Rab3A/Rab27A System Silencing Ameliorates High Glucose-Induced Injury in Podocytes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Podocyte Cell Culture
2.2. Cell Transfection and Glucose Treatment
2.3. Exosome Isolation from Podocyte Cultures
2.4. Nanoparticle Tracking Analyses (NTA)
2.5. Transmission Electron Microscopy (TEM)
2.6. RNA Extraction and cDNA Synthesis
2.7. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.8. Cell Pellet and Exosome Homogenization, Electrophoresis and Western Blot Analyses
2.9. Immunocytochemistry
2.10. Cell Apoptosis Assay
2.11. Statistical Analysis
3. Results
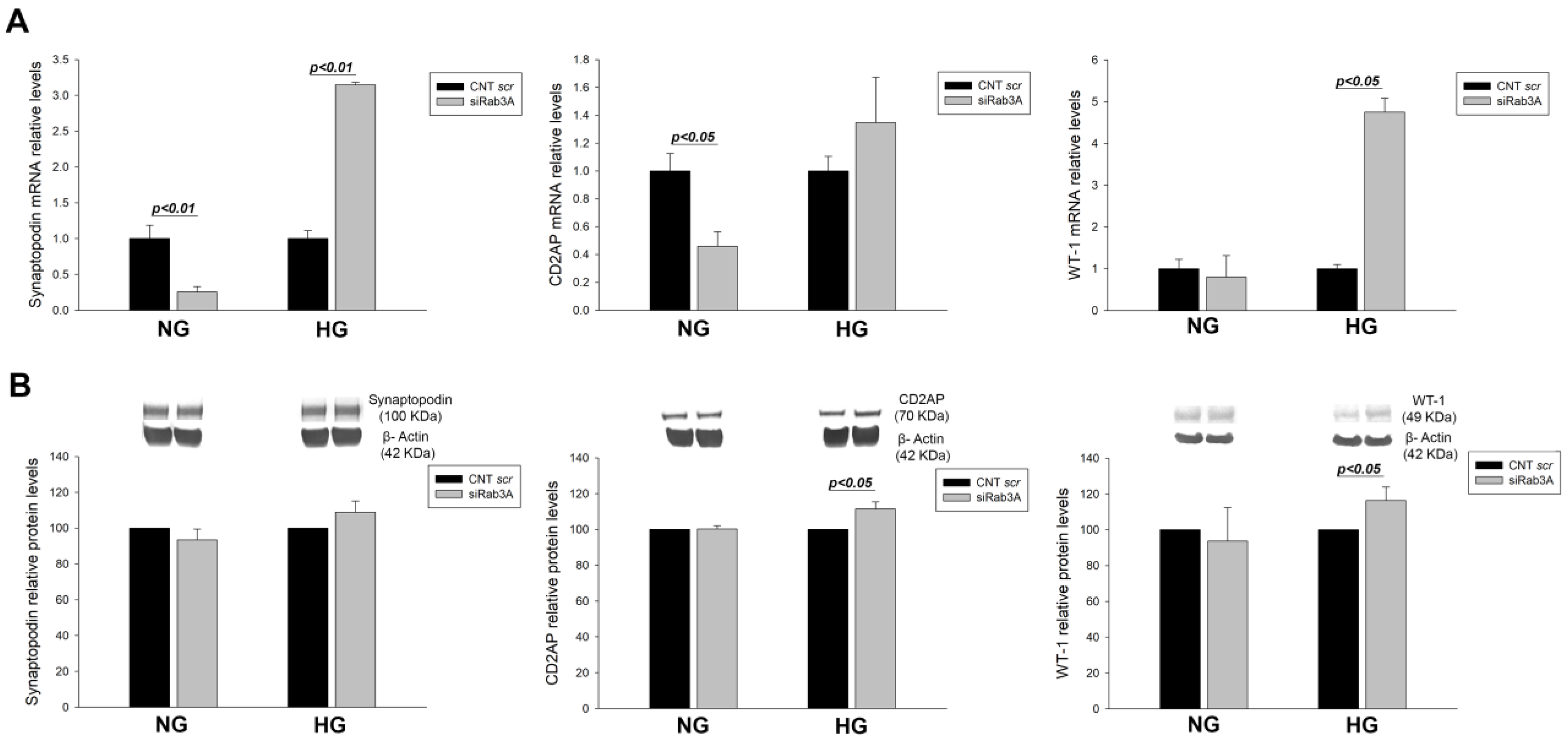
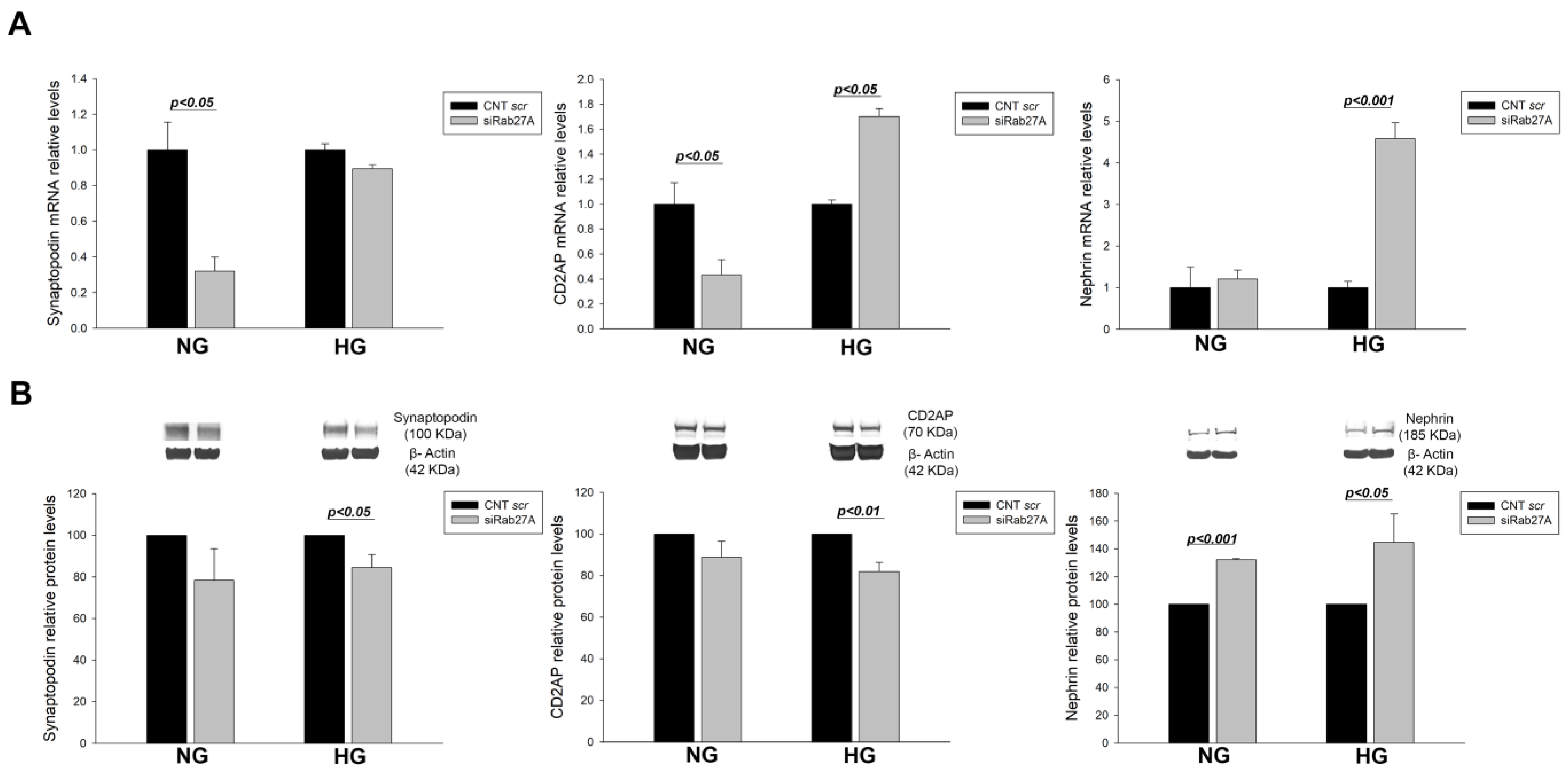
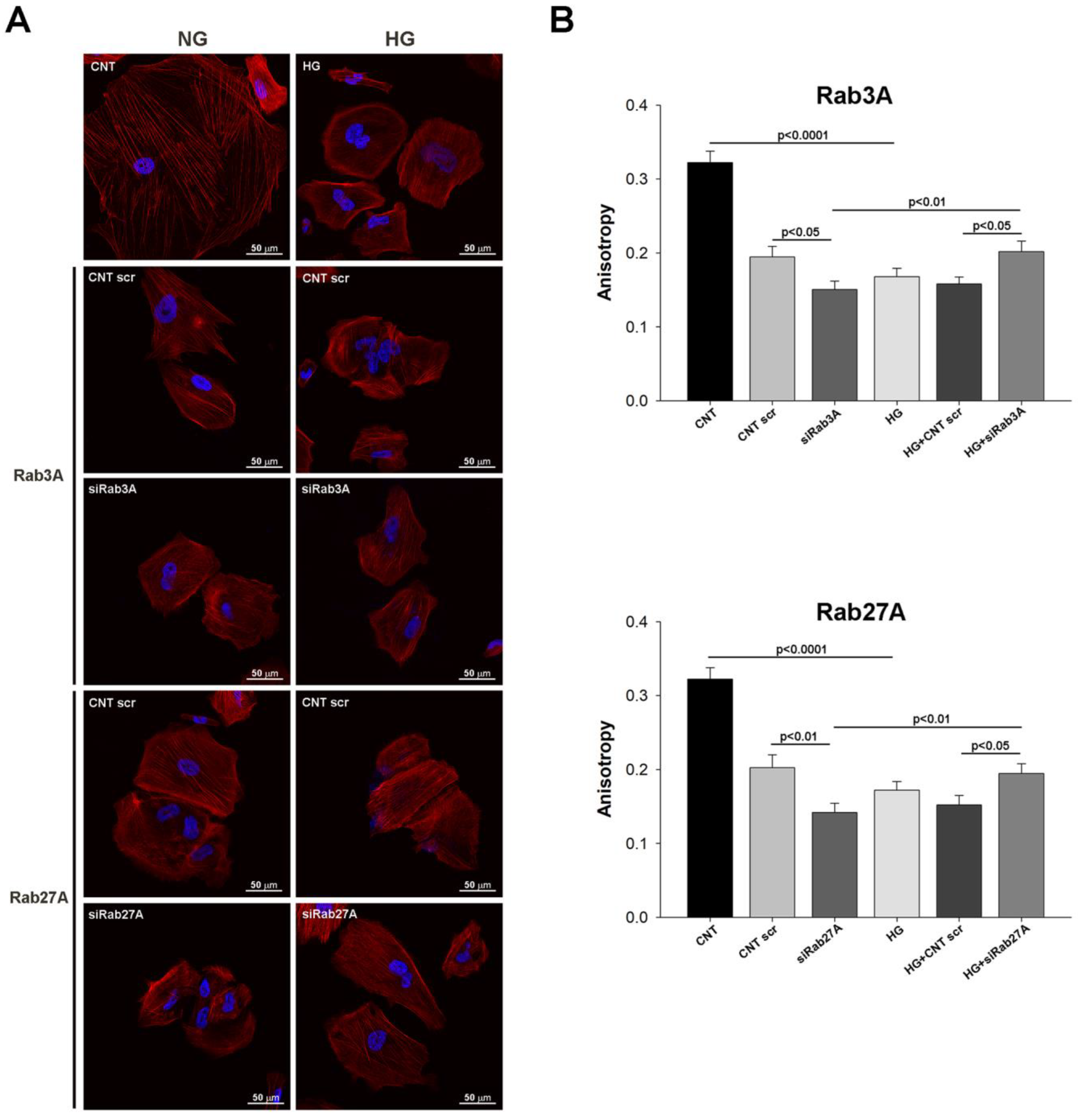
3.1. Effect of RAB3A and RAB27A Gene Silencing and Treatment with High Glucose on the Rab3A/27A System and Podocyte Markers
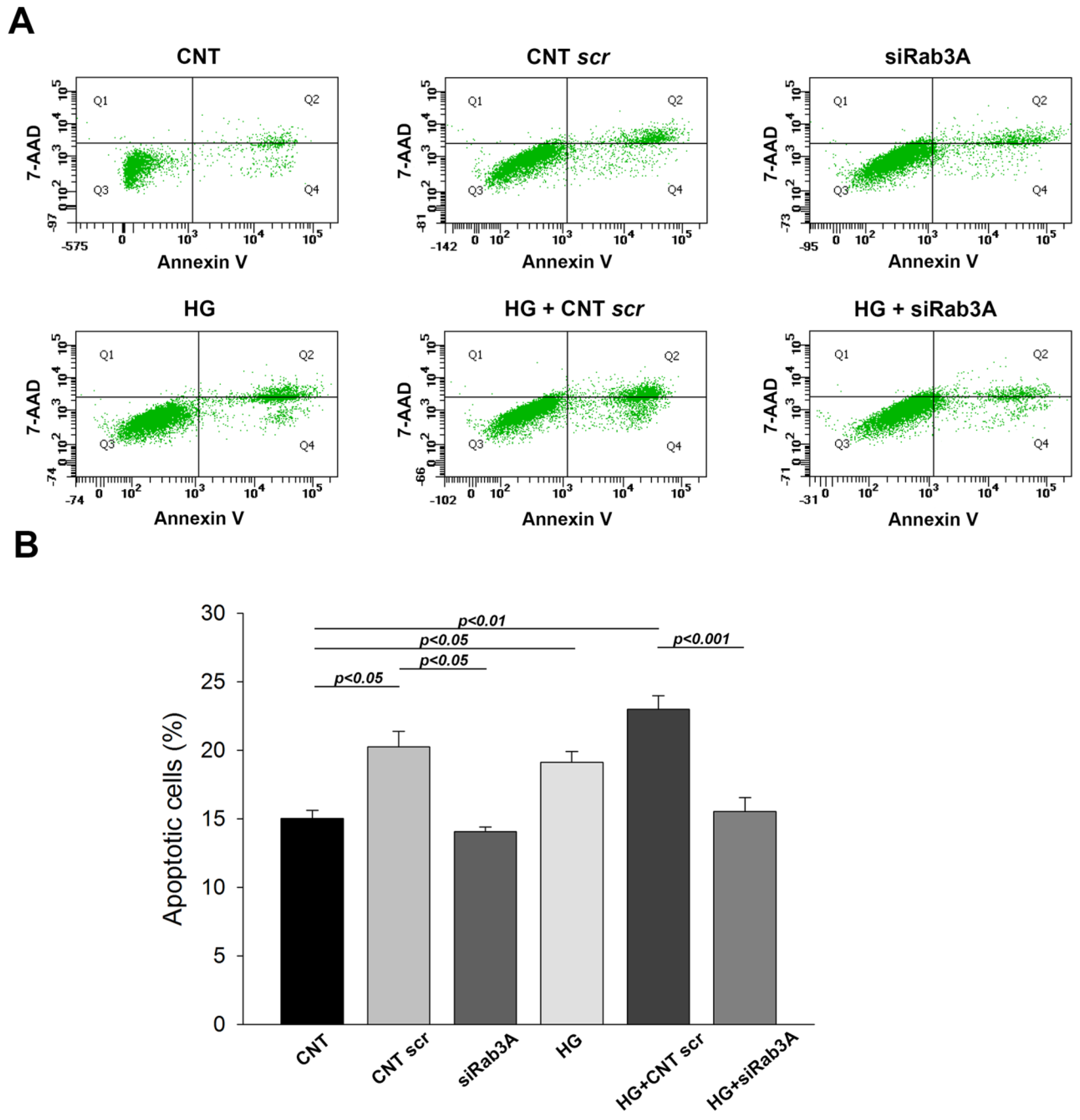
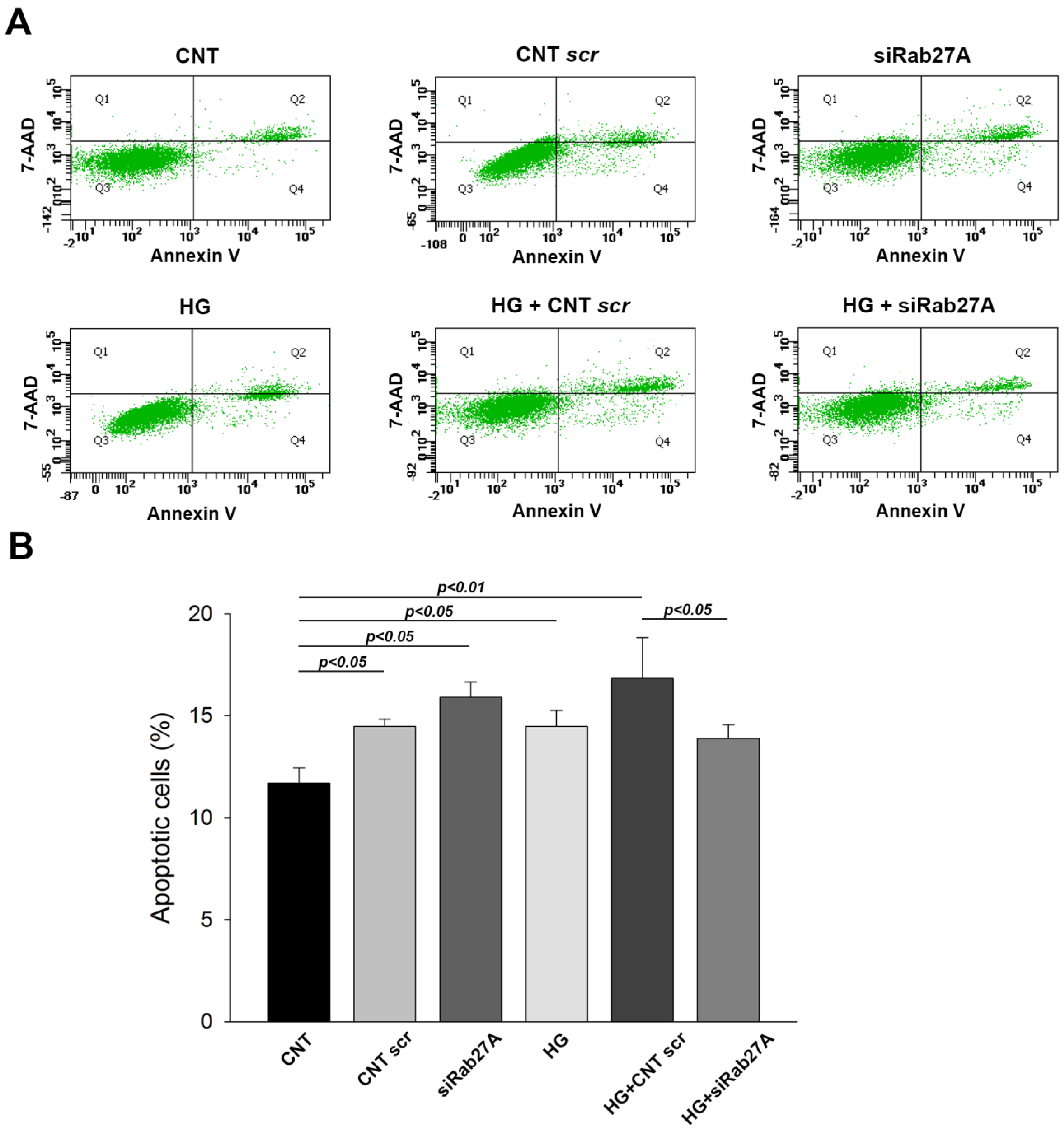
3.2. Influence of Rab3A/27A System Silencing and Glucose Overload on Podocyte Apoptosis and Structure
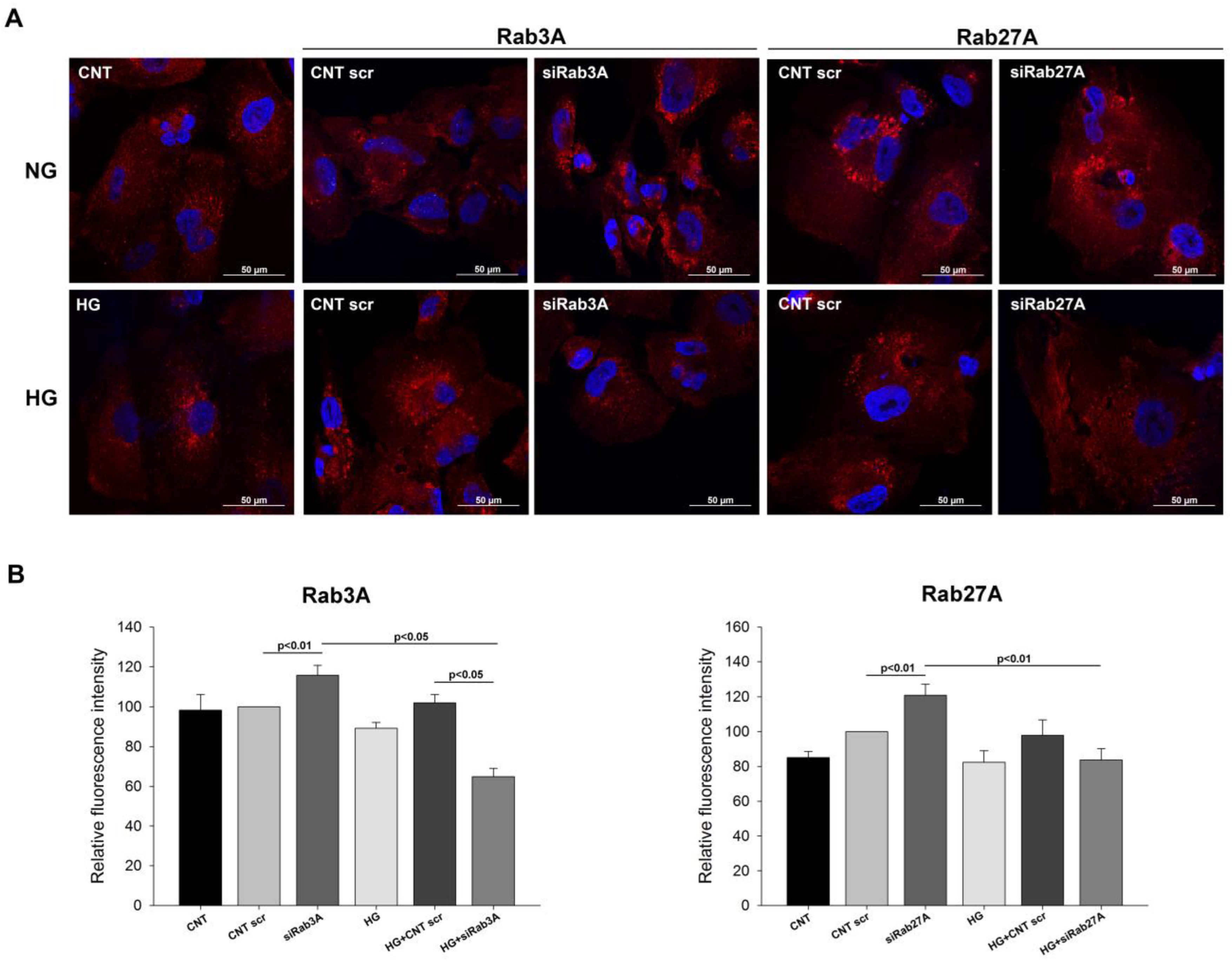
3.3. Analysis of Vesicular Transport Markers under Rab3A/27A System Silencing and Glucose Treatment
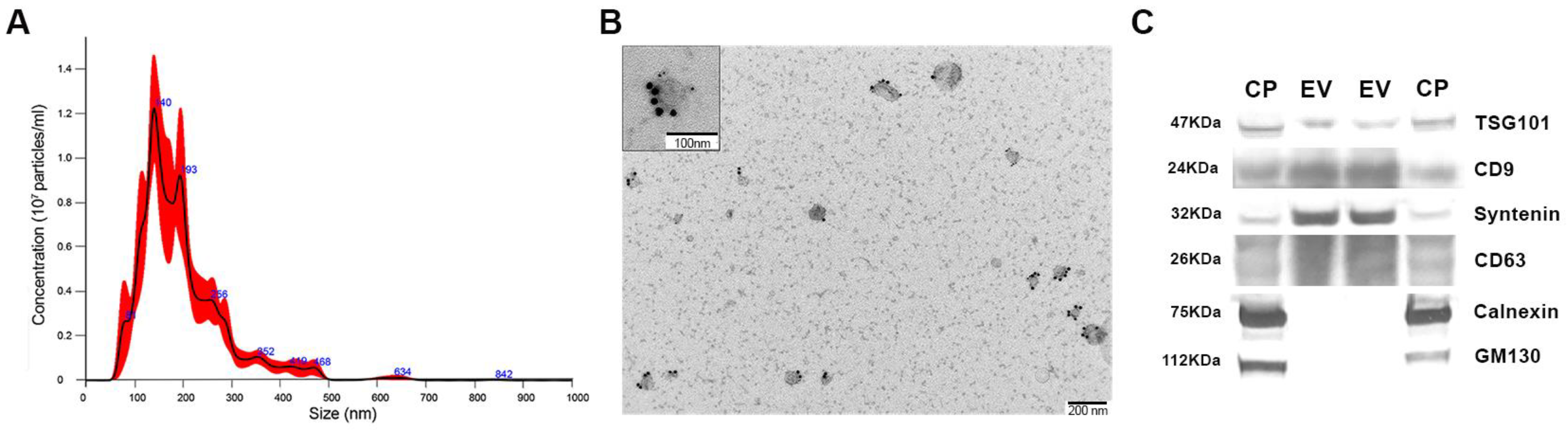
3.4. Isolation and Characterization of Exosomes from Podocyte Culture Medium
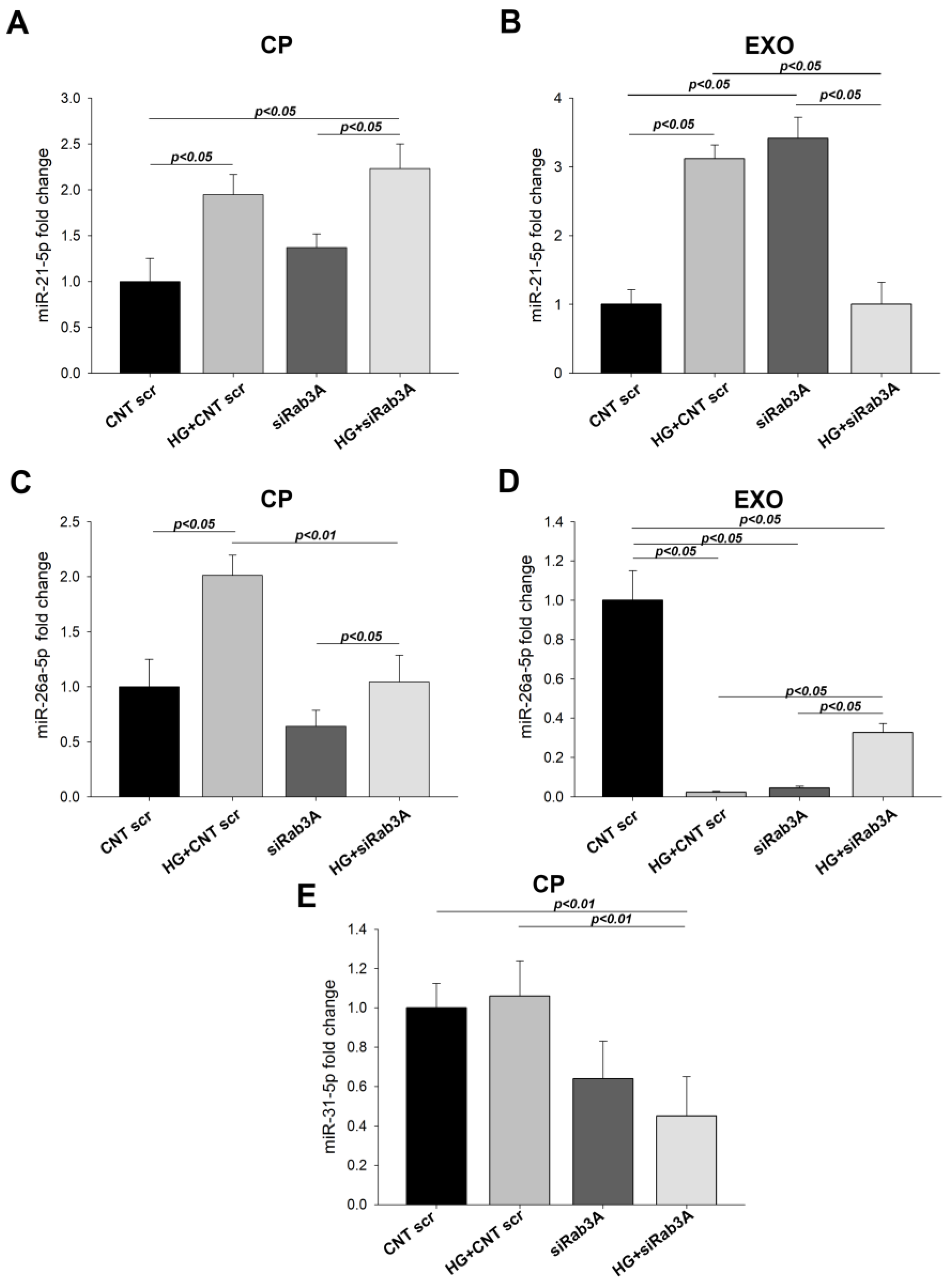
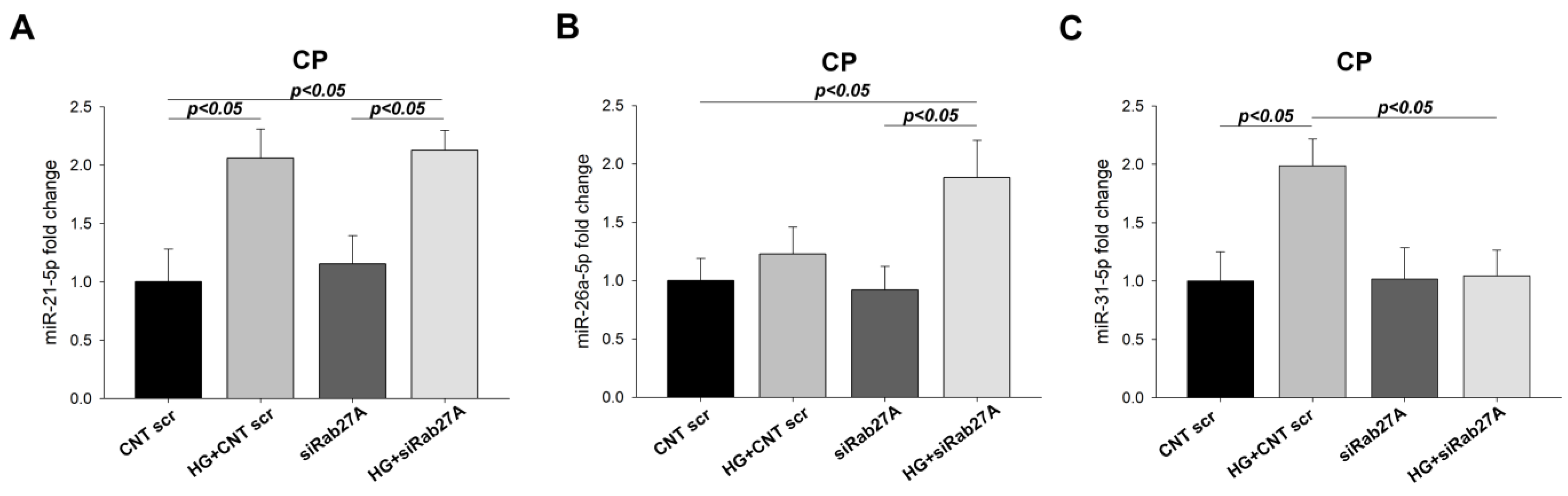
3.5. Effect of Rab3A/27A System Silencing on miRNA Expression in Cell Pellets and Secreted Exosomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chowdhury, T.A.; Ali, O. Diabetes and the kidney. Clin. Med. 2021, 21, e318–e322. [Google Scholar] [CrossRef] [PubMed]
- Kainz, A.; Hronsky, M.; Stel, V.S.; Jager, K.J.; Geroldinger, A.; Dunkler, D.; Heinze, G.; Tripepi, G.; Oberbauer, R. Prediction of prevalence of chronic kidney disease in diabetic patients in countries of the European Union up to 2025. Nephrol. Dial. Transplant. 2015, 30 (Suppl. S4), iv113–iv118. [Google Scholar] [CrossRef] [PubMed]
- Koye, D.N.; Shaw, J.E.; Reid, C.M.; Atkins, R.C.; Reutens, A.T.; Magliano, D.J. Incidence of chronic kidney disease among people with diabetes: A systematic review of observational studies. Diabet. Med. 2017, 34, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C. Targeting the Pathobiology of Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2021, 28, 282–289. [Google Scholar] [CrossRef]
- Reiser, J.; Altintas, M.M. Podocytes. F1000Research 2016, 5, 114. [Google Scholar] [CrossRef]
- Lewko, B.; Stepinski, J. Hyperglycemia and mechanical stress: Targeting the renal podocyte. J. Cell. Physiol. 2009, 221, 288–295. [Google Scholar] [CrossRef]
- Podgorski, P.; Konieczny, A.; Lis, L.; Witkiewicz, W.; Hruby, Z. Glomerular podocytes in diabetic renal disease. Adv. Clin. Exp. Med. 2019, 28, 1711–1715. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, Z.; Han, L.; Zheng, Y.; Wei, Y.; Wang, X.; Wang, Q.; Fang, X.; Zhao, L.; Tong, X. Research Progress on the Pathological Mechanisms of Podocytes in Diabetic Nephropathy. J. Diabetes Res. 2020, 2020, 7504798. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Chandrashekar, K.; Juncos, L.A. Molecular Interactions Between Reactive Oxygen Species and Autophagy in Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3791. [Google Scholar] [CrossRef]
- Wu, X.; Gao, Y.; Xu, L.; Dang, W.; Yan, H.; Zou, D.; Zhu, Z.; Luo, L.; Tian, N.; Wang, X.; et al. Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci. Rep. 2017, 7, 9371. [Google Scholar] [CrossRef]
- Das, F.; Ghosh-Choudhury, N.; Lee, D.Y.; Gorin, Y.; Kasinath, B.S.; Choudhury, G.G. Akt2 causes TGFbeta-induced deptor downregulation facilitating mTOR to drive podocyte hypertrophy and matrix protein expression. PLoS ONE 2018, 13, e0207285. [Google Scholar] [CrossRef] [PubMed]
- Bible, E. Diabetic nephropathy: Sirt1 attenuates diabetic albuminuria. Nat. Rev. Nephrol. 2013, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Arroyo, O.; Ortega, A.; Galera, M.; Solaz, E.; Martinez-Hervas, S.; Redon, J.; Cortes, R. Decreased Urinary Levels of SIRT1 as Non-Invasive Biomarker of Early Renal Damage in Hypertension. Int. J. Mol. Sci. 2020, 21, 6390. [Google Scholar] [CrossRef]
- Miaomiao, W.; Chunhua, L.; Xiaochen, Z.; Xiaoniao, C.; Hongli, L.; Zhuo, Y. Autophagy is involved in regulating VEGF during high-glucose-induced podocyte injury. Mol. Biosyst. 2016, 12, 2202–2212. [Google Scholar] [CrossRef]
- Susztak, K.; Raff, A.C.; Schiffer, M.; Bottinger, E.P. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 2006, 55, 225–233. [Google Scholar] [CrossRef]
- Chen, S.; Lee, J.S.; Iglesias-de la Cruz, M.C.; Wang, A.; Izquierdo-Lahuerta, A.; Gandhi, N.K.; Danesh, F.R.; Wolf, G.; Ziyadeh, F.N. Angiotensin II stimulates alpha3(IV) collagen production in mouse podocytes via TGF-beta and VEGF signalling: Implications for diabetic glomerulopathy. Nephrol. Dial. Transplant. 2005, 20, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Nishad, R.; Mukhi, D.; Singh, A.K.; Motrapu, M.; Chintala, K.; Tammineni, P.; Pasupulati, A.K. Growth hormone induces mitotic catastrophe of glomerular podocytes and contributes to proteinuria. Cell Death Dis. 2021, 12, 342. [Google Scholar] [CrossRef]
- Mukhi, D.; Kolligundla, L.P.; Maruvada, S.; Nishad, R.; Pasupulati, A.K. Growth hormone induces transforming growth factor-beta1 in podocytes: Implications in podocytopathy and proteinuria. Biochim. Biophys. Acta. Mol. Cell Res. 2023, 1870, 119391. [Google Scholar] [CrossRef]
- Assady, S.; Wanner, N.; Skorecki, K.L.; Huber, T.B. New Insights into Podocyte Biology in Glomerular Health and Disease. J. Am. Soc. Nephrol. 2017, 28, 1707–1715. [Google Scholar] [CrossRef]
- Yin, L.; Yu, L.; He, J.C.; Chen, A. Controversies in Podocyte Loss: Death or Detachment? Front. Cell Dev. Biol. 2021, 9, 771931. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Bazzan, E.; Tine, M.; Casara, A.; Biondini, D.; Semenzato, U.; Cocconcelli, E.; Balestro, E.; Damin, M.; Radu, C.M.; Turato, G.; et al. Critical Review of the Evolution of Extracellular Vesicles’ Knowledge: From 1946 to Today. Int. J. Mol. Sci. 2021, 22, 6417. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Tolmachova, T.; Anders, R.; Stinchcombe, J.; Bossi, G.; Griffiths, G.M.; Huxley, C.; Seabra, M.C. A general role for Rab27a in secretory cells. Mol. Biol. Cell 2004, 15, 332–344. [Google Scholar] [CrossRef]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef]
- Quevedo, M.F.; Bustos, M.A.; Masone, D.; Roggero, C.M.; Bustos, D.M.; Tomes, C.N. Grab recruitment by Rab27A-Rabphilin3a triggers Rab3A activation in human sperm exocytosis. Biochim. Biophys. Acta. Mol. Cell Res. 2019, 1866, 612–622. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, X.; Wu, X. The Rab GTPase in the heart: Pivotal roles in development and disease. Life Sci. 2022, 306, 120806. [Google Scholar] [CrossRef]
- Martinez-Arroyo, O.; Ortega, A.; Perez-Hernandez, J.; Chaves, F.J.; Redon, J.; Cortes, R. The Rab-Rabphilin system in injured human podocytes stressed by glucose overload and angiotensin II. Am. J. Physiol. Ren. Physiol. 2020, 319, F178–F191. [Google Scholar] [CrossRef]
- Rastaldi, M.P.; Armelloni, S.; Berra, S.; Li, M.; Pesaresi, M.; Poczewski, H.; Langer, B.; Kerjaschki, D.; Henger, A.; Blattner, S.M.; et al. Glomerular podocytes possess the synaptic vesicle molecule Rab3A and its specific effector rabphilin-3a. Am. J. Pathol. 2003, 163, 889–899. [Google Scholar] [CrossRef]
- Rastaldi, M.P.; Armelloni, S.; Berra, S.; Calvaresi, N.; Corbelli, A.; Giardino, L.A.; Li, M.; Wang, G.Q.; Fornasieri, A.; Villa, A.; et al. Glomerular podocytes contain neuron-like functional synaptic vesicles. FASEB J. 2006, 20, 976–978. [Google Scholar] [CrossRef]
- Armelloni, S.; Calvaresi, N.; Ikehata, M.; Corbelli, A.; Mattinzoli, D.; Giardino, L.A.; Li, M.; Messa, P.; Rastaldi, M.P. Proteinuria and glomerular damage in Rab3A knockout mice chronically fed a high-glucose diet. Nephron. Exp. Nephrol. 2012, 120, e69–e80. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Arroyo, O.; Selma-Soriano, E.; Ortega, A.; Cortes, R.; Redon, J. Small Rab GTPases in Intracellular Vesicle Trafficking: The Case of Rab3A/Raphillin-3A Complex in the Kidney. Int. J. Mol. Sci. 2021, 22, 7679. [Google Scholar] [CrossRef]
- Saleem, M.A.; O’Hare, M.J.; Reiser, J.; Coward, R.J.; Inward, C.D.; Farren, T.; Xing, C.Y.; Ni, L.; Mathieson, P.W.; Mundel, P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002, 13, 630–638. [Google Scholar] [CrossRef]
- Perez-Hernandez, J.; Riffo-Campos, A.L.; Ortega, A.; Martinez-Arroyo, O.; Perez-Gil, D.; Olivares, D.; Solaz, E.; Martinez, F.; Martinez-Hervas, S.; Chaves, F.J.; et al. Urinary- and Plasma-Derived Exosomes Reveal a Distinct MicroRNA Signature Associated With Albuminuria in Hypertension. Hypertension 2021, 77, 960–971. [Google Scholar] [CrossRef]
- Boudaoud, A.; Burian, A.; Borowska-Wykret, D.; Uyttewaal, M.; Wrzalik, R.; Kwiatkowska, D.; Hamant, O. FibrilTool, an ImageJ plug-in to quantify fibrillar structures in raw microscopy images. Nat. Protoc. 2014, 9, 457–463. [Google Scholar] [CrossRef]
- Li, S.; Jia, Y.; Xue, M.; Hu, F.; Zheng, Z.; Zhang, S.; Ren, S.; Yang, Y.; Si, Z.; Wang, L.; et al. Inhibiting Rab27a in renal tubular epithelial cells attenuates the inflammation of diabetic kidney disease through the miR-26a-5p/CHAC1/NF-kB pathway. Life Sci. 2020, 261, 118347. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell. Mol. Life Sci. 2008, 65, 2801–2813. [Google Scholar] [CrossRef]
- Darchen, F.; Goud, B. Multiple aspects of Rab protein action in the secretory pathway: Focus on Rab3 and Rab6. Biochimie 2000, 82, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Geppert, M.; Goda, Y.; Stevens, C.F.; Sudhof, T.C. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature 1997, 387, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Lledo, P.M.; Roa, M.; Vincent, J.D.; Henry, J.P.; Darchen, F. The GTPase Rab3a negatively controls calcium-dependent exocytosis in neuroendocrine cells. EMBO J. 1994, 13, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Bustos, M.A.; Lucchesi, O.; Ruete, M.C.; Mayorga, L.S.; Tomes, C.N. Rab27 and Rab3 sequentially regulate human sperm dense-core granule exocytosis. Proc. Natl. Acad. Sci. USA 2012, 109, E2057–E2066. [Google Scholar] [CrossRef]
- Cabaco, L.C.; Bento-Lopes, L.; Neto, M.V.; Ferreira, A.; Staubli, W.B.L.; Ramalho, J.S.; Seabra, M.C.; Barral, D.C. RAB3A Regulates Melanin Exocytosis and Transfer Induced by Keratinocyte-Conditioned Medium. JID Innov. 2022, 2, 100139. [Google Scholar] [CrossRef]
- Handley, M.T.; Haynes, L.P.; Burgoyne, R.D. Differential dynamics of Rab3A and Rab27A on secretory granules. J. Cell Sci. 2007, 120, 973–984. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Z.; Hu, J.; Feng, J.; Zhu, Z.; Fan, Y.; Lin, Q.; Ding, G. Mfn2 Regulates High Glucose-Induced MAMs Dysfunction and Apoptosis in Podocytes via PERK Pathway. Front. Cell Dev. Biol. 2021, 9, 769213. [Google Scholar] [CrossRef] [PubMed]
- Dimuccio, V.; Bellucci, L.; Genta, M.; Grange, C.; Brizzi, M.F.; Gili, M.; Gallo, S.; Centomo, M.L.; Collino, F.; Bussolati, B. Upregulation of miR145 and miR126 in EVs from Renal Cells Undergoing EMT and Urine of Diabetic Nephropathy Patients. Int. J. Mol. Sci. 2022, 23, 12098. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, A.; Gao, W.; Gui, F.; Zou, Y.; Zhou, X.; Hong, Z. miR-5590-3p inhibits the proliferation and metastasis of renal cancer cells by targeting ROCK2 to inhibit proliferation, migration and invasion. Oncol. Lett. 2022, 24, 377. [Google Scholar] [CrossRef]
- Perez-Hernandez, J.; Martinez-Arroyo, O.; Ortega, A.; Galera, M.; Solis-Salguero, M.A.; Chaves, F.J.; Redon, J.; Forner, M.J.; Cortes, R. Urinary exosomal miR-146a as a marker of albuminuria, activity changes and disease flares in lupus nephritis. J. Nephrol. 2021, 34, 1157–1167. [Google Scholar] [CrossRef]
- Krichevsky, A.M.; Gabriely, G. miR-21: A small multi-faceted RNA. J. Cell. Mol. Med. 2009, 13, 39–53. [Google Scholar] [CrossRef]
- Larrue, R.; Fellah, S.; Van der Hauwaert, C.; Hennino, M.F.; Perrais, M.; Lionet, A.; Glowacki, F.; Pottier, N.; Cauffiez, C. The Versatile Role of miR-21 in Renal Homeostasis and Diseases. Cells 2022, 11, 3525. [Google Scholar] [CrossRef]
- Zhong, X.; Chung, A.C.; Chen, H.Y.; Dong, Y.; Meng, X.M.; Li, R.; Yang, W.; Hou, F.F.; Lan, H.Y. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia 2013, 56, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y.; Luo, J.; O’Connor, C.; Jing, X.; Nair, V.; Ju, W.; Randolph, A.; Ben-Dov, I.Z.; Matar, R.N.; Briskin, D.; et al. MicroRNA-21 in glomerular injury. J. Am. Soc. Nephrol. 2015, 26, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Dong, B.; Tian, Y.; Lefebvre, P.; Meng, Z.; Wang, X.; Pattou, F.; Han, W.; Wang, X.; Lou, F.; et al. MicroRNA-26a regulates insulin sensitivity and metabolism of glucose and lipids. J. Clin. Investig. 2015, 125, 2497–2509. [Google Scholar] [CrossRef]
- Zheng, Z.; Guan, M.; Jia, Y.; Wang, D.; Pang, R.; Lv, F.; Xiao, Z.; Wang, L.; Zhang, H.; Xue, Y. The coordinated roles of miR-26a and miR-30c in regulating TGFbeta1-induced epithelial-to-mesenchymal transition in diabetic nephropathy. Sci. Rep. 2016, 6, 37492. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zong, Y.; Ma, X.; Jiang, C.; Shan, H.; Lin, Y.; Xia, W.; Yin, F.; Wang, N.; Zhou, L.; et al. miR-26a Attenuated Bone-Specific Insulin Resistance and Bone Quality in Diabetic Mice. Mol. Ther. Nucleic Acids 2020, 20, 459–467. [Google Scholar] [CrossRef]
- Koga, K.; Yokoi, H.; Mori, K.; Kasahara, M.; Kuwabara, T.; Imamaki, H.; Ishii, A.; Mori, K.P.; Kato, Y.; Ohno, S.; et al. MicroRNA-26a inhibits TGF-beta-induced extracellular matrix protein expression in podocytes by targeting CTGF and is downregulated in diabetic nephropathy. Diabetologia 2015, 58, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, P.; Chen, W.; Bi, J. Mechanism of bone marrow mesenchymal stem cells secreting miR-26a exosomes affecting high glucose-induced skin fibroblasts function by regulating TLR4/NF-kappaB signaling. Inflamm. Res. 2021, 70, 811–821. [Google Scholar] [CrossRef]
- Asangani, I.A.; Harms, P.W.; Dodson, L.; Pandhi, M.; Kunju, L.P.; Maher, C.A.; Fullen, D.R.; Johnson, T.M.; Giordano, T.J.; Palanisamy, N.; et al. Genetic and epigenetic loss of microRNA-31 leads to feed-forward expression of EZH2 in melanoma. Oncotarget 2012, 3, 1011–1025. [Google Scholar] [CrossRef]
- Rovira-Llopis, S.; Escribano-Lopez, I.; Diaz-Morales, N.; Iannantuoni, F.; Lopez-Domenech, S.; Andujar, I.; Jover, A.; Pantoja, J.; Pallardo, L.M.; Banuls, C.; et al. Downregulation of miR-31 in Diabetic Nephropathy and its Relationship with Inflammation. Cell. Physiol. Biochem. 2018, 50, 1005–1014. [Google Scholar] [CrossRef]

| Target (Gene Name) | Size (bp) | Sequence 5′ → 3′ |
|---|---|---|
| ACTB | 20 | F TGGAGAAAATCTGGCACCAC |
| 22 | R CATGATCTGGGTCATCTTCTCG | |
| B2MG | 21 | F TCCAGCGTACTCCAAAGATTC |
| 21 | R GTCAACTTCAATGTCGGATGG | |
| CD2AP | 20 | F AGGCATGGGAATGTAGCAAG |
| 21 | R TGACGCTTCTTGGTCTTCTTC | |
| NPHS1 | 20 | F TGCAGTTTCCCCCAACTAAC |
| 19 | R ACGCTGACGCATGTCAAGT | |
| RAB3A | 23 | F CCTCATGTATGACATCACCAACG |
| 22 | R CCTCAAAGAACTCGAACCCAAG | |
| RAB27A | 24 | F AAAGAGTGGTGTACAGAGCCAGTG |
| 24 | R GTCGTTAAGCTACGAAACCTCTCC | |
| SYNPO | 20 | F GCCGCAAATCCATGTTTACT |
| 20 | R CTCATCCGCTGTCTGTACCA | |
| WT-1 | 20 | F TTCGCAATCAGGGTTACAGC |
| 20 | R AATGAGTGGTTGGGGAACTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Arroyo, O.; Flores-Chova, A.; Sanchez-Garcia, B.; Redon, J.; Cortes, R.; Ortega, A. Rab3A/Rab27A System Silencing Ameliorates High Glucose-Induced Injury in Podocytes. Biology 2023, 12, 690. https://doi.org/10.3390/biology12050690
Martinez-Arroyo O, Flores-Chova A, Sanchez-Garcia B, Redon J, Cortes R, Ortega A. Rab3A/Rab27A System Silencing Ameliorates High Glucose-Induced Injury in Podocytes. Biology. 2023; 12(5):690. https://doi.org/10.3390/biology12050690
Chicago/Turabian StyleMartinez-Arroyo, Olga, Ana Flores-Chova, Belen Sanchez-Garcia, Josep Redon, Raquel Cortes, and Ana Ortega. 2023. "Rab3A/Rab27A System Silencing Ameliorates High Glucose-Induced Injury in Podocytes" Biology 12, no. 5: 690. https://doi.org/10.3390/biology12050690
APA StyleMartinez-Arroyo, O., Flores-Chova, A., Sanchez-Garcia, B., Redon, J., Cortes, R., & Ortega, A. (2023). Rab3A/Rab27A System Silencing Ameliorates High Glucose-Induced Injury in Podocytes. Biology, 12(5), 690. https://doi.org/10.3390/biology12050690








