocyte 2019, 8, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.-Y.M.; Allen, K.J.; Turner, P.C.; El-Nezami, H. Modulation of Mucin mRNA (MUC5AC and MUC5B) Expression and Protein Production and Secretion in Caco-2/HT29-MTX Co-cultures Following Exposure to Individual and Combined Fusarium Mycotoxins. Toxicol. Sci. 2014, 139, 83–98. [Google Scholar] [CrossRef] [PubMed]
- van Laar, A.; Grootaert, C.; Van Nieuwerburgh, F.; Deforce, D.; Desmet, T.; Beerens, K.; Van Camp, J. Metabolism and Health Effects of Rare Sugars in a CACO-2/HepG2 Coculture Model. Nutrients 2022, 14, 611. [Google Scholar] [CrossRef] [PubMed]
- Dugardin, C.; Briand, O.; Touche, V.; Schonewille, M.; Moreau, F.; Le May, C.; Groen, A.K.; Staels, B.; Lestavel, S. Retrograde cholesterol transport in the human Caco-2/TC7 cell line: A model to study trans-intestinal cholesterol excretion in atherogenic and diabetic dyslipidemia. Acta Diabetol. 2017, 54, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Hoki, T.; Miyanishi, K.; Tanaka, S.; Takada, K.; Kawano, Y.; Sakurada, A.; Sato, M.; Kubo, T.; Sato, T.; Sato, Y.; et al. Increased duodenal iron absorption through up-regulation of divalent metal transporter 1 from enhancement of iron regulatory protein 1 activity in patients with nonalcoholic steatohepatitis. Hepatology 2015, 62, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Shek, D.; Chen, D.; Read, S.A.; Ahlenstiel, G. Examining the gut-liver axis in liver cancer using organoid models. Cancer Lett. 2021, 510, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Deng, P.; Wang, Y.; Zhang, X.; Guo, Y.; Chen, W.; Qin, J. Microengineered Multi-Organoid System from hiPSCs to Recapitulate Human Liver-Islet Axis in Normal and Type 2 Diabetes. Adv. Sci. 2022, 9, e2103495. [Google Scholar] [CrossRef] [PubMed]
- Safari, Z.; Gerard, P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell. Mol. Life Sci. CMLS 2019, 76, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Hoyles, L.; Fernández-Real, J.-M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, H.; Yang, X.; Xue, X.; Deng, L.; Shen, J.; Zhang, M.; Zhao, L.; Zhang, C. Genetically Obese Human Gut Microbiota Induces Liver Steatosis in Germ-Free Mice Fed on Normal Diet. Front. Microbiol. 2018, 9, 1602. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; De Stefanis, C.; Gnani, D.; Furlanello, C.; Zandona, A.; Paci, P.; Capuani, G.; et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017, 65, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Ahmed, I.; Gillevet, P.M.; Probert, C.S.; Ratcliffe, N.M.; Smith, S.; Greenwood, R.; Sikaroodi, M.; Lam, V.; Crotty, P.; et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2013, 11, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clement, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.A.; Rocha, R.; Costa, P.R.F.; Almeida, N.S.; Cotrim, H.P. Probiotic, Prebiotic or Symbiotic Supplementation Impacts on Intestinal Microbiota in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review. Arq. De Gastroenterol. 2022, 59, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Bakhshimoghaddam, F.; Alizadeh, M. Contribution of gut microbiota to nonalcoholic fatty liver disease: Pathways of mechanisms. Clin. Nutr. ESPEN 2021, 44, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Qu, F.; Chen, L.; Liu, C.; Zhang, M.; Ren, F.; Guo, H.; Zhang, H.; Ge, S.; Wu, C.; et al. SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. J. Endocrinol. 2020, 245, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Avagliano, C.; Calignano, A.; Berni Canani, R. The Protective Role of Butyrate against Obesity and Obesity-Related Diseases. Molecules 2021, 26, 682. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Khalil, A.A.; Rahman, U.U.; Khalid, A.; Naz, S.; Shariati, M.A.; Rebezov, M.; Urtecho, E.Z.; de Albuquerque, R.; Anwar, S.; et al. Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): An updated review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6034–6054. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Olofsson, L.E. Gut Microbiota-Dependent Modulation of Energy Metabolism. J. Innate Immun. 2018, 10, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Ray, K. NAFLD. Leaky guts: Intestinal permeability and NASH. Nature reviews. Gastroenterol. Hepatol. 2015, 12, 123. [Google Scholar]
- Moonwiriyakit, A.; Pathomthongtaweechai, N.; Steinhagen, P.R.; Chantawichitwong, P.; Satianrapapong, W.; Pongkorpsakol, P. Tight junctions: From molecules to gastrointestinal diseases. Tissue Barriers 2023, 11, 2077620. [Google Scholar] [CrossRef] [PubMed]
- Muto, H.; Honda, T.; Tanaka, T.; Yokoyama, S.; Yamamoto, K.; Ito, T.; Imai, N.; Ishizu, Y.; Maeda, K.; Ishikawa, T.; et al. Proteomic Analysis Reveals Changes in Tight Junctions in the Small Intestinal Epithelium of Mice Fed a High-Fat Diet. Nutrients 2023, 15, 1473. [Google Scholar] [CrossRef] [PubMed]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut Microbiota and Intestinal Trans-Epithelial Permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef] [PubMed]
- Lastname, A.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Nieß, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2022, 26, 671–683. [Google Scholar]
- Wang, Y.; Liu, Y. Gut-liver-axis: Barrier function of liver sinusoidal endothelial cell. J. Gastroenterol. Hepatol. 2021, 36, 2706–2714. [Google Scholar] [CrossRef]
- Soppert, J.; Brandt, E.F.; Heussen, N.M.; Barzakova, E.; Blank, L.M.; Kuepfer, L.; Hornef, M.W.; Trebicka, J.; Jankowski, J.; Berres, M.L.; et al. Blood Endotoxin Levels as Biomarker of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2023, 21, 2746–2758. [Google Scholar] [CrossRef] [PubMed]
- Guohong, L.; Qingxi, Z.; Hongyun, W. Characteristics of intestinal bacteria with fatty liver diseases and cirrhosis. Ann. Hepatol. 2019, 18, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takeda, K. Current views of toll-like receptor signaling pathways. Gastroenterol. Res. Pract. 2010, 2010, 240365. [Google Scholar] [CrossRef] [PubMed]
- Pontarollo, G.; Kollar, B.; Mann, A.; Khuu, M.P.; Kiouptsi, K.; Bayer, F.; Brandão, I.; Zinina, V.V.; Hahlbrock, J.; Malinarich, F.; et al. Commensal bacteria weaken the intestinal barrier by suppressing epithelial neuropilin-1 and Hedgehog signaling. Nat. Metab. 2023, 5, 1174–1187. [Google Scholar] [CrossRef] [PubMed]
- Burgueño, J.F.; Abreu, M.T. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.S.; Seki, E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S1), 38–42. [Google Scholar] [CrossRef] [PubMed]
- Csak, T.; Velayudham, A.; Hritz, I.; Petrasek, J.; Levin, I.; Lippai, D.; Catalano, D.; Mandrekar, P.; Dolganiuc, A.; Kurt-Jones, E.; et al. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. American journal of physiology. Gastrointest. Liver Physiol. 2011, 300, G433–G441. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, C.; Wang, X.; Kim, K.; Bartolome, A.; Dongiovanni, P.; Yates, K.P.; Valenti, L.; Carrer, M.; Sadowski, T.; et al. Hepatocyte TLR4 triggers inter-hepatocyte Jagged1/Notch signaling to determine NASH-induced fibrosis. Sci. Transl. Med. 2021, 13, eabe1692. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Kodama, Y.; Inokuchi, S.; Schnabl, B.; Aoyama, T.; Ohnishi, H.; Olefsky, J.M.; Brenner, D.A.; Seki, E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology 2010, 139, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Yang, L.; van Rooijen, N.; Brenner, D.A.; Ohnishi, H.; Seki, E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology 2013, 57, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Sun, Y.; Jiang, Z.; Du, K.; Xia, N.; Zhong, G. Key genes associated with non-alcoholic fatty liver disease and acute myocardial infarction. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e922492. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Capurso, L. FAO/WHO guidelines on probiotics: 10 years later. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.R.; Park, H.J.; Kang, D.; Chung, H.; Nam, M.H.; Lee, Y.; Park, J.H.; Lee, H.Y. A protective mechanism of probiotic Lactobacillus against hepatic steatosis via reducing host intestinal fatty acid absorption. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, S.H.; Sin, H.S.; Jang, S.H.; Lee, S.W.; Kim, S.Y.; Kwon, B.; Yu, K.Y.; Kim, S.Y.; Yang, D.K. Hypocholesterolemic Effects of Probiotic Mixture on Diet-Induced Hypercholesterolemic Rats. Nutrients 2017, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Zeng, D.; Wang, H.; Ni, X.; Yi, D.; Pan, K.; Jing, B. Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl. Microbiol. Biotechnol. 2014, 98, 6817–6829. [Google Scholar] [CrossRef]
- Yao, F.; Jia, R.; Huang, H.; Yu, Y.; Mei, L.; Bai, L.; Ding, Y.; Zheng, P. Effect of Lactobacillus paracasei N1115 and fructooligosaccharides in nonalcoholic fatty liver disease. Arch. Med. Sci. AMS 2019, 15, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.; Jun, D.W.; Lee, K.N.; Lee, H.L.; Lee, O.Y.; Choi, H.S.; Yoon, B.C. Lactobacillus paracasei Induces M2-Dominant Kupffer Cell Polarization in a Mouse Model of Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2015, 60, 3340–3350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Li, Y.Q.; Wang, Y.Z. Protective effect of Saccharomyces boulardii against intestinal mucosal barrier injury in rats with nonalcoholic fatty liver disease. Chin. J. Hepatol. 2016, 24, 921–926. [Google Scholar]
- Briskey, D.; Heritage, M.; Jaskowski, L.A.; Peake, J.; Gobe, G.; Subramaniam, V.N.; Crawford, D.; Campbell, C.; Vitetta, L. Probiotics modify tight-junction proteins in an animal model of nonalcoholic fatty liver disease. Ther. Adv. Gastroenterol. 2016, 9, 463–472. [Google Scholar] [CrossRef]
- Singh, T.P.; Natraj, B.H. Next-generation probiotics: A promising approach towards designing personalized medicine. Crit. Rev. Microbiol. 2021, 47, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Qv, L.; Lu, Y.; Wang, B.; Berglund, B.; Li, L. An Update on the Efficacy and Functionality of Probiotics for the Treatment of Non-Alcoholic Fatty Liver Disease. Engineering 2021, 7, 679–686. [Google Scholar] [CrossRef]
- Ouwerkerk, J.P.; Aalvink, S.; Belzer, C.; de Vos, W.M. Akkermansia glycaniphila sp. nov., an anaerobic mucin-degrading bacterium isolated from reticulated python faeces. Int. J. Syst. Evol. Microbiol. 2016, 66, 4614–4620. [Google Scholar] [CrossRef] [PubMed]
- Moreira, G.V.; Azevedo, F.F.; Ribeiro, L.M.; Santos, A.; Guadagnini, D.; Gama, P.; Liberti, E.A.; Saad, M.; Carvalho, C. Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J. Nutr. Biochem. 2018, 62, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; de Vos, W.M.; et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Lukovac, S.; Belzer, C.; Pellis, L.; Keijser, B.J.; de Vos, W.M.; Montijn, R.C.; Roeselers, G. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio 2014, 5, e01438-14. [Google Scholar] [CrossRef] [PubMed]
- Remely, M.; Aumueller, E.; Merold, C.; Dworzak, S.; Hippe, B.; Zanner, J.; Pointner, A.; Brath, H.; Haslberger, A.G. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene 2014, 537, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Gauffin Cano, P.; Santacruz, A.; Moya, A.; Sanz, Y. Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS ONE 2012, 7, e41079. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.R.; Khoruts, A.; Staley, C.; Sadowsky, M.J.; Abd, M.; Alani, M.; Bakow, B.; Curran, P.; McKenney, J.; Tisch, A.; et al. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection: A Randomized Trial. Ann. Intern. Med. 2016, 165, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zou, G.; Li, B.; Du, X.; Sun, Z.; Sun, Y.; Jiang, X. Fecal Microbiota Transplantation (FMT) Alleviates Experimental Colitis in Mice by Gut Microbiota Regulation. J. Microbiol. Biotechnol. 2020, 30, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lv, L.; Wang, C. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Meta-Analysis of Randomized Controlled Trials. Front. Cell. Infect. Microbiol. 2022, 12, 827395. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Deng, Z.; Luo, W.; He, X.; Chen, Y. Effect of Fecal Microbiota Transplantation on Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Front. Cell. Infect. Microbiol. 2022, 12, 759306. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Y.; Bai, J.; Li, G.; Liu, Y.; Deng, S.; Zhou, R.; Tao, K.; Xia, Z. Increased plasma genistein after bariatric surgery could promote remission of NAFLD in patients with obesity. Front. Endocrinol. 2022, 13, 1024769. [Google Scholar] [CrossRef] [PubMed]
- Smits, L.P.; Bouter, K.E.; de Vos, W.M.; Borody, T.J.; Nieuwdorp, M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology 2013, 145, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojarvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916 e7. [Google Scholar] [CrossRef] [PubMed]
- Rotman, Y.; Sanyal, A.J. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut 2017, 66, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Megerlin, F.; Fouassier, E. Faecal microbiota transplantation in France: What applicable law? Ann. Pharm. Fr. 2014, 72, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; de Vos, W.M. How to Manipulate the Microbiota: Fecal Microbiota Transplantation. Adv. Exp. Med. Biol. 2016, 902, 143–153. [Google Scholar] [PubMed]
- Zhang, L.; Liu, Y.; Sun, Y.; Zhang, X. Combined Physical Exercise and Diet: Regulation of Gut Microbiota to Prevent and Treat of Metabolic Disease: A Review. Nutrients 2022, 14, 4774. [Google Scholar] [CrossRef] [PubMed]
- Donati Zeppa, S.; Agostini, D.; Gervasi, M.; Annibalini, G.; Amatori, S.; Ferrini, F.; Sisti, D.; Piccoli, G.; Barbieri, E.; Sestili, P.; et al. Mutual Interactions among Exercise, Sport Supplements and Microbiota. Nutrients 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liu, S.; Chen, L.; Shen, J.; Niu, Y.; Wang, T.; Zhang, W.; Fu, L. Effect of exercise and butyrate supplementation on microbiota composition and lipid metabolism. J. Endocrinol. 2019, 243, 125–135. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L. A Review of the Role of the Gut Microbiome in Personalized Sports Nutrition. Front. Nutr. 2019, 6, 191. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.W.; Park, Y.M.; Holscher, H.D.; Padilla, J.; Scroggins, R.J.; Welly, R.; Britton, S.L.; Koch, L.G.; Vieira-Potter, V.J.; Swanson, K.S. Physical Activity Differentially Affects the Cecal Microbiota of Ovariectomized Female Rats Selectively Bred for High and Low Aerobic Capacity. PLoS ONE 2015, 10, e0136150. [Google Scholar] [CrossRef]
- Luo, B.; Xiang, D.; Nieman, D.C.; Chen, P. The effects of moderate exercise on chronic stress-induced intestinal barrier dysfunction and antimicrobial defense. Brain Behav. Immun. 2014, 39, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.P.; Burini, R.C. The impact of physical exercise on the gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, A.; Mennitti, C.; Falcone, N.; La Monica, I.; Di Iorio, M.R.; Tripodi, L.; Gentile, A.; Vitale, M.; Pero, R.; Pastore, L.; et al. Positive effects of physical activity in autism spectrum disorder: How influences behavior, metabolic disorder and gut microbiota. Front. Psychiatry 2023, 14, 1238797. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Margolis, L.M.; Madslien, E.H.; Murphy, N.E.; Castellani, J.W.; Gundersen, Y.; Hoke, A.V.; Levangie, M.W.; Kumar, R.; Chakraborty, N.; et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. American journal of physiology. Gastrointest. Liver Physiol. 2017, 312, G559–G571. [Google Scholar] [CrossRef] [PubMed]
- Gutekunst, K.; Krüger, K.; August, C.; Diener, M.; Mooren, F.C. Acute exercises induce disorders of the gastrointestinal integrity in a murine model. Eur. J. Appl. Physiol. 2014, 114, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Kirpich, I.A.; Marsano, L.S.; McClain, C.J. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin. Biochem. 2015, 48, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.E.; Kim, D.K.; Seo, W.; Gao, B.; Yoo, S.H.; Song, B.J. Fructose Promotes Leaky Gut, Endotoxemia, and Liver Fibrosis Through Ethanol-Inducible Cytochrome P450-2E1-Mediated Oxidative and Nitrative Stress. Hepatology 2021, 73, 2180–2195. [Google Scholar] [CrossRef] [PubMed]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat-The Relationship between Diet, Microbiota, and Metabolic Disorders-A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef] [PubMed]
- Hijova, E. Gut bacterial metabolites of indigestible polysaccharides in intestinal fermentation as mediators of public health. Bratisl. Lek. Listy 2019, 120, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yue, H.; Wang, Y.; Guo, C.; Du, Z.; Jin, C.; Ding, K. Intestinal microbes derived butyrate is related to the immunomodulatory activities of Dendrobium officinale polysaccharide. Int. J. Biol. Macromol. 2020, 149, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Li, Q.M.; Zha, X.Q.; Luo, J.P. Dendrobium fimbriatum Hook polysaccharide ameliorates dextran-sodium-sulfate-induced colitis in mice via improving intestinal barrier function, modulating intestinal microbiota, and reducing oxidative stress and inflammatory responses. Food Funct. 2022, 13, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Yang, Y.; Luo, Z.; Guan, L.; Zhu, W. The Colonic Microbiome and Epithelial Transcriptome Are Altered in Rats Fed a High-Protein Diet Compared with a Normal-Protein Diet. J. Nutr. 2016, 146, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Johns, D.J.; Hartmann-Boyce, J.; Jebb, S.A.; Aveyard, P. Diet or exercise interventions vs combined behavioral weight management programs: A systematic review and meta-analysis of direct comparisons. J. Acad. Nutr. Diet. 2014, 114, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef] [PubMed]
- Usuda, H.; Okamoto, T.; Wada, K. Leaky Gut: Effect of Dietary Fiber and Fats on Microbiome and Intestinal Barrier. Int. J. Mol. Sci. 2021, 22, 7613. [Google Scholar] [CrossRef]
- Parolini, C.; Bjorndal, B.; Busnelli, M.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Ramsvik, M.; Bruheim, I.; Berge, R.K.; Chiesa, G. Effect of Dietary Components from Antarctic Krill on Atherosclerosis in apoE-Deficient Mice. Mol. Nutr. Food Res. 2017, 61, 1700098. [Google Scholar] [CrossRef]


| Methods/Models | Advantages | Disadvantages | Reference | |
|---|---|---|---|---|
| In Vivo | ||||
| Exogenous inert probes | Polyethylene glycols (PEG), 51Chromium-ethylenediaminetetraacetic acid (51Cr-EDTA), FITC-dextran 4000, monosaccharides, disaccharides |
|
| [43,47,49,53] |
| Multi-sugar | [52] | |||
| Endogenous biomarkers | Zonulin | Easily assessed by low equipment and method requirements | Non-specific and easily interfered with by other factors. | [43] |
| Calprotectin |
| Independent of the hepatic inflammatory state and fibrosis stage of NASH. | [64,65,66] | |
| Fatty acid binding proteins (FABPs) |
| Not recommended for assessing intestinal barrier function in NAFLD and a low limit of detection, as well as a short half-life time. | [67,73] | |
| Citrulline | Higher specificityand sensitivity to small intestinal permeability | Mainly for small intestinal permeability and easily interfered with by other factors. | [75,76] | |
| Trefoil factor 3 (TFF3) | Mechanisms closely related to intestinal barrier maintenance | Low specificity and easily interfered with by other factors. | [82] | |
| Monoculture | Caco-2 cells | Simple structure, broad applicability, and low cost | Must be cultured for a certain time and to reach a certain density before being used and cannot simulate the dynamic process of the intestinal barrier. | [93] |
| Co-culture | Caco-2/HT29-MTX cells Caco-2/HepG2 cells Caco-2/TC7 cells | More accurate replication of the structural and functional characteristics of the human intestinal barrier and its physiological environment | Strict culture conditions, complex procedures, many uncertain factors, limited to horizontal interactions and cannot fully replicate the three-dimensional barrier function of the intestine. | [96,98,99,100] |
| Organoids | Derived from obese/fatty liver patients or laboratory animal | Form complex three-dimensional structures resembling important histological and functional aspects of living tissues |
| [101] |
| Organism | Effects | References |
|---|---|---|
| Conventional probiotics (CPs) | ||
| Lactobacillus rhamnosus GG | hepatic steatosis ↓ | [138] |
| Probiotic blend (B. longum, B. lactis, and B. breve and L. reuteri and L. plantarum) | body weight ↓ triglycerides ↓ total cholesterol ↓ LDL-C ↓and HDL-C ↑ | [139] |
| L. johnsonii BS15 | intestinal permeability ↓ LPS levels ↓ liver inflammatory factors ↓ | [140] |
| Lactobacillus paracasei N1115 | hepatic steatosis ↓ the inflammatory factors ↓ | [141] |
| Lactobacillus paracasei | pro-inflammatory cytokines ↓ | [142] |
| Saccharomyces boulardii | hepatic steatosis ↓ TNF-α ↓ | [143] |
| Akkermansia muciniphila | adipose tissue mass ↓ metabolic endotoxaemia ↓ adipose tissue inflammation ↓ insulin resistance ↓ | [148] |
| Bacteroides | SCFAs ↑ | [151] |
| Mycobacterium anisopliae CECT 7771 | body weight ↓ steatosis ↓ | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, C.; Zhou, X.; Xia, F.; Zhou, B. Intestinal Barrier Dysfunction and Gut Microbiota in Non-Alcoholic Fatty Liver Disease: Assessment, Mechanisms, and Therapeutic Considerations. Biology 2024, 13, 243. https://doi.org/10.3390/biology13040243
Long C, Zhou X, Xia F, Zhou B. Intestinal Barrier Dysfunction and Gut Microbiota in Non-Alcoholic Fatty Liver Disease: Assessment, Mechanisms, and Therapeutic Considerations. Biology. 2024; 13(4):243. https://doi.org/10.3390/biology13040243
Chicago/Turabian StyleLong, Changrui, Xiaoyan Zhou, Fan Xia, and Benjie Zhou. 2024. "Intestinal Barrier Dysfunction and Gut Microbiota in Non-Alcoholic Fatty Liver Disease: Assessment, Mechanisms, and Therapeutic Considerations" Biology 13, no. 4: 243. https://doi.org/10.3390/biology13040243
APA StyleLong, C., Zhou, X., Xia, F., & Zhou, B. (2024). Intestinal Barrier Dysfunction and Gut Microbiota in Non-Alcoholic Fatty Liver Disease: Assessment, Mechanisms, and Therapeutic Considerations. Biology, 13(4), 243. https://doi.org/10.3390/biology13040243






