Aldanella attleborensis (Mollusca) from Cambrian Stage 2 of the Three Gorges Area and Its Stratigraphic Implications
Abstract
Simple Summary
Abstract
1. Introduction
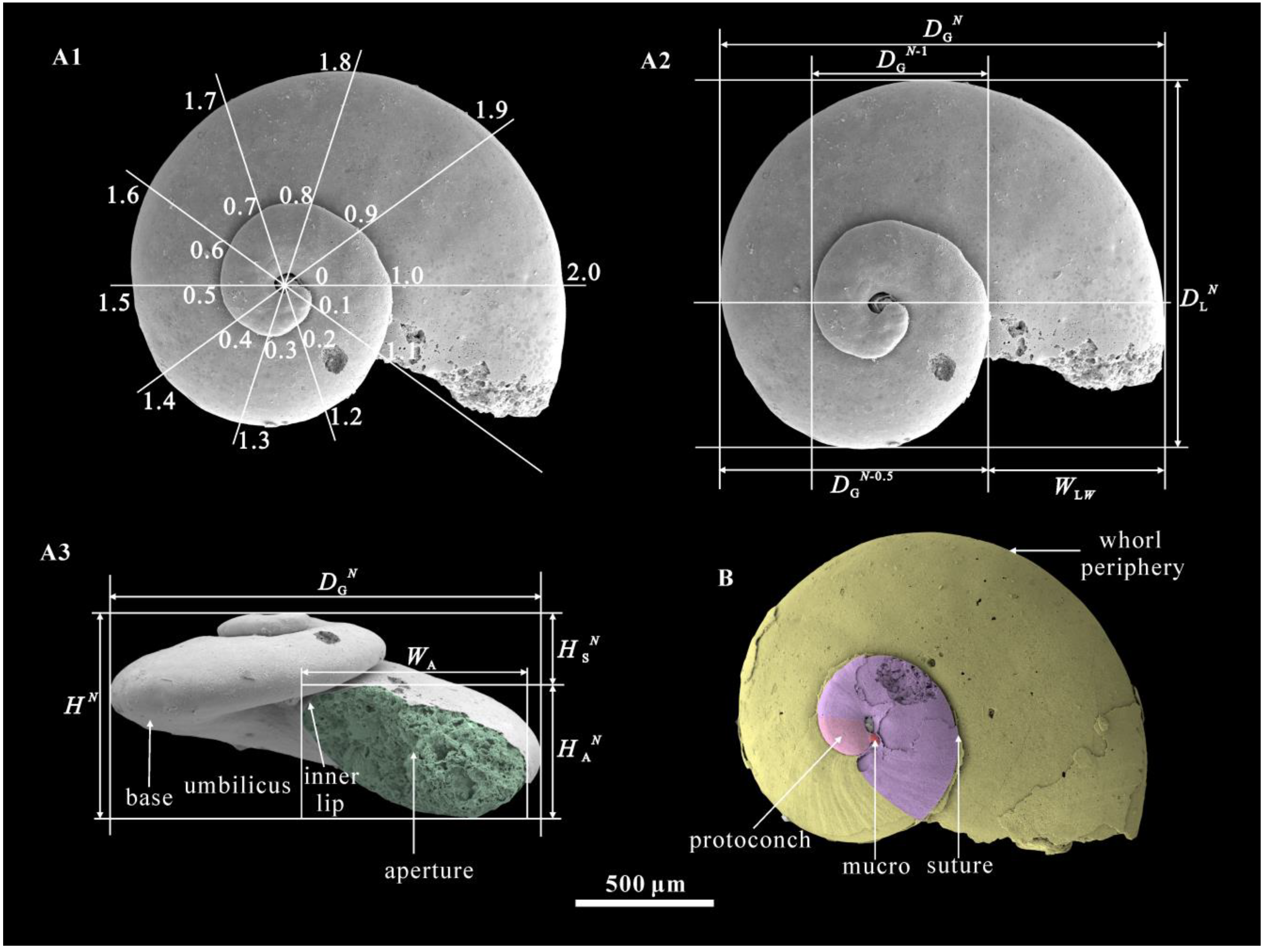
2. Geological Setting, Materials, and Methods
3. Results
Systematic Palaeontology
- 1962 Aldanella attleborensis Vostokova, p. 66–67, pl. 2, figure 12a,b [23].
- 2011 Aldanella attleborensis; Parkhaev and Karlova, p. 1187, 1189, 1191, pl. 1, figures 1–6; pl. 2, figures 1–8; pl. 3, figures 1–10; pl. 4, figures 1–10; pl. 5, figures 1–8; pl. 17, figures 1 and 2 (and the synonymy list therein) [13].
- 2013 Aldanella attleborensis; Dzik and Mazurek, figures 2A–G, 3A–F and 5A–I [66].
- 2014 Aldanella yanjiaheensis; Guo et al., figure 4a [19].
- 2014a Aldanella attleborensis; Parkhaev, figure 1 [67].
- 2017 Aldanella attleborensis; Kouchinsky et al., pp. 347–351, figures 20A–F, 21A–G and 22A,B [21].
- 2020 Aldanella attleborensis; Steiner et al., figure 6A–E [46].
- 2021 Aldanella attleborensis; Guo et al., figure 3A–D [20].
4. Discussion
4.1. Shell Microstructures of Aldanella attleborensis
4.2. Muscle Scars
4.3. Systematic Position of Aldanella
4.4. Ontogeny of Aldanella attleborensis
4.5. Stratigraphic Implications of Aldanella attleborensis

5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shu, D.G. Cambrian explosion: Birth of tree of animals. Gondwana Res. 2008, 14, 219–240. [Google Scholar] [CrossRef]
- Maloof, A.C.; Porter, S.M.; Moore, J.L.; Dudás, F.Ö.; Bowring, S.A.; Hoggins, J.A.; Fike, D.A.; Eddy, M.P. The earliest Cambrian record of animals and ocean geochemical change. Geol. Soc. Am. Bull. 2010, 122, 1731–1774. [Google Scholar] [CrossRef]
- Erwin, D.H.; Laflamme, M.; Tweedt, S.M.; Sperling, E.A.; Pisani, D.; Peterson, K.J. The Cambrian conundrum: Early divergence and later ecological success in the early history of animals. Science 2011, 334, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Chen, M.E.; He, T.G.; Zhu, M.Y.; Yin, G.Z.; Feng, W.M.; Xu, J.T.; Jiang, Z.W.; Liu, D.Y.; Li, G.X.; et al. Taxonomy and Biostratigraphy of Small Shelly Fossils in China; Science Press: Beijing, China, 1999; pp. 1–247. (In Chinese) [Google Scholar]
- Parkhaev, P.Y. The Functional Morphology of the Cambrian Univalved Mollusks–Helcionellids: 1. Paleontol. J. 2000, 34, 32–38. [Google Scholar]
- Parkhaev, P.Y. The Functional Morphology of the Cambrian Univalved Mollusks–Helcionellids: 2. Paleontol. J. 2001, 35, 470–475. [Google Scholar]
- Parkhaev, P.Y. Phylogenesis and the System of the Cambrian Univalved Mollusks. Paleontol. J. 2002, 36, 25–36. [Google Scholar]
- Parkhaev, P.Y. The Early Cambrian radiation of Mollusca. In Phylogeny and Evolution of the Mollusca; Ponder, W.F., Lindberg, D.R., Eds.; University California Press: Berkeley, CA, USA, 2008; pp. 33–69. [Google Scholar]
- Steiner, M.; Li, G.X.; Qian, Y.; Zhu, M.Y.; Erdtmann, B.D. Neoproterozoic to early Cambrian small shelly fossil assemblages and a revised biostratigraphic correlation of the Yangtze Platform (China). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 254, 67–99. [Google Scholar] [CrossRef]
- Shaler, N.S.; Foerste, A.F. Preliminary description of North Attleborough fossils. Bull. Museum Compar. Zool. 1888, 16, 27–41. [Google Scholar]
- Grabau, A.W. Palaeontology of the Cambrian terranes of the Boston Basin. Occas. Pap. Boston Soc. Natur. Hist. 1900, 4, 601–694. [Google Scholar]
- Li, G.X.; Zhao, X.; Gubanov, A.P.; Zhu, M.Y.; Na, L. Early Cambrian mollusc Watsonella crosbyi: A potential GSSP index fossil for the base of the Cambrian Stage 2. Acta Geol. Sin. 2011, 85, 309–319. [Google Scholar] [CrossRef]
- Parkhaev, P.Y.; Karlova, G.A. Taxonomic revision and evolution of Cambrian mollusks of the genus Aldanella Vostokova, 1962 (Gastropoda: Archaeobranchia). Paleontol. J. 2011, 45, 1145–1205. [Google Scholar] [CrossRef]
- Parkhaev, P.Y.; Karlova, G.A.; Rozanov, A.Y. Taxonomy, stratigraphy and biogeography of Aldanella attleborensis–A possible candidate for defining the base of Cambrian Stage 2. Bull. Museum Northern Arizona 2011, 67, 298–300. [Google Scholar]
- Devaere, L.; Clausen, S.; Steiner, M.; Álvaro, J.J.; Vachard, D. Chronostratigraphic and palaeogeographic significance of an early Cambrian microfauna from the Heraultia Limestone, northern Montagne Noire, France. Palaeontol. Electron. 2013, 16, 1–91. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Steiner, M.; Li, G.X.; Keupp, H. Terreneuvian small shelly faunas of East Yunnan (South China) and their biostratigraphic implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 398, 28–58. [Google Scholar] [CrossRef]
- Betts, M.J.; Paterson, J.R.; Jago, J.B.; Jacquet, S.M.; Skovsted, C.B.; Topper, T.P.; Brock, G.A. New lower Cambrian shelly fossil assemblage zones in the lower Hawker Group, Arrowie Basin, South Australia. Gondwana Res. 2016, 36, 163–195. [Google Scholar] [CrossRef]
- Betts, M.J.; Paterson, J.R.; Jacquet, S.M.; Andrew, A.S.; Hall, P.A.; Jago, J.B.; Jagodzinski, E.A.; Preiss, W.V.; Crowley, J.L.; Brougham, T.; et al. Early Cambrian chronostratigraphy and geochronology of South Australia. Earth-Sci. Rev. 2018, 185, 498–543. [Google Scholar] [CrossRef]
- Guo, J.F.; Li, Y.; Li, G.X. Small shelly fossils from the Early Cambrian Yanjiahe Formation, Yichang, Hubei, China. Gondwana Res. 2014, 25, 999–1007. [Google Scholar] [CrossRef]
- Guo, J.F.; Li, G.X.; Qiang, Y.Q.; Song, Z.C.; Zhang, Z.F.; Han, J.; Wang, W.Z. Watsonella crosbyi from the lower Cambrian (Terreneuvian, Stage 2) Yanjiahe Formation in Three Gorges Area, South China. Palaeoworld 2021, 30, 1–19. [Google Scholar] [CrossRef]
- Kouchinsky, A.; Bengtson, S.; Landing, E.; Steiner, M.; Vendrasco, M.; Ziegler, K. Terreneuvian stratigraphy and faunas from the Anabar Uplift, Siberia. Acta Palaeontol. Pol. 2017, 62, 311–440. [Google Scholar] [CrossRef]
- Jacquet, S.M.; Brougham, T.; Skovsted, C.B.; Jago, J.B.; Laurie, J.R.; Betts, M.J.; Topper, T.P.; Brock, G.A. Watsonella crosbyi from the lower Cambrian (Terreneuvian, Stage 2) Normanville Group in South Australia. Geol. Mag. 2017, 154, 1088–1104. [Google Scholar]
- Vostokova, V.A. Cambrian Gastropods of the Siberian Platform and Taimyr. Sbornik Statei Paleontol. Biostratigrafii 1962, 28, 51–74. (In Russian) [Google Scholar]
- Runnegar, B.; Pojeta, J.J. Molluscan phylogeny: The paleontological viewpoint. Science 1974, 186, 311–317. [Google Scholar] [CrossRef]
- Pojeta, J.J.; Runnegar, B. The paleontology of rostroconch mollusks and the early history of the phylum Mollusca. US Geol. Sur. Prof. Pap. 1976, 968, 1–88. [Google Scholar]
- Parkhaev, P.Y. New data on the morphology of ancient gastropods of the genus Aldanella vostokova, 1962 (archaeobranchia, pelagielliformes). Paleontol. J. 2006, 40, 244–252. [Google Scholar] [CrossRef]
- Thomas, R.D.K.; Runnegar, B.; Matt, K. Pelagiella exigua, an early Cambrian stem gastropod with chaetae: Lophotrochozoan heritage and conchiferan novelty. Palaeontology 2020, 63, 1–27. [Google Scholar] [CrossRef]
- Öpik, A. Über den estländischen blauen Ton. Publ. Geol. Inst. Univ. Tartu 1926, 6, 39–46. [Google Scholar]
- Rozanov, A.Y.; Missarzhevsky, V.V. Biostratigraphy and fauna of Lower Cambrian horizons. Tr. Geol. Inst. Akad. Nauk SSSR 1966, 148, 1–126. (In Russian) [Google Scholar]
- Rozanov, A.Y.; Missarzhevsky, V.V.; Volkova, N.A.; Voronova, L.G.; Krylov, I.N.; Keller, B.M.; Korolyuk, I.K.; Lendzion, K.; Michniak, R.; Pyhova, N.G.; et al. The Tommotian Stage and the Cambrian lower boundary problem. Tr. Geol. Inst. Akad. Nauk SSSR 1969, 206, 1–380. (In Russian) [Google Scholar]
- Golubev, S.N. Ontogenetic changes and evolutionary trends in Early Cambrian spiral gastropods of the superfamily Pelagiellacea. Paleontol. J. 1976, 10, 143–149. [Google Scholar]
- Lendzion, K. First gastropod fauna from the Klimontovian Stage (Lower Cambrian) of the South-Eastern Poland. Kwartalnik Geol. 1977, 21, 239–243. [Google Scholar]
- Chen, P. Discovery of Lower Cambrian small shelly fossils from Jijiapo, Yichang, west Hubei and its significance. Prof. Pap. Stratigr. Palaeontol. 1984, 13, 49–64. (In Chinese) [Google Scholar]
- Barskova, M.I. New Mollusks from the Lower Cambrian Deposits of the Kolyma Uplift. Paleontol. J. 1988, 1, 101–105. (In Russian) [Google Scholar]
- Missarzhevsky, V.V. Oldest skeletal fossils and stratigraphy of Precambrian and Cambrian boundary beds. Tr. Geol. Inst. Akad. Nauk SSSR 1989, 443, 1–237. (In Russian) [Google Scholar]
- Bokova, A.R. New Lower Cambrian gastropods from the Siberian Platform. Paleontol. J. 1990, 24, 134–136. [Google Scholar]
- Gubanov, A.P.; Fernández Remolar, D.C.; Peel, J.S. Early Cambrian molluscs from Sierra de Córdoba. Geobios 2004, 37, 199–215. (In Spain) [Google Scholar] [CrossRef]
- Parkhaev, P.Y. Shell chirality in Cambrian gastropods and sinistral members of the genus Aldanella Vostokova, 1962. Paleontol. J. 2007, 41, 233–240. [Google Scholar] [CrossRef]
- Landing, E. Lower Cambrian of eastern Massachusetts: Stratigraphy and small shelly fossils. J. Paleontol. 1988, 62, 661–695. [Google Scholar]
- Isakar, M.; Peel, J.S. Lower Cambrian helcionelloid molluscs from Estonia. GFF 2007, 129, 255–262. [Google Scholar] [CrossRef]
- Landing, E.; Geyer, G.; Brasier, M.D.; Bowring, S.A. Cambrian evolutionary radiation: Context, correlation, and chronostratigraphy-Overcoming deficiencies of the first appearance datum (FAD) concept. Earth-Sci. Rev. 2013, 123, 133–172. [Google Scholar] [CrossRef]
- Li, G.X.; Xiao, S.H. Micrina and Tannuolina (Tannuolinidae) from the lower Cambrian of eastern Yunnan, South China. J. Paleontol. 2004, 78, 900–913. [Google Scholar] [CrossRef]
- Xing, Y.S.; Ding, Q.X.; Luo, H.L.; He, T.G.; Wang, Y.G. The Sinian-Cambrian boundary of China; Geological Publishing House: Beijing, China, 1984; pp. 1–262. (In Chinese) [Google Scholar]
- Ding, L.F.; Zhang, L.Y.; Li, Y.; Dong, J.S.; Chen, H.X.; Tian, S.H. The Study of Late Sinian–Early Cambrian Biotas from the Northern Margin of Yangtze Platform; Scientific and Technical Documents Publishing House: Beijing, China, 1992; pp. 1–156. (In Chinese) [Google Scholar]
- Guo, J.F.; Li, Y.; Han, J.; Zhang, X.L.; Zhang, Z.F.; Ou, Q.; Liu, J.N.; Shu, D.G.; Maruyama, S.; Komiya, T. Fossil association from the lower Cambrian Yanjiahe Formation in the Yangtze Gorges area, Hubei, South China. Acta Geol. Sin. 2008, 82, 1124–1132. [Google Scholar]
- Steiner, M.; Yang, B.; Hohl, S.; Zhang, L.; Chang, S. Cambrian small skeletal fossil and carbon isotope records of the southern Huangling Anticline, Hubei (China) and implications for chemostratigraphy of the Yangtze Platform. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 554, 1–19. [Google Scholar] [CrossRef]
- Yu, W. New molluscan naterials of the Tethys. In 2nd International Symposium on Shallow Tethys; Mckenzie, K.G., Ed.; A A Balkema Publisher: Rotterdam, The Netherlands, 1987; pp. 51–59. [Google Scholar]
- Qian, J.X.; Xiao, B. An Early Cambrian Small Shelly Fauna from Aksu-Wushi Region, Xinjiang. Prof. Pap. Stratigr. Palaeontol. 1984, 13, 65–90. (In Chinese) [Google Scholar]
- Qian, Y.; Feng, W.M.; Li, G.X.; Yang, A.H.; Feng, M.; Zhao, X.; Xiao, B. Taxonomy and biostratigraphy of the early Cambrian univalved Mollusc fossils from Xinjiang. Acta Micropalaeontol. Sin. 2009, 26, 193–210. (In Chinese) [Google Scholar]
- Yang, B.; Steiner, M. Terreneuvian bio- and chemostratigraphy of the South Sichuan Region (South China). J. Geol. Soc. London 2021, 178, 1–15. [Google Scholar] [CrossRef]
- Guo, J.F.; Han, J.; Van Iten, H.; Wang, X.; Qiang, Y.Q.; Song, Z.C.; Wang, W.Z.; Zhang, Z.F.; Li, G.X. A fourteen-faced hexangulaconulariid from the early Cambrian (Stage 2) Yanjiahe Formation, South China. J. Paleontol. 2020, 94, 45–55. [Google Scholar] [CrossRef]
- Guo, J.F.; Li, Y.; Han, H.P.; Ou, Q.; Zhou, J.R.; Zheng, Y.J. New macroscopic problematic fossil from the early Cambrian Yanjiahe biota, Yichang, Hubei, China. Acta Geol. Sin. 2012, 86, 791–798. [Google Scholar]
- Guo, J.F.; Chen, Y.L.; Song, Z.C.; Zhang, Z.F.; Qiang, Y.Q.; Betts, M.J.; Zheng, Y.J.; Yao, X.Y. Geometric morphometric analysis of Protoconites minor from the Cambrian (Terreneuvian) Yanjiahe Formation in Three Gorges, South China. Palaeontol. Electron. 2020, 23, a48. [Google Scholar] [CrossRef]
- Topper, T.P.; Guo, J.F.; Clausen, S.; Skovsted, C.B.; Zhang, Z.F. A stem group echinoderm from the basal Cambrian of China and the origins of Ambulacraria. Nat. Commun. 2019, 10, 1366. [Google Scholar] [CrossRef]
- Okada, Y.; Sawaki, Y.; Komiya, T.; Hirata, T.; Takahata, N.; Sano, Y.; Han, J.; Maruyama, S. New chronological constraints for cryogenian to Cambrian rocks in the Three Gorges, Weng’an and Chengjiang areas, South China. Gondwana Res. 2014, 25, 1027–1044. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Zhu, M.Y. Lowermost Cambrian acritarchs from the Yanjiahe Formation, South China: Implication for defining the base of the Cambrian in the Yangtze Platform. Geol. Mag. 2017, 1, 1–15. [Google Scholar] [CrossRef]
- Guo, J.; Han, J.; Iten, H.V.; Song, Z.C.; Qiang, Y.Q.; Wang, W.Z.; Zhang, Z.F.; Li, G.X.; Sun, Y.F.; Sun, j. A new tetraradial olivooid (Medusozoa) from the lower Cambrian (Stage 2) Yanjiahe Formation, South China. J. Paleontol. 2020, 94, 457–466. [Google Scholar] [CrossRef]
- Guo, J.F.; Han, J.; Iten, H.V.; Song, Z.C.; Qiang, Y.Q.; Wang, W.Z.; Zhang, Z.F.; Li, G.X. A ten-faced hexangulaconulariid from Cambrian Stage 2 of South China. J. Paleontol. 2021, 95, 957–964. [Google Scholar] [CrossRef]
- Song, Z.C.; Guo, J.F.; Han, J.; Iten, H.V.; Qiang, Y.Q.; Peng, J.X.; Sun, J.; Zhang, Z.F. A new species of Septuconularia (Hexangulaconulariidae, Cnidaria) from Cambrian Stage 2, South China. Acta Geol. Sin. 2022, 96, 757–765. [Google Scholar] [CrossRef]
- Qiang, Y.Q.; Peng, J.X.; Song, Z.C.; Sun, J.; Zhao, X.F.; Li, G.X.; Han, J.; Guo, J.F. Early Cambrian Anabarella plana from Three Gorges area, South China. Front. Earth Sci. 2023, 10, 2591. [Google Scholar] [CrossRef]
- Song, Z.C.; Guo, J.F.; Pan, B.; Qiang, Y.Q.; Li, G.X.; Peng, J.X.; Sun, J.; Han, J. Ocruranus–Eohalobia Sclerites from the Cambrian Stage 2 Yanjiahe Formation in South China: Scleritome Reconstruction and Zoological Affinity. Biology 2022, 11, 1648. [Google Scholar] [CrossRef]
- Cuvier, G. Tableau Élementaire de L’historie Naturelle des Animaux; Baudouin: Paris, France, 1797; p. 710. [Google Scholar]
- Mackinnon, D.I. New Zealand late Middle Cambrian molluscs and the origin of Rostroconchia and Bivalvia. Alcheringa 1985, 9, 65–81. [Google Scholar] [CrossRef]
- Linsley, R.M.; Kier, W.M. The Paragastropoda: A proposal for a new Class of Paleozoic Mollusca. Malacologia 1984, 25, 241–254. [Google Scholar]
- Matthew, G.F. The Protolenus fauna. Trans. N. Y. Acad. Sci. 1895, 14, 101–153. [Google Scholar] [CrossRef]
- Dzik, J.; Mazurek, D. Affinities of the alleged earliest Cambrian gastropod Aldanella. Can. J. Zool. 2013, 91, 914–923. [Google Scholar] [CrossRef]
- Parkhaev, P.Y. On the stratigraphy of Aldanella attleborensis–potential index species for defining the base of Cambrian Stage 2. In Extended Summary, IGCP Project 591 Field Workshop; Zhan, R.B., Huang, B., Eds.; Nanjing University Press: Nanjing, China, 2014; pp. 102–105. [Google Scholar]
- Krajewski, K.P. Early diagenetic phosphate cements in the Albian condensed glauconitic limestone of the Tatra Mountains, Western Carpathians. Sedimentology 1984, 31, 443–470. [Google Scholar] [CrossRef]
- Yue, Z.; Gao, L.Z. Paleontology, biostratigraphy and geological significance of the early Cambrian Protoconodonts and other skeletal microfossils from Aksu-Wushi region, Xinjiang, China. Bull. Inst. Geol. Chinese Acad. Geol. Sci. 1992, 23, 133–160. (In Chinese) [Google Scholar]
- Vendrasco, M.J.; Checa, A.G.; Kouchinsky, A.V. Shell microstructure of the early bivalve Pojetaia and the independent origin of nacre within the mollusca. Palaeontology 2011, 54, 825–850. [Google Scholar] [CrossRef]
- Kouchinsky, A. Shell microstructures of the Early Cambrian Anabarella and Watsonella as new evidence on the origin of the Rostroconchia. Lethaia 1999, 32, 173–180. [Google Scholar] [CrossRef]
- Kouchinsky, A.; Alexander, R.; Bengtson, S.; Bowyer, F.; Clausen, S.; Holmer, L.E.; Kolesnikov, K.A.; Korovnikov, I.V.; Pavlov, V.; Skovsted, C.B.; et al. Early–middle Cambrian stratigraphy and faunas from northern Siberia. Acta Palaeontol. Pol. 2022, 67, 341–464. [Google Scholar] [CrossRef]
- Kouchinsky, A. Shell microstructures in Early Cambrian molluscs. Acta Palaeontol. Pol. 2000, 45, 119–150. [Google Scholar]
- Ushatinskaya, G.T.; Parkhaev, P.Y. Preservation of imprints and casts of Cambrian brachiopods, mollusks, and problematics. Paleontol. J. 2005, 39, 29–39. [Google Scholar]
- Vendrasco, M.J.; Porter, S.M.; Kouchinsky, A.V.; Li, G.X.; Fernandez, C.Z. Shell microstructures in early mollusks. Festivus. 2010, 42, 43–53. [Google Scholar]
- Li, L.Y.; Zhang, X.L.; Yun, H.; Li, G.X. Complex hierarchical microstructures of Cambrian mollusk Pelagiella: Insight into early biomineralization and evolution. Sci. Rep. 2017, 7, 1935. [Google Scholar] [CrossRef]
- Parkhaev, P.Y. Muscle scars of the Cambrian univalved mollusks and their significance for systematics. Paleontol. J. 2002, 36, 453–459. [Google Scholar]
- Parkhaev, P.Y. Structure of shell muscles in the Cambrian gastropod genus Bemella (Gastropoda: Archaeobranchia: Helcionellidae). Paleontol. J. 2014, 48, 17–25. [Google Scholar] [CrossRef]
- Peel, J.S. Muscle scars in euomphaline gastropods from the Ordovician of Baltica. Est. J. Earth Sci. 2019, 68, 88–100. [Google Scholar] [CrossRef]
- Horný, R.J. Circumbilical retractor muscle attachment areas found in Tropidodiscus (Gastropoda, Bellerophontoidea). J. Czech Geol. Soc. 2019, 44, 126–130. [Google Scholar]
- Peel, J.S. Muscle scars and mode of life of Carinaropsis (Bellerophontoidea, Gastropoda) from the Ordovician of Tennessee. J. Paleont. 1993, 67, 528–534. [Google Scholar] [CrossRef]
- Peel, J.S. Muscle scars in Porcellia (Gastropoda; Pleurotomariacea) from the Carboniferous of England. Bull. geol. Soc. Denmark. 1986, 35, 53–58. [Google Scholar] [CrossRef]
- Runnegar, B.; Jell, P.A. Australian Middle Cambrian molluscs and their bearing on early molluscan evolution. Alcheringa 1976, 1, 109–138. [Google Scholar] [CrossRef]
- Runnegar, B. Muscle scars, shell form and torsion in Cambrian and Ordovician univalved molluscs. Lethaia 1981, 14, 311–322. [Google Scholar] [CrossRef]
- Gubanov, A.P.; Peel, J.S. Cambrian monoplacophoran molluscs (Class Helcionelloida). Am. Malacol. Bull. 2000, 15, 139–145. [Google Scholar]
- Yochelson, E. Discussion of early Cambrian ‘‘molluscs”. J. Geol. Soc. 1975, 131, 661–662. [Google Scholar]
- Yochelson, E. An alternative approach to the interpretation of the phylogeny of ancient mollusks. Malacologia 1978, 17, 165–191. [Google Scholar]
- Runnegar, B. Shell microstructures of Cambrian molluscs replicated by phosphate. Alcheringa 1985, 9, 245–257. [Google Scholar] [CrossRef]
- Li, L.Y.; Zhang, X.L.; Skovsted, C.B.; Yun, H.; Li, G.X.; Pan, B. Shell microstructures of the helcionelloid mollusc Anabarella australis from the lower Cambrian (Series 2) Xinji Formation of North China. J. Syst. Palaeontol. 2019, 1, 1–11. [Google Scholar]
- Li, L.Y.; Skovsted, C.B.; Topper, T.P. Deep origin of the crossed-lamellar microstructure in early Cambrian molluscs. Palaeontology 2022, 65, 1–14. [Google Scholar] [CrossRef]
- Nützel, A.; Frýda, J. Paleozoic plankton revolution: Evidence from early gastropod ontogeny. Geology 2003, 31, 829–831. [Google Scholar] [CrossRef]
- Dzik, J. Decline in diversity of early Palaeozoic loosely coiled gastropod protoconchs. Lethaia 2019, 53, 32–46. [Google Scholar] [CrossRef]
- Parkhaev, P.Y. Protoconch morphology and peculiarities of the early ontogeny of the Cambrian Helcionelloid Mollusks. Paleontol. J. 2014, 48, 369–379. [Google Scholar] [CrossRef]
- Jablonski, D.; Lutz, R.A. Larval ecology of marine benthic invertebrates: Paleobiological implications. Biol. Rev. 1983, 58, 21–89. [Google Scholar] [CrossRef]
- Dzik, J. Evolution of “small shelly fossils” assemblages of the early Paleozoic. Acta Palaeontol. Pol. 1994, 39, 247–313. [Google Scholar]
- Zhu, M.Y.; Yang, A.H.; Yuan, J.L.; Li, G.X.; Zhang, J.M.; Zhao, F.C.; Ahn, S.Y.; Miao, L.Y. Cambrian integrative stratigraphy and timescale of China. Sci. China: Earth Sci. 2019, 62, 25–60. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, M.E.; Chen, Y.Y. Hyolithids and other small shelly fossils from the lower Cambrian Huangshandong formation in the eastern part of the Yangtze Gorge. Acta Palaeontol. Sin. 1979, 18, 207–229. (In Chinese) [Google Scholar]
- Torsvik, T.H.; Cocks, L.R.M. New global palaeogeographical reconstructions for the Early Palaeozoic and their generation. In Early Palaeozoic Biogeography and Palaeogeography; Harper, D.A.T., Servais, T., Eds.; Geological Society: London, UK, 2013; pp. 5–24. [Google Scholar]
- Bengtson, S.; Fletcher, T.P. The oldest sequence of skeletal fossils in the Lower Cambrian of southeastern Newfoundland. Can. J. Earth Sci. 1983, 20, 525–536. [Google Scholar] [CrossRef]
- Esakova, N.V.; Zhegallo, E.A. Biostratigraphy and fauna of the lower Cambrian of Mongolia. Tr. Sovm. Sovet. Mongol. Paleontol. Eksped. 1996, 46, 1–216. (In Russian) [Google Scholar]
- Gubanov, A.P. Early Cambrian palaeogeography and the probable Iberia–Siberia connection. Tectonophysics 2002, 352, 153–168. [Google Scholar] [CrossRef]
- Smith, E.F.; Macdonald, F.A.; Petach, T.A.; Bold, U.; Schragg, D.P. Integrated stratigraphic, geochemical, and paleontological late Ediacaran to early Cambrian records from southwestern Mongolia. Geol. Soc. Am. Bull. 2016, 128, 442–468. [Google Scholar] [CrossRef]
- Li, G.X.; Zhu, M.Y.; Zhang, J.M.; Steiner, M. Bio- and carbon isotope stratigraphy of the lower Cambrian in eastern Yunnna and the subdivision of the 1st Series of the Cambrian. In Proceedings of the Geological Society of Australia, XI International Conference of the Cambrian Stage Subdivision Working Group, South Australia, Australia, 14–24 August 2006; Jago, J., Ed.; p. 20. [Google Scholar]
- Landing, E.; Kouchinsky, A. Correlation of the Cambrian evolutionary radiation: Geochronology, evolutionary stasis of earliest Cambrian (Terreneuvian) small shelly fossil (SSF) taxa, and chronostratigraphic significance. Geol. Mag. 2016, 153, 750–756. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiang, Y.; Guo, J.; Li, G.; Song, Z.; Peng, J.; Sun, J.; Han, J.; Zhang, Z. Aldanella attleborensis (Mollusca) from Cambrian Stage 2 of the Three Gorges Area and Its Stratigraphic Implications. Biology 2023, 12, 261. https://doi.org/10.3390/biology12020261
Qiang Y, Guo J, Li G, Song Z, Peng J, Sun J, Han J, Zhang Z. Aldanella attleborensis (Mollusca) from Cambrian Stage 2 of the Three Gorges Area and Its Stratigraphic Implications. Biology. 2023; 12(2):261. https://doi.org/10.3390/biology12020261
Chicago/Turabian StyleQiang, Yaqin, Junfeng Guo, Guoxiang Li, Zuchen Song, Jiaxin Peng, Jie Sun, Jian Han, and Zhifei Zhang. 2023. "Aldanella attleborensis (Mollusca) from Cambrian Stage 2 of the Three Gorges Area and Its Stratigraphic Implications" Biology 12, no. 2: 261. https://doi.org/10.3390/biology12020261
APA StyleQiang, Y., Guo, J., Li, G., Song, Z., Peng, J., Sun, J., Han, J., & Zhang, Z. (2023). Aldanella attleborensis (Mollusca) from Cambrian Stage 2 of the Three Gorges Area and Its Stratigraphic Implications. Biology, 12(2), 261. https://doi.org/10.3390/biology12020261







