Neurobiology of Aggression—Review of Recent Findings and Relationship with Alcohol and Trauma
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Neuroanatomy of Aggression
2.1. Frontal Cortical Structures
2.2. Temporal Cortical Structures
2.3. The Striatum
2.4. The Limbic System
2.5. The Parietal and Occipital Lobe
3. The Neurotransmission and Genetics of Aggression
3.1. Serotonin, Dopamine and Their Degradation
3.2. Glutamate
3.3. γ-Aminobutyric Acid (GABA)
3.4. Inflammatory Markers
3.5. Opioid Receptors
3.6. Orexin
3.7. Oxytocin/Vasopressin
4. Alcohol and Aggression: Insight from Preclinical Studies
4.1. Alcohol and GABA
4.2. Alcohol and Serotonin
4.3. Alcohol, Dopamine, and Behavioral Sensitization
4.4. Alcohol and Glutamate
5. Alcohol and Aggression: Insight from Clinical Studies
5.1. Behavioral Effects and Risk Factors of Alcohol
5.2. Effect of Alcohol on Sensory, Emotional, and Cognitive Processes
5.3. Alcohol, Neurotransmitter Systems, and Brain Regions
6. Role of Trauma in Alcohol Consumption and Aggressive Behavior
6.1. Trauma and Alcohol Consumption
6.2. Trauma and Aggressive Behavior
6.3. Genetic Factors in Trauma, Alcohol Consumption, and Aggression
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Austerman, J. Violence and Aggressive Behavior. Pediatr. Rev. 2017, 38, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Helmy, M.; Zhang, J.; Wang, H. Neurobiology and Neural Circuits of Aggression. Adv. Exp. Med. Biol. 2020, 1284, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Jantzen, D.; Brinker, U.; Orschiedt, J.; Heinemeier, J.; Piek, J.; Hauenstein, K.; Krüger, J.; Lidke, G.; Lübke, H.; Lampe, R.; et al. A Bronze Age battlefield? Weapons and trauma in the Tollense Valley, north-eastern Germany. Antiquity 2011, 85, 417–433. [Google Scholar] [CrossRef]
- Roy, K. Chariot War in the Bronze Age: 3000-800 BCE. In A Global History of Warfare and Technology; Roy, K., Ed.; Springer Nature Singapore: Singapore, 2022; pp. 19–33. ISBN 978-981-19-3477-3. [Google Scholar]
- Kempes, M.; Matthys, W.; de Vries, H.; van Engeland, H. Reactive and proactive aggression in children—A review of theory, findings and the relevance for child and adolescent psychiatry. Eur. Child Adolesc. Psychiatry 2005, 14, 11–19. [Google Scholar] [CrossRef]
- Del Vecchio, T. Instrumental Aggression. In Encyclopedia of Child Behavior and Development; Goldstein, S., Naglieri, J.A., Eds.; Springer: Boston, MA, USA, 2011; pp. 823–824. ISBN 978-0-387-77579-1. [Google Scholar]
- Weierstall, R.; Elbert, T. The Appetitive Aggression Scale-development of an instrument for the assessment of human’s attraction to violence. Eur. J. Psychotraumatol. 2011, 2, 8430. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, N.L.; Nilsson, S.R.O.; Golden, S.A. Rage Against the Machine: Advancing the study of aggression ethology via machine learning. Psychopharmacology 2020, 237, 2569–2588. [Google Scholar] [CrossRef]
- de Almeida, R.M.M.; Cabral, J.C.C.; Narvaes, R. Behavioural, hormonal and neurobiological mechanisms of aggressive behaviour in human and nonhuman primates. Physiol. Behav. 2015, 143, 121–135. [Google Scholar] [CrossRef]
- Been, L.E.; Gibbons, A.B.; Meisel, R.L. Towards a neurobiology of female aggression. Neuropharmacology 2019, 156, 107451. [Google Scholar] [CrossRef]
- Björkqvist, K. Gender differences in aggression. Curr. Opin. Psychol. 2018, 19, 39–42. [Google Scholar] [CrossRef]
- Parrott, D.J.; Eckhardt, C.I. Effects of Alcohol on Human Aggression. Curr. Opin. Psychol. 2018, 19, 1–5. [Google Scholar] [CrossRef]
- Florek, S.; Dębski, P.; Piegza, M.; Gorczyca, P.; Pudlo, R. Relationship between the Severity of Anxiety Symptoms, Aggression and Alcohol Consumption during the COVID-19 Pandemic Period. Medicina 2021, 57, 959. [Google Scholar] [CrossRef] [PubMed]
- Beiderbeck, D.I.; Reber, S.O.; Havasi, A.; Bredewold, R.; Veenema, A.H.; Neumann, I.D. High and abnormal forms of aggression in rats with extremes in trait anxiety—Involvement of the dopamine system in the nucleus accumbens. Psychoneuroendocrinology 2012, 37, 1969–1980. [Google Scholar] [CrossRef] [PubMed]
- Augsburger, M.; Dohrmann, K.; Schauer, M.; Elbert, T. Relations between traumatic stress, dimensions of impulsivity, and reactive and appetitive aggression in individuals with refugee status. Psychol. Trauma 2017, 9, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Augsburger, M.; Maercker, A. Associations between trauma exposure, posttraumatic stress disorder, and aggression perpetrated by women. A meta-analysis. Clin. Psychol. Sci. Pract. 2020, 27, e12322. [Google Scholar] [CrossRef] [Green Version]
- Caspi, A.; McClay, J.; Moffitt, T.E.; Mill, J.; Martin, J.; Craig, I.W.; Taylor, A.; Poulton, R. Role of genotype in the cycle of violence in maltreated children. Science 2002, 297, 851–854. [Google Scholar] [CrossRef]
- Nordman, J.; Li, Z. The Dorsal Raphe Regulates the Duration of Attack through the Medial Orbitofrontal Cortex and Medial Amygdala. eNeuro 2020, 7, 1–15. [Google Scholar] [CrossRef]
- Yang, Y.; Raine, A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: A meta-analysis. Psychiatry Res. 2009, 174, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Bertsch, K.; Florange, J.; Herpertz, S.C. Understanding Brain Mechanisms of Reactive Aggression. Curr. Psychiatry Rep. 2020, 22, 81. [Google Scholar] [CrossRef]
- Alegria, A.A.; Radua, J.; Rubia, K. Meta-Analysis of fMRI Studies of Disruptive Behavior Disorders. Am. J. Psychiatry 2016, 173, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- de Brito, S.A.; Mechelli, A.; Wilke, M.; Laurens, K.R.; Jones, A.P.; Barker, G.J.; Hodgins, S.; Viding, E. Size matters: Increased grey matter in boys with conduct problems and callous-unemotional traits. Brain 2009, 132, 843–852. [Google Scholar] [CrossRef] [Green Version]
- van Heukelum, S.; Tulva, K.; Geers, F.E.; van Dulm, S.; Ruisch, I.H.; Mill, J.; Viana, J.F.; Beckmann, C.F.; Buitelaar, J.K.; Poelmans, G.; et al. A central role for anterior cingulate cortex in the control of pathological aggression. Curr. Biol. 2021, 31, 2321–2333.e5. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Raine, A.; Chou, K.-H.; Chen, I.-Y.; Hung, D.; Lin, C.-P. Abnormal white matter integrity in rapists as indicated by diffusion tensor imaging. BMC Neurosci. 2016, 17, 45. [Google Scholar] [CrossRef] [Green Version]
- Dambacher, F.; Schuhmann, T.; Lobbestael, J.; Arntz, A.; Brugman, S.; Sack, A.T. Reducing proactive aggression through non-invasive brain stimulation. Soc. Cogn. Affect. Neurosci. 2015, 10, 1303–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hortensius, R.; Schutter, D.J.L.G.; Harmon-Jones, E. When anger leads to aggression: Induction of relative left frontal cortical activity with transcranial direct current stimulation increases the anger-aggression relationship. Soc. Cogn. Affect. Neurosci. 2012, 7, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Dambacher, F.; Schuhmann, T.; Lobbestael, J.; Arntz, A.; Brugman, S.; Sack, A.T. No Effects of Bilateral tDCS over Inferior Frontal Gyrus on Response Inhibition and Aggression. PLoS ONE 2015, 10, e0132170. [Google Scholar] [CrossRef] [Green Version]
- Choy, O.; Raine, A.; Hamilton, R.H. Stimulation of the Prefrontal Cortex Reduces Intentions to Commit Aggression: A Randomized, Double-Blind, Placebo-Controlled, Stratified, Parallel-Group Trial. J. Neurosci. 2018, 38, 6505–6512. [Google Scholar] [CrossRef]
- Cristofori, I.; Zhong, W.; Mandoske, V.; Chau, A.; Krueger, F.; Strenziok, M.; Grafman, J. Brain Regions Influencing Implicit Violent Attitudes: A Lesion-Mapping Study. J. Neurosci. 2016, 36, 2757–2768. [Google Scholar] [CrossRef] [Green Version]
- Perach-Barzilay, N.; Tauber, A.; Klein, E.; Chistyakov, A.; Ne’eman, R.; Shamay-Tsoory, S.G. Asymmetry in the dorsolateral prefrontal cortex and aggressive behavior: A continuous theta-burst magnetic stimulation study. Soc. Neurosci. 2013, 8, 178–188. [Google Scholar] [CrossRef]
- Konikkou, K.; Kostantinou, N.; Fanti, K.A. Transcranial magnetic stimulation over the dorsolateral prefrontal cortex affects emotional processing: Accounting for individual differences in antisocial behavior. J. Exp. Criminol. 2020, 16, 349–366. [Google Scholar] [CrossRef]
- Chester, D.S.; Lynam, D.R.; Milich, R.; DeWall, C.N. Physical aggressiveness and gray matter deficits in ventromedial prefrontal cortex. Cortex 2017, 97, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Chester, D.S.; Lynam, D.R.; Milich, R.; DeWall, C.N. Neural mechanisms of the rejection-aggression link. Soc. Cogn. Affect. Neurosci. 2018, 13, 501–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chester, D.S.; Bell, S.B.; DeWall, C.N.; West, S.J.; Romero-Lopez, M.; Craig, A.W. Neural correlates of intertemporal choice in aggressive behavior. Aggress. Behav. 2019, 45, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Thijssen, S.; Ringoot, A.P.; Wildeboer, A.; Bakermans-Kranenburg, M.J.; El Marroun, H.; Hofman, A.; Jaddoe, V.W.V.; Verhulst, F.C.; Tiemeier, H.; van IJzendoorn, M.H.; et al. Brain morphology of childhood aggressive behavior: A multi-informant study in school-age children. Cogn. Affect. Behav. Neurosci. 2015, 15, 564–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miczek, K.A.; Takahashi, A.; Gobrogge, K.L.; Hwa, L.S.; de Almeida, R.M.M. Escalated Aggression in Animal Models: Shedding New Light on Mesocorticolimbic Circuits. Curr. Opin. Behav. Sci. 2015, 3, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, A.; Nagayasu, K.; Nishitani, N.; Kaneko, S.; Koide, T. Control of intermale aggression by medial prefrontal cortex activation in the mouse. PLoS ONE 2014, 9, e94657. [Google Scholar] [CrossRef] [Green Version]
- Thiebaut de Schotten, M.; Dell’Acqua, F.; Ratiu, P.; Leslie, A.; Howells, H.; Cabanis, E.; Iba-Zizen, M.T.; Plaisant, O.; Simmons, A.; Dronkers, N.F.; et al. From Phineas Gage and Monsieur Leborgne to H.M.: Revisiting Disconnection Syndromes. Cereb. Cortex 2015, 25, 4812–4827. [Google Scholar] [CrossRef] [Green Version]
- Bannon, S.M.; Salis, K.L.; Daniel O’Leary, K. Structural brain abnormalities in aggression and violent behavior. Aggress. Violent Behav. 2015, 25, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Beyer, F.; Münte, T.F.; Göttlich, M.; Krämer, U.M. Orbitofrontal Cortex Reactivity to Angry Facial Expression in a Social Interaction Correlates with Aggressive Behavior. Cereb. Cortex 2015, 25, 3057–3063. [Google Scholar] [CrossRef]
- Kuniishi, H.; Ichisaka, S.; Matsuda, S.; Futora, E.; Harada, R.; Hata, Y. Chronic Inactivation of the Orbitofrontal Cortex Increases Anxiety-Like Behavior and Impulsive Aggression, but Decreases Depression-Like Behavior in Rats. Front. Behav. Neurosci. 2016, 10, 250. [Google Scholar] [CrossRef] [Green Version]
- Seidenwurm, D.; Pounds, T.R.; Globus, A.; Valk, P.E. Abnormal temporal lobe metabolism in violent subjects: Correlation of imaging and neuropsychiatric findings. AJNR Am. J. Neuroradiol. 1997, 18, 625–631. [Google Scholar]
- Kruesi, M.J.P.; Casanova, M.F.; Mannheim, G.; Johnson-Bilder, A. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Res. 2004, 132, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bufkin, J.L.; Luttrell, V.R. Neuroimaging studies of aggressive and violent behavior: Current findings and implications for criminology and criminal justice. Trauma Violence Abus. 2005, 6, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Buades-Rotger, M.; Engelke, C.; Beyer, F.; Keevil, B.G.; Brabant, G.; Krämer, U.M. Endogenous testosterone is associated with lower amygdala reactivity to angry faces and reduced aggressive behavior in healthy young women. Sci. Rep. 2016, 6, 38538. [Google Scholar] [CrossRef] [Green Version]
- Raine, A. The neuromoral theory of antisocial, violent, and psychopathic behavior. Psychiatry Res. 2019, 277, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; Pezzoli, P.; Jaworska, N.; Seto, M.C. Brain responses in aggression-prone individuals: A systematic review and meta-analysis of functional magnetic resonance imaging (fMRI) studies of anger- and aggression-eliciting tasks. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 119, 110596. [Google Scholar] [CrossRef]
- Robbins, T.W.; Everitt, B.J. Functions of dopamine in the dorsal and ventral striatum. Semin. Neurosci. 1992, 4, 119–127. [Google Scholar] [CrossRef]
- Da Cunha-Bang, S.; Fisher, P.M.; Hjordt, L.V.; Perfalk, E.; Persson Skibsted, A.; Bock, C.; Ohlhues Baandrup, A.; Deen, M.; Thomsen, C.; Sestoft, D.M.; et al. Violent offenders respond to provocations with high amygdala and striatal reactivity. Soc. Cogn. Affect. Neurosci. 2017, 12, 802–810. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Joshi, S.H.; Jahanshad, N.; Thompson, P.M.; Baker, L.A. Neural correlates of proactive and reactive aggression in adolescent twins. Aggress. Behav. 2017, 43, 230–240. [Google Scholar] [CrossRef]
- White, S.F.; Brislin, S.J.; Sinclair, S.; Blair, J.R. Punishing unfairness: Rewarding or the organization of a reactively aggressive response? Hum. Brain Mapp. 2014, 35, 2137–2147. [Google Scholar] [CrossRef] [Green Version]
- Golden, S.A.; Jin, M.; Shaham, Y. Animal Models of (or for) Aggression Reward, Addiction, and Relapse: Behavior and Circuits. J. Neurosci. 2019, 39, 3996–4008. [Google Scholar] [CrossRef] [Green Version]
- Golden, S.A.; Jin, M.; Heins, C.; Venniro, M.; Michaelides, M.; Shaham, Y. Nucleus Accumbens Drd1-Expressing Neurons Control Aggression Self-Administration and Aggression Seeking in Mice. J. Neurosci. 2019, 39, 2482–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golden, S.A.; Aleyasin, H.; Heins, R.; Flanigan, M.; Heshmati, M.; Takahashi, A.; Russo, S.J.; Shaham, Y. Persistent conditioned place preference to aggression experience in adult male sexually-experienced CD-1 mice. Genes Brain Behav. 2017, 16, 44–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falkner, A.L.; Grosenick, L.; Davidson, T.J.; Deisseroth, K.; Lin, D. Hypothalamic control of male aggression-seeking behavior. Nat. Neurosci. 2016, 19, 596–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krizan, Z.; Johar, O. Narcissistic rage revisited. J. Pers. Soc. Psychol. 2015, 108, 784–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiegel, E.A.; Miller, H.R.; Oppenheimer, M.J. forebrain and rage reactions. J. Neurophysiol. 1940, 3, 538–548. [Google Scholar] [CrossRef]
- Siegel, A.; Victoroff, J. Understanding human aggression: New insights from neuroscience. Int. J. Law Psychiatry 2009, 32, 209–215. [Google Scholar] [CrossRef]
- Wong, L.C.; Wang, L.; D’Amour, J.A.; Yumita, T.; Chen, G.; Yamaguchi, T.; Chang, B.C.; Bernstein, H.; You, X.; Feng, J.E.; et al. Effective Modulation of Male Aggression through Lateral Septum to Medial Hypothalamus Projection. Curr. Biol. 2016, 26, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lischinsky, J.E.; Lin, D. Neural mechanisms of aggression across species. Nat. Neurosci. 2020, 23, 1317–1328. [Google Scholar] [CrossRef]
- Cupaioli, F.A.; Zucca, F.A.; Caporale, C.; Lesch, K.-P.; Passamonti, L.; Zecca, L. The neurobiology of human aggressive behavior: Neuroimaging, genetic, and neurochemical aspects. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110059. [Google Scholar] [CrossRef]
- Leroy, F.; Park, J.; Asok, A.; Brann, D.H.; Meira, T.; Boyle, L.M.; Buss, E.W.; Kandel, E.R.; Siegelbaum, S.A. A circuit from hippocampal CA2 to lateral septum disinhibits social aggression. Nature 2018, 564, 213–218. [Google Scholar] [CrossRef]
- Mahadevia, D.; Saha, R.; Manganaro, A.; Chuhma, N.; Ziolkowski-Blake, A.; Morgan, A.A.; Dumitriu, D.; Rayport, S.; Ansorge, M.S. Dopamine promotes aggression in mice via ventral tegmental area to lateral septum projections. Nat. Commun. 2021, 12, 6796. [Google Scholar] [CrossRef]
- Menon, R.; Süß, T.; Oliveira, V.E.d.M.; Neumann, I.D.; Bludau, A. Neurobiology of the lateral septum: Regulation of social behavior. Trends Neurosci. 2022, 45, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Hashikawa, Y.; Hashikawa, K.; Falkner, A.L.; Lin, D. Ventromedial Hypothalamus and the Generation of Aggression. Front. Syst. Neurosci. 2017, 11, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roeling, T.A.; Veening, J.G.; Kruk, M.R.; Peters, J.P.; Vermelis, M.E.; Nieuwenhuys, R. Efferent connections of the hypothalamic “aggression area” in the rat. Neuroscience 1994, 59, 1001–1024. [Google Scholar] [CrossRef]
- Lin, D.; Boyle, M.P.; Dollar, P.; Lee, H.; Lein, E.S.; Perona, P.; Anderson, D.J. Functional identification of an aggression locus in the mouse hypothalamus. Nature 2011, 470, 221–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biro, L.; Sipos, E.; Bruzsik, B.; Farkas, I.; Zelena, D.; Balazsfi, D.; Toth, M.; Haller, J. Task Division within the Prefrontal Cortex: Distinct Neuron Populations Selectively Control Different Aspects of Aggressive Behavior via the Hypothalamus. J. Neurosci. 2018, 38, 4065–4075. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, F.V.; Hamani, C.; Fonoff, E.T.; Brentani, H.; Alho, E.J.L.; de Morais, R.M.C.B.; de Souza, A.L.; Rigonatti, S.P.; Martinez, R.C.R. Amygdala and Hypothalamus: Historical Overview with Focus on Aggression. Neurosurgery 2019, 85, 11–30. [Google Scholar] [CrossRef] [Green Version]
- Sano, K.; Mayanagi, Y. Posteromedial hypothalamotomy in the treatment of violent, aggressive behaviour. Acta Neurochir. Suppl. 1988, 44, 145–151. [Google Scholar] [CrossRef]
- Maley, J.H.; Alvernia, J.E.; Valle, E.P.; Richardson, D. Deep brain stimulation of the orbitofrontal projections for the treatment of intermittent explosive disorder. Neurosurg. Focus 2010, 29, E11. [Google Scholar] [CrossRef] [Green Version]
- Franzini, A.; Broggi, G.; Cordella, R.; Dones, I.; Messina, G. Deep-brain stimulation for aggressive and disruptive behavior. World Neurosurg. 2013, 80, S29.e11–S29.e14. [Google Scholar] [CrossRef]
- Torres, C.V.; Sola, R.G.; Pastor, J.; Pedrosa, M.; Navas, M.; García-Navarrete, E.; Ezquiaga, E.; García-Camba, E. Long-term results of posteromedial hypothalamic deep brain stimulation for patients with resistant aggressiveness. J. Neurosurg. 2013, 119, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.V.; Blasco, G.; Navas García, M.; Ezquiaga, E.; Pastor, J.; Vega-Zelaya, L.; Pulido Rivas, P.; Pérez Rodrigo, S.; Manzanares, R. Deep brain stimulation for aggressiveness: Long-term follow-up and tractography study of the stimulated brain areas. J. Neurosurg. 2020, 134, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Gean, P.-W. The Ventral Hippocampus Controls Stress-Provoked Impulsive Aggression through the Ventromedial Hypothalamus in Post-Weaning Social Isolation Mice. Cell Rep. 2019, 28, 1195–1205.e3. [Google Scholar] [CrossRef] [Green Version]
- Coccaro, E.F.; Lee, R.; McCloskey, M.; Csernansky, J.G.; Wang, L. Morphometric analysis of amygdla and hippocampus shape in impulsively aggressive and healthy control subjects. J. Psychiatr. Res. 2015, 69, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, M.; Forbes, E.E.; Gianaros, P.J.; Erickson, K.I.; Brennan, L.M.; Shaw, D.S. Maternal depression in childhood and aggression in young adulthood: Evidence for mediation by offspring amygdala-hippocampal volume ratio. J. Child Psychol. Psychiatry 2015, 56, 1083–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dailey, N.S.; Smith, R.; Vanuk, J.R.; Raikes, A.C.; Killgore, W.D.S. Resting-state functional connectivity as a biomarker of aggression in mild traumatic brain injury. Neuroreport 2018, 29, 1413–1417. [Google Scholar] [CrossRef]
- Haller, J. The role of central and medial amygdala in normal and abnormal aggression: A review of classical approaches. Neurosci. Biobehav. Rev. 2018, 85, 34–43. [Google Scholar] [CrossRef]
- Unger, E.K.; Burke, K.J.; Yang, C.F.; Bender, K.J.; Fuller, P.M.; Shah, N.M. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep. 2015, 10, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Wei, D.; Song, S.C.; Lim, B.; Tritsch, N.X.; Lin, D. Posterior amygdala regulates sexual and aggressive behaviors in male mice. Nat. Neurosci. 2020, 23, 1111–1124. [Google Scholar] [CrossRef]
- Abellán-Álvaro, M.; Martínez-García, F.; Lanuza, E.; Agustín-Pavón, C. Inhibition of the medial amygdala disrupts escalated aggression in lactating female mice after repeated exposure to male intruders. Commun. Biol. 2022, 5, 980. [Google Scholar] [CrossRef]
- Da Cunha-Bang, S.; Fisher, P.M.; Hjordt, L.V.; Holst, K.; Knudsen, G.M. Amygdala reactivity to fearful faces correlates positively with impulsive aggression. Soc. Neurosci. 2019, 14, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Pardini, D.A.; Raine, A.; Erickson, K.; Loeber, R. Lower amygdala volume in men is associated with childhood aggression, early psychopathic traits, and future violence. Biol. Psychiatry 2014, 75, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, C.; Tesli, N.; Gurholt, T.P.; Rokicki, J.; Hjell, G.; Fischer-Vieler, T.; Melle, I.; Agartz, I.; Andreassen, O.A.; Rasmussen, K.; et al. Associations between amygdala nuclei volumes, psychosis, psychopathy, and violent offending. Psychiatry Res. Neuroimaging 2022, 319, 111416. [Google Scholar] [CrossRef] [PubMed]
- Saxbe, D.; Lyden, H.; Gimbel, S.I.; Sachs, M.; Del Piero, L.B.; Margolin, G.; Kaplan, J.T. Longitudinal Associations Between Family Aggression, Externalizing Behavior, and the Structure and Function of the Amygdala. J. Res. Adolesc. 2018, 28, 134–149. [Google Scholar] [CrossRef]
- Raine, A.; Meloy, J.R.; Bihrle, S.; Stoddard, J.; Lacasse, L.; Buchsbaum, M.S. Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behav. Sci. Law 1998, 16, 319–332. [Google Scholar] [CrossRef]
- Heesink, L.; Edward Gladwin, T.; Terburg, D.; van Honk, J.; Kleber, R.; Geuze, E. Proximity alert! Distance related cuneus activation in military veterans with anger and aggression problems. Psychiatry Res. Neuroimaging 2017, 266, 114–122. [Google Scholar] [CrossRef]
- Varkevisser, T.; Gladwin, T.E.; Heesink, L.; van Honk, J.; Geuze, E. Resting-state functional connectivity in combat veterans suffering from impulsive aggression. Soc. Cogn. Affect. Neurosci. 2017, 12, 1881–1889. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, K.; Kalvin, C.; Morand-Beaulieu, S.; He, G.; Pelphrey, K.A.; McCarthy, G.; Sukhodolsky, D.G. Amygdala-prefrontal connectivity in children with maladaptive aggression is modulated by social impairment. Cereb. Cortex 2022, 32, 4371–4385. [Google Scholar] [CrossRef]
- Saxbe, D.; Khoddam, H.; Del Piero, L.; Stoycos, S.A.; Gimbel, S.I.; Margolin, G.; Kaplan, J.T. Community violence exposure in early adolescence: Longitudinal associations with hippocampal and amygdala volume and resting state connectivity. Dev. Sci. 2018, 21, e12686. [Google Scholar] [CrossRef]
- Sukhodolsky, D.G.; Ibrahim, K.; Kalvin, C.B.; Jordan, R.P.; Eilbott, J.; Hampson, M. Increased amygdala and decreased frontolimbic r esting- s tate functional connectivity in children with aggressive behavior. Soc. Cogn. Affect. Neurosci. 2022, 17, 634–644. [Google Scholar] [CrossRef]
- Beyer, F.; Münte, T.F.; Wiechert, J.; Heldmann, M.; Krämer, U.M. Trait aggressiveness is not related to structural connectivity between orbitofrontal cortex and amygdala. PLoS ONE 2014, 9, e101105. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Teixeira, C.M.; Mahadevia, D.; Huang, Y.-Y.; Balsam, D.; Mann, J.J.; Gingrich, J.A.; Ansorge, M.S. Optogenetic stimulation of DAergic VTA neurons increases aggression. Mol. Psychiatry 2014, 19, 635. [Google Scholar] [CrossRef]
- Fuxjager, M.J.; Forbes-Lorman, R.M.; Coss, D.J.; Auger, C.J.; Auger, A.P.; Marler, C.A. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc. Natl. Acad. Sci. USA 2010, 107, 12393–12398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckholtz, J.W.; Treadway, M.T.; Cowan, R.L.; Woodward, N.D.; Benning, S.D.; Li, R.; Ansari, M.S.; Baldwin, R.M.; Schwartzman, A.N.; Shelby, E.S.; et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat. Neurosci. 2010, 13, 419–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Zhang, Y.; Zhou, J.; Wang, X. Altered Resting-State Functional Connectivity in the Default Mode Network in Male Juvenile Violent Offenders. Brain Imaging Behav. 2022, 16, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Werhahn, J.E.; Mohl, S.; Willinger, D.; Smigielski, L.; Roth, A.; Hofstetter, C.; Stämpfli, P.; Naaijen, J.; Mulder, L.M.; Glennon, J.C.; et al. Aggression subtypes relate to distinct resting state functional connectivity in children and adolescents with disruptive behavior. Eur. Child Adolesc. Psychiatry 2021, 30, 1237–1249. [Google Scholar] [CrossRef]
- Coccaro, E.F.; Keedy, S.; Malina, M.; Lee, R.; Phan, K.L. Neuronal responses in social-emotional information processing in impulsive aggressive individuals. Neuropsychopharmacology 2022, 47, 1249–1255. [Google Scholar] [CrossRef]
- Beyer, F.; Münte, T.F.; Krämer, U.M. Increased neural reactivity to socio-emotional stimuli links social exclusion and aggression. Biol. Psychol. 2014, 96, 102–110. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, X.; Xia, L.-X. Brain structures and functional connectivity associated with individual differences in trait proactive aggression. Sci. Rep. 2019, 9, 7731. [Google Scholar] [CrossRef] [Green Version]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Hoyer, D.; Clarke, D.E.; Fozard, J.R.; Hartig, P.R.; Martin, G.R.; Mylecharane, E.J.; Saxena, P.R.; Humphrey, P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994, 46, 157–203. [Google Scholar]
- Linnoila, M.; Virkkunen, M.; Scheinin, M.; Nuutila, A.; Rimon, R.; Goodwin, F.K. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983, 33, 2609–2614. [Google Scholar] [CrossRef]
- Duke, A.A.; Bègue, L.; Bell, R.; Eisenlohr-Moul, T. Revisiting the serotonin-aggression relation in humans: A meta-analysis. Psychol. Bull. 2013, 139, 1148–1172. [Google Scholar] [CrossRef] [Green Version]
- Walther, D.J.; Peter, J.-U.; Bashammakh, S.; Hörtnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef]
- Kästner, N.; Richter, S.H.; Urbanik, S.; Kunert, J.; Waider, J.; Lesch, K.-P.; Kaiser, S.; Sachser, N. Brain serotonin deficiency affects female aggression. Sci. Rep. 2019, 9, 1366. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Grandjean, J.; Sbrini, G.; Schipper, P.; Hofwijks, N.; Stoop, J.; Calabrese, F.; Homberg, J. Tryptophan Hydroxylase 2 Knockout Male Rats Exhibit a Strengthened Oxytocin System, Are Aggressive, and Are Less Anxious. ACS Chem. Neurosci. 2022, 13, 2974–2981. [Google Scholar] [CrossRef]
- Svirin, E.; Veniaminova, E.; Costa-Nunes, J.P.; Gorlova, A.; Umriukhin, A.; Kalueff, A.V.; Proshin, A.; Anthony, D.C.; Nedorubov, A.; Tse, A.C.K.; et al. Predation Stress Causes Excessive Aggression in Female Mice with Partial Genetic Inactivation of Tryptophan Hydroxylase-2: Evidence for Altered Myelination-Related Processes. Cells 2022, 11, 1036. [Google Scholar] [CrossRef]
- Gorlova, A.; Ortega, G.; Waider, J.; Bazhenova, N.; Veniaminova, E.; Proshin, A.; Kalueff, A.V.; Anthony, D.C.; Lesch, K.-P.; Strekalova, T. Stress-induced aggression in heterozygous TPH2 mutant mice is associated with alterations in serotonin turnover and expression of 5-HT6 and AMPA subunit 2A receptors. J. Affect. Disord. 2020, 272, 440–451. [Google Scholar] [CrossRef]
- Peeters, D.G.A.; de Boer, S.F.; Terneusen, A.; Newman-Tancredi, A.; Varney, M.A.; Verkes, R.-J.; Homberg, J.R. Enhanced aggressive phenotype of Tph2 knockout rats is associated with diminished 5-HT1A receptor sensitivity. Neuropharmacology 2019, 153, 134–141. [Google Scholar] [CrossRef]
- Takahashi, A.; Shiroishi, T.; Koide, T. Genetic mapping of escalated aggression in wild-derived mouse strain MSM/Ms: Association with serotonin-related genes. Front. Neurosci. 2014, 8, 156. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Lee, M.-S.; Lee, S.-H.; Lee, B.-C.; Kim, S.-H.; Joe, S.-H.; Jung, I.-K.; Choi, I.-G.; Ham, B.-J. Association between tryptophan hydroxylase 2 polymorphism and anger-related personality traits among young Korean women. Neuropsychobiology 2010, 62, 158–163. [Google Scholar] [CrossRef]
- Yoon, H.-K.; Lee, H.-J.; Kim, L.; Lee, M.-S.; Ham, B.-J. Impact of tryptophan hydroxylase 2 G-703T polymorphism on anger-related personality traits and orbitofrontal cortex. Behav. Brain Res. 2012, 231, 105–110. [Google Scholar] [CrossRef]
- Booij, L.; Turecki, G.; Leyton, M.; Gravel, P.; Lopez De Lara, C.; Diksic, M.; Benkelfat, C. Tryptophan hydroxylase(2) gene polymorphisms predict brain serotonin synthesis in the orbitofrontal cortex in humans. Mol. Psychiatry 2012, 17, 809–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laas, K.; Kiive, E.; Mäestu, J.; Vaht, M.; Veidebaum, T.; Harro, J. Nice guys: Homozygocity for the TPH2 -703G/T (rs4570625) minor allele promotes low aggressiveness and low anxiety. J. Affect. Disord. 2017, 215, 230–236. [Google Scholar] [CrossRef]
- Studer, E.; Näslund, J.; Andersson, E.; Nilsson, S.; Westberg, L.; Eriksson, E. Serotonin depletion-induced maladaptive aggression requires the presence of androgens. PLoS ONE 2015, 10, e0126462. [Google Scholar] [CrossRef]
- Audero, E.; Mlinar, B.; Baccini, G.; Skachokova, Z.K.; Corradetti, R.; Gross, C. Suppression of serotonin neuron firing increases aggression in mice. J. Neurosci. 2013, 33, 8678–8688. [Google Scholar] [CrossRef] [Green Version]
- Tan, O.; Martin, L.J.; Bowen, M.T. Divergent pathways mediate 5-HT1A receptor agonist effects on close social interaction, grooming and aggressive behaviour in mice: Exploring the involvement of the oxytocin and vasopressin systems. J. Psychopharmacol. 2020, 34, 795–805. [Google Scholar] [CrossRef]
- Terranova, J.I.; Song, Z.; Larkin, T.E.; Hardcastle, N.; Norvelle, A.; Riaz, A.; Albers, H.E. Serotonin and arginine-vasopressin mediate sex differences in the regulation of dominance and aggression by the social brain. Proc. Natl. Acad. Sci. USA 2016, 113, 13233–13238. [Google Scholar] [CrossRef] [Green Version]
- Stein, D.J.; Miczek, K.A.; Lucion, A.B.; de Almeida, R.M.M. Aggression-reducing effects of F15599, a novel selective 5-HT1A receptor agonist, after microinjection into the ventral orbital prefrontal cortex, but not in infralimbic cortex in male mice. Psychopharmacology 2013, 230, 375–387. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Xu, H.; Xue, Y.; An, D.; Li, H.; Chen, W.; Yu, D.; Sun, Y.; Ma, J.; Tang, Y.; et al. 5-HT2CR antagonist/5-HT2CR inverse agonist recovered the increased isolation-induced aggressive behavior of BALB/c mice mediated by ADAR1 (p110) expression and Htr2c RNA editing. Brain Behav. 2018, 8, e00929. [Google Scholar] [CrossRef]
- Butovskaya, M.L.; Butovskaya, P.R.; Vasilyev, V.A.; Sukhodolskaya, J.M.; Fekhredtinova, D.I.; Karelin, D.V.; Fedenok, J.N.; Mabulla, A.Z.P.; Ryskov, A.P.; Lazebny, O.E. Serotonergic gene polymorphisms (5-HTTLPR, 5HTR1A, 5HTR2A), and population differences in aggression: Traditional (Hadza and Datoga) and industrial (Russians) populations compared. J. Physiol. Anthropol. 2018, 37, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedic Erjavec, G.; Tudor, L.; Nikolac Perkovic, M.; Podobnik, J.; Dodig Curkovic, K.; Curkovic, M.; Svob Strac, D.; Cusek, M.; Bortolato, M.; Pivac, N. Serotonin 5-HT2A receptor polymorphisms are associated with irritability and aggression in conduct disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 117, 110542. [Google Scholar] [CrossRef] [PubMed]
- Braccagni, G.; Scheggi, S.; Bortolato, M. Elevated levels of serotonin 5-HT2A receptors in the orbitofrontal cortex of antisocial individuals. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha-Bang, S.; Stenbæk, D.S.; Holst, K.; Licht, C.L.; Jensen, P.S.; Frokjaer, V.G.; Mortensen, E.L.; Knudsen, G.M. Trait aggression and trait impulsivity are not related to frontal cortex 5-HT2A receptor binding in healthy individuals. Psychiatry Res. 2013, 212, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha-Bang, S.; Hjordt, L.V.; Perfalk, E.; Beliveau, V.; Bock, C.; Lehel, S.; Thomsen, C.; Sestoft, D.; Svarer, C.; Knudsen, G.M. Serotonin 1B Receptor Binding Is Associated with Trait Anger and Level of Psychopathy in Violent Offenders. Biol. Psychiatry 2017, 82, 267–274. [Google Scholar] [CrossRef]
- Hakulinen, C.; Jokela, M.; Hintsanen, M.; Merjonen, P.; Pulkki-Råback, L.; Seppälä, I.; Lyytikäinen, L.-P.; Lehtimäki, T.; Kähönen, M.; Viikari, J.; et al. Serotonin receptor 1B genotype and hostility, anger and aggressive behavior through the lifespan: The Young Finns study. J. Behav. Med. 2013, 36, 583–590. [Google Scholar] [CrossRef]
- Da Cunha-Bang, S.; Mc Mahon, B.; Fisher, P.M.; Jensen, P.S.; Svarer, C.; Knudsen, G.M. High trait aggression in men is associated with low 5-HT levels, as indexed by 5-HT4 receptor binding. Soc. Cogn. Affect. Neurosci. 2016, 11, 548–555. [Google Scholar] [CrossRef]
- Beaulieu, J.-M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [Green Version]
- Aleyasin, H.; Flanigan, M.E.; Golden, S.A.; Takahashi, A.; Menard, C.; Pfau, M.L.; Multer, J.; Pina, J.; McCabe, K.A.; Bhatti, N.; et al. Cell-Type-Specific Role of ΔFosB in Nucleus Accumbens in Modulating Intermale Aggression. J. Neurosci. 2018, 38, 5913–5924. [Google Scholar] [CrossRef]
- Felippe, R.M.; Oliveira, G.M.; Barbosa, R.S.; Esteves, B.D.; Gonzaga, B.M.S.; Horita, S.I.M.; Garzoni, L.R.; Beghini, D.G.; Araújo-Jorge, T.C.; Fragoso, V.M.S. Experimental Social Stress: Dopaminergic Receptors, Oxidative Stress, and c-Fos Protein Are Involved in Highly Aggressive Behavior. Front. Cell. Neurosci. 2021, 15, 696834. [Google Scholar] [CrossRef]
- Patki, G.; Atrooz, F.; Alkadhi, I.; Solanki, N.; Salim, S. High aggression in rats is associated with elevated stress, anxiety-like behavior, and altered catecholamine content in the brain. Neurosci. Lett. 2015, 584, 308–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smagin, D.A.; Babenko, V.N.; Redina, O.E.; Kovalenko, I.L.; Galyamina, A.G.; Kudryavtseva, N.N. Reduced Expression of Slc Genes in the VTA and NAcc of Male Mice with Positive Fighting Experience. Genes 2021, 12, 1099. [Google Scholar] [CrossRef]
- Zoratto, F.; Franchi, F.; Macrì, S.; Laviola, G. Methylphenidate administration promotes sociability and reduces aggression in a mouse model of callousness. Psychopharmacology 2019, 236, 2593–2611. [Google Scholar] [CrossRef] [PubMed]
- Studer, E.; Näslund, J.; Westman, A.; Carlsson, A.; Eriksson, E. The effects of the dopamine stabilizer (-)-OSU6162 on aggressive and sexual behavior in rodents. Transl. Psychiatry 2016, 6, e762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pape, L.; van Lith, K.; Veltman, D.; Cohn, M.; Marhe, R.; van den Brink, W.; Doreleijers, T.; Popma, A. Effect of Methylphenidate on Resting-State Connectivity in Adolescents with a Disruptive Behavior Disorder: A Double-Blind Randomized Placebo-Controlled fMRI Study. Front. Psychiatry 2021, 12, 662652. [Google Scholar] [CrossRef]
- Farbiash, T.; Berger, A.; Atzaba-Poria, N.; Auerbach, J.G. Prediction of preschool aggression from DRD4 risk, parental ADHD symptoms, and home chaos. J. Abnorm. Child Psychol. 2014, 42, 489–499. [Google Scholar] [CrossRef]
- Buchmann, A.F.; Zohsel, K.; Blomeyer, D.; Hohm, E.; Hohmann, S.; Jennen-Steinmetz, C.; Treutlein, J.; Becker, K.; Banaschewski, T.; Schmidt, M.H.; et al. Interaction between prenatal stress and dopamine D4 receptor genotype in predicting aggression and cortisol levels in young adults. Psychopharmacology 2014, 231, 3089–3097. [Google Scholar] [CrossRef]
- Wu, T.; Barnes, J.C. Two dopamine receptor genes (DRD2 and DRD4) predict psychopathic personality traits in a sample of American adults. J. Crim. Justice 2013, 41, 188–195. [Google Scholar] [CrossRef]
- Cherepkova, E.V.; Maksimov, V.N.; Kushnarev, A.P.; Shakhmatov, I.I.; Aftanas, L.I. The polymorphism of dopamine receptor D4 (DRD4) and dopamine transporter (DAT) genes in the men with antisocial behaviour and mixed martial arts fighters. World J. Biol. Psychiatry 2019, 20, 402–415. [Google Scholar] [CrossRef]
- Qadeer, M.I.; Amar, A.; Mann, J.J.; Hasnain, S. Polymorphisms in dopaminergic system genes; association with criminal behavior and self-reported aggression in violent prison inmates from Pakistan. PLoS ONE 2017, 12, e0173571. [Google Scholar] [CrossRef] [Green Version]
- Kolla, N.J.; Bortolato, M. The role of monoamine oxidase A in the neurobiology of aggressive, antisocial, and violent behavior: A tale of mice and men. Prog. Neurobiol. 2020, 194, 101875. [Google Scholar] [CrossRef] [PubMed]
- Iofrida, C.; Palumbo, S.; Pellegrini, S. Molecular genetics and antisocial behavior: Where do we stand? Exp. Biol. Med. 2014, 239, 1514–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qayyum, A.; Zai, C.C.; Hirata, Y.; Tiwari, A.K.; Cheema, S.; Nowrouzi, B.; Beitchman, J.H.; Kennedy, J.L. The Role of the Catechol-o-Methyltransferase (COMT) GeneVal158Met in Aggressive Behavior, a Review of Genetic Studies. Curr. Neuropharmacol. 2015, 13, 802–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ficks, C.A.; Waldman, I.D. Candidate genes for aggression and antisocial behavior: A meta-analysis of association studies of the 5HTTLPR and MAOA-uVNTR. Behav. Genet. 2014, 44, 427–444. [Google Scholar] [CrossRef] [PubMed]
- McSwiggan, S.; Elger, B.; Appelbaum, P.S. The forensic use of behavioral genetics in criminal proceedings: Case of the MAOA-L genotype. Int. J. Law Psychiatry 2017, 50, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Shih, J.C.; Chen, K. MAO-A and -B gene knock-out mice exhibit distinctly different behavior. Neurobiology 1999, 7, 235–246. [Google Scholar]
- Labonté, B.; Abdallah, K.; Maussion, G.; Yerko, V.; Yang, J.; Bittar, T.; Quessy, F.; Golden, S.A.; Navarro, L.; Checknita, D.; et al. Regulation of impulsive and aggressive behaviours by a novel lncRNA. Mol. Psychiatry 2021, 26, 3751–3764. [Google Scholar] [CrossRef]
- Yu, Q.; Teixeira, C.M.; Mahadevia, D.; Huang, Y.; Balsam, D.; Mann, J.J.; Gingrich, J.A.; Ansorge, M.S. Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice. Mol. Psychiatry 2014, 19, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Godar, S.C.; Bortolato, M.; Castelli, M.P.; Casti, A.; Casu, A.; Chen, K.; Ennas, M.G.; Tambaro, S.; Shih, J.C. The aggression and behavioral abnormalities associated with monoamine oxidase A deficiency are rescued by acute inhibition of serotonin reuptake. J. Psychiatr. Res. 2014, 56, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Smeijers, D.; Bulten, E.; Franke, B.; Buitelaar, J.; Verkes, R.-J. Associations of multiple trauma types and MAOA with severe aggressive behavior and MAOA effects on training outcome. Eur. Neuropsychopharmacol. 2020, 30, 66–74. [Google Scholar] [CrossRef]
- Martínez, R.M.; Liao, T.-T.; Fan, Y.-T.; Chen, Y.-C.; Chen, C. Interaction effects of the 5-HTT and MAOA-uVNTR gene variants on pre-attentive EEG activity in response to threatening voices. Commun. Biol. 2022, 5, 340. [Google Scholar] [CrossRef] [PubMed]
- Kuepper, Y.; Grant, P.; Wielpuetz, C.; Hennig, J. MAOA-uVNTR genotype predicts interindividual differences in experimental aggressiveness as a function of the degree of provocation. Behav. Brain Res. 2013, 247, 73–78. [Google Scholar] [CrossRef]
- Kolla, N.J.; Patel, R.; Meyer, J.H.; Chakravarty, M.M. Association of monoamine oxidase-A genetic variants and amygdala morphology in violent offenders with antisocial personality disorder and high psychopathic traits. Sci. Rep. 2017, 7, 9607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stetler, D.A.; Davis, C.; Leavitt, K.; Schriger, I.; Benson, K.; Bhakta, S.; Wang, L.C.; Oben, C.; Watters, M.; Haghnegahdar, T.; et al. Association of low-activity MAOA allelic variants with violent crime in incarcerated offenders. J. Psychiatr. Res. 2014, 58, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ming, Q.; Wang, X.; Yao, S. The interactive effect of the MAOA-VNTR genotype and childhood abuse on aggressive behaviors in Chinese male adolescents. Psychiatr. Genet. 2016, 26, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Holz, N.; Boecker, R.; Buchmann, A.F.; Blomeyer, D.; Baumeister, S.; Hohmann, S.; Jennen-Steinmetz, C.; Wolf, I.; Rietschel, M.; Witt, S.H.; et al. Evidence for a Sex-Dependent MAOA× Childhood Stress Interaction in the Neural Circuitry of Aggression. Cereb. Cortex 2016, 26, 904–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorodetsky, E.; Bevilacqua, L.; Carli, V.; Sarchiapone, M.; Roy, A.; Goldman, D.; Enoch, M.-A. The interactive effect of MAOA-LPR genotype and childhood physical neglect on aggressive behaviors in Italian male prisoners. Genes Brain Behav. 2014, 13, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ming, Q.-S.; Yi, J.-Y.; Wang, X.; Chai, Q.-L.; Yao, S.-Q. Gene-Gene-Environment Interactions of Serotonin Transporter, Monoamine Oxidase A and Childhood Maltreatment Predict Aggressive Behavior in Chinese Adolescents. Front. Behav. Neurosci. 2017, 11, 17. [Google Scholar] [CrossRef] [Green Version]
- Kant, T.; Koyama, E.; Zai, C.C.; Beitchman, J.H.; Kennedy, J.L. COMT Val/Met and Psychopathic Traits in Children and Adolescents: A Systematic Review and New Evidence of a Developmental Trajectory toward Psychopathy. Int. J. Mol. Sci. 2022, 23, 1782. [Google Scholar] [CrossRef]
- Gutleb, D.R.; Roos, C.; Noll, A.; Ostner, J.; Schülke, O. COMT Val158 Met moderates the link between rank and aggression in a non-human primate. Genes Brain Behav. 2018, 17, e12443. [Google Scholar] [CrossRef]
- Hygen, B.W.; Belsky, J.; Stenseng, F.; Lydersen, S.; Guzey, I.C.; Wichstrøm, L. Child exposure to serious life events, COMT, and aggression: Testing differential susceptibility theory. Dev. Psychol. 2015, 51, 1098–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Goozen, S.H.M.; Langley, K.; Northover, C.; Hubble, K.; Rubia, K.; Schepman, K.; O’Donovan, M.C.; Thapar, A. Identifying mechanisms that underlie links between COMT genotype and aggression in male adolescents with ADHD. J. Child Psychol. Psychiatry 2016, 57, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Tuvblad, C.; Narusyte, J.; Comasco, E.; Andershed, H.; Andershed, A.-K.; Colins, O.F.; Fanti, K.A.; Nilsson, K.W. Physical and verbal aggressive behavior and COMT genotype: Sensitivity to the environment. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016, 171, 708–718. [Google Scholar] [CrossRef] [Green Version]
- Fritz, M.; Rösel, F.; Dobler, H.; Streb, J.; Dudeck, M. Childhood Trauma, the Combination of MAO-A and COMT Genetic Polymorphisms and the Joy of Being Aggressive in Forensic Psychiatric Patients. Brain Sci. 2021, 11, 1008. [Google Scholar] [CrossRef] [PubMed]
- Nordman, J.C. Anger management: Mechanisms of glutamate receptor-mediated synaptic plasticity underlying animal aggression. Int. J. Biochem. Cell Biol. 2022, 142, 106120. [Google Scholar] [CrossRef]
- Vekovischeva, O.Y.; Aitta-Aho, T.; Echenko, O.; Kankaanpää, A.; Seppälä, T.; Honkanen, A.; Sprengel, R.; Korpi, E.R. Reduced aggression in AMPA-type glutamate receptor GluR-A subunit-deficient mice. Genes Brain Behav. 2004, 3, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Araki, R.; Ago, Y.; Hasebe, S.; Nishiyama, S.; Tanaka, T.; Oka, S.; Takuma, K.; Matsuda, T. Involvement of prefrontal AMPA receptors in encounter stimulation-induced hyperactivity in isolation-reared mice. Int. J. Neuropsychopharmacol. 2014, 17, 883–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, K.; Kurosawa, N.; Seki, K. The role of the AMPA receptor and 5-HT(3) receptor on aggressive behavior and depressive-like symptoms in chronic social isolation-reared mice. Physiol. Behav. 2016, 153, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.; Wang, L.; Jiao, Z.-L.; Yang, R.-R.; Xu, C.; Xu, X.-H. VMHvl-Projecting Vglut1+ Neurons in the Posterior Amygdala Gate Territorial Aggression. Cell Rep. 2020, 31, 107517. [Google Scholar] [CrossRef]
- Shin, S.Y.; Baek, N.J.; Han, S.H.; Min, S.S. Chronic administration of ketamine ameliorates the anxiety- and aggressive-like behavior in adolescent mice induced by neonatal maternal separation. Korean J. Physiol. Pharmacol. 2019, 23, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-H.; Su, C.-L.; Gean, P.-W. Mechanism underlying NMDA blockade-induced inhibition of aggression in post-weaning socially isolated mice. Neuropharmacology 2018, 143, 95–105. [Google Scholar] [CrossRef]
- Frau, R.; Pardu, A.; Godar, S.; Bini, V.; Bortolato, M. Combined Antagonism of 5-HT2 and NMDA Receptors Reduces the Aggression of Monoamine Oxidase a Knockout Mice. Pharmaceuticals 2022, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Halene, T.B.; Ehrlichman, R.S.; Liang, Y.; Christian, E.P.; Jonak, G.J.; Gur, T.L.; Blendy, J.A.; Dow, H.C.; Brodkin, E.S.; Schneider, F.; et al. Assessment of NMDA receptor NR1 subunit hypofunction in mice as a model for schizophrenia. Genes Brain Behav. 2009, 8, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Bacq, A.; Astori, S.; Gebara, E.; Tang, W.; Silva, B.A.; Sanchez-Mut, J.; Grosse, J.; Guillot de Suduiraut, I.; Zanoletti, O.; Maclachlan, C.; et al. Amygdala GluN2B-NMDAR dysfunction is critical in abnormal aggression of neurodevelopmental origin induced by St8sia2 deficiency. Mol. Psychiatry 2020, 25, 2144–2161. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.F.; Postigo, D.; Martín, M.; Burón, E. Antiaggressive effects of MPEP, a selective antagonist of mGlu5 receptors, in agonistic interactions between male mice. Eur. J. Pharmacol. 2006, 551, 67–70. [Google Scholar] [CrossRef]
- Been, L.E.; Moore, K.M.; Kennedy, B.C.; Meisel, R.L. Metabotropic Glutamate Receptor and Fragile X Signaling in a Female Model of Escalated Aggression. Biol. Psychiatry 2016, 79, 685–692. [Google Scholar] [CrossRef] [Green Version]
- Barbano, M.F.; Wang, H.-L.; Zhang, S.; Miranda-Barrientos, J.; Estrin, D.J.; Figueroa-González, A.; Liu, B.; Barker, D.J.; Morales, M. VTA Glutamatergic Neurons Mediate Innate Defensive Behaviors. Neuron 2020, 107, 368–382.e8. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.C.; Mulder, L.M.; Zwiers, M.P.; Sethi, A.; Hoekstra, P.J.; Dietrich, A.; Baumeister, S.; Aggensteiner, P.M.; Banaschewski, T.; Brandeis, D.; et al. Distinct associations between fronto-striatal glutamate concentrations and callous-unemotional traits and proactive aggression in disruptive behavior. Cortex 2019, 121, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Smaragdi, A.; Chavez, S.; Lobaugh, N.J.; Meyer, J.H.; Kolla, N.J. Differential levels of prefrontal cortex glutamate+glutamine in adults with antisocial personality disorder and bipolar disorder: A proton magnetic resonance spectroscopy study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 93, 250–255. [Google Scholar] [CrossRef]
- Ende, G.; Cackowski, S.; van Eijk, J.; Sack, M.; Demirakca, T.; Kleindienst, N.; Bohus, M.; Sobanski, E.; Krause-Utz, A.; Schmahl, C. Impulsivity and Aggression in Female BPD and ADHD Patients: Association with ACC Glutamate and GABA Concentrations. Neuropsychopharmacology 2016, 41, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Frangaj, A.; Fan, Q.R. Structural biology of GABAB receptor. Neuropharmacology 2018, 136, 68–79. [Google Scholar] [CrossRef]
- Olsen, R.W.; Tobin, A.J. Molecular biology of GABAA receptors. FASEB J. 1990, 4, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Narvaes, R.; Martins de Almeida, R.M. Aggressive behavior and three neurotransmitters: Dopamine, GABA, and serotonin—A review of the last 10 years. Psychol. Neurosci. 2014, 7, 601–607. [Google Scholar] [CrossRef]
- Jager, A.; Amiri, H.; Bielczyk, N.; van Heukelum, S.; Heerschap, A.; Aschrafi, A.; Poelmans, G.; Buitelaar, J.K.; Kozicz, T.; Glennon, J.C. Cortical control of aggression: GABA signalling in the anterior cingulate cortex. Eur. Neuropsychopharmacol. 2020, 30, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Chaibi, I.; Bennis, M.; Ba-M’Hamed, S. GABA-A receptor signaling in the anterior cingulate cortex modulates aggression and anxiety-related behaviors in socially isolated mice. Brain Res. 2021, 1762, 147440. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.M.; Markham, C.M.; Norvelle, A.; Albers, H.E.; Huhman, K.L. GABAA receptor activation in the lateral septum reduces the expression of conditioned defeat and increases aggression in Syrian hamsters. Brain Res. 2012, 1439, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Borland, J.M.; Walton, J.C.; Norvelle, A.; Grantham, K.N.; Aiani, L.M.; Larkin, T.E.; McCann, K.E.; Albers, H.E. Social experience and sex-dependent regulation of aggression in the lateral septum by extrasynaptic δGABAA receptors. Psychopharmacology 2020, 237, 329–344. [Google Scholar] [CrossRef]
- Newman, E.L.; Smith, K.S.; Takahashi, A.; Chu, A.; Hwa, L.S.; Chen, Y.; DeBold, J.F.; Rudolph, U.; Miczek, K.A. α2-containing GABA(A) receptors: A requirement for midazolam-escalated aggression and social approach in mice. Psychopharmacology 2015, 232, 4359–4369. [Google Scholar] [CrossRef] [Green Version]
- Hong, W.; Kim, D.-W.; Anderson, D.J. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell 2014, 158, 1348–1361. [Google Scholar] [CrossRef] [Green Version]
- Baleisyte, A.; Schneggenburger, R.; Kochubey, O. Stimulation of medial amygdala GABA neurons with kinetically different channelrhodopsins yields opposite behavioral outcomes. Cell Rep. 2022, 39, 110850. [Google Scholar] [CrossRef]
- Gulsun, M.; Oznur, T.; Aydemir, E.; Ozcelik, F.; Erdem, M.; Zincir, S.; Akgul, O.; Kurt, Y. Possible relationship between amino acids, aggression and psychopathy. Int. J. Psychiatry Clin. Pract. 2016, 20, 91–100. [Google Scholar] [CrossRef]
- Kiive, E.; Laas, K.; Vaht, M.; Veidebaum, T.; Harro, J. Stressful life events increase aggression and alcohol use in young carriers of the GABRA2 rs279826/rs279858 A-allele. Eur. Neuropsychopharmacol. 2017, 27, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Haroon, E.; Raison, C.L.; Felger, J.C. Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits. Depress. Anxiety 2013, 30, 297–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klawonn, A.M.; Fritz, M.; Castany, S.; Pignatelli, M.; Canal, C.; Similä, F.; Tejeda, H.A.; Levinsson, J.; Jaarola, M.; Jakobsson, J.; et al. Microglial activation elicits a negative affective state through prostaglandin-mediated modulation of striatal neurons. Immunity 2021, 54, 225–234.e6. [Google Scholar] [CrossRef] [PubMed]
- Hassanain, M.; Zalcman, S.; Bhatt, S.; Siegel, A. Interleukin-1 beta in the hypothalamus potentiates feline defensive rage: Role of serotonin-2 receptors. Neuroscience 2003, 120, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Hassanain, M.; Bhatt, S.; Zalcman, S.; Siegel, A. Potentiating role of interleukin-1beta (IL-1beta) and IL-1beta type 1 receptors in the medial hypothalamus in defensive rage behavior in the cat. Brain Res. 2005, 1048, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Bhatt, R.; Zalcman, S.S.; Siegel, A. Role of IL-1 beta and 5-HT2 receptors in midbrain periaqueductal gray (PAG) in potentiating defensive rage behavior in cat. Brain Behav. Immun. 2008, 22, 224–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.; Siegel, A.; Zalcman, S.S. Lack of aggression and anxiolytic-like behavior in TNF receptor (TNF-R1 and TNF-R2) deficient mice. Brain Behav. Immun. 2010, 24, 1276–1280. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, A.; Aleyasin, H.; Stavarache, M.A.; Li, L.; Cathomas, F.; Parise, L.F.; Lin, H.-Y.; Burnett, C.J.; Aubry, A.; Flanigan, M.E.; et al. Neuromodulatory effect of interleukin 1β in the dorsal raphe nucleus on individual differences in aggression. Mol. Psychiatry 2022, 27, 2563–2579. [Google Scholar] [CrossRef]
- Alperina, E.; Idova, G.; Zhukova, E.; Zhanaeva, S.; Kozhemyakina, R. Cytokine variations within brain structures in rats selected for differences in aggression. Neurosci. Lett. 2019, 692, 193–198. [Google Scholar] [CrossRef]
- Coccaro, E.F.; Lee, R.; Coussons-Read, M. Elevated plasma inflammatory markers in individuals with intermittent explosive disorder and correlation with aggression in humans. JAMA Psychiatry 2014, 71, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Coccaro, E.F.; Lee, R.; Coussons-Read, M. Cerebrospinal fluid inflammatory cytokines and aggression in personality disordered subjects. Int. J. Neuropsychopharmacol. 2015, 18, pyv001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provençal, N.; Suderman, M.J.; Vitaro, F.; Szyf, M.; Tremblay, R.E. Childhood chronic physical aggression associates with adult cytokine levels in plasma. PLoS ONE 2013, 8, e69481. [Google Scholar] [CrossRef] [Green Version]
- Pouget, J.G.; Bryushkova, L.; Koyama, E.; Zai, C.C.; Fonseka, T.M.; Mueller, D.; Kennedy, J.L.; Beitchman, J.H. Exploring the association of interleukin polymorphisms with aggression and internalizing behaviors in children and adolescents. Brain Behav. 2022, 12, e2753. [Google Scholar] [CrossRef]
- Miller, G.M.; Bendor, J.; Tiefenbacher, S.; Yang, H.; Novak, M.A.; Madras, B.K. A mu-opioid receptor single nucleotide polymorphism in rhesus monkey: Association with stress response and aggression. Mol. Psychiatry 2004, 9, 99–108. [Google Scholar] [CrossRef]
- Driscoll, C.A.; Lindell, S.G.; Schwandt, M.L.; Suomi, S.J.; Higley, J.D.; Heilig, M.; Barr, C.S. OPRM1 genotype interacts with serotonin system dysfunction to predict alcohol-heightened aggression in primates. Addict. Biol. 2017, 22, 1655–1664. [Google Scholar] [CrossRef]
- Farah Naquiah, M.Z.; James, R.J.; Suratman, S.; Lee, L.S.; Mohd Hafidz, M.I.; Salleh, M.Z.; Teh, L.K. Transgenerational effects of paternal heroin addiction on anxiety and aggression behavior in male offspring. Behav. Brain Funct. 2016, 12, 23. [Google Scholar] [CrossRef] [Green Version]
- Weidler, C.; Wagels, L.; Regenbogen, C.; Hofhansel, L.; Blendy, J.A.; Clemens, B.; Montag, C.; Habel, U. The influence of the OPRM1 (A118G) polymorphism on behavioral and neural correlates of aggression in healthy males. Neuropharmacology 2019, 156, 107467. [Google Scholar] [CrossRef] [PubMed]
- Cimino, S.; Carola, V.; Cerniglia, L.; Bussone, S.; Bevilacqua, A.; Tambelli, R. The μ-opioid receptor gene A118G polymorphism is associated with insecure attachment in children with disruptive mood regulation disorder and their mothers. Brain Behav. 2020, 10, e01659. [Google Scholar] [CrossRef]
- Flanigan, M.E.; Aleyasin, H.; Li, L.; Burnett, C.J.; Chan, K.L.; LeClair, K.B.; Lucas, E.K.; Matikainen-Ankney, B.; Durand-de Cuttoli, R.; Takahashi, A.; et al. Orexin signaling in GABAergic lateral habenula neurons modulates aggressive behavior in male mice. Nat. Neurosci. 2020, 23, 638–650. [Google Scholar] [CrossRef]
- Flanigan, M.; Aleyasin, H.; Takahashi, A.; Golden, S.A.; Russo, S.J. An emerging role for the lateral habenula in aggressive behavior. Pharmacol. Biochem. Behav. 2017, 162, 79–86. [Google Scholar] [CrossRef]
- Harro, J.; Laas, K.; Eensoo, D.; Kurrikoff, T.; Sakala, K.; Vaht, M.; Parik, J.; Mäestu, J.; Veidebaum, T. Orexin/hypocretin receptor gene (HCRTR1) variation is associated with aggressive behaviour. Neuropharmacology 2019, 156, 107527. [Google Scholar] [CrossRef] [Green Version]
- Pulver, A.; Kiive, E.; Kanarik, M.; Harro, J. Association of orexin/hypocretin receptor gene (HCRTR1) with reward sensitivity, and interaction with gender. Brain Res. 2020, 1746, 147013. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, V.; Maltese, F.; Contarini, G.; Nigro, M.; Bonavia, A.; Huang, H.; Gigliucci, V.; Morelli, G.; Scheggia, D.; Managò, F.; et al. Oxytocin Signaling in the Central Amygdala Modulates Emotion Discrimination in Mice. Curr. Biol. 2019, 29, 1938–1953.e6. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Albers, H.E. Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Front. Neuroendocrinol. 2018, 51, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Froemke, R.C.; Young, L.J. Oxytocin, Neural Plasticity, and Social Behavior. Annu. Rev. Neurosci. 2021, 44, 359–381. [Google Scholar] [CrossRef]
- Haller, J. The neurobiology of abnormal manifestations of aggression—A review of hypothalamic mechanisms in cats, rodents, and humans. Brain Res. Bull. 2013, 93, 97–109. [Google Scholar] [CrossRef]
- Beiderbeck, D.I.; Neumann, I.D.; Veenema, A.H. Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. Eur. J. Neurosci. 2007, 26, 3597–3605. [Google Scholar] [CrossRef]
- Carrillo, M.; Ricci, L.A.; Melloni, R.H. Glutamate-vasopressin interactions and the neurobiology of anabolic steroid-induced offensive aggression. Neuroscience 2011, 185, 85–96. [Google Scholar] [CrossRef]
- Wersinger, S.R.; Ginns, E.I.; O’Carroll, A.-M.; Lolait, S.J.; Young, W.S. Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol. Psychiatry 2002, 7, 975–984. [Google Scholar] [CrossRef] [Green Version]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.E.d.M.; Lukas, M.; Wolf, H.N.; Durante, E.; Lorenz, A.; Mayer, A.-L.; Bludau, A.; Bosch, O.J.; Grinevich, V.; Egger, V.; et al. Oxytocin and vasopressin within the ventral and dorsal lateral septum modulate aggression in female rats. Nat. Commun. 2021, 12, 2900. [Google Scholar] [CrossRef]
- Tan, O.; Musullulu, H.; Raymond, J.S.; Wilson, B.; Langguth, M.; Bowen, M.T. Oxytocin and vasopressin inhibit hyper-aggressive behaviour in socially isolated mice. Neuropharmacology 2019, 156, 107573. [Google Scholar] [CrossRef] [PubMed]
- Kawada, A.; Nagasawa, M.; Murata, A.; Mogi, K.; Watanabe, K.; Kikusui, T.; Kameda, T. Vasopressin enhances human preemptive strike in both males and females. Sci. Rep. 2019, 9, 9664. [Google Scholar] [CrossRef] [Green Version]
- Akkoc Altinok, D.C.; Votinov, M.; Henzelmann, F.; Jo, H.; Eisert, A.; Habel, U.; Wagels, L. A Combined Administration of Testosterone and Arginine Vasopressin Affects Aggressive Behavior in Males. Brain Sci. 2021, 11, 1623. [Google Scholar] [CrossRef] [PubMed]
- Brunnlieb, C.; Münte, T.F.; Krämer, U.; Tempelmann, C.; Heldmann, M. Vasopressin modulates neural responses during human reactive aggression. Soc. Neurosci. 2013, 8, 148–164. [Google Scholar] [CrossRef]
- Yudko, E.; Blanchard, D.C.; Henrie, J.A.; Blanchard, R.J. Emerging themes in preclinical research on alcohol and aggression. Recent Dev. Alcohol. 1997, 13, 123–138. [Google Scholar] [CrossRef]
- Duncan, E.A.; Tamashiro, K.L.K.; Nguyen, M.M.N.; Gardner, S.R.; Woods, S.C.; Sakai, R.R. The impact of moderate daily alcohol consumption on aggression and the formation of dominance hierarchies in rats. Psychopharmacology 2006, 189, 83–94. [Google Scholar] [CrossRef]
- Miczek, K.A.; de Boer, S.F.; Haller, J. Excessive aggression as model of violence: A critical evaluation of current preclinical methods. Psychopharmacology 2013, 226, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Weerts, E.M.; Tornatzky, W.; Miczek, K.A. Prevention of the pro-aggressive effects of alcohol in rats and squirrel monkeys by benzodiazepine receptor antagonists. Psychopharmacology 1993, 111, 144–152. [Google Scholar] [CrossRef]
- Fish, E.W.; DeBold, J.F.; Miczek, K.A. Repeated alcohol: Behavioral sensitization and alcohol-heightened aggression in mice. Psychopharmacology 2002, 160, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Faccidomo, S.; Bannai, M.; Miczek, K.A. Escalated aggression after alcohol drinking in male mice: Dorsal raphé and prefrontal cortex serotonin and 5-HT(1B) receptors. Neuropsychopharmacology 2008, 33, 2888–2899. [Google Scholar] [CrossRef] [Green Version]
- Fish, E.W.; Faccidomo, S.; DeBold, J.F.; Miczek, K.A. Alcohol, allopregnanolone and aggression in mice. Psychopharmacology 2001, 153, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Miczek, K.A.; Barros, H.M.; Sakoda, L.; Weerts, E.M. Alcohol and heightened aggression in individual mice. Alcohol. Clin. Exp. Res. 1998, 22, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Miczek, K.A.; DeBold, J.F.; van Erp, A.M.; Tornatzky, W. Alcohol, GABAA-benzodiazepine receptor complex, and aggression. Recent Dev. Alcohol. 1997, 13, 139–171. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Kwa, C.; DeBold, J.F.; Miczek, K.A. GABA(A) receptors in the dorsal raphé nucleus of mice: Escalation of aggression after alcohol consumption. Psychopharmacology 2010, 211, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Miczek, K.A.; O’Donnell, J.M. Alcohol and chlordiazepoxide increase suppressed aggression in mice. Psychopharmacology 1980, 69, 39–44. [Google Scholar] [CrossRef]
- Venuti, L.S.; Pena-Flores, N.L.; Herberholz, J. Cellular interactions between social experience, alcohol sensitivity, and GABAergic inhibition in a crayfish neural circuit. J. Neurophysiol. 2021, 125, 256–272. [Google Scholar] [CrossRef]
- Miczek, K.A.; Hussain, S.; Faccidomo, S. Alcohol-heightened aggression in mice: Attenuation by 5-HT1A receptor agonists. Psychopharmacology 1998, 139, 160–168. [Google Scholar] [CrossRef]
- Miczek, K.A.; de Almeida, R.M. Oral drug self-administration in the home cage of mice: Alcohol-heightened aggression and inhibition by the 5-HT1B agonist anpirtoline. Psychopharmacology 2001, 157, 421–429. [Google Scholar] [CrossRef]
- Mehlman, P.T.; Higley, J.D.; Faucher, I.; Lilly, A.A.; Taub, D.M.; Vickers, J.; Suomi, S.J.; Linnoila, M. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am. J. Psychiatry 1994, 151, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Mosienko, V.; Bert, B.; Beis, D.; Matthes, S.; Fink, H.; Bader, M.; Alenina, N. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl. Psychiatry 2012, 2, e122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeMarquand, D.; Pihl, R.O.; Benkelfat, C. Serotonin and alcohol intake, abuse, and dependence: Findings of animal studies. Biol. Psychiatry 1994, 36, 395–421. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.E.; Ziani, P.R.; Fontana, B.D.; Duarte, T.; Stefanello, F.V.; Canzian, J.; Santos, A.R.S.; Rosemberg, D.B. Role of the serotonergic system in ethanol-induced aggression and anxiety: A pharmacological approach using the zebrafish model. Eur. Neuropsychopharmacol. 2020, 32, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Bocchio, M.; McHugh, S.B.; Bannerman, D.M.; Sharp, T.; Capogna, M. Serotonin, Amygdala and Fear: Assembling the Puzzle. Front. Neural Circuits 2016, 10, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowry, C.A.; Johnson, P.L.; Hay-Schmidt, A.; Mikkelsen, J.; Shekhar, A. Modulation of anxiety circuits by serotonergic systems. Stress 2005, 8, 233–246. [Google Scholar] [CrossRef]
- Mathur, P.; Guo, S. Differences of acute versus chronic ethanol exposure on anxiety-like behavioral responses in zebrafish. Behav. Brain Res. 2011, 219, 234–239. [Google Scholar] [CrossRef] [Green Version]
- Kalinichenko, L.S.; Mühle, C.; Eulenburg, V.; Praetner, M.; Reichel, M.; Gulbins, E.; Kornhuber, J.; Müller, C.P. Enhanced Alcohol Preference and Anxiolytic Alcohol Effects in Niemann-Pick Disease Model in Mice. Front. Neurol. 2019, 10, 731. [Google Scholar] [CrossRef] [Green Version]
- Kiryanova, V.; Dyck, R.H. Increased aggression, improved spatial memory, and reduced anxiety-like behaviour in adult male mice exposed to fluoxetine early in life. Dev. Neurosci. 2014, 36, 396–408. [Google Scholar] [CrossRef]
- Clarke, R.B.C.; Adermark, L.; Chau, P.; Söderpalm, B.; Ericson, M. Increase in nucleus accumbens dopamine levels following local ethanol administration is not mediated by acetaldehyde. Alcohol Alcohol 2014, 49, 498–504. [Google Scholar] [CrossRef] [Green Version]
- Doherty, J.M.; Schier, C.J.; Vena, A.A.; Dilly, G.A.; Gonzales, R.A. Medial Prefrontal Cortical Dopamine Responses During Operant Self-Administration of Sweetened Ethanol. Alcohol. Clin. Exp. Res. 2016, 40, 1662–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimoto, K.; Ueda, S.; Kato, B.; Takeuchi, Y.; Kawai, Y.; Noritake, K.; Yasuhara, M. Alcohol enhances characteristic releases of dopamine and serotonin in the central nucleus of the amygdala. Neurochem. Int. 2000, 37, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Van Erp, A.M.; Miczek, K.A. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J. Neurosci. 2000, 20, 9320–9325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Erp, A.M.M.; Miczek, K.A. Increased accumbal dopamine during daily alcohol consumption and subsequent aggressive behavior in rats. Psychopharmacology 2007, 191, 679–688. [Google Scholar] [CrossRef]
- Zapata, A.; Gonzales, R.A.; Shippenberg, T.S. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology 2006, 31, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Booij, L.; Welfeld, K.; Leyton, M.; Dagher, A.; Boileau, I.; Sibon, I.; Baker, G.B.; Diksic, M.; Soucy, J.-P.; Pruessner, J.C.; et al. Dopamine cross-sensitization between psychostimulant drugs and stress in healthy male volunteers. Transl. Psychiatry 2016, 6, e740. [Google Scholar] [CrossRef] [Green Version]
- Renard, J.; Loureiro, M.; Rosen, L.G.; Zunder, J.; de Oliveira, C.; Schmid, S.; Rushlow, W.J.; Laviolette, S.R. Cannabidiol Counteracts Amphetamine-Induced Neuronal and Behavioral Sensitization of the Mesolimbic Dopamine Pathway through a Novel mTOR/p70S6 Kinase Signaling Pathway. J. Neurosci. 2016, 36, 5160–5169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefevre, E.M.; Pisansky, M.T.; Toddes, C.; Baruffaldi, F.; Pravetoni, M.; Tian, L.; Kono, T.J.Y.; Rothwell, P.E. Interruption of continuous opioid exposure exacerbates drug-evoked adaptations in the mesolimbic dopamine system. Neuropsychopharmacology 2020, 45, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- Domino, E.F.; Tsukada, H. Nicotine sensitization of monkey striatal dopamine release. Eur. J. Pharmacol. 2009, 607, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Fennell, A.M.; Pitts, E.G.; Sexton, L.L.; Ferris, M.J. Phasic Dopamine Release Magnitude Tracks Individual Differences in Sensitization of Locomotor Response following a History of Nicotine Exposure. Sci. Rep. 2020, 10, 173. [Google Scholar] [CrossRef] [Green Version]
- Bahi, A.; Dreyer, J.-L. Involvement of nucleus accumbens dopamine D1 receptors in ethanol drinking, ethanol-induced conditioned place preference, and ethanol-induced psychomotor sensitization in mice. Psychopharmacology 2012, 222, 141–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Z.-M.; Rodd, Z.A.; Engleman, E.A.; McBride, W.J. Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol. Clin. Exp. Res. 2009, 33, 1571–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nona, C.N.; Hendershot, C.S.; Lê, A.D. Behavioural sensitization to alcohol: Bridging the gap between preclinical research and human models. Pharmacol. Biochem. Behav. 2018, 173, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.L.; Terunuma, M.; Wang, T.L.; Hewage, N.; Bicakci, M.B.; Moss, S.J.; DeBold, J.F.; Miczek, K.A. A Role for Prefrontal Cortical NMDA Receptors in Murine Alcohol-Heightened Aggression. Neuropsychopharmacology 2018, 43, 1224–1234. [Google Scholar] [CrossRef] [Green Version]
- Newman, E.L.; Chu, A.; Bahamón, B.; Takahashi, A.; DeBold, J.F.; Miczek, K.A. NMDA receptor antagonism: Escalation of aggressive behavior in alcohol-drinking mice. Psychopharmacology 2012, 224, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Hwa, L.S.; Nathanson, A.J.; Shimamoto, A.; Tayeh, J.K.; Wilens, A.R.; Holly, E.N.; Newman, E.L.; DeBold, J.F.; Miczek, K.A. Aggression and increased glutamate in the mPFC during withdrawal from intermittent alcohol in outbred mice. Psychopharmacology 2015, 232, 2889–2902. [Google Scholar] [CrossRef] [Green Version]
- Becker, A.; Peters, B.; Schroeder, H.; Mann, T.; Huether, G.; Grecksch, G. Ketamine-induced changes in rat behaviour: A possible animal model of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 687–700. [Google Scholar] [CrossRef]
- Darke, S. The toxicology of homicide offenders and victims: A review. Drug Alcohol Rev. 2010, 29, 202–215. [Google Scholar] [CrossRef]
- Pridemore, W.A. Vodka and violence: Alcohol consumption and homicide rates in Russia. Am. J. Public Health 2002, 92, 1921–1930. [Google Scholar] [CrossRef]
- Cherpitel, C.J.; Ye, Y.; Bond, J.; Room, R.; Borges, G. Attribution of alcohol to violence-related injury: Self and other’s drinking in the event. J. Stud. Alcohol Drugs 2012, 73, 277–284. [Google Scholar] [CrossRef]
- Caetano, R.; Schafer, J.; Cunradi, C.B. Alcohol-Related Intimate Partner Violence Among White, Black, and Hispanic Couples in the United States. Alcohol Res. Health 2001, 25, 58–65. [Google Scholar] [PubMed]
- Yalch, M.M.; Christodoulou, J.; Rotheram-Borus, M.J.; Tomlinson, M. Longitudinal Association Between Intimate Partner Violence and Alcohol Use in a Population Cohort of South African Women. J. Interpers. Violence 2023, 38, NP1718–NP1737. [Google Scholar] [CrossRef] [PubMed]
- Sabia, J.J. Alcohol consumption and domestic violence against mothers. J. Ment. Health Policy Econ. 2004, 7, 191–205. [Google Scholar] [PubMed]
- Yu, R.; Nevado-Holgado, A.J.; Molero, Y.; D’Onofrio, B.M.; Larsson, H.; Howard, L.M.; Fazel, S. Mental disorders and intimate partner violence perpetrated by men towards women: A Swedish population-based longitudinal study. PLoS Med. 2019, 16, e1002995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duke, A.A.; Giancola, P.R.; Morris, D.H.; Holt, J.C.D.; Gunn, R.L. Alcohol dose and aggression: Another reason why drinking more is a bad idea. J. Stud. Alcohol Drugs 2011, 72, 34–43. [Google Scholar] [CrossRef]
- Dougherty, D.M.; Cherek, D.R.; Bennett, R.H. The effects of alcohol on the aggressive responding of women. J. Stud. Alcohol 1996, 57, 178–186. [Google Scholar] [CrossRef]
- Kuypers, K.; Verkes, R.J.; van den Brink, W.; van Amsterdam, J.; Ramaekers, J.G. Intoxicated aggression: Do alcohol and stimulants cause dose-related aggression? A review. Eur. Neuropsychopharmacol. 2020, 30, 114–147. [Google Scholar] [CrossRef]
- Miller, M.A.; Fillmore, M.T. Protracted impairment of impulse control under an acute dose of alcohol: A time-course analysis. Addict. Behav. 2014, 39, 1589–1596. [Google Scholar] [CrossRef] [Green Version]
- Fillmore, M.T.; Weafer, J. Acute tolerance to alcohol in at-risk binge drinkers. Psychol. Addict. Behav. 2012, 26, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Hoaken, P.N.S.; Stewart, S.H. Drugs of abuse and the elicitation of human aggressive behavior. Addict. Behav. 2003, 28, 1533–1554. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.L.; Mattick, R.P.; Jamadar, S.D.; Iredale, J.M. Deficits in behavioural inhibition in substance abuse and addiction: A meta-analysis. Drug Alcohol Depend. 2014, 145, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, K.; Halgren, E.; Klopp, J.; Maltzman, I. Alcohol effects on movement-related potentials: A measure of impulsivity? J. Stud. Alcohol 2000, 61, 24–31. [Google Scholar] [CrossRef]
- Hasegawa, A.; Matsumoto, N.; Yamashita, Y.; Tanaka, K.; Kawaguchi, J.; Yamamoto, T. Response inhibition deficits are positively associated with trait rumination, but attentional inhibition deficits are not: Aggressive behaviors and interpersonal stressors as mediators. Psychol. Res. 2022, 86, 858–870. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Niu, G.; Zhang, L.; Chang, H. Reactive Aggression Affects Response Inhibition to Angry Expressions in Adolescents: An Event-Related Potential Study Using the Emotional Go/No-Go Paradigm. Front. Psychol. 2020, 11, 558461. [Google Scholar] [CrossRef] [PubMed]
- Weafer, J.; Fillmore, M.T. Comparison of alcohol impairment of behavioral and attentional inhibition. Drug Alcohol Depend. 2012, 126, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Giancola, P.R. Alcohol-related aggression in men and women: The influence of dispositional aggressivity. J. Stud. Alcohol 2002, 63, 696–708. [Google Scholar] [CrossRef]
- Beck, A.; Heinz, A. Alcohol-related aggression-social and neurobiological factors. Dtsch. Arztebl. Int. 2013, 110, 711–715. [Google Scholar] [CrossRef] [Green Version]
- Wells, S.; Mihic, L.; Tremblay, P.F.; Graham, K.; Demers, A. Where, with whom, and how much alcohol is consumed on drinking events involving aggression? Event-level associations in a Canadian national survey of university students. Alcohol. Clin. Exp. Res. 2008, 32, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Abbey, A. Alcohol’s role in sexual violence perpetration: Theoretical explanations, existing evidence and future directions. Drug Alcohol Rev. 2011, 30, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Heinz, A.J.; Beck, A.; Meyer-Lindenberg, A.; Sterzer, P.; Heinz, A. Cognitive and neurobiological mechanisms of alcohol-related aggression. Nat. Rev. Neurosci. 2011, 12, 400–413. [Google Scholar] [CrossRef]
- Attwood, A.S.; Munafò, M.R. Effects of acute alcohol consumption and processing of emotion in faces: Implications for understanding alcohol-related aggression. J. Psychopharmacol. 2014, 28, 719–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Berre, A.-P. Emotional processing and social cognition in alcohol use disorder. Neuropsychology 2019, 33, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, K.; Rickenbacher, E.; Azma, S. Effects of Alcohol Intoxication on Response Conflict in a Flanker Task. J. Addict. Res. Ther. 2012, (Suppl. 3), 002. [Google Scholar] [CrossRef] [Green Version]
- Magrys, S.A.; Olmstead, M.C. Alcohol intoxication alters cognitive skills mediated by frontal and temporal brain regions. Brain Cogn. 2014, 85, 271–276. [Google Scholar] [CrossRef]
- Giancola, P.R. Executive functioning: A conceptual framework for alcohol-related aggression. Exp. Clin. Psychopharmacol. 2000, 8, 576–597. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.H.G.; Perrett, D.I.; Waiter, G.D.; Pechey, S. Differential effects of tryptophan depletion on emotion processing according to face direction. Soc. Cogn. Affect. Neurosci. 2007, 2, 264–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daly, E.; Deeley, Q.; Hallahan, B.; Craig, M.; Brammer, M.; Lamar, M.; Cleare, A.; Giampietro, V.; Ecker, C.; Page, L.; et al. Effects of acute tryptophan depletion on neural processing of facial expressions of emotion in humans. Psychopharmacology 2010, 210, 499–510. [Google Scholar] [CrossRef]
- Fairchild, G.; van Goozen, S.H.M.; Calder, A.J.; Stollery, S.J.; Goodyer, I.M. Deficits in facial expression recognition in male adolescents with early-onset or adolescence-onset conduct disorder. J. Child Psychol. Psychiatry 2009, 50, 627–636. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Liu, X.; Cheng, L. Facial Emotion Perceptual Tendency in Violent and Non-violent Offenders. J. Interpers. Violence 2022, 37, NP15058–NP15074. [Google Scholar] [CrossRef]
- Marinkovic, K.; Oscar-Berman, M.; Urban, T.; O’Reilly, C.E.; Howard, J.A.; Sawyer, K.; Harris, G.J. Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol. Clin. Exp. Res. 2009, 33, 1880–1892. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Ge, T.T.; Yin, G.; Cui, R.; Zhao, G.; Yang, W. Stress-Induced Functional Alterations in Amygdala: Implications for Neuropsychiatric Diseases. Front. Neurosci. 2018, 12, 367. [Google Scholar] [CrossRef] [Green Version]
- Eichenbaum, H. The role of the hippocampus in navigation is memory. J. Neurophysiol. 2017, 117, 1785–1796. [Google Scholar] [CrossRef] [Green Version]
- Sher, L.; Oquendo, M.A.; Grunebaum, M.F.; Burke, A.K.; Huang, Y.; Mann, J.J. CSF monoamine metabolites and lethality of suicide attempts in depressed patients with alcohol dependence. Eur. Neuropsychopharmacol. 2007, 17, 12–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strac, D.S.; Erjavec, G.N.; Perkovic, M.N.; Sviglin, K.N.; Borovecki, F.; Pivac, N. Association of GABAA receptor α2 subunit gene (GABRA2) with alcohol dependence-related aggressive behavior. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 63, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, M.; Klawonn, A.M.; Zahr, N.M. Neuroimaging in alcohol use disorder: From mouse to man. J. Neurosci. Res. 2022, 100, 1140–1158. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Durazzo, T.C.; Meyerhoff, D.J. Regional Brain Volume Changes in Alcohol-Dependent Individuals During Short-Term and Long-Term Abstinence. Alcohol. Clin. Exp. Res. 2018, 42, 1062–1072. [Google Scholar] [CrossRef]
- Gan, G.; Sterzer, P.; Marxen, M.; Zimmermann, U.S.; Smolka, M.N. Neural and Behavioral Correlates of Alcohol-Induced Aggression Under Provocation. Neuropsychopharmacology 2015, 40, 2886–2896. [Google Scholar] [CrossRef] [Green Version]
- Collins, A.L.; Saunders, B.T. Heterogeneity in striatal dopamine circuits: Form and function in dynamic reward seeking. J. Neurosci. Res. 2020, 98, 1046–1069. [Google Scholar] [CrossRef]
- Menegas, W.; Bergan, J.F.; Ogawa, S.K.; Isogai, Y.; Umadevi Venkataraju, K.; Osten, P.; Uchida, N.; Watabe-Uchida, M. Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. Elife 2015, 4, e10032. [Google Scholar] [CrossRef]
- Benjet, C.; Bromet, E.; Karam, E.G.; Kessler, R.C.; McLaughlin, K.A.; Ruscio, A.M.; Shahly, V.; Stein, D.J.; Petukhova, M.; Hill, E.; et al. The epidemiology of traumatic event exposure worldwide: Results from the World Mental Health Survey Consortium. Psychol. Med. 2016, 46, 327–343. [Google Scholar] [CrossRef] [Green Version]
- Costello, E.J.; Erkanli, A.; Fairbank, J.A.; Angold, A. The prevalence of potentially traumatic events in childhood and adolescence. J. Trauma. Stress 2002, 15, 99–112. [Google Scholar] [CrossRef]
- Nugent, N.R.; Sumner, J.A.; Amstadter, A.B. Resilience after trauma: From surviving to thriving. Eur. J. Psychotraumatol. 2014, 5, 25339. [Google Scholar] [CrossRef] [Green Version]
- Do, T.T.H.; Correa-Velez, I.; Dunne, M.P. Trauma Exposure and Mental Health Problems Among Adults in Central Vietnam: A Randomized Cross-Sectional Survey. Front. Psychiatry 2019, 10, 31. [Google Scholar] [CrossRef]
- Yehuda, R.; Hoge, C.W.; McFarlane, A.C.; Vermetten, E.; Lanius, R.A.; Nievergelt, C.M.; Hobfoll, S.E.; Koenen, K.C.; Neylan, T.C.; Hyman, S.E. Post-traumatic stress disorder. Nat. Rev. Dis. Primers 2015, 1, 15057. [Google Scholar] [CrossRef]
- Kessler, R.C.; Aguilar-Gaxiola, S.; Alonso, J.; Benjet, C.; Bromet, E.J.; Cardoso, G.; Degenhardt, L.; de Girolamo, G.; Dinolova, R.V.; Ferry, F.; et al. Trauma and PTSD in the WHO World Mental Health Surveys. Eur. J. Psychotraumatol. 2017, 8, 1353383. [Google Scholar] [CrossRef] [Green Version]
- Alisic, E.; Zalta, A.K.; van Wesel, F.; Larsen, S.E.; Hafstad, G.S.; Hassanpour, K.; Smid, G.E. Rates of post-traumatic stress disorder in trauma-exposed children and adolescents: Meta-analysis. Br. J. Psychiatry 2014, 204, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 0-89042-555-8. [Google Scholar]
- Debell, F.; Fear, N.T.; Head, M.; Batt-Rawden, S.; Greenberg, N.; Wessely, S.; Goodwin, L. A systematic review of the comorbidity between PTSD and alcohol misuse. Soc. Psychiatry Psychiatr. Epidemiol. 2014, 49, 1401–1425. [Google Scholar] [CrossRef]
- Khoury, L.; Tang, Y.L.; Bradley, B.; Cubells, J.F.; Ressler, K.J. Substance use, childhood traumatic experience, and Posttraumatic Stress Disorder in an urban civilian population. Depress. Anxiety 2010, 27, 1077–1086. [Google Scholar] [CrossRef]
- Bellos, S.; Petrikis, P.; Malliori, M.; Mavreas, V.; Skapinakis, P. Prevalence of Alcohol Use Disorders and Their Association with Sociodemographic Determinants and Depression/Anxiety Disorders in a Representative Sample of the Greek General Population. Psychiatry J. 2020, 2020, 4841050. [Google Scholar] [CrossRef] [Green Version]
- Skobić, H.; Sinanović, O.; Skobić Bovan, N.; Ivanković, A.; Pejanović Skobić, N. Prevalence of alcohol abuse and alcoholism in general population of Mostar region, Bosnia and Herzegovina. Coll. Antropol. 2010, 34 (Suppl. 1), 29–31. [Google Scholar]
- Stewart, S.H.; Conrod, P.J.; Samoluk, S.B.; Pihl, R.O.; Dongier, M. Posttraumatic Stress Disorder Symptoms and Situation-Specific Drinking in Women Substance Abusers. Alcohol. Treat. Q. 2000, 18, 31–47. [Google Scholar] [CrossRef]
- Jakupcak, M.; Tull, M.T.; McDermott, M.J.; Kaysen, D.; Hunt, S.; Simpson, T. PTSD symptom clusters in relationship to alcohol misuse among Iraq and Afghanistan war veterans seeking post-deployment VA health care. Addict. Behav. 2010, 35, 840–843. [Google Scholar] [CrossRef]
- Golier, J.A.; Yehuda, R.; Schmeidler, J.; Siever, L.J. Variability and severity of depression and anxiety in post traumatic stress disorder and major depressive disorder. Depress. Anxiety 2001, 13, 97–100. [Google Scholar] [CrossRef]
- Flory, J.D.; Yehuda, R. Comorbidity between post-traumatic stress disorder and major depressive disorder: Alternative explanations and treatment considerations. Dialogues Clin. Neurosci. 2015, 17, 141–150. [Google Scholar] [CrossRef]
- Leeies, M.; Pagura, J.; Sareen, J.; Bolton, J.M. The use of alcohol and drugs to self-medicate symptoms of posttraumatic stress disorder. Depress. Anxiety 2010, 27, 731–736. [Google Scholar] [CrossRef]
- Chang, C.; Kaczkurkin, A.N.; McLean, C.P.; Foa, E.B. Emotion regulation is associated with PTSD and depression among female adolescent survivors of childhood sexual abuse. Psychol. Trauma 2018, 10, 319–326. [Google Scholar] [CrossRef]
- Norman, S.B.; Haller, M.; Hamblen, J.L.; Southwick, S.M.; Pietrzak, R.H. The burden of co-occurring alcohol use disorder and PTSD in U.S. Military veterans: Comorbidities, functioning, and suicidality. Psychol. Addict. Behav. 2018, 32, 224–229. [Google Scholar] [CrossRef]
- Petrakis, I.L.; Desai, N.; Gueorguieva, R.; Arias, A.; O’Brien, E.; Jane, J.S.; Sevarino, K.; Southwick, S.; Ralevski, E. Prazosin for Veterans with Posttraumatic Stress Disorder and Comorbid Alcohol Dependence: A Clinical Trial. Alcohol. Clin. Exp. Res. 2016, 40, 178–186. [Google Scholar] [CrossRef]
- Flanagan, J.C.; Jones, J.L.; Jarnecke, A.M.; Back, S.E. Behavioral Treatments for Alcohol Use Disorder and Post-Traumatic Stress Disorder. Alcohol Res. 2018, 39, 181–192. [Google Scholar]
- Hudson, P. Effective treatments for PTSD: Practice guidelines from the International Society for Traumatic Stress Studies. Br. J. Guid. Couns. 2011, 39, 194–195. [Google Scholar] [CrossRef]
- Taft, C.T.; Creech, S.K.; Murphy, C.M. Anger and aggression in PTSD. Curr. Opin. Psychol. 2017, 14, 67–71. [Google Scholar] [CrossRef]
- Taft, C.T.; Watkins, L.E.; Stafford, J.; Street, A.E.; Monson, C.M. Posttraumatic stress disorder and intimate relationship problems: A meta-analysis. J. Consult. Clin. Psychol. 2011, 79, 22–33. [Google Scholar] [CrossRef]
- Misca, G.; Forgey, M.A. The Role of PTSD in Bi-directional Intimate Partner Violence in Military and Veteran Populations: A Research Review. Front. Psychol. 2017, 8, 1394. [Google Scholar] [CrossRef]
- Grattan, R.E.; Lara, N.; Botello, R.M.; Tryon, V.L.; Maguire, A.M.; Carter, C.S.; Niendam, T.A. A History of Trauma is Associated with Aggression, Depression, Non-Suicidal Self-Injury Behavior, and Suicide Ideation in First-Episode Psychosis. J. Clin. Med. 2019, 8, 1082. [Google Scholar] [CrossRef] [Green Version]
- Elbogen, E.B.; Johnson, S.C. The intricate link between violence and mental disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry 2009, 66, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Bomyea, J.; Risbrough, V.; Lang, A.J. A consideration of select pre-trauma factors as key vulnerabilities in PTSD. Clin. Psychol. Rev. 2012, 32, 630–641. [Google Scholar] [CrossRef] [Green Version]
- Korpel, P.O.J.; Varkevisser, T.; Hoppenbrouwers, S.S.; van Honk, J.; Geuze, E. The Predictive Value of Early-Life Trauma, Psychopathy, and the Testosterone-Cortisol Ratio for Impulsive Aggression Problems in Veterans. Chronic Stress 2019, 3, 2470547019871901. [Google Scholar] [CrossRef] [Green Version]
- Brady, K.T.; Killeen, T.K.; Brewerton, T.; Lucerini, S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J. Clin. Psychiatry 2000, 61 (Suppl. 7), 22–32. [Google Scholar]
- Jacobsen, L.K.; Southwick, S.M.; Kosten, T.R. Substance use disorders in patients with posttraumatic stress disorder: A review of the literature. Am. J. Psychiatry 2001, 158, 1184–1190. [Google Scholar] [CrossRef]
- Dyer, K.F.W.; Dorahy, M.J.; Shannon, M.; Corry, M. Trauma typology as a risk factor for aggression and self-harm in a complex PTSD population: The mediating role of alterations in self-perception. J. Trauma Dissociation 2013, 14, 56–68. [Google Scholar] [CrossRef]
- Watkins, L.E.; Sprang, K.R.; Rothbaum, B.O. Treating PTSD: A Review of Evidence-Based Psychotherapy Interventions. Front. Behav. Neurosci. 2018, 12, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, W. Pharmacotherapy for Post-traumatic Stress Disorder in Combat Veterans: Focus on Antidepressants and Atypical Antipsychotic Agents. Pharm. Ther. 2012, 37, 32–38. [Google Scholar]
- Monnelly, E.P.; Ciraulo, D.A.; Knapp, C.; Keane, T. Low-dose risperidone as adjunctive therapy for irritable aggression in posttraumatic stress disorder. J. Clin. Psychopharmacol. 2003, 23, 193–196. [Google Scholar] [CrossRef]
- Almli, L.M.; Fani, N.; Smith, A.K.; Ressler, K.J. Genetic approaches to understanding post-traumatic stress disorder. Int. J. Neuropsychopharmacol. 2014, 17, 355–370. [Google Scholar] [CrossRef] [Green Version]
- Verhulst, B.; Neale, M.C.; Kendler, K.S. The heritability of alcohol use disorders: A meta-analysis of twin and adoption studies. Psychol. Med. 2015, 45, 1061–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuvblad, C.; Baker, L.A. Human aggression across the lifespan: Genetic propensities and environmental moderators. Adv. Genet. 2011, 75, 171–214. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.Y.; Chung, H.G.; Shin, H.-S.; Kim, S.J.; Choi, J.H.; Chung, M.Y.; An, S.K.; Choi, T.K.; So, H.S.; Cho, H.-S. Apolipoprotein e gene polymorphism, alcohol use, and their interactions in combat-related posttraumatic stress disorder. Depress. Anxiety 2013, 30, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.E.; Han, S.; Harpaz-Rotem, I.; Mota, N.P.; Southwick, S.M.; Krystal, J.H.; Gelernter, J.; Pietrzak, R.H. FKBP5 polymorphisms, childhood abuse, and PTSD symptoms: Results from the National Health and Resilience in Veterans Study. Psychoneuroendocrinology 2016, 69, 98–105. [Google Scholar] [CrossRef]
- Xie, P.; Kranzler, H.R.; Poling, J.; Stein, M.B.; Anton, R.F.; Farrer, L.A.; Gelernter, J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology 2010, 35, 1684–1692. [Google Scholar] [CrossRef] [Green Version]
- Frazzetto, G.; Di Lorenzo, G.; Carola, V.; Proietti, L.; Sokolowska, E.; Siracusano, A.; Gross, C.; Troisi, A. Early trauma and increased risk for physical aggression during adulthood: The moderating role of MAOA genotype. PLoS ONE 2007, 2, e486. [Google Scholar] [CrossRef] [Green Version]
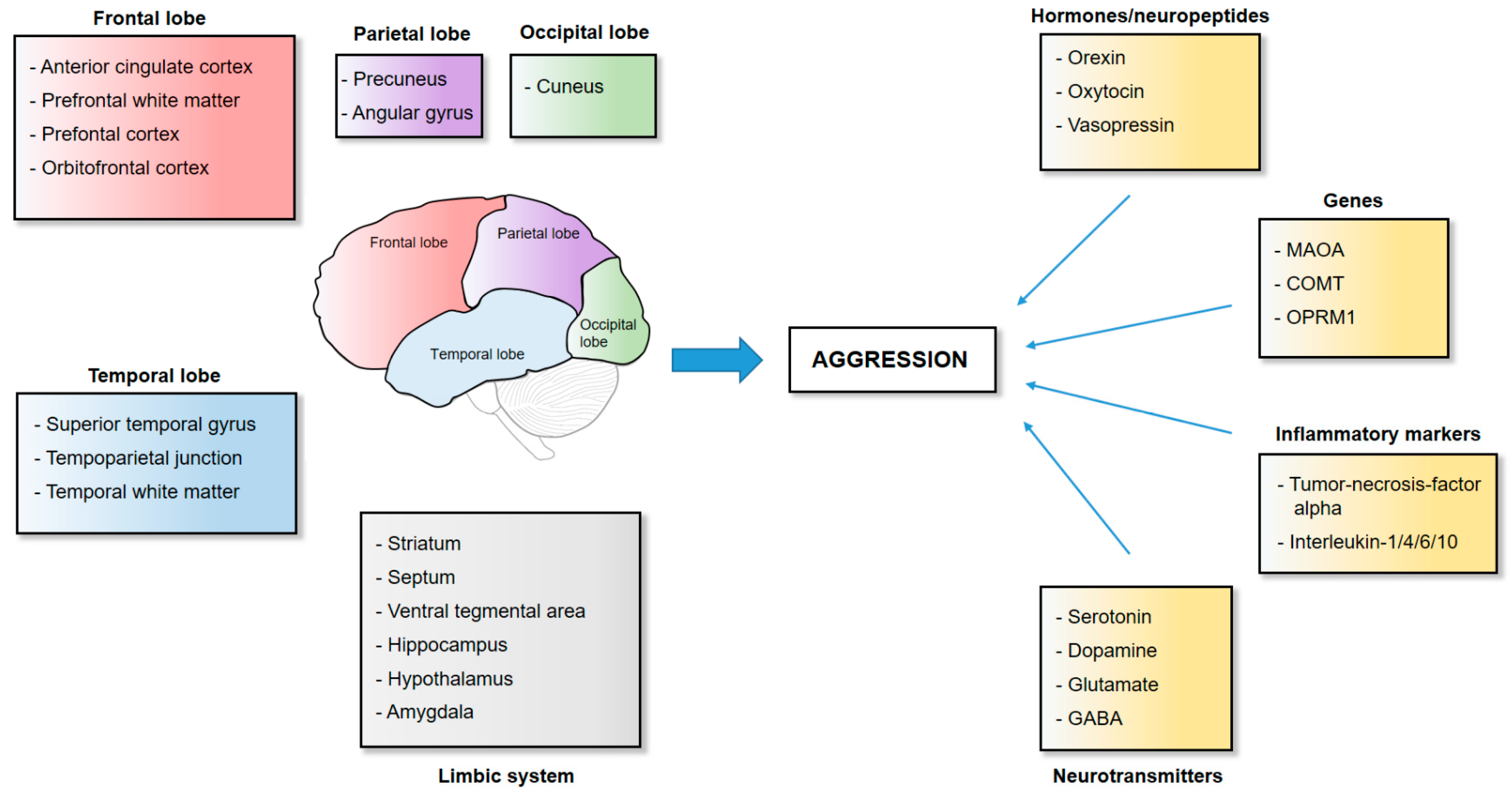

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritz, M.; Soravia, S.-M.; Dudeck, M.; Malli, L.; Fakhoury, M. Neurobiology of Aggression—Review of Recent Findings and Relationship with Alcohol and Trauma. Biology 2023, 12, 469. https://doi.org/10.3390/biology12030469
Fritz M, Soravia S-M, Dudeck M, Malli L, Fakhoury M. Neurobiology of Aggression—Review of Recent Findings and Relationship with Alcohol and Trauma. Biology. 2023; 12(3):469. https://doi.org/10.3390/biology12030469
Chicago/Turabian StyleFritz, Michael, Sarah-Maria Soravia, Manuela Dudeck, Layal Malli, and Marc Fakhoury. 2023. "Neurobiology of Aggression—Review of Recent Findings and Relationship with Alcohol and Trauma" Biology 12, no. 3: 469. https://doi.org/10.3390/biology12030469







