Systemic Prenatal Stress Exposure through Corticosterone Application Adversely Affects Avian Embryonic Skin Development
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chicken Embryo Treatment
2.2. Histological Analysis
2.3. Immunohistochemical Analysis
2.4. Whole-Mount In Situ Hybridization (ISH)
2.5. TUNEL Analysis
2.6. RNA Isolation, Reverse Transcription, and Real-Time PCR (RT-PCR)
2.7. Statistical Analysis
3. Results
3.1. Prenatal Stress Impairs Angiogenesis of the Chorioallantoic Membrane (CAM)
3.2. Prenatal Stress Increases the Risk of Developmental Deficits
3.3. Prenatal Stress Reduces Embryonic Growth
3.4. Prenatal Stress Reduces the Expression of Dermo-1
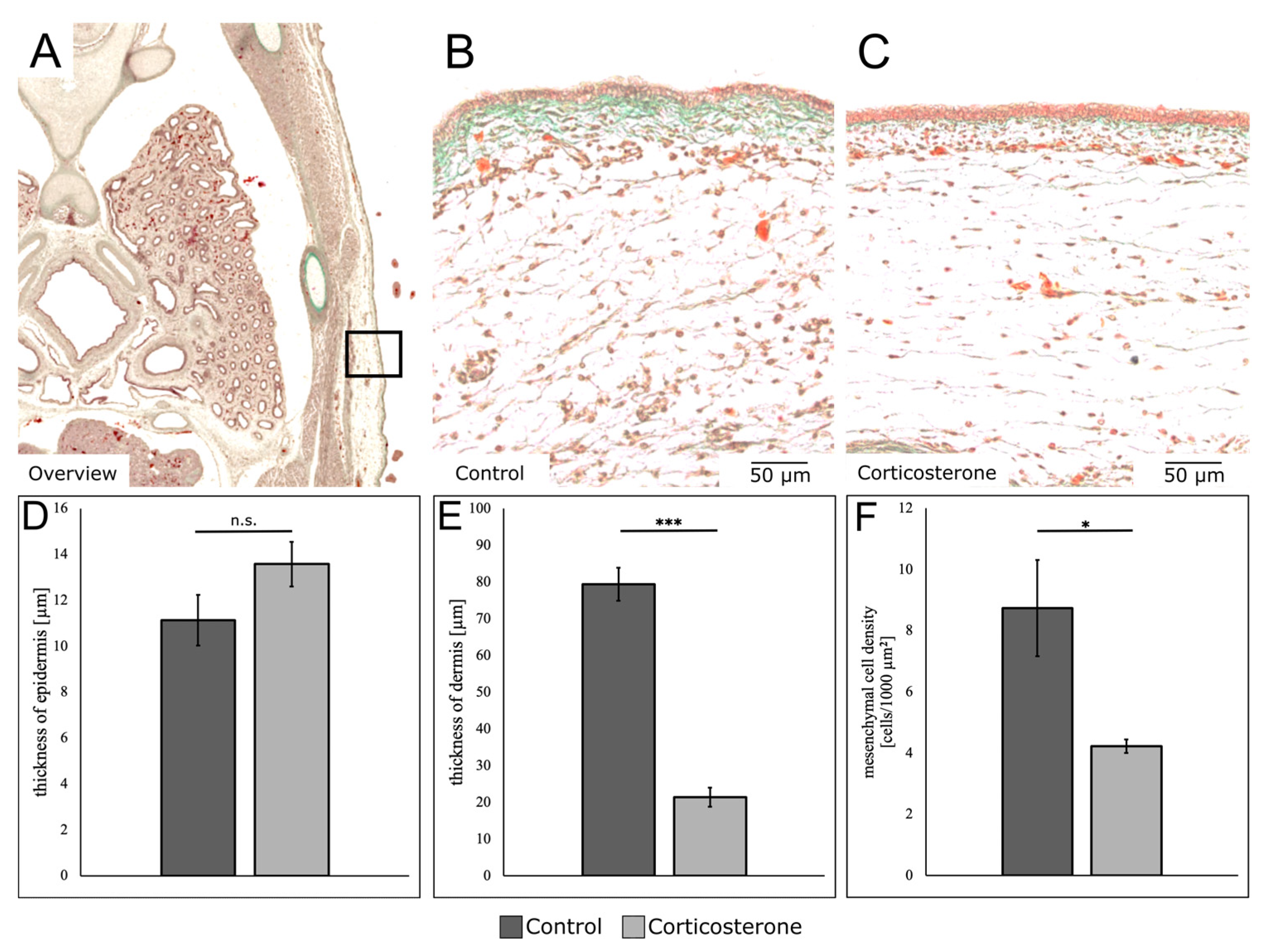
3.5. Prenatal Stress Alters the Composition of the Different Skin Layers
3.6. Prenatal Stress Reduces the Expression of Mesenchymal Markers
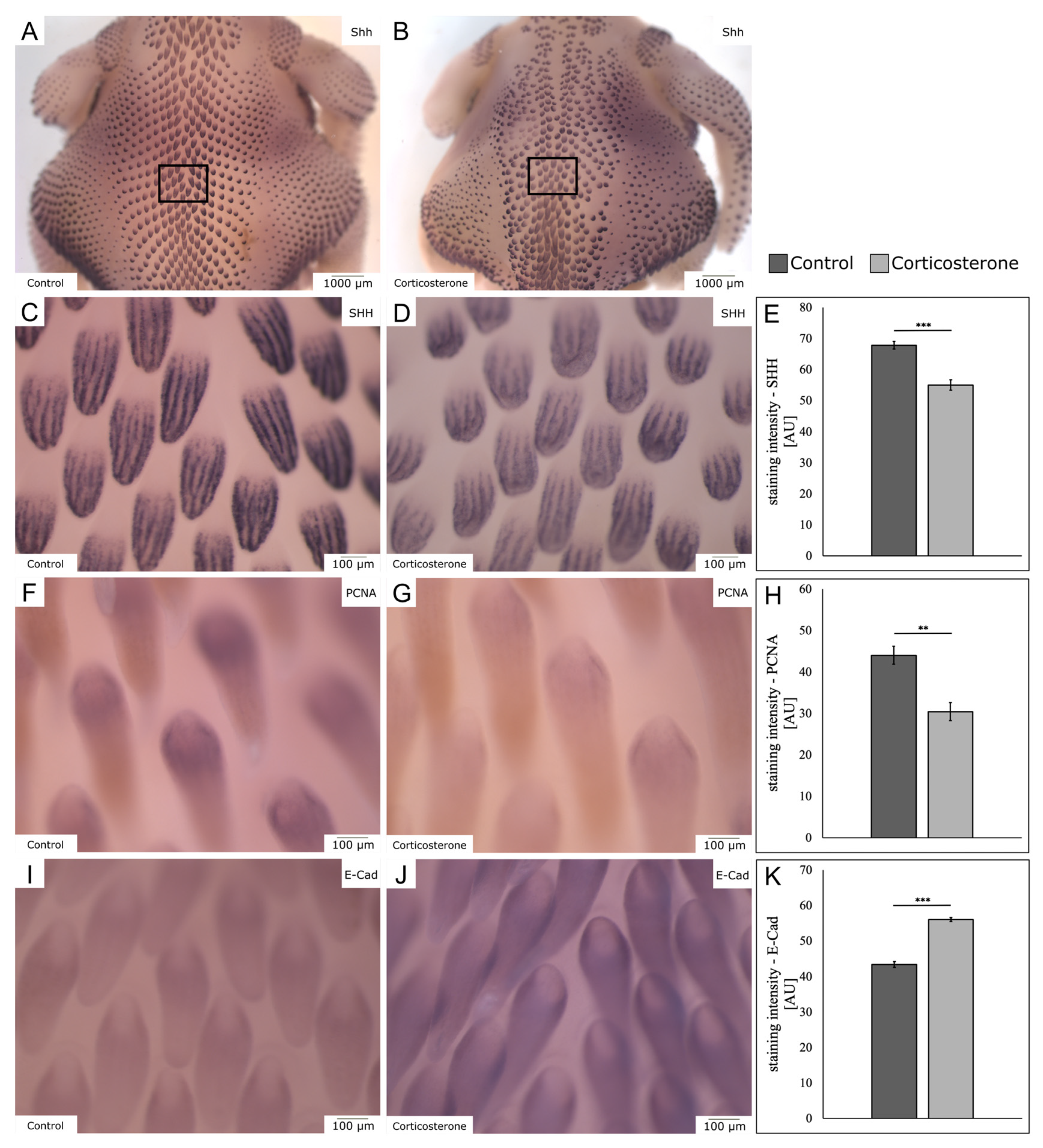
3.7. Prenatal Stress Reduces the Mitotic Activity in the Skin
3.8. Prenatal Stress Impairs Embryonic Skin Appendage Formation
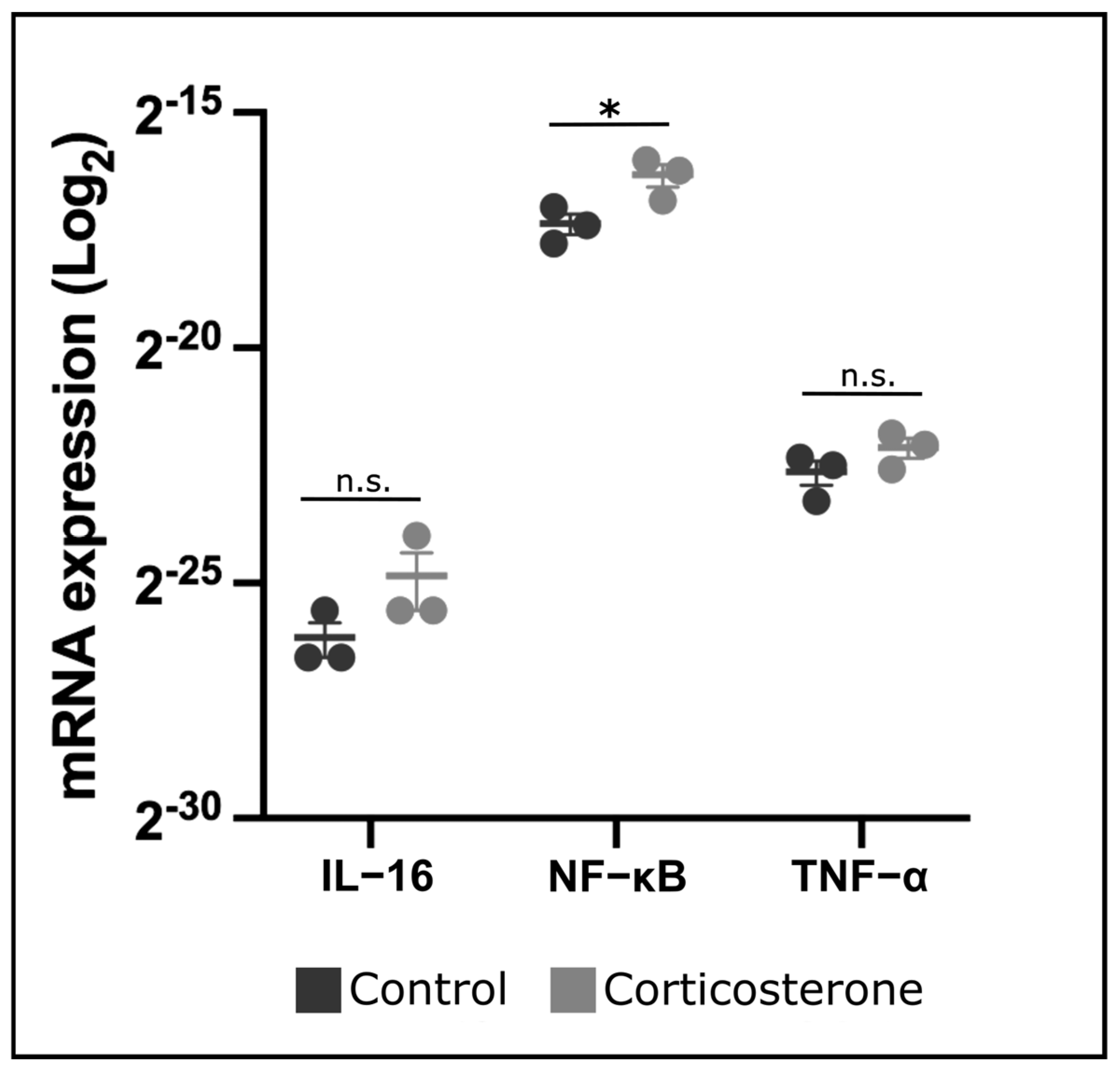
3.9. Prenatal Stress Increases Expression of NF-κB in the Skin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Li, N.; Chen, R.; Lee, T.; Gao, Y.; Yuan, Z.; Nie, Y.; Sun, T. Prenatal Stress Leads to Deficits in Brain Development, Mood Related Behaviors and Gut Microbiota in Offspring. Neurobiol. Stress. 2021, 15, 100333. [Google Scholar] [CrossRef]
- Brannigan, R.; Tanskanen, A.; Huttunen, M.O.; Cannon, M.; Leacy, F.P.; Clarke, M.C. The Role of Prenatal Stress as a Pathway to Personality Disorder: Longitudinal Birth Cohort Study. Br. J. Psychiatry 2020, 216, 190. [Google Scholar] [CrossRef]
- Walsh, K.; McCormack, C.A.; Webster, R.; Pinto, A.; Lee, S.; Feng, T.; Sloan Krakovsky, H.; O’Grady, S.M.; Tycko, B.; Champagne, F.A.; et al. Maternal Prenatal Stress Phenotypes Associate with Fetal Neurodevelopment and Birth Outcomes. Proc. Natl. Acad. Sci. USA 2019, 116, 90589. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, N.C.; Kyratzi, E.; Lamprokostopoulou, A.; Chrousos, G.P.; Charmandari, E. Stress, the Stress System and the Role of Glucocorticoids. Neuroimmunomodulation 2014, 2, 89. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, B.R.H.; Mulder, E.J.H.; Mennes, M.; Glover, V. Antenatal Maternal Anxiety and Stress and the Neurobehavioural Development of the Fetus and Child: Links and Possible Mechanisms. A Review. Neurosci. Biobehav. Rev. 2005, 29, 237–258. [Google Scholar] [CrossRef]
- Gitau, R.; Cameron, A.; Fisk, N.M.; Glover, V. Fetal Exposure to Maternal Cortisol. Lancet 1998, 352, 707–708. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The Biology of the Glucocorticoid Receptor: New Signaling Mechanisms in Health and Disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Topete, D.; Cidlowski, J.A. One Hormone, Two Actions: Anti- and pro-Inflammatory Effects of Glucocorticoids. Neuroimmunomodulation 2014, 22, 20–32. [Google Scholar] [CrossRef]
- De Bosscher, K.; Haegeman, G. Minireview: Latest Perspectives on Antiinflammatory Actions of Glucocorticoids. Mol. Endocrinol. 2009, 23, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Vegiopoulos, A.; Herzig, S. Glucocorticoids, Metabolism and Metabolic Diseases. Mol. Cell. Endocrinol. 2007, 275, 43–61. [Google Scholar] [CrossRef]
- Nussinovitch, U.; Freire de Carvalho, J.; Pereira, M.R.R.; Shoenfeld, Y. Glucocorticoids and the Cardiovascular System: State of the Art. Curr. Pharm. Des. 2010, 16, 3574–3585. [Google Scholar] [CrossRef]
- Fowden, A.L.; Forhead, A.J. Glucocorticoids as Regulatory Signals during Intrauterine Development. Exp. Physiol. 2015, 100, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Rog-Zielinska, E.A.; Richardson, R.V.; Denvir, M.A.; Chapman, K.E. Glucocorticoids and Foetal Heart Maturation; Implications for Prematurity and Foetal Programming. J. Mol. Endocrinol. 2014, 52, R125–R135. [Google Scholar] [CrossRef]
- Oitzl, M.S.; Champagne, D.L.; van der Veen, R.; de Kloet, E.R. Brain Development under Stress: Hypotheses of Glucocorticoid Actions Revisited. Neurosci. Biobehav. Rev. 2010, 34, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Gokulakrishnan, G.; Chang, X.; Fleischmann, R.; Fiorotto, M.L. Precocious Glucocorticoid Exposure Reduces Skeletal Muscle Satellite Cells in the Fetal Rat. J. Endocrinol. 2017, 232, 561–572. [Google Scholar] [CrossRef][Green Version]
- Abdo, J.M.; Sopko, N.A.; Milner, S.M. The Applied Anatomy of Human Skin: A Model for Regeneration. Wound Medicine 2020, 28, 86. [Google Scholar] [CrossRef]
- Niculet, E.; Bobeica, C.; Tatu, A.L. Glucocorticoid-Induced Skin Atrophy: The Old and the New. Clin. Cosmet. Investig. Dermatol. 2020, 13, 1041–1050. [Google Scholar] [CrossRef]
- Arima, M.; Plitt, J.; Stellato, C.; Bickel, C.; Motojima, S.; Makino, S.; Fukuda, T.; Schleimer, R.P. Expression of Interleukin-16 by Human Epithelial Cells. Inhibition by Dexamethasone. Am. J. Respir. Cell Mol. Biol. 1999, 21, 684–692. [Google Scholar] [CrossRef]
- Auphan, N.; DiDonato, J.A.; Rosette, C.; Helmberg, A.; Karin, M. Immunosuppression by Glucocorticoids: Inhibition of NF-Kappa B Activity through Induction of I Kappa B Synthesis. Science 1995, 270, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, I. The Effects of the Glucocorticoids on Connective Tissue. Acta Med. Scand. 1969, 185, 17–20. [Google Scholar] [CrossRef]
- Houck, J.C.; Patel, Y.M. Proposed Mode of Action of Corticosteroids on the Connective Tissue. Nature 1965, 2, 12. [Google Scholar]
- Asboe-Hansen, G. Influence of Corticosteroids on Connective Tissue. Dermatology 1976, 152, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A. Glucocorticoids Inhibit Wound Healing: Novel Mechanism of Action. J. Investig. Dermatol. 2017, 137, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Bablok, M.; Gellisch, M.; Brand-Saberi, B.; Morosan-Puopolo, G. Local Glucocorticoid Administration Impairs Embryonic Wound Healing. Biomedicines 2022, 10, 3125. [Google Scholar] [CrossRef] [PubMed]
- Gruver-Yates, A.L.; Cidlowski, J.A. Tissue-Specific Actions of Glucocorticoids on Apoptosis: A Double-Edged Sword. Cells 2013, 2, 202–223. [Google Scholar] [CrossRef]
- le Douarin, N.M.; Ziller, C.; Couly, G.F. Patterning of Neural Crest Derivatives in the Avian Embryo: In Vivo and In Vitro Studies. Dev. Biol. 1993, 159, 1219. [Google Scholar] [CrossRef]
- Christ, B.; Huang, R.; Scaal, M. Amniote Somite Derivatives. Dev. Dyn. 2007, 236, 2382–2396. [Google Scholar] [CrossRef] [PubMed]
- Mauger, A. Role of Neural Tube in the Development of the Dorsal Plumage in the Chick Embryo. Wilhelm. Roux Arch. Entwickl. Mech. Org. 1972, 170, 680. [Google Scholar] [CrossRef]
- Christ, B.; Jacob, M.; Jacob, H.J. On the Origin and Development of the Ventrolateral Abdominal Muscles in the Avian Embryo. Anat. Embryol. 1983, 166, 7946. [Google Scholar] [CrossRef]
- Zhi, Q.; Huang, R.; Christ, B.; Brand-Saberi, B. Participation of Individual Brachial Somites in Skeletal Muscles of the Avian Distal Wing. Anat. Embryol. 1996, 194, 8534. [Google Scholar] [CrossRef]
- Wessells, N.K. Morphology and Proliferation during Early Feather Development. Dev. Biol. 1965, 12, 131–153. [Google Scholar] [CrossRef]
- Christ, B.; Scaal, M. Formation and Differentiation of Avian Somite Derivatives. In Somitogenesis; Springer: New York, NY, USA, 2008; pp. 1–41. [Google Scholar]
- Li, L.; Cserjesi, P.; Olson, E.N. Dermo-1: A Novel Twist-Related BHLH Protein Expressed in the Developing Dermis. Dev. Biol. 1995, 172, 280–292. [Google Scholar] [CrossRef]
- Scaal, M.; Füchtbauer, E.-M.; Brand-Saberi, B. CDermo-1 Expression Indicates a Role in Avian Skin Development. Anat. Embryol. 2001, 203, 1–7. [Google Scholar] [CrossRef]
- Eckes, B.; Colucci-Guyon, E.; Smola, H.; Nodder, S.; Babinet, C.; Krieg, T.; Martin, P. Impaired Wound Healing in Embryonic and Adult Mice Lacking Vimentin. J. Cell Sci. 2000, 113, 2455–2462. [Google Scholar] [CrossRef]
- Pankov, R.; Yamada, K.M. Fibronectin at a Glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef]
- Kurki, P.; Vanderlaan, M.; Dolbeare, F.; Gray, J.; Tan, E.M. Expression of Proliferating Cell Nuclear Antigen (PCNA)/Cyclin during the Cell Cycle. Exp. Cell Res. 1986, 166, 209–219. [Google Scholar] [CrossRef]
- Elmaci, İ.; Altinoz, M.A.; Sari, R.; Bolukbasi, F.H. Phosphorylated Histone H3 (PHH3) as a Novel Cell Proliferation Marker and Prognosticator for Meningeal Tumors: A Short Review. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 627–631. [Google Scholar] [CrossRef]
- Nohno, T.; Kawakami, Y.; Ohuchi, H.; Fujiwara, A.; Yoshioka, H.; Noji, S. Involvement of the Sonic Hedgehog Gene in Chick Feather Formation. Biochem. Biophys. Res. Commun. 1995, 206, 33–39. [Google Scholar] [CrossRef]
- Ting-Berreth, S.A.; Chuong, C.-M. Sonic Hedgehogin Feather Morphogenesis: Induction of Mesenchymal Condensation and Association with Cell Death. Dev. Dyn. 1996, 207, 157–170. [Google Scholar] [CrossRef]
- Young, P.; Boussadia, O.; Halfter, H.; Grose, R.; Berger, P.; Leone, D.P.; Robenek, H.; Charnay, P.; Kemler, R.; Suter, U. E-Cadherin Controls Adherens Junctions in the Epidermis and the Renewal of Hair Follicles. EMBO J. 2003, 22, 5723–5733. [Google Scholar] [CrossRef]
- de Groef, B.; Grommen, S.V.H.; Darras, V.M. The Chicken Embryo as a Model for Developmental Endocrinology: Development of the Thyrotropic, Corticotropic, and Somatotropic Axes. Mol. Cell. Endocrinol. 2008, 293, 17–24. [Google Scholar] [CrossRef]
- Austdal, L.P.E.; Bjørnstad, S.; Mathisen, G.H.; Aden, P.K.; Mikkola, I.; Paulsen, R.E.; Rakkestad, K.E. Glucocorticoid Effects on Cerebellar Development in a Chicken Embryo Model: Exploring Changes in PAX6 and Metalloproteinase-9 After Exposure to Dexamethasone. J. Neuroendocrinol. 2016, 28, 12438. [Google Scholar] [CrossRef]
- Ribeiro, L.N.M.; Schlemper, A.E.; da Silva, M.V.; Fonseca, B.B. Chicken Embryo: A Useful Animal Model for Drug Testing? Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4828–4839. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Essa, M.E.A. In Ovo Injection Corticosterone Method for Physiological and Behavioral Studies in Chickens. MethodsX 2020, 7, 100908. [Google Scholar] [CrossRef]
- Bablok, M.; Gellisch, M.; Scharf, M.; Brand-Saberi, B.; Morosan-Puopolo, G. Spatiotemporal Expression Pattern of the Chicken Glucocorticoid Receptor during Early Embryonic Development. Ann. Anat. 2023, 247, 152056. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A Series of Normal Stages in the Development of the Chick Embryo. J. Morphol. 1951, 88, 404. [Google Scholar] [CrossRef]
- Heiblum, R.; Arnon, E.; Chazan, G.; Robinzon, B.; Gvaryahu, G.; Snapir, N. Glucocorticoid Administration During Incubation: Embryo Mortality and Posthatch Growth in Chickens. Poult. Sci. 2001, 80, 1357–1363. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Nieto, M.A.; Patel, K.; Wilkinson, D.G. In Situ Hybridization Analysis of Chick Embryos in Whole Mount and Tissue Sections. Methods Cell Biol. 1996, 51, 219–235. [Google Scholar] [CrossRef]
- Yahya, I.; Böing, M.; Brand-Saberi, B.; Morosan-Puopolo, G. How to Distinguish between Different Cell Lineages Sharing Common Markers Using Combinations of Double In-Situ-Hybridization and Immunostaining in Avian Embryos: CXCR4-Positive Mesodermal and Neural Crest-Derived Cells. Histochem. Cell Biol. 2021, 155, 145–155. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; di Leo, F.; Giardina, L. Prenatal Stress Induces Body Weight Deficit and Behavioural Alterations in Rats: The Effect of Diazepam. Eur. Neuropsychopharmacol. 1999, 9, 239–245. [Google Scholar] [CrossRef]
- McNatt, L.G.; Weimer, L.; Yanni, J.; Clark, A.F. Angiostatic Activity of Steroids in the Chick Embryo CAM and Rabbit Cornea Models of Neovascularization. J. Ocul. Pharmacol. Ther. 1999, 15, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Clausen, C.F.; Morton, J.S.; Davidge, S.T. Effects of Hypoxia-Induced Intrauterine Growth Restriction on Cardiopulmonary Structure and Function during Adulthood. Cardiovasc. Res. 2009, 81, 713–722. [Google Scholar] [CrossRef]
- Nalivaeva, N.N.; Turner, A.J.; Zhuravin, I.A. Role of Prenatal Hypoxia in Brain Development, Cognitive Functions, and Neurodegeneration. Front. Neurosci. 2018, 12, 825. [Google Scholar] [CrossRef]
- Xu, S.; He, X.; Shi, J.; Li, Z.; Song, J.; Wang, J.; Wang, G.; Brand-Saberi, B.; Cheng, X.; Yang, X. Interaction between Retinoic Acid and FGF/ERK Signals Are Involved in Dexamethasone-Induced Abnormal Myogenesis during Embryonic Development. Toxicology 2021, 461, 152917. [Google Scholar] [CrossRef] [PubMed]
- Hornik, C.; Krishan, K.; Yusuf, F.; Scaal, M.; Brand-Saberi, B. CDermo-1 Misexpression Induces Dense Dermis, Feathers, and Scales. Dev. Biol. 2005, 277, 42–50. [Google Scholar] [CrossRef]
- Jacob, T.; Chakravarty, A.; Panchal, A.; Patil, M.; Ghodadra, G.; Sudhakaran, J.; Nuesslein-Volhard, C. Zebrafish Twist2/Dermo1 Regulates Scale Shape and Scale Organization during Skin Development and Regeneration. Cells Dev. 2021, 166, 203684. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.; Unemori, E.; Hunt, T.K.; Amento, E. Transforming Growth Factor Beta (TGF-β and Dexamethasone Have Direct Opposing Effects on Collagen Metabolism in Low Passage Human Dermal Fibroblasts in Vitro. Growth Factors 1994, 11, 205–213. [Google Scholar] [CrossRef]
- Pratt, W.B. The Mechanism of Glucocorticoid Effects in Fibroblasts. J. Investig. Dermatol. 1978, 71, 24–35. [Google Scholar] [CrossRef]
- Zhang, L.; Lei, W.; Wang, X.; Tang, Y.; Song, J. Glucocorticoid Induces Mesenchymal-to-Epithelial Transition and Inhibits TGF-Β1-Induced Epithelial-to-Mesenchymal Transition and Cell Migration. FEBS Lett. 2010, 584, 38. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Yang, H.-W.; Lee, S.A.; Park, J.-H.; Heo, M.; Shin, J.-M. Inhibitory Effect of Glucocorticoids on TGF-Β1-Mediated Epithelial-to-Mesenchymal Transition of Airway Epithelium via MAPK and Snail/Slug Signaling Pathways. J. Allergy Clin. Immunol. 2018, 141, AB66. [Google Scholar] [CrossRef]
- Pérez, P.; Page, A.; Bravo, A.; Río, M.; Giménez-Conti, I.; Budunova, I.; Slaga, T.J.; Jorcano, J.L. Altered Skin Development and Impaired Proliferative and Inflammatory Responses in Transgenic Mice Overexpressing the Glucocorticoid Receptor. FASEB J. 2001, 15, 2030–2032. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, A.; Hirai, A.; Thompson, E.A. Glucocorticoid Inhibition of Fibroblast Proliferation and Regulation of the Cyclin Kinase Inhibitor P21Cip1. Mol. Endocrinol. 1997, 11, 577–586. [Google Scholar] [CrossRef]
- Schacke, H. Mechanisms Involved in the Side Effects of Glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Sur, I.; Ulvmar, M.; Toftgård, R. The Two-Faced NF-ΚB in the Skin. Int. Rev. Immunol. 2008, 27, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Ullrich, R.; Aebischer, T.; Hülsken, J.; Birchmeier, W.; Klemm, U.; Scheidereit, C. Requirement of NF-ΚB/Rel for the Development of Hair Follicles and Other Epidermal Appendices. Development 2001, 128, 3843–3853. [Google Scholar] [CrossRef]
- Coussons-Read, M.E. Effects of Prenatal Stress on Pregnancy and Human Development: Mechanisms and Pathways. Obstet. Med. 2013, 6, 52–57. [Google Scholar] [CrossRef]
- Diz-Chaves, Y.; Astiz, M.; Bellini, M.J.; Garcia-Segura, L.M. Prenatal Stress Increases the Expression of Proinflammatory Cytokines and Exacerbates the Inflammatory Response to LPS in the Hippocampal Formation of Adult Male Mice. Brain Behav. Immun. 2013, 28, 196–206. [Google Scholar] [CrossRef]
- Rao, N.A.S.; McCalman, M.T.; Moulos, P.; Francoijs, K.-J.; Chatziioannou, A.; Kolisis, F.N.; Alexis, M.N.; Mitsiou, D.J.; Stunnenberg, H.G. Coactivation of GR and NFKB Alters the Repertoire of Their Binding Sites and Target Genes. Genome Res. 2011, 21, 1404–1416. [Google Scholar] [CrossRef]
- Bekhbat, M.; Rowson, S.A.; Neigh, G.N. Checks and Balances: The Glucocorticoid Receptor and NFĸB in Good Times and Bad. Front. Neuroendocrinol. 2017, 46, 15–31. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gellisch, M.; Bablok, M.; Divvela, S.S.K.; Morosan-Puopolo, G.; Brand-Saberi, B. Systemic Prenatal Stress Exposure through Corticosterone Application Adversely Affects Avian Embryonic Skin Development. Biology 2023, 12, 656. https://doi.org/10.3390/biology12050656
Gellisch M, Bablok M, Divvela SSK, Morosan-Puopolo G, Brand-Saberi B. Systemic Prenatal Stress Exposure through Corticosterone Application Adversely Affects Avian Embryonic Skin Development. Biology. 2023; 12(5):656. https://doi.org/10.3390/biology12050656
Chicago/Turabian StyleGellisch, Morris, Martin Bablok, Satya Srirama Karthik Divvela, Gabriela Morosan-Puopolo, and Beate Brand-Saberi. 2023. "Systemic Prenatal Stress Exposure through Corticosterone Application Adversely Affects Avian Embryonic Skin Development" Biology 12, no. 5: 656. https://doi.org/10.3390/biology12050656
APA StyleGellisch, M., Bablok, M., Divvela, S. S. K., Morosan-Puopolo, G., & Brand-Saberi, B. (2023). Systemic Prenatal Stress Exposure through Corticosterone Application Adversely Affects Avian Embryonic Skin Development. Biology, 12(5), 656. https://doi.org/10.3390/biology12050656





