Fibrous or Prismatic? A Comparison of the Lamello-Fibrillar Nacre in Early Cambrian and Modern Lophotrochozoans
Abstract
:Simple Summary
Abstract
1. Introduction
2. Fiber and Lamello-Fibrillar Nacre in Lophotrochozoans
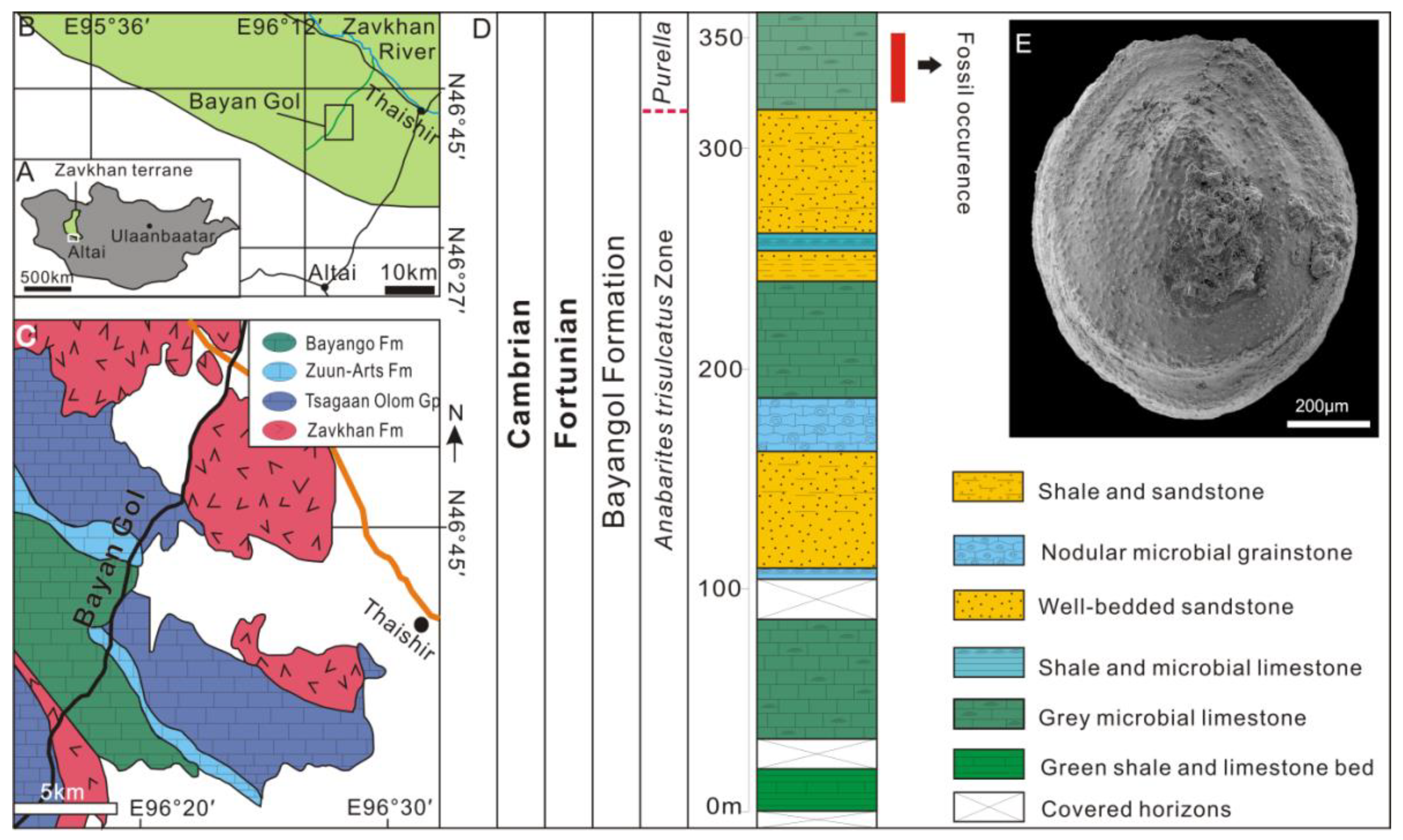
3. Geological Settings, Materials and Methods
4. Results
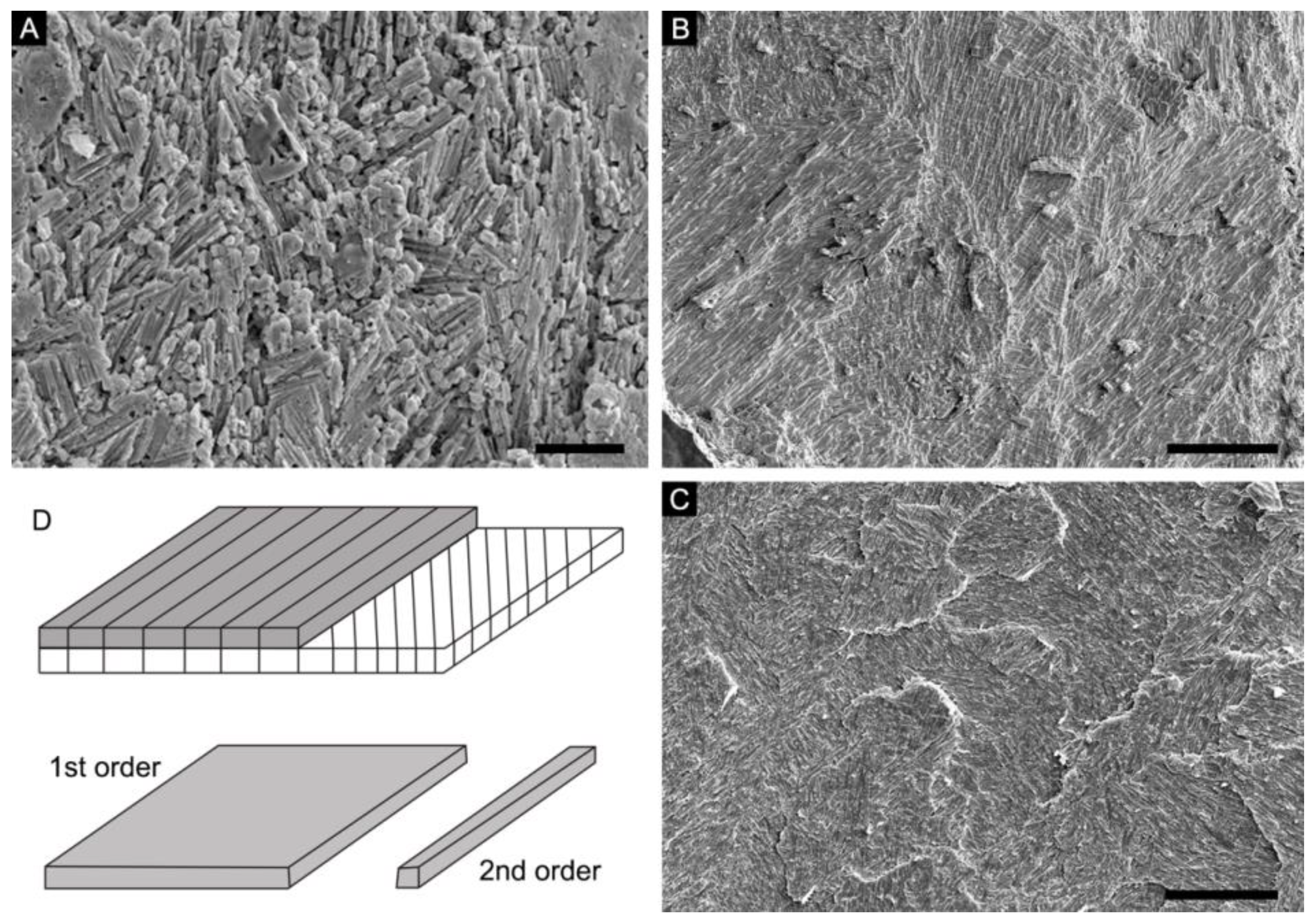
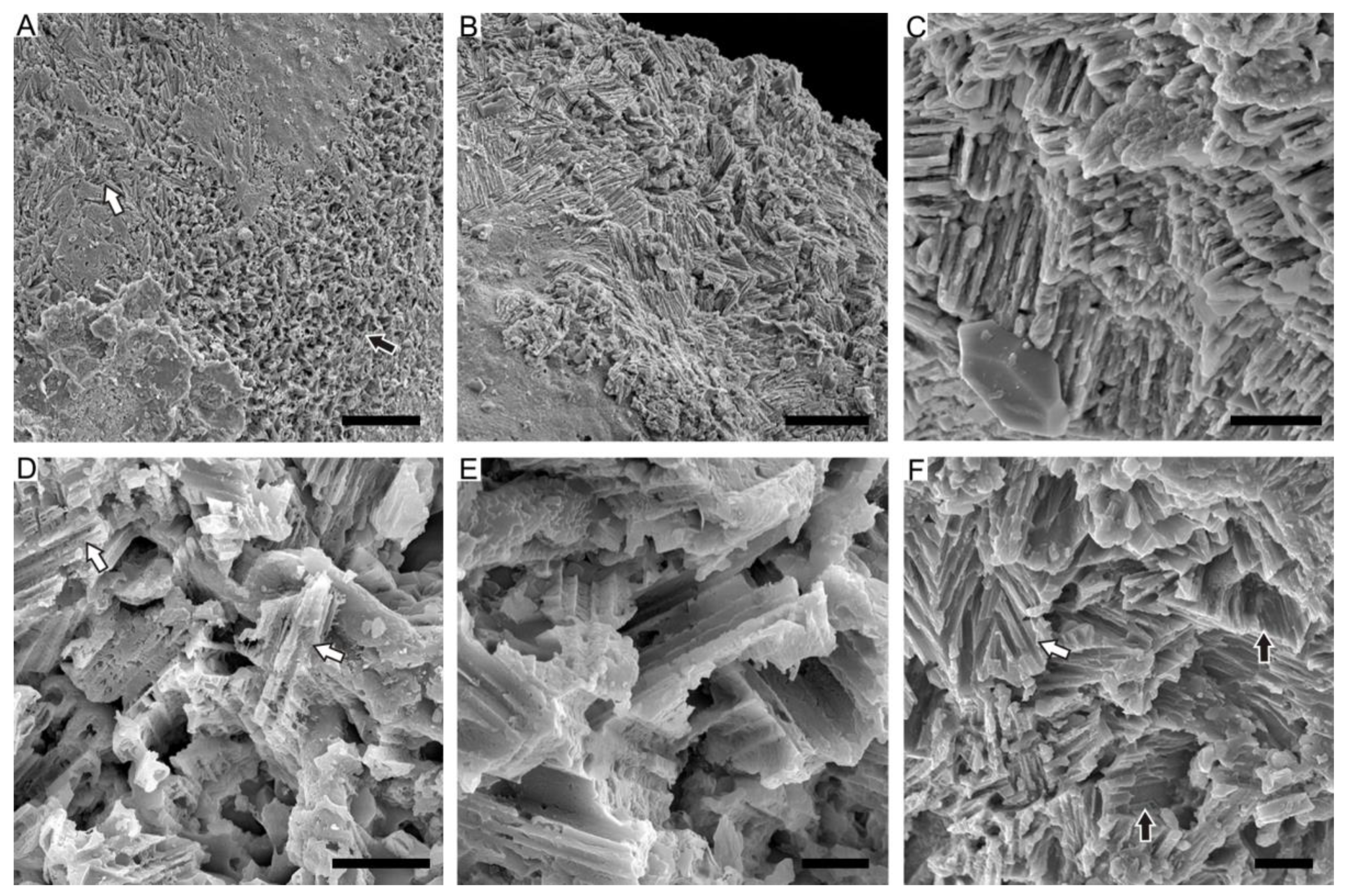
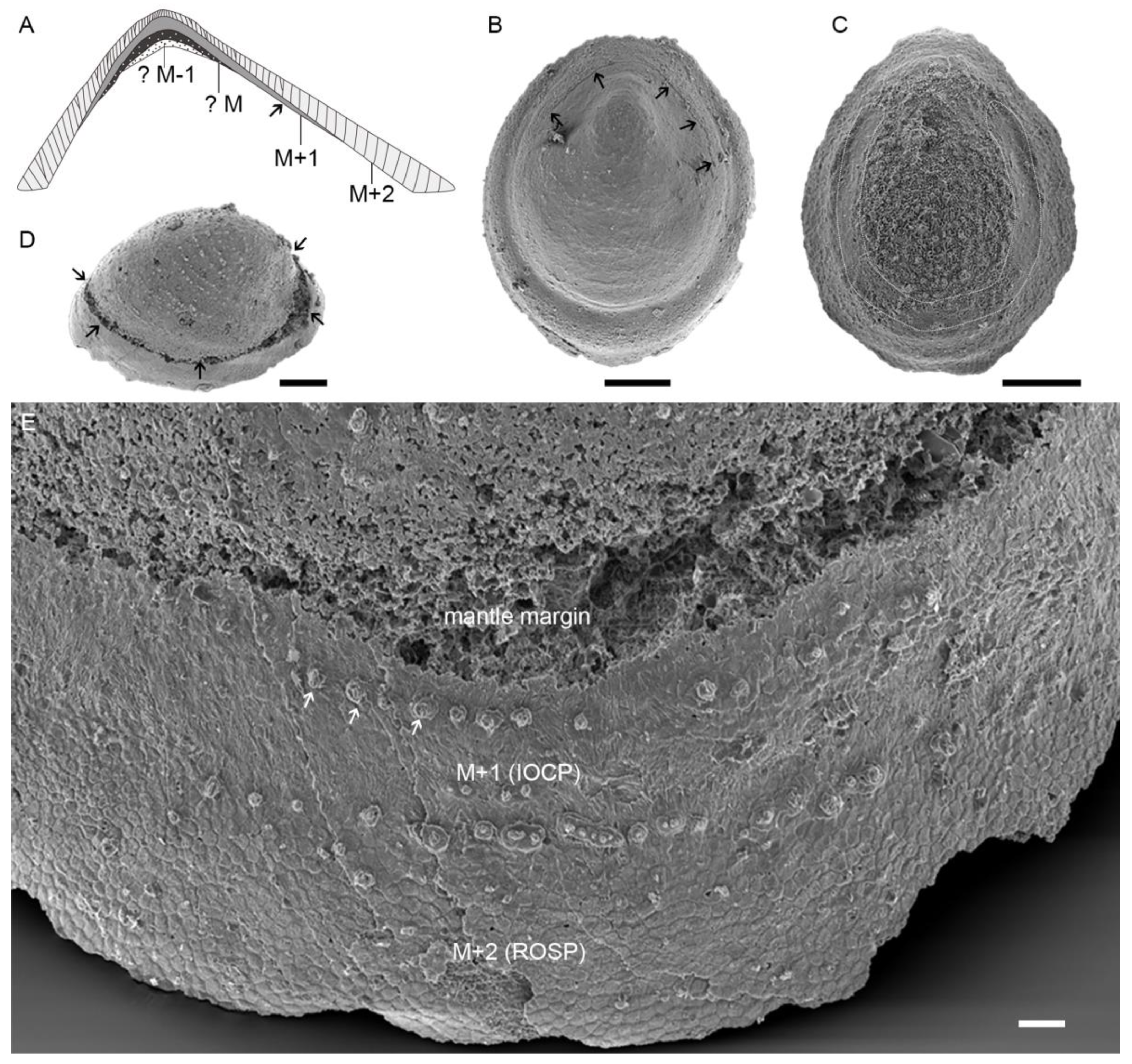
4.1. Lamello-Fibrillar Nacre of Extant Coleoid Cuttlebones
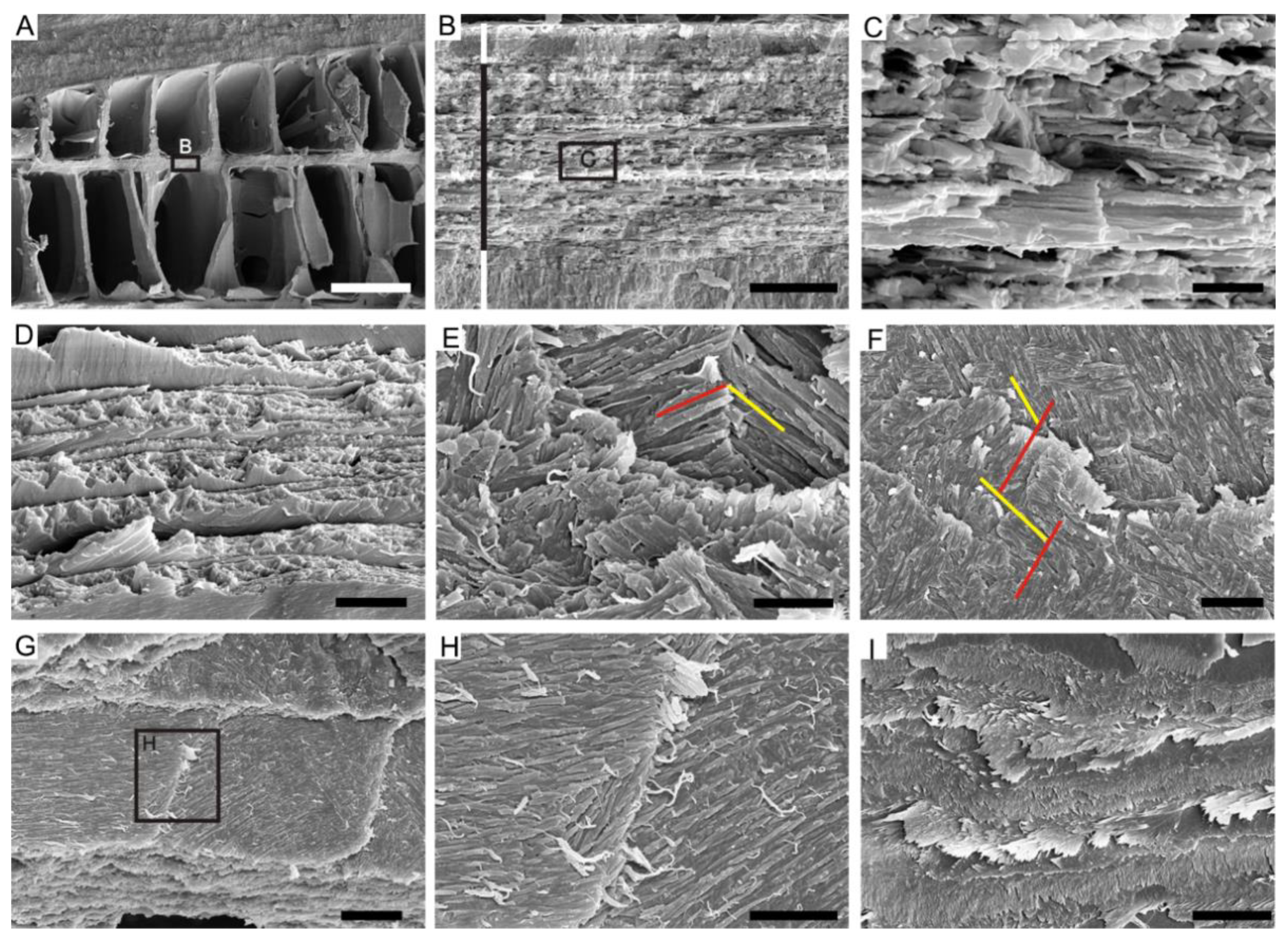
4.2. Ordered-Chevron Microstructure of Serpulid Tubes
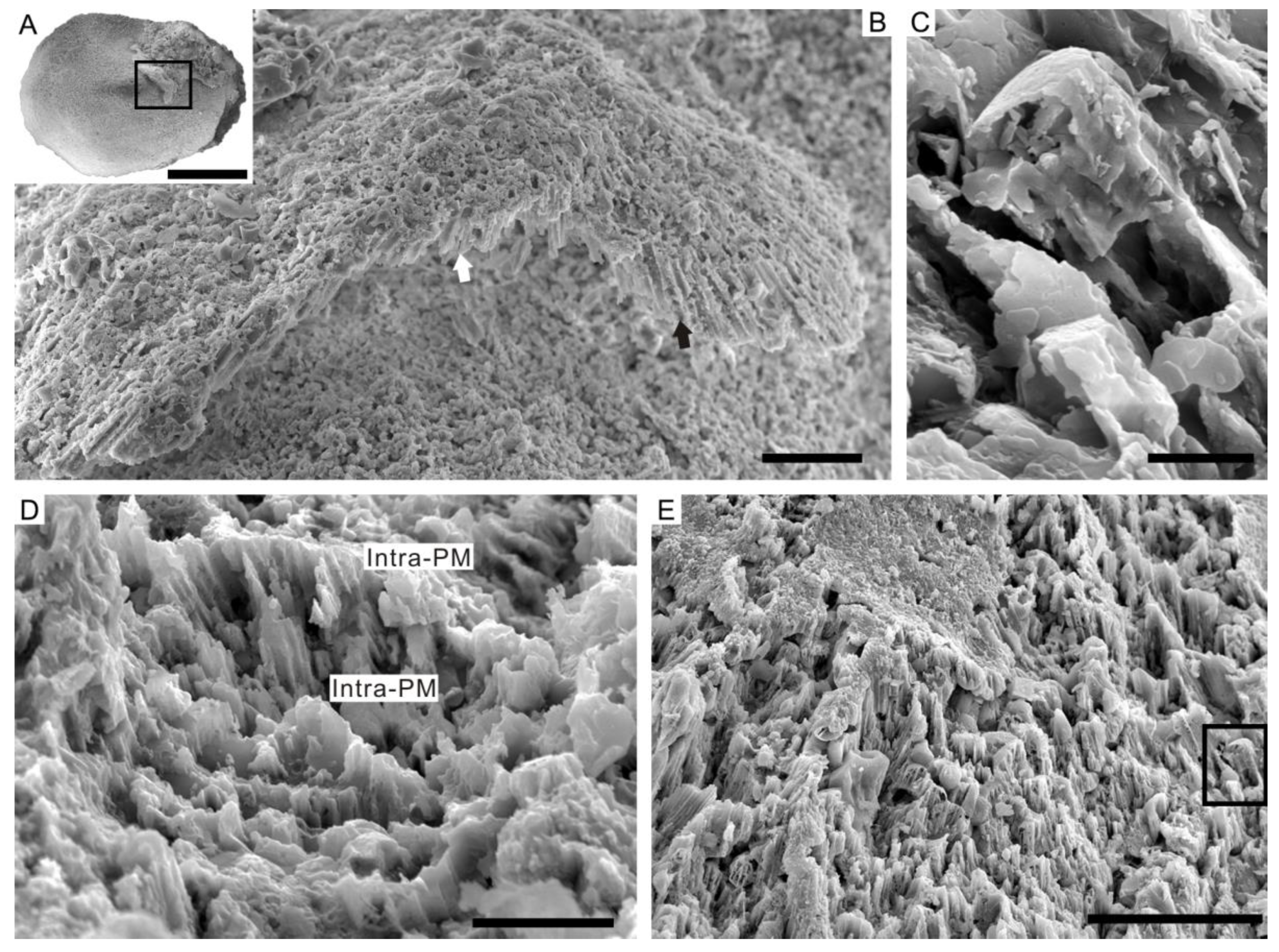
4.3. Prismatic Microstructure of Cambrian Postacanthella Shells
5. Discussion
5.1. Preservation and Reconstruction of Postacanthella Shells
5.2. Lamello-Fibrillar or Prismatic?
5.3. Biomineralizing a Prismatic Shell in the Terreneuvian Aragonite Sea
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vendrasco, M.J.; Rodríguez-Navarro, A.B.; Checa, A.G.; Devaere, L.; Porter, S.M. To infer the early evolution of mollusc shell microstructures. Key Eng. Mater. 2016, 672, 113–133. [Google Scholar] [CrossRef]
- Janiszewska, K.; Mazur, M.; Machalski, M.; Stolarski, J. From pristine aragonite to blocky calcite: Exceptional preservation and diagenesis of cephalopod nacre in porous Cretaceous limestones. PLoS ONE 2018, 13, e0208598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pederson, C.; Mavromatis, V.; Dietzel, M.; Rollion-Bard, C.; Nehrke, G.; Jöns, N.; Jochum, K.P.; Immenhauser, A. Diagenesis of mollusc aragonite and the role of fluid reservoirs. Earth Planet. Sci. Lett. 2019, 514, 130–142. [Google Scholar] [CrossRef]
- Knoll, A.H. Biomineralization and evolutionary history. Rev. Miner. Geochem. 2003, 54, 329–356. [Google Scholar] [CrossRef] [Green Version]
- Vendrasco, M.J.; Porter, S.M.; Kouchinsky, A.; Li, G.; Fernandez, C.Z. New data on molluscs and their shell microstructures from the Middle Cambrian Gowers Formation, Australia. Palaeontology 2010, 53, 97–135. [Google Scholar] [CrossRef]
- Murdock, D.J.E. The ‘biomineralization toolkit’ and the origin of animal skeletons. Biol. Rev. 2020, 95, 1372–1392. [Google Scholar] [CrossRef]
- Li, L.; Skovsted, C.B.; Topper, T.P. Deep origin of the crossed-lamellar microstructure in early Cambrian molluscs. Palaeontology 2022, 65, e12620. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Skovsted, C.B.; Yun, H.; Pan, B.; Li, G. Homologous shell microstructures in Cambrian hyoliths and molluscs. Palaeontology 2019, 62, 515–532. [Google Scholar] [CrossRef]
- Li, L.; Skovsted, C.B.; Yun, H.; Betts, M.J.; Zhang, X. New insight into the soft anatomy and shell microstructures of early Cambrian orthothecids (Hyolitha). Proc. R. Soc. B Biol. Sci. 2020, 287, 20201467. [Google Scholar] [CrossRef] [PubMed]
- Balthasar, U.; Skovsted, C.B.; Holmer, L.E.; Brock, G.A. Homologous skeletal secretion in tommotiids and brachiopods. Geology 2019, 37, 1143–1146. [Google Scholar] [CrossRef]
- Yun, H.; Zhang, X.; Brock, G.A.; Li, L.; Li, G. Biomineralization of the Cambrian chancelloriids. Geology 2021, 49, 623–628. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Yun, H.; Li, G. Complex hierarchical microstructures of Cambrian mollusk Pelagiella: Insight into early biomineralization and evolution. Sci. Rep. 2017, 7, 1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.; Betts, M.J.; Holmer, L.E.; Chen, Y.; Liu, F.; Liang, Y.; Zhang, Z. Early Cambrian (Stage 4) brachiopods from the Shipai Formation in the Three Gorges area of South China. J. Paleontol. 2021, 95, 497–526. [Google Scholar] [CrossRef]
- Checa, A.G. Physical and biological determinants of the fabrication of molluscan shell microstructures. Front. Mar. Sci. 2018, 5, 353. [Google Scholar] [CrossRef] [Green Version]
- Stanley, S.M. Influence of seawater chemistry on biomineralization throughout phanerozoic time: Paleontological and experimental evidence. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 232, 214–236. [Google Scholar] [CrossRef]
- Ries, J.B. Review: Geological and experimental evidence for secular variation in seawater Mg/Ca (calcite-aragonite seas) and its effects on marine biological calcification. Biogeosciences 2010, 7, 2795–2849. [Google Scholar] [CrossRef] [Green Version]
- Zhuravlev, A.Y.; Wood, R.A. Eve of biomineralization: Controls on skeletal mineralogy. Geology 2008, 36, 923–926. [Google Scholar] [CrossRef]
- Wood, R.A.; Zhuravlev, A.Y. Escalation and ecological selectively of mineralogy in the Cambrian Radiation of skeletons. Earth Sci. Rev. 2012, 115, 249–261. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, D. Current understanding on the Cambrian Explosion: Questions and answers. PalZ 2021, 95, 641–660. [Google Scholar] [CrossRef]
- Gilbert, P.U.; Bergmann, K.D.; Boekelheide, N.; Tambutté, S.; Mass, T.; Marin, F.; Adkins, J.F.; Erez, J.; Gilbert, B.; Knutson, V.; et al. Biomineralization: Integrating mechanism and evolutionary history. Sci. Adv. 2022, 8, eabl9653. [Google Scholar] [CrossRef]
- Conci, N.; Griesshaber, E.; Rivera-Vicéns, R.E.; Schmahl, W.W.; Vargas, S.; Wörheide, G. Molecular and mineral responses of corals grown under artificial Calcite Sea conditions. bioRxiv 2022. [Google Scholar] [CrossRef]
- Moore, J.L.; Porter, S.M. Plywood-like shell microstructures in hyoliths from the middle Cambrian (Drumian) Gowers Formation, Georgina Basin, Australia. Palaeontology 2018, 61, 441–467. [Google Scholar] [CrossRef]
- Checa, A.G.; Pina, C.M.; Osuna-Mascaró, A.J.; Rodríguez-Navarro, A.B.; Harper, E.M. Crystalline organization of the fibrous prismatic calcitic layer of the Mediterranean mussel Mytilus galloprovincialis. Eur. J. Miner. 2014, 26, 495–505. [Google Scholar] [CrossRef]
- Ye, F.; Garbelli, C.; Shen, S.; Angiolini, L. The shell fabric of Palaeozoic brachiopods: Patterns and trends. Lethaia 2021, 54, 419–439. [Google Scholar] [CrossRef]
- Roda, M.S.; Griesshaber, E.; Angiolini, L.; Rollion-Bard, C.; Harper, E.M.; Bitner, M.A.; Milner Garcia, S.; Ye, F.; Henkel, D.; Häussermann, V.; et al. The architecture of Recent brachiopod shells: Diversity of biocrystal and biopolymer assemblages in rhynchonellide, terebratulide, thecideide and craniide shells. Mar. Biol. 2022, 169, 4. [Google Scholar] [CrossRef]
- van de Locht, R.; Verch, A.; Saunders, M.; Dissard, D.; Rixen, T.; Moya, A.; Kröger, R. Microstructural evolution and nanoscale crystallography in scleractinian coral spherulites. J. Struct. Biol. 2013, 183, 57–65. [Google Scholar] [CrossRef]
- Chandran, R.; Williams, L.; Hung, A.; Nowlin, K.; LaJeunesse, D. SEM characterization of anatomical variation in chitin organization in insect and arthropod cuticles. Micron 2016, 82, 74–85. [Google Scholar] [CrossRef]
- Carter, J.G.; Clark, G.R. Classification and phylogenetic significance of molluscan shell microstructure. Series Geol. Notes Short Course 1985, 13, 50–71. [Google Scholar] [CrossRef]
- Doguzhaeva, L.A. Two early Cretaceous spirulid coleoids of the north-western Caucasus: Their shell ultrastructure and evolutionary implications. Palaeontology 1996, 39, 681–707. [Google Scholar]
- Checa, A.G.; Cartwright, J.H.; Sánchez-Almazo, I.; Andrade, J.P.; Ruiz-Raya, F. The cuttlefish Sepia officinalis (Sepiidae, Cephalopoda) constructs cuttlebone from a liquid-crystal precursor. Sci. Rep. 2015, 5, 11513. [Google Scholar] [CrossRef] [Green Version]
- Doguzhaeva, L.A.; Dunca, E. Siphonal zone structure in the cuttlebone of Sepia officinalis. Swiss J. Palaeontol. 2015, 134, 167–176. [Google Scholar] [CrossRef]
- Dauphin, Y.; Luquet, G.; Percot, A.; Bonnaud-Ponticelli, L. Comparison of embryonic and adult shells of Sepia officinalis (Cephalopoda, Mollusca). Zoomorphology 2020, 139, 151–169. [Google Scholar] [CrossRef]
- Hedegaard, C. Shell structures of the recent Vetigastropoda. J. Molluscan Stud. 1997, 63, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Buckman, J.O.; Harries, D.B. Reef forming Serpula vermicularis from Scotland and Ireland: Tube structure, composition and implications. Zool. Anz. 2020, 288, 53–65. [Google Scholar] [CrossRef]
- Taylor, P.D.; Vinn, O.; Wilson, M.A. Evolution of biomineralization in ‘Lophophorates’. Palaeontology 2010, 84, 317–333. [Google Scholar]
- Vinn, O. Biomineralization in polychaete annelids: A review. Minerals 2021, 11, 1151. [Google Scholar] [CrossRef]
- Vinn, O.; ten Hove, H.A.; Mutvei, H.; Kirsimaee, K. Ultrastructure and mineral composition of serpulid tubes (Polychaeta, Annelida). Zool. J. Linn. Soc. 2008, 154, 633–650. [Google Scholar] [CrossRef]
- Vinn, O.; Kirsimäe, K.; ten Hove, H.A. Tube ultrastructure of Pomatoceros americanus (Polychaeta, Serpulidae): Implications for the tube formation of serpulids. Estonian J. Earth Sci. 2009, 58, 148–152. [Google Scholar] [CrossRef]
- Vinn, O. Occurrence, formation and function of organic sheets in the mineral tube structures of Serpulidae (polychaeta, Annelida). PLoS ONE 2013, 8, e75330. [Google Scholar] [CrossRef] [Green Version]
- Vendrasco, M.J.; Checa, A.G.; Kouchinsky, A.V. Shell microstructure of the early bivalve Pojetaia and the independent origin of nacre within the Mollusca. Palaeontology 2011, 54, 825–850. [Google Scholar] [CrossRef]
- Kouchinsky, A.V. Shell microstructures in Early Cambrian molluscs. Acta Palaeontol. Polonica 2000, 45, 119–150. [Google Scholar]
- Feng, W.; Sun, W.; Qian, Y. Skeletalization characters, classification and evolutionary significance of Early Cambrian monoplacophoran maikhanellids. Acta Palaeontol. Sin. 2001, 40, 205–216, (In Chinese with Einglish Summary). [Google Scholar]
- Feng, W.; Sun, W. Phosphate replicated and replaced microstructure of molluscan shells from the earliest Cambrian of China. Acta Palaeontol. Polonica 2003, 48, 21–30. [Google Scholar]
- Vendrasco, M.J.; Li, G.; Porter, S.M.; Fernandez, C.Z. New data on the enigmatic Ocruranus–Eohalobia group of early Cambrian small skeletal fossils. Palaeontology 2009, 52, 1373–1396. [Google Scholar] [CrossRef]
- Malinky, J.M.; Yochelson, E.L. On the systematic position of the Hyolitha (Kingdom Animalia). Mem. Assoc. Australas. Palaeontol. 2007, 34, 521–536. [Google Scholar]
- Mus, M.M.; Jeppsson, L.; Malinky, J.M. A complete reconstruction of the hyolithid skeleton. J. Paleontol. 2014, 88, 160–170. [Google Scholar]
- Skovsted, C.B.; Mus, M.M.; Zhang, Z.; Pan, B.; Li, L.; Liu, F.; Li, G.; Zhang, Z. On the origin of hyolith helens. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 555, 109848. [Google Scholar] [CrossRef]
- Moysiuk, J.; Smith, M.R.; Caron, J.B. Hyoliths are Palaeozoic lophophorates. Nature 2017, 541, 394–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouchinsky, A.V.; Bengtson, S.; Landing, E.; Steiner, M.; Vendrasco, M.J.; Ziegler, K. Terreneuvian stratigraphy and faunas from the Anabar Uplift, Siberia. Acta Palaeontol. Polonica 2017, 62, 311–440. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.L.; Skovsted, C.B.; Zhang, Z.F. A hyolithid without helens preserving the oldest hyolith muscle scars; palaeobiology of Paramicrocornus from the Shujingtuo Formation (Cambrian Series 2) of South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 489, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Vendrasco, M.J.; Checa, A.G.; Porter, S.M. Periostracum and fibrous shell microstructure in the unusual Cambrian hyolith Cupitheca. Span. J. Paleontol. 2020, 32, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Peel, J.S. In-place operculum demonstrates that the Middle Cambrian Protowenella is a hyolith and not a mollusc. Alcheringa 2021, 45, 385–394. [Google Scholar] [CrossRef]
- Peel, J.S.; Kouchinsky, A.V. Middle Cambrian (Miaolingian Series, Wuliuan Stage) molluscs and mollusc-like microfossils from North Greenland (Laurentia). Bull. Geol. Soc. Den. 2022, 70, 69–104. [Google Scholar] [CrossRef]
- Kouchinsky, A.V.; Alexander, R.; Bengtson, S.; Bowyer, F.; Clausen, S.; Holmer, L.E.; Kolesnikov, K.A.; Korovnikov, I.V.; Pavlov, V.; Skovsted, C.B.; et al. Early-middle Cambrian stratigraphy and faunas from northern Siberia. Acta Palaeontol. Polonica 2022, 67, 341–464. [Google Scholar] [CrossRef]
- Topper, T.P.; Betts, M.J.; Dorjnamjaa, D.; Li, G.; Li, L.; Altanshagai, G.; Enkhbaatar, B.; Skovsted, C.B. Locating the BACE of the Cambrian: Bayan Gol in southwestern Mongolia and global correlation of the Ediacaran–Cambrian boundary. Earth Sci. Rev. 2022, 229, 104017. [Google Scholar] [CrossRef]
- Li, L.; Topper, T.P.; Betts, M.J.; Dorjnamjaa, D.; Altanshagai, G.; Enkhbaatar, B.; Li, G.; Skovsted, C.B. Calcitic shells in the aragonite sea of the earliest Cambrian. Geology 2022, 51, 8–12. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Skovsted, C.B.; Yun, H.; Pan, B.; Li, G. Revisiting the molluscan fauna from the Cambrian (Series 2, stages 3–4) Xinji Formation of North China. Pap. Palaeontol. 2021, 7, 521–564. [Google Scholar] [CrossRef]
- Claybourn, T.M.; Jacquet, S.M.; Skovsted, C.B.; Topper, T.P.; Holmer, L.E.; Brock, G.A. Mollusks from the upper Shackleton Limestone (Cambrian Series 2), Central Transantarctic Mountains, East Antarctica. J. Paleontol. 2019, 93, 437–459. [Google Scholar] [CrossRef] [Green Version]
- Bengtson, S.; Conway Morris, S.; Cooper, B.J.; Jell, P.A.; Runnegar, B.N. Early Cambrian fossils from South Australia. Mem. Assoc. Australas. Palaeontol. 1990, 9, 1–364. [Google Scholar]
- Checa, A.G.; Rodríguez-Navarro, A.B.; Esteban-Delgado, F.J. The nature and formation of calcitic columnar prismatic shell layers in pteriomorphian bivalves. Biomaterials 2005, 26, 6404–6414. [Google Scholar] [CrossRef]
- Cuif, J.P.; Dauphin, Y.; Luquet, G.; Medjoubi, K.; Somogyi, A.; Perez-Huerta, A. Revisiting the organic template model through the microstructural study of shell development in Pinctada margaritifera, the Polynesian pearl oyster. Minerals 2018, 8, 370. [Google Scholar] [CrossRef]
- Cuif, J.P.; Belhadj, O.; Borensztajn, S.; Gèze, M.; Trigos-Santos, S.; Prado, P.; Dauphin, Y. Prism substructures in the shell of Pinna nobilis (Linnaeus, 1758), Mollusca—Evidence for a three-dimensional pulsed-growth model. Heliyon 2020, 6, e04513. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.D.; Runnegar, B.; Matt, K. Pelagiella exigua, an early Cambrian stem gastropod with chaetae: Lophotrochozoan heritage and conchiferan novelty. Palaeontology 2020, 63, 601–627. [Google Scholar] [CrossRef] [Green Version]
- Runnegar, B. Shell microstructures of Cambrian molluscs replicated by phosphate. Alcheringa Australas. J. Palaeontol. 2008, 9, 245–257. [Google Scholar] [CrossRef]
- Wei, G.; Hood, A.V.S.; Planavsky, N.J.; Li, D.; Ling, H.F.; Tarhan, L.G. Calcium isotopic constraints on the transition from aragonite seas to calcite seas in the Cambrian. Glob. Biogeochem. Cycles 2022, 36, e2021GB007235. [Google Scholar] [CrossRef]
- Porter, S.M. Calcite and aragonite seas and the de novo acquisition of carbonate skeletons. Geobiology 2010, 8, 256–277. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Betts, M.J.; Yun, H.; Pan, B.; Topper, T.P.; Li, G.; Zhang, X.; Skovsted, C.B. Fibrous or Prismatic? A Comparison of the Lamello-Fibrillar Nacre in Early Cambrian and Modern Lophotrochozoans. Biology 2023, 12, 113. https://doi.org/10.3390/biology12010113
Li L, Betts MJ, Yun H, Pan B, Topper TP, Li G, Zhang X, Skovsted CB. Fibrous or Prismatic? A Comparison of the Lamello-Fibrillar Nacre in Early Cambrian and Modern Lophotrochozoans. Biology. 2023; 12(1):113. https://doi.org/10.3390/biology12010113
Chicago/Turabian StyleLi, Luoyang, Marissa J. Betts, Hao Yun, Bing Pan, Timothy P. Topper, Guoxiang Li, Xingliang Zhang, and Christian B. Skovsted. 2023. "Fibrous or Prismatic? A Comparison of the Lamello-Fibrillar Nacre in Early Cambrian and Modern Lophotrochozoans" Biology 12, no. 1: 113. https://doi.org/10.3390/biology12010113
APA StyleLi, L., Betts, M. J., Yun, H., Pan, B., Topper, T. P., Li, G., Zhang, X., & Skovsted, C. B. (2023). Fibrous or Prismatic? A Comparison of the Lamello-Fibrillar Nacre in Early Cambrian and Modern Lophotrochozoans. Biology, 12(1), 113. https://doi.org/10.3390/biology12010113





