Plastid Phylogenomic Insights into the Inter-Tribal Relationships of Plantaginaceae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, DNA Extraction, Sequencing, Assembly and Annotation
2.2. Plastome Comparative Analyses
2.3. Phylogenetic Analyses
3. Results
3.1. Features of the Plastomes
3.2. Plastome Structural Variation
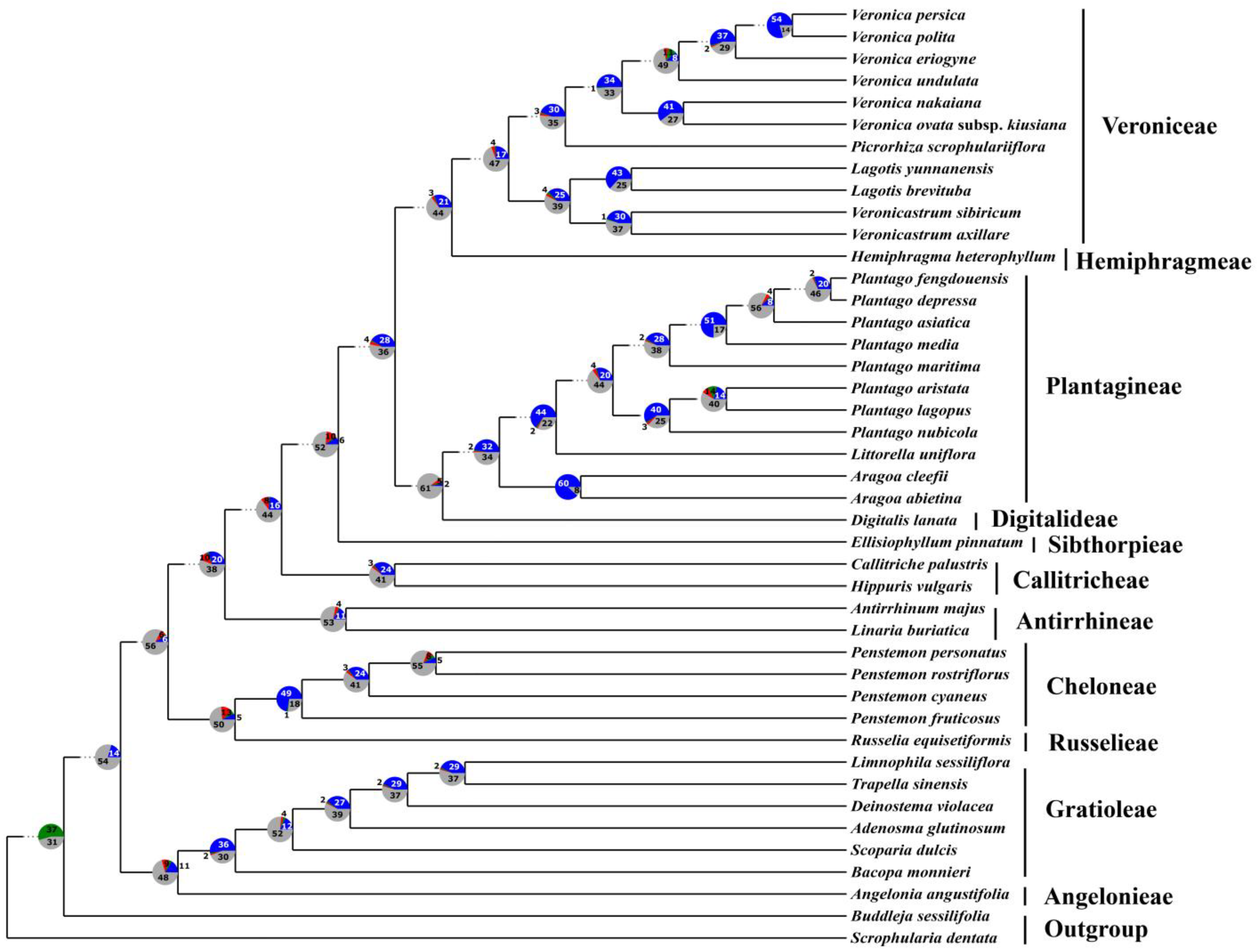
3.3. Phylogenetic Analyses
3.4. Concordance and Conflict of the Gene Tree
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, P.F. Angiosperm Phylogeny Website. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 9 October 2022).
- Olmstead, R.G.; Reeves, P.A. Evidence for the Polyphyly of the Scrophulariaceae Based on Chloroplast RbcL and NdhF Sequences. Ann. Mo. Bot. Gard. 1995, 82, 176–193. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Albach, D.C.; Chase, M.W. Incongruence in Veroniceae (Plantaginaceae): Evidence from Two Plastid and a Nuclear Ribosomal DNA Region. Mol. Phylogenet. Evol. 2004, 32, 183–197. [Google Scholar] [CrossRef]
- Albach, D.C.; Montserrat, M.; Fischer, M.A.; Chase, M.W. Evolution of Veroniceae: A Phylogenetic Perspective. Ann. Mo. Bot. Gard. 2001, 91, 275–302. [Google Scholar]
- Albach, D.C.; Martínez-Ortega, M.M.; Fischer, M.A.; Chase, M.W. A New Classification of the Tribe Veroniceae-Problems and a Possible Solution. TAXON 2004, 53, 429–452. [Google Scholar] [CrossRef]
- Albach, D.C.; Meudt, H.M.; Oxelman, B. Piecing Together the “New” Plantaginaceae. Am. J. Bot. 2005, 92, 297–315. [Google Scholar] [CrossRef]
- Bello, M.A.; Chase, M.W.; Olmstead, R.G.; Ronsted, N.; Albach, D. The Paramo Endemic Aragoa Is the Sister Genus of Plantago (Plantaginaceae; Lamiales): Evidence from Plastid RbcL and Nuclear Ribosomal ITS Sequence Data. Kew Bull. 2002, 57, 585. [Google Scholar] [CrossRef]
- Rahmanzadeh, R.; Müller, K.; Fischer, E.; Bartels, D.; Borsch, T. The Linderniaceae and Gratiolaceae Are Further Lineages Distinct from the Scrophulariaceae (Lamiales). Plant Biol. 2005, 7, 67–78. [Google Scholar] [CrossRef]
- Oxelman, B.; Kornhall, P.; Olmstead, R.G.; Bremer, B. Further Disintegration of Scrophulariaceae. TAXON 2005, 54, 411–425. [Google Scholar] [CrossRef]
- Estes, D.; Small, R.L. Phylogenetic Relationships of the Monotypic Genus Amphianthus (Plantaginaceae Tribe Gratioleae) Inferred from Chloroplast DNA Sequences. Syst. Bot. 2008, 33, 176–182. [Google Scholar] [CrossRef]
- Gormley, I.C.; Bedigian, D.; Olmstead, R.G. Phylogeny of Pedaliaceae and Martyniaceae and the Placement of Trapella in Plantaginaceae s. l. Syst. Bot. 2015, 40, 259–268. [Google Scholar] [CrossRef]
- Albach, D.C.; Tsymbalyuk, Z.M.; Mosyakin, S.L. Pollen Morphology of Ellisiophyllum and Sibthorpia (Plantaginaceae, Tribe Sibthorpieae) and Phylogenetics of the Tribe. Plant Syst. Evol. 2021, 307, 66. [Google Scholar] [CrossRef]
- Mower, J.P.; Hanley, L.; Wolff, K.; Pabón-Mora, N.; González, F. Complete Mitogenomes of Two Aragoa Species and Phylogeny of Plantagineae (Plantaginaceae, Lamiales) Using Mitochondrial Genes and the Nuclear Ribosomal RNA Repeat. Plants 2021, 10, 2673. [Google Scholar] [CrossRef]
- Wolfe, A.D.; Datwyler, S.L.; Randle, C.P. A Phylogenetic and Biogeographic Analysis of the Cheloneae (Scrophulariaceae) Based on ITS and MatK Sequence Data. Syst. Bot. 2002, 27, 138–148. [Google Scholar] [CrossRef]
- Tank, D.C.; Beardsley, P.M.; Kelchner, S.A.; Olmstead, R.G. Review of the Systematics of Scrophulariaceae s.l. and Their Current Disposition. Aust. Syst. Bot. 2006, 19, 289–307. [Google Scholar] [CrossRef]
- Mower, J.P.; Guo, W.; Partha, R.; Fan, W.; Levsen, N.; Wolff, K.; Nugent, J.M.; Pabón-Mora, N.; González, F. Plastomes from Tribe Plantagineae (Plantaginaceae) Reveal Infrageneric Structural Synapormorphies and Localized Hypermutation for Plantago and Functional Loss of Ndh Genes from Littorella. Mol. Phylogenet. Evol. 2021, 162, 107217. [Google Scholar] [CrossRef] [PubMed]
- Rokas, A.; Williams, B.L.; King, N.; Carroll, S.B. Genome-Scale Approaches to Resolving Incongruence in Molecular Phylogenies. Nature 2003, 425, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, A.R.; Sarkar, I.N. The Impact of Taxon Sampling on Phylogenetic Inference: A Review of Two Decades of Controversy. Brief. Bioinf. 2012, 13, 122–134. [Google Scholar] [CrossRef]
- Ravi, V.; Khurana, J.P.; Tyagi, A.K.; Khurana, P. An Update on Chloroplast Genomes. Plant Syst. Evol. 2008, 271, 101–122. [Google Scholar] [CrossRef]
- Jansen, R.K.; Ruhlman, T.A. Plastid Genomes of Seed Plants. In Genomics of Chloroplasts and Mitochondria; Bock, R., Knoop, V., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 35, pp. 103–126. [Google Scholar]
- Jung, J.; Kim, C.; Kim, J.H. Insights into Phylogenetic Relationships and Genome Evolution of Subfamily Commelinoideae (Commelinaceae Mirb.) Inferred from Complete Chloroplast Genomes. BMC Genomics 2021, 22, 231. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, C.; Landis, J.B.; Deng, M.; Chen, J. Plastome Phylogenomics of Cephalotaxus (Cephalotaxaceae) and Allied Genera. Ann. Bot. 2021, 127, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Chen, Y.P.; Salmaki, Y.; Drew, B.T.; Wilson, T.C.; Scheen, A.-C.; Celep, F.; Bräuchler, C.; Bendiksby, M.; Wang, Q.; et al. An Updated Tribal Classification of Lamiaceae Based on Plastome Phylogenomics. BMC Biol. 2021, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Mower, J.P.; Vickrey, T.L. Structural Diversity Among Plastid Genomes of Land Plants. Adv. Bot. Res. 2018, 85, 263–292. [Google Scholar] [CrossRef]
- Lyko, P.; Wicke, S. Genomic Reconfiguration in Parasitic Plants Involves Considerable Gene Losses alongside Global Genome Size Inflation and Gene Births. Plant Physiol. 2021, 186, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Rabiee, M.; Sayyari, E.; Mirarab, S. ASTRAL-III: Polynomial Time Species Tree Reconstruction from Partially Resolved Gene Trees. BMC Bioinf. 2018, 19, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Moore, M.J.; Brown, J.W.; Yang, Y. Analysis of Phylogenomic Datasets Reveals Conflict, Concordance, and Gene Duplications with Examples from Animals and Plants. BMC Evol. Biol. 2015, 15, 150. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Jin, J.J.; Yu, W.B.; Yang, J.B.; Song, Y.; dePamphilis, C.W.; Yi, T.S.; Li, D.Z. GetOrganelle: A Fast and Versatile Toolkit for Accurate de Novo Assembly of Organelle Genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Wick, R.R.; Schultz, M.B.; Zobel, J.; Holt, K.E. Bandage: Interactive Visualization of de Novo Genome Assemblies. Bioinformatics 2015, 31, 3350–3352. [Google Scholar] [CrossRef]
- Qu, X.J.; Moore, M.J.; Li, D.Z.; Yi, T.S. PGA: A Software Package for Rapid, Accurate, and Flexible Batch Annotation of Plastomes. Plant Methods 2019, 15, 50. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences. Methods Mol. Biol. 2019, 1962, 1–14. [Google Scholar] [CrossRef]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) Version 1.3.1: Expanded Toolkit for the Graphical Visualization of Organellar Genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. IRscope: An Online Program to Visualize the Junction Sites of Chloroplast Genomes. Bioinformatics 2018, 34, 3030–3031. [Google Scholar] [CrossRef]
- Darling, A.C.E.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple Alignment of Conserved Genomic Sequence with Rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef]
- Walker, J.F.; Walker-Hale, N.; Vargas, O.M.; Larson, D.A.; Stull, G.W. Characterizing Gene Tree Conflict in Plastome-Inferred Phylogenies. PeerJ 2019, 7, e7747. [Google Scholar] [CrossRef]
- Gonçalves, D.J.P.; Simpson, B.B.; Ortiz, E.M.; Shimizu, G.H.; Jansen, R.K. Incongruence between Gene Trees and Species Trees and Phylogenetic Signal Variation in Plastid Genes. Mol. Phylogenet. Evol. 2019, 138, 219–232. [Google Scholar] [CrossRef]
- Mirarab, S.; Reaz, R.; Bayzid, M.S.; Zimmermann, T.; Swenson, M.S.; Warnow, T. ASTRAL: Genome-Scale Coalescent-Based Species Tree Estimation. Bioinformatics 2014, 30, i541–i548. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An Integrated and Scalable Desktop Platform for Streamlined Molecular Sequence Data Management and Evolutionary Phylogenetics Studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A Tool for Phylogenetic Analysis and Post-analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Zhang, C.; Sayyari, E.; Mirarab, S. ASTRAL-III: Increased Scalability and Impacts of Contracting Low Support Branches. In Comparative Genomics; Meidanis, J., Nakhleh, L., Eds.; Springer: Cham, Switzerland, 2017; Volume 10562, pp. 53–75. [Google Scholar] [CrossRef]
- Silva, S.R.; Diaz, Y.C.A.; Penha, H.A.; Pinheiro, D.G.; Fernandes, C.C.; Miranda, V.F.O.; Michael, T.P.; Varani, A.M. The Chloroplast Genome of Utricularia Reniformis Sheds Light on the Evolution of the Ndh Gene Complex of Terrestrial Carnivorous Plants from the Lentibulariaceae Family. PLoS ONE 2016, 11, e0165176. [Google Scholar] [CrossRef]
- Martínez-Alberola, F.; del Campo, E.M.; Lázaro-Gimeno, D.; Mezquita-Claramonte, S.; Molins, A.; Mateu-Andrés, I.; Pedrola-Monfort, J.; Casano, L.M.; Barreno, E. Balanced Gene Losses, Duplications and Intensive Rearrangements Led to an Unusual Regularly Sized Genome in Arbutus Unedo Chloroplasts. PLoS ONE 2013, 8, e79685. [Google Scholar] [CrossRef]
- Millen, R.S.; Olmstead, R.G.; Adams, K.L.; Palmer, J.D.; Lao, N.T.; Heggie, L.; Kavanagh, T.A.; Hibberd, J.M.; Gray, J.C.; Morden, C.W.; et al. Many parallel losses of infA from chloroplast DNA during angiosperm evolution with multiple independent transfers to the nucleus. Plant Cell 2001, 13, 645–658. [Google Scholar] [CrossRef]
- Braukmann, T.; Kuzmina, M.; Stefanović, S. Plastid genome evolution across the genus Cuscuta (Convolvulaceae): Two clades within subgenus Grammica exhibit extensive gene loss. J. Exp. Bot. 2013, 64, 977–989. [Google Scholar] [CrossRef]
- Albalat, R.; Cañestro, C. Evolution by Gene Loss. Nat. Rev. Genet. 2016, 17, 379–391. [Google Scholar] [CrossRef]
- Jiao, Y.; Feng, G.; Huang, L.; Nie, G.; Li, Z.; Peng, Y.; Li, D.; Xiong, Y.; Hu, Z.; Zhang, X. Complete Chloroplast Genomes of 14 Subspecies of D. Glomerata: Phylogenetic and Comparative Genomic Analyses. Genes 2022, 13, 1621. [Google Scholar] [CrossRef]
- Liu, C.K.; Lei, J.Q.; Jiang, Q.P.; Zhou, S.-D.; He, X.J. The Complete Plastomes of Seven Peucedanum Plants: Comparative and Phylogenetic Analyses for the Peucedanum Genus. BMC Plant Biol. 2022, 22, 101. [Google Scholar] [CrossRef]
- Landau, A.; Diaz Paleo, A.; Civitillo, R.; Jaureguialzo, M.; Prina, A.R. Two InfA Gene Mutations Independently Originated from a Mutator Genotype in Barley. J. Hered. 2007, 98, 272–276. [Google Scholar] [CrossRef]
- Jeffroy, O.; Brinkmann, H.; Delsuc, F.; Philippe, H. Phylogenomics: The Beginning of Incongruence? Trends Genet. 2006, 22, 225–231. [Google Scholar] [CrossRef]
- Philippe, H.; Brinkmann, H.; Lavrov, D.V.; Littlewood, D.T.J.; Manuel, M.; Wörheide, G.; Baurain, D. Resolving Difficult Phylogenetic Questions: Why More Sequences Are Not Enough. PLoS Biol. 2011, 9, e1000602. [Google Scholar] [CrossRef]
- Richards, E.J.; Brown, J.M.; Barley, A.J.; Chong, R.A.; Thomson, R.C. Variation Across Mitochondrial Gene Trees Provides Evidence for Systematic Error: How Much Gene Tree Variation Is Biological? Syst. Biol. 2018, 67, 847–860. [Google Scholar] [CrossRef]
- Erixon, P.; Oxelman, B. Whole-Gene Positive Selection, Elevated Synonymous Substitution Rates, Duplication, and Indel Evolution of the Chloroplast ClpP1 Gene. PLoS ONE 2008, 3, e1386. [Google Scholar] [CrossRef]
- Bouillé, M.; Senneville, S.; Bousquet, J. Discordant MtDNA and CpDNA Phylogenies Indicate Geographic Speciation and Reticulation as Driving Factors for the Diversification of the Genus Picea. Tree Genet. Genomes 2011, 7, 469–484. [Google Scholar] [CrossRef]
- D’Alelio, D.; Ruggiero, M.V. Interspecific Plastidial Recombination in the Diatom Genus Pseudo-Nitzschia. J. Phycol. 2015, 51, 1024–1028. [Google Scholar] [CrossRef]
- Walker, J.F.; Brown, J.W.; Smith, S.A. Analyzing Contentious Relationships and Outlier Genes in Phylogenomics. Syst. Biol. 2018, 67, 916–924. [Google Scholar] [CrossRef]
- Sancho, R.; Cantalapiedra, C.P.; López-Alvarez, D.; Gordon, S.P.; Vogel, J.P.; Catalán, P.; Contreras-Moreira, B. Comparative Plastome Genomics and Phylogenomics of Brachypodium: Flowering Time Signatures, Introgression and Recombination in Recently Diverged Ecotypes. New Phytol. 2018, 218, 1631–1644. [Google Scholar] [CrossRef] [PubMed]
- Weber, A. Pair-Flowered Cymes in the Lamiales: Structure, Distribution and Origin. Ann. Bot. 2013, 112, 1577–1595. [Google Scholar] [CrossRef]
- Wolfe, A.D.; Randle, C.P.; Datwyler, S.L.; Morawetz, J.J.; Arguedas, N.; Diaz, J. Phylogeny, Taxonomic Affinities, and Biogeography of Penstemon (Plantaginaceae) Based on ITS and CpDNA Sequence Data. Am. J. Bot. 2006, 93, 1699–1713. [Google Scholar] [CrossRef]
- Fischer, E. Scrophulariaceae. In Flowering Plants· Dicotyledons; Kadereit, J.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 7, pp. 333–432. [Google Scholar]
- Tsymbalyuk, Z.M. Comparative palynomorphological investigation of the representatives of the tribe Antirrhineae Dumort. (Veronicaceae Durande). Mod. Phytomorphol. 2013, 3, 189–194. [Google Scholar] [CrossRef]
- Tsymbalyuk, Z.M. Palynomorphological Peculiarities of Representatives of the Order Lamiales s. l.: Phylogenetic Significance and Main Trends of Evolution. Presented at. Ph.D. Thesis, M.G. Kholodny Institute of Botany NAS of Ukraine, Kyiv, Ukraine, 2016. [Google Scholar] [CrossRef]
- Tsymbalyuk, Z.M.; Mosyakin, S.L. Atlas of Pollen Grains of Representatives of Plantaginaceae and Scrophulariaceae; M.G. Kholodny Institute of Botany, National Academy of Science of Ukraine: Kyiv, Ukraine, 2013; ISBN 978-966-02-7066-4. [Google Scholar] [CrossRef]
- Tsymbalyuk, Z.M.; Mosyakin, S.L. Evolutionary-palynomorphologycal analysis of some tribes of the family Plantaginaceae. Ukr. Bot. J. 2014, 71, 442–448. [Google Scholar] [CrossRef]
- Taskova, R.M.; Gotfredsen, C.H.; Jensen, S.R. Chemotaxonomy of Veroniceae and Its Allies in the Plantaginaceae. Phytochemistry 2006, 67, 286–301. [Google Scholar] [CrossRef]
- Olmstead, R.G.; Pamphilis, C.W.; Wolfe, A.D.; Young, N.D.; Elisons, W.J.; Reeves, P.A. Disintegration of the Scrophulariaceae. Am. J. Bot. 2001, 88, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.A.; Rudall, P.J.; González, F.; Fernández-Alonso, J.L. Floral Morphology and Development in Aragoa (Plantaginaceae) and Related Members of the Order Lamiales. Int. J. Plant Sci. 2004, 165, 723–738. [Google Scholar] [CrossRef]
- Schäferhoff, B.; Fleischmann, A.; Fischer, E.; Albach, D.C.; Borsch, T.; Heubl, G.; Müller, K.F. Towards Resolving Lamiales Relationships: Insights from Rapidly Evolving Chloroplast Sequences. BMC Evol. Biol. 2010, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Saunders, E.R. A study of Veronica from the viewpoint of certain floral characters. Bot. J. Linn. Soc. 1934, 49, 453–493. [Google Scholar] [CrossRef]
- Taskova, R.M.; Gotfredsen, C.H.; Jensen, S.R. Chemotaxonomic Markers in Digitalideae (Plantaginaceae). Phytochemistry 2005, 66, 1440–1447. [Google Scholar] [CrossRef]
- Rønsted, N.; Franzyk, H.; Mølgaard, P.; Jaroszewski, J.W.; Jensen, S.R. Chemotaxonomy and Evolution of Plantago L. Plant Syst. Evol. 2003, 242, 63–82. [Google Scholar] [CrossRef]
- Rønsted, N.; Bello, M.A.; Jensen, S.R. Aragoside and iridoid glucosides from Aragoa cundinamarcensis. Phytochemistry 2003, 64, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.Y. Taxonomy and evolution of the Veroniceae (Scrophulariaceae) with special reference to palynology. Opera Bot. 1984, 75, 5–60. [Google Scholar]
- Kampny, C.M.; Dengler, N.G. Evolution of flower shape in Veroniceae (Scrophulariaceae). Pl. Syst. Evol. 1997, 205, 1–25. [Google Scholar] [CrossRef]
- Li, G.D.; Kim, C.; Zha, H.G.; Zhou, Z.; Nie, Z.L.; Sun, H. Molecular Phylogeny and Biogeography of the Arctic-alpine Genus Lagotis (Plantaginaceae). TAXON 2014, 63, 103–115. [Google Scholar] [CrossRef]
- Surina, B.; Pfanzelt, S.; Einzmann, H.J.R.; Albach, D.C. Bridging the Alps and the Middle East: Evolution, Phylogeny and Systematics of the Genus Wulfenia (Plantaginaceae). TAXON 2014, 63, 843–858. [Google Scholar] [CrossRef]





| Tribes | Species | GC Content (%) | Size (bp) | Gene Number (Unique) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genome | LSC | IR | SSC | Total | PCGs | rRNA | tRNA | |||
| Angelonieae | Angelonia angustifolia | 37.7 | 154,316 | 84,110 | 27,128 | 15,950 | 112 | 78 | 4 | 30 |
| Antirrhineae | Antirrhinum majus | 37.9 | 152,606 | 83,205 | 25,734 | 17,933 | 114 | 80 | 4 | 30 |
| Linaria buriatica | 37.8 | 150,665 | 81,766 | 25,648 | 17,603 | 114 | 80 | 4 | 30 | |
| Callitricheae | Callitriche palustris | 37.8 | 150,138 | 81,432 | 25,667 | 17,372 | 114 | 80 | 4 | 30 |
| Hippuris vulgaris | 37.6 | 152,763 | 82,983 | 25,743 | 18,294 | 114 | 80 | 4 | 30 | |
| Cheloneae | Penstemon cyaneus | 37.9 | 152,604 | 83,724 | 25,534 | 17,812 | 114 | 80 | 4 | 30 |
| Penstemon fruticosus | 37.9 | 152,704 | 83,684 | 25,599 | 17,822 | 114 | 80 | 4 | 30 | |
| Penstemon personatus | 37.9 | 152,602 | 83,728 | 25,528 | 17,818 | 114 | 80 | 4 | 30 | |
| Penstemon rostriflorus | 37.9 | 152,598 | 83,651 | 25,564 | 17,819 | 114 | 80 | 4 | 30 | |
| Digitalideae | Digitalis lanata | 38.6 | 153,108 | 83,936 | 25,743 | 17,686 | 114 | 80 | 4 | 30 |
| Gratioleae | Deinostema violacea | 37.5 | 154,280 | 85,713 | 25,179 | 18,209 | 114 | 80 | 4 | 30 |
| Adenosma glutinosum | 37.5 | 154,220 | 84,820 | 25,636 | 18,128 | 114 | 80 | 4 | 30 | |
| Bacopa monnieri | 37.6 | 152,495 | 83,765 | 25,668 | 17,394 | 114 | 80 | 4 | 30 | |
| Limnophila sessiliflora | 37.4 | 152,395 | 83,163 | 25,545 | 18,142 | 114 | 80 | 4 | 30 | |
| Scoparia dulcis | 37.5 | 153,701 | 85,029 | 25,273 | 18,126 | 113 | 79 | 4 | 30 | |
| Trapella sinensis | 37.4 | 152,297 | 83,830 | 25,010 | 18,447 | 114 | 80 | 4 | 30 | |
| Hemiphragmeae | Hemiphragma heterophyllum | 38.1 | 152,707 | 83,268 | 25,808 | 17,823 | 114 | 80 | 4 | 30 |
| Plantagineae | Littorella uniflora | 39.0 | 130,833 | 77,490 | 21,404 | 10,535 | 103 | 69 | 4 | 30 |
| Aragoa cleefii | 38.3 | 150,285 | 81,865 | 25,347 | 17,726 | 113 | 79 | 4 | 30 | |
| Aragoa abietina | 38.2 | 150,320 | 81,899 | 25,347 | 17,727 | 113 | 79 | 4 | 30 | |
| Plantago lagopus | 38.3 | 150,088 | 82,574 | 24,542 | 18,430 | 113 | 79 | 4 | 30 | |
| Plantago nubicola | 38.2 | 151,611 | 83,657 | 24,955 | 18,044 | 113 | 79 | 4 | 30 | |
| Plantago maritima | 38.6 | 158,358 | 82,223 | 33,735 | 8665 | 114 | 79 | 4 | 31 | |
| Plantago aristata | 38.4 | 149,910 | 82450 | 24,582 | 18,296 | 113 | 79 | 4 | 30 | |
| Plantago media | 38.0 | 164,130 | 82,757 | 38,398 | 4577 | 113 | 79 | 4 | 30 | |
| Plantago depressa | 38.0 | 164,617 | 82,933 | 38,388 | 4908 | 113 | 79 | 4 | 30 | |
| Plantago fengdouensis | 38.0 | 164,976 | 82,972 | 38,644 | 4716 | 113 | 79 | 4 | 30 | |
| Plantago asiatica | 38.1 | 165,045 | 82,964 | 38,724 | 4633 | 113 | 79 | 4 | 30 | |
| Russelieae | Russelia equisetiformis | 38.2 | 153,337 | 83,844 | 25,738 | 18,017 | 114 | 80 | 4 | 30 |
| Sibthorpieae | Ellisiophyllum pinnatum | 38.2 | 152,424 | 83,232 | 25,787 | 17,618 | 114 | 80 | 4 | 30 |
| Veroniceae | Veronica polita | 37.9 | 150,191 | 81,847 | 25,465 | 17,414 | 113 | 79 | 4 | 30 |
| Veronica persica | 37.9 | 150,198 | 81,850 | 25,465 | 17,418 | 113 | 79 | 4 | 30 | |
| Veronica eriogyne | 38.0 | 151,083 | 82,302 | 25,666 | 17,449 | 113 | 79 | 4 | 30 | |
| Veronica undulata | 38.1 | 151,178 | 82,644 | 25,566 | 17,402 | 114 | 80 | 4 | 30 | |
| Veronica ovata | 38.0 | 152,249 | 83,187 | 25,679 | 17,704 | 114 | 80 | 4 | 30 | |
| Veronica nakaiana | 37.9 | 152,319 | 83,195 | 25,711 | 17,702 | 114 | 80 | 4 | 30 | |
| Picrorhiza scrophulariiflora | 38.1 | 152,643 | 83,191 | 25,829 | 17,794 | 114 | 80 | 4 | 30 | |
| Veronicastrum axillare | 38.3 | 152,691 | 83,559 | 25,765 | 17,602 | 114 | 80 | 4 | 30 | |
| Veronicastrum sibiricum | 38.3 | 152,930 | 83,616 | 25,757 | 17,800 | 114 | 80 | 4 | 30 | |
| Lagotis brevituba | 38.3 | 152,967 | 83,740 | 25,691 | 17,845 | 114 | 80 | 4 | 30 | |
| Lagotis yunnanensis | 38.4 | 152,979 | 83,641 | 25,677 | 17,794 | 114 | 80 | 4 | 30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, P.; Tang, L.; Luo, Y.; Liu, C.; Yan, H. Plastid Phylogenomic Insights into the Inter-Tribal Relationships of Plantaginaceae. Biology 2023, 12, 263. https://doi.org/10.3390/biology12020263
Xie P, Tang L, Luo Y, Liu C, Yan H. Plastid Phylogenomic Insights into the Inter-Tribal Relationships of Plantaginaceae. Biology. 2023; 12(2):263. https://doi.org/10.3390/biology12020263
Chicago/Turabian StyleXie, Pingxuan, Lilei Tang, Yanzhen Luo, Changkun Liu, and Hanjing Yan. 2023. "Plastid Phylogenomic Insights into the Inter-Tribal Relationships of Plantaginaceae" Biology 12, no. 2: 263. https://doi.org/10.3390/biology12020263
APA StyleXie, P., Tang, L., Luo, Y., Liu, C., & Yan, H. (2023). Plastid Phylogenomic Insights into the Inter-Tribal Relationships of Plantaginaceae. Biology, 12(2), 263. https://doi.org/10.3390/biology12020263






