Crop–Weed Introgression Plays Critical Roles in Genetic Differentiation and Diversity of Weedy Rice: A Case Study of Human-Influenced Weed Evolution
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Historical Changes in Rice Cultivation from Indica to Japonica Varieties in Jiangsu Province
2.2. Collection of Plant Materials
2.3. DNA Extraction, Amplification, and Genotyping
2.4. Data Analysis
2.4.1. Indica–Japonica Characterization of Weedy Rice and Cultivated Rice
2.4.2. Estimate of Crop-to-Weed Introgression Using the Frequency of Japonica-Specific Alleles (Fj)
2.4.3. Correlation between Genetic Differentiation and Crop-to-Weed Introgression in JS Weedy Rice
2.4.4. Correlation between Genetic Diversity and Crop-to-Weed Introgression in JS Weedy Rice
3. Results
3.1. Rapid Alteration of Rice Cultivation from Indica to Japonica Varieties in Jiangsu Province
3.2. Patterns of Introgression from Japonica Rice Varieties to Weedy Rice
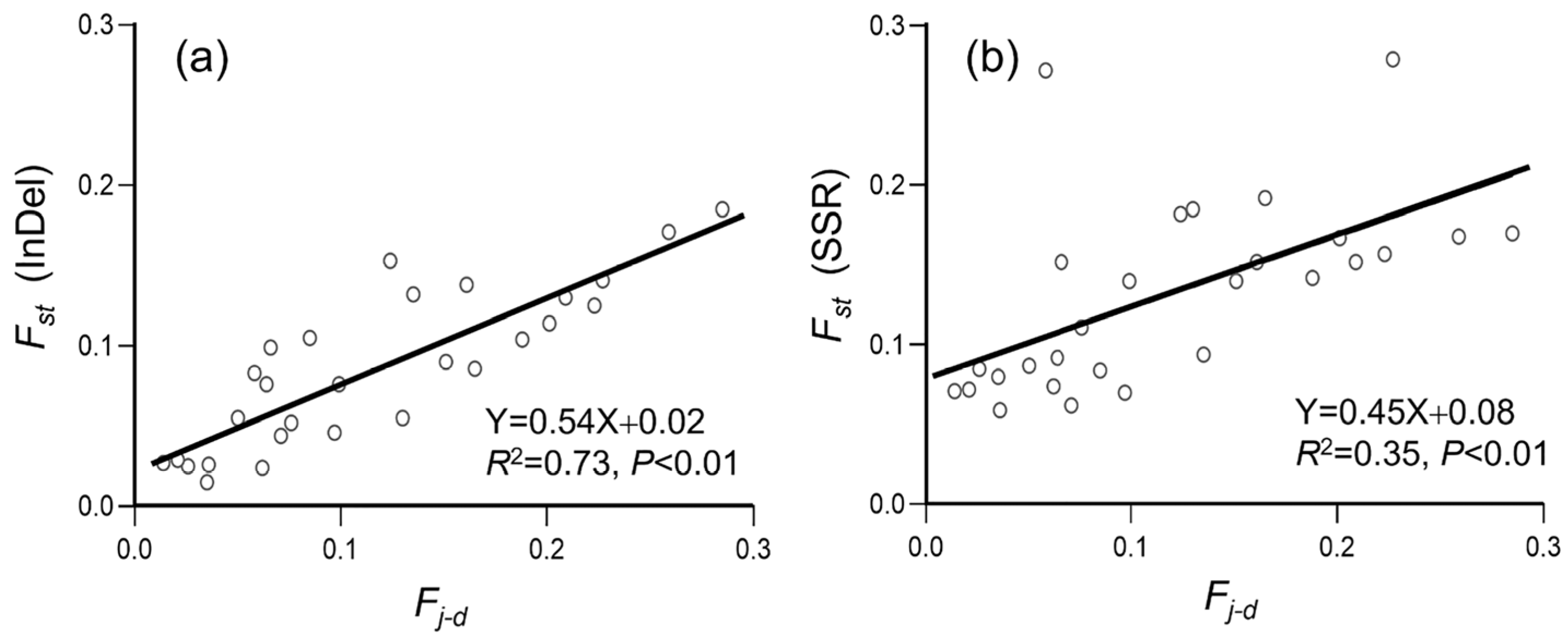
3.3. Genetic Differentiation in Weedy Rice Associated with Crop-to-Weed Introgression
3.4. Genetic Diversity of Weedy Rice Associated with Crop-to-Weed Introgression
4. Discussion
4.1. The Change in Rice Varieties Greatly Influences Indica–Japonica Characteristics of Weedy Rice through Crop-to-Weedy Introgression
4.2. Crop-to-Weed Introgression Impacts Genetic Differentiation and Genetic Diversity in Weedy Rice through Accumilated Crop-Specific Alleles
4.3. Human Activities Can Accelerate the Evolution of Conspecific Weeds in Agroecosystems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slarkin, M. Gene flow in natural populations. Annu. Rev. Ecol. Syst. 1985, 16, 393–430. [Google Scholar] [CrossRef]
- Hartl, D.L.; Clark, A.G. Principles of Population Genetics, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 1997; pp. 5–307. [Google Scholar]
- Templeton, A.R. Population Genetics and Microevolutionary Theory, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 77–474. [Google Scholar]
- Slatkin, M. Gene flow and the geographic structure of natural populations. Science 1987, 236, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Rieseberg, L.H.; Wendel, J.F. Introgression and its consequences in plants. In Hybrid Zones and the Evolutionary Process, 1st ed.; Harrison, R.G., Ed.; Oxford University Press, Inc.: New York, NY, USA, 1993; pp. 70–110. [Google Scholar]
- Ellstrand, N.C. Is gene flow the most important evolutionary force in plants? Am. J. Bot. 2014, 101, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Ellstrand, N.C.; Prentice, H.C.; Hancock, J.F. Gene flow and introgression from domesticated plants into their wild relatives. Annu. Rev. Ecol. Syst. 1999, 30, 539–563. [Google Scholar] [CrossRef]
- Morrell, P.L.; Williams Coplin, T.D.; Lattu, A.L.; Bowers, J.E.; Chandler, J.M.; Paterson, A.H. Crop-to-weed introgression has impacted allelic composition of johnsongrass populations with and without recent exposure to cultivated sorghum. Mol. Ecol. 2005, 14, 2143–2154. [Google Scholar] [CrossRef]
- Mallet, J. Hybridization as an invasion of the genome. TREE 2005, 20, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Abbott, R.; Albach, D.; Ansell, S.; Arntzen, J.W.; Baird, S.J.; Bierne, N.; Boughman, J.; Brelsford, A.; Buerkle, C.A.; Buggs, R.; et al. Hybridization and speciation. J. Evol. Biol. 2013, 26, 229–246. [Google Scholar] [CrossRef]
- Nettel, A.; Dodd, R.S.; Afzal-Rafii, Z.; Tovilla-Hernández, C. Genetic diversity enhanced by ancient introgression and secondary contact in East Pacific black mangroves. Mol. Ecol. 2008, 17, 2680–2690. [Google Scholar] [CrossRef]
- Hegstad, J.M.; Nelson, R.L.; Renny-Byfield, S.; Feng, L.; Chaky, J.M. Introgression of novel genetic diversity to improve soybean yield. Theor. Appl. Genet. 2019, 132, 2541–2552. [Google Scholar] [CrossRef]
- Rhymer, J.M.; Simberloff, D. Extinction by hybridization and introgression. Annu. Rev. Ecol. Syst. 1996, 27, 83–109. [Google Scholar] [CrossRef]
- Hübner, S.; Bercovich, N.; Todesco, M.; Mandel, J.R.; Odenheimer, J.; Ziegler, E.; Lee, J.S.; Baute, G.J.; Owens, G.L.; Grassa, C.J. Sunflower pan-genome analysis shows that hybridization altered gene content and disease resistance. Nat. Plants 2019, 5, 54–62. [Google Scholar] [CrossRef]
- Snow, A.A.; Culley, T.M.; Campbell, L.G.; Sweeney, P.M.; Hegde, S.G.; Ellstrand, N.C. Long-term persistence of crop alleles in weedy populations of wild radish (Raphanus raphanistrum). New Phytol. 2010, 186, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Ellstrand, N.C.; Meirmans, P.; Rong, J.; Bartsch, D.; Ghosh, A.; De Jong, T.J.; Haccou, P.; Lu, B.-R.; Snow, A.A.; Neal Stewart, C., Jr.; et al. Introgression of crop alleles into wild or weedy populations. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 325–345. [Google Scholar] [CrossRef]
- Wedger, M.J.; Roma-Burgos, N.; Olsen, K.M. Genomic revolution of US weedy rice in response to 21st century agricultural technologies. Commun. Biol. 2022, 5, 885. [Google Scholar] [CrossRef] [PubMed]
- Reagon, M.; Thurber, C.S.; Olsen, K.M.; Jia, Y.; Caicedo, A.L. The long and the short of it: SD1 polymorphism and the evolution of growth trait divergence in U.S. weedy rice. Mol. Ecol. 2011, 20, 3743–3756. [Google Scholar] [CrossRef]
- Xia, H.B.; Wang, W.; Xia, H.; Zhao, W.; Lu, B.-R. Conspecific crop-weed introgression influences evolution of weedy rice (Oryza sativa f. spontanea) across a geographical range. PLoS ONE 2011, 6, e16189. [Google Scholar] [CrossRef]
- Jiang, Z.; Xia, H.B.; Basso, B.; Lu, B.-R. Introgression from cultivated rice influences genetic differentiation of weedy rice populations at a local spatial scale. Theor. Appl. Genet. 2012, 124, 309–322. [Google Scholar] [CrossRef]
- Sun, J.; Qian, Q.; Ma, D.R.; Xu, Z.J.; Liu, D.; Du, H.B.; Chen, W.F. Introgression and selection shaping the genome and adaptive loci of weedy rice in northern China. New Phytol. 2013, 197, 290–299. [Google Scholar] [CrossRef]
- Song, B.K.; Chuah, T.S.; Tam, S.M.; Olsen, K.M. Malaysian weedy rice shows its true stripes: Wild Oryza and elite rice cultivars shape agricultural weed evolution in Southeast Asia. Mol. Ecol. 2014, 23, 5003–5017. [Google Scholar] [CrossRef]
- Song, Z.J.; Wang, Z.; Feng, Y.; Yao, N.; Yang, J.; Lu, B.-R. Genetic divergence of weedy rice populations associated with their geographic location and coexisting conspecific crop: Implications on adaptive evolution of agricultural weeds. J. Syst. Evol. 2015, 53, 330–338. [Google Scholar] [CrossRef]
- Ellstrand, N.C. Does introgression of crop alleles into wild and weedy living populations create cryptic in situ germplasm banks? Mol. Ecol. 2018, 27, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Delouche, J.C.; Burgos, N.R.; Gealy, D.R.; de San Martín, G.Z.; Labrada, R.; Larinde, M.; Rosell, C. Weedy Rices-Origin, Biology, Ecology and Control, 1st ed.; FAO: Rome, Italy, 2007; pp. 1–131. [Google Scholar]
- Chauhan, S.B. Strategies to manage weedy rice in Asia. Crop Prot. 2013, 48, 51–56. [Google Scholar] [CrossRef]
- Nadir, S.; Xiong, H.-B.; Zhu, Q.; Zhang, X.-L.; Xu, H.-Y.; Li, J.; Dongchen, W.; Henry, D.; Guo, X.-Q.; Khan, S.; et al. Weedy rice in sustainable rice production. A review. Agron. Sustain. Dev. 2017, 37, 46. [Google Scholar] [CrossRef]
- Ishikawa, R.; Toki, N.; Imai, K.; Sato, Y.I.; Yamagishi, H.; Shimamoto, Y.; Ueno, K.; Morishima, H.; Sato, T. Origin of weedy rice grown in Bhutan and the force of genetic diversity. Genet. Resour. Crop Evol. 2005, 52, 395–403. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, W.; Wu, C.; Song, X.; Qiang, S. Genetic diversity and origin of japonica- and indica-like rice biotypes of weedy rice in the Guangdong and Liaoning provinces of China. Genet. Resour. Crop Evol. 2012, 59, 399–410. [Google Scholar] [CrossRef]
- Zhang, S.L.; Li, J.; Lee, D.S.; Xu, H.; Zhang, L.D.; Dongchen, W.H.; Xiong, H.B.; Zhu, Q.; Zhang, X.; Lu, B.-R.; et al. Genetic differentiation of Asian weedy rice revealed with InDel markers. Crop Sci. 2014, 54, 2499–2508. [Google Scholar] [CrossRef]
- Cao, Q.J.; Lu, B.-R.; Xia, H.; Rong, J.; Sala, F.; Spada, A.; Grassi, F. Genetic diversity and origin of weedy Rice (Oryza sativa f. spontanea) populations found in north-eastern China revealed by simple sequence repeat (SSR) markers. Ann. Bot. 2006, 98, 1241–1252. [Google Scholar] [CrossRef]
- Li, L.-F.; Olsen, K.M. Population genomics of weedy crop relatives: Insights from weedy rice. In Population Genomics: Crop Plants, 1st ed.; Rajora, O.P., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 1–25. [Google Scholar]
- Zhang, B.H.; Dong, E.J.; Zhang, H.; Shi, Z.H.; Song, X.L.; Qiang, S.; Dai, W.M. Use of hybrid rice and the proliferation of weedy rice: A case in Jiangsu, China. Crop Sci. 2016, 56, 673–681. [Google Scholar] [CrossRef]
- Shao, J.; Dai, W.M.; Zhang, L.J.; Song, X.L.; Qiang, S. Genetic diversity and origin of weedy rice in central region of Jiangsu Province, China. Acta Agron. Sin. 2011, 37, 1324–1332. (In Chinese) [Google Scholar] [CrossRef]
- Gong, J.; Xing, Z.; Hu, Y.; Zhang, H.; Dai, Q.; Huo, Z.; Xu, K.; Wei, H.; Gao, H. Relative advantages of “indica to japonica” and production development strategies. China Rice 2013, 19, 1–6. (In Chinese) [Google Scholar]
- Xu, D.; Xu, X.; Li, C. Investigation and study on the shift of “indica to japonica” rice cultivation and the extension of Nongken 58 in the southern rice regions in the middle of the 20th century. Ancient Mod. Agric. 2016, 1, 1–17. (In Chinese) [Google Scholar]
- Deng, J.P.; Du, Y.L. The production situation and development countermeasures of japonica in Jiangsu province. China Rice 2006, 4, 8–11. (In Chinese) [Google Scholar]
- Li, L.-F.; Li, Y.-L.; Jia, Y.; Caicedo, A.L.; Olsen, K.M. Signatures of adaptation in the weedy rice genome. Nat. Genet. 2017, 49, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhou, Y.; Mao, L.; Ye, C.; Wang, W.; Zhang, J.; Yu, Y.; Fu, F.; Wang, Y.; Qian, F.; et al. Genomic variation associated with local adaptation of weedy rice during de-domestication. Nat. Commun. 2017, 8, 15323. [Google Scholar] [CrossRef]
- Qiu, J.; Jia, L.; Wu, D.; Weng, X.; Chen, L.; Sun, J.; Chen, M.; Mao, L.; Jiang, B.; Ye, C.; et al. Diverse genetic mechanisms underlie worldwide convergent rice feralization. Genome Biol. 2020, 21, 70. [Google Scholar] [CrossRef]
- Zhu, Y.-Q.; Fang, J.; Wang, Y.; Pang, L.-H.; Lu, B.-R. Key roles of de-domesticaiton and novel mutation in origin and diversification of global weedy rice. Biology 2021, 10, 828. [Google Scholar] [CrossRef]
- Chen, X.; Qiang, S.; Yang, J.; Zhang, B.H.; Zhang, Z.; Song, X.L.; Dai, W.M. Hierarchical clustering and indica–japonica classification: Uncover mutual spread and indica–japonica differentiation for weedy rice in Jiangsu Province. Chin. J. Rice Sci. 2015, 29, 82–90. (In Chinese) [Google Scholar]
- Lu, B.-R.; Cai, X.X.; Jin, X. Efficient indica and japonica rice identification based on the InDel molecular method: Its implication in rice breeding and evolutionary research. Prog. Nat. Sci. 2009, 19, 1241–1252. [Google Scholar] [CrossRef]
- Liu, P.; Cai, X.X.; Lu, B.-R. Single-seeded InDel fingerprints in rice: An effective tool for indica–japonica rice classification and evolutionary studies. J. Syst. Evol. 2012, 50, 1–11. [Google Scholar] [CrossRef]
- Agriculture and Forestry Department of Jiangsu Province. Agricultural Statistics of Jiangsu Province; Agriculture and Forestry Department of Jiangsu Province: Nanjing, China, 1982; pp. 1–220. (In Chinese)
- Jiangsu Provincial Bureau of Statistics; Agriculture and Forestry Department of Jiangsu Province. The Year Book of Jiangsu Rural Economic Statistics; Jiangsu Provincial Bureau of Statistics: Nanjing, China, 1998; (In Chinese, one book per year from 1988–1998).
- Liu, G.P.; Kang, C.J. The Yearbook of Jiangsu Rural Statistical; Jiangsu Provincial Bureau of Statistics: Nanjing, China, 2018; (In Chinese, one book per year from 2000–2018).
- Yang, L.J.; Cui, J.L.; Tang, Y.G. The Science of Rice Cultivation in Jiangsu Province; Jiangsu Science & Technology Press: Nanjing, China, 1990; pp. 5–188. (In Chinese) [Google Scholar]
- Wang, Q.Q.; Zhang, Y.B.; Shi, Y.; Xia, Y.L.; Chen, Z.W.; Chen, D.; Huang, S.S.; Lu, J.D.; Zhang, Y.Y.; Zhao, H.Y.; et al. The History of Agricultural Development in Jiangsu Province; Jiangsu Science & Technology Press: Nanjing, China, 1992; pp. 8–132. (In Chinese) [Google Scholar]
- Jiangsu Provincial Local Records Compilation Committee. Jiangsu Provincial Annals (Agricultural Annals); Jiangsu Classics Publishing House: Nanjing, China, 1997; pp. 10–120.
- Esri. Available online: https://www.esri.com/en-us/home (accessed on 20 April 2023).
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Gramene Markers Database. Available online: https://archive.gramene.org/markers/ (accessed on 20 April 2023).
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- GraphPad. Available online: https://www.graphpad.com/ (accessed on 20 April 2023).
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research: An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Wright, S. The genetical structure of populations. Ann. Eugen. 1951, 15, 323–354. [Google Scholar] [CrossRef]
- Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 1978, 89, 583–590. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Cao, Q.; Chen, Z.D.; Tang, L.H.; Wang, Y.P.; Fang, X.W.; Wang, C.L.; Zhong, W.G. Indica–japonica differentiation of chloroplast DNA of weedy rice in the Changjiang and Huaihe River valley of China. Chin. J. Rice Sci. 2009, 4, 391–397. (In Chinese) [Google Scholar]
- Chen, L.J.; Lee, D.S.; Song, Z.P.; Suh, H.S.; Lu, B.-R. Gene flow from cultivated rice (Oryza sativa) to its weedy and wild relatives. Ann. Bot. 2004, 93, 67–73. [Google Scholar] [CrossRef]
- Zuo, J.; Zhang, L.J.; Song, X.L.; Dai, W.M.; Qiang, S. Innate factors causing differences in gene flow frequency from transgenic rice to different weedy rice biotypes. Pest Manag. Sci. 2011, 67, 677–690. [Google Scholar] [CrossRef]
- Gealy, D.R.; Burgos, N.R.; Yeater, K.M.; Jackson, A.K. Outcrossing potential between US blackhull red rice and indica rice cultivars. Weed Sci. 2015, 63, 647–657. [Google Scholar] [CrossRef]
- Shivrain, V.K.; Burgos, N.R.; Anders, M.M.; Rajguru, S.N.; Moore, J.; Sales, M.A. Gene flow between ClearfieldTM rice and red rice. Crop Prot. 2007, 26, 349–356. [Google Scholar] [CrossRef]
- Shivrain, V.K.; Burgos, N.R.; Gealy, D.R.; Moldenhauer, K.A.K.; Baquireza, C.J. Maximum outcrossing rate and genetic compatibility between red rice (Oryza sativa) biotypes and ClearfieldTM rice. Weed Sci. 2008, 56, 807–813. [Google Scholar] [CrossRef]
- Goulart, I.C.G.R.; Menezes, V.G.; Bortoly, E.D.; Kupas, V.; Merotto, A. Detecting gene flow from ALS-resistant hybrid and inbred rice to weedy rice using single plant pollen donors. Exp. Agric. 2015, 52, 237–250. [Google Scholar] [CrossRef]
- Nam, K.-H.; Kim, D.Y.; Moon, Y.S.; Pack, I.S.; Jeong, S.-C.; Park, K.W.; Kim, C.-G. Gene flow from transgenic PPO-inhibiting herbicide-resistant rice to weedy rice, and agronomic performance by their hybrids. J. Plant Biol. 2019, 62, 286–296. [Google Scholar] [CrossRef]
- Dai, W.M.; Song, X.L.; Wu, C.; Zhang, L.J.; Zuo, R.L.; Zhang, Z.; Li, S.S.; Cao, D.; Zuo, J.; Yang, L.; et al. Investigation of weedy rice (Oryza sativa f. spontantea) occurrence in Jiangsu Province. Jiangsu J. Agric. Sci. 2009, 25, 712–714. (In Chinese) [Google Scholar]
- Barnaud, A.; Deu, M.; Garine, E.; Chantereau, J.; Bolteu, J.; Koïda, E.O.; Mckey, D.; Joly, H.I. A weed-crop complex in sorghum: The dynamics of genetic diversity in a traditional farming system. Am. J. Bot. 2009, 96, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.G.; Lee, D.; Shukla, K.; Waite, T.A.; Bartsch, D. An ecological approach to measuring the evolutionary consequences of gene flow from crops to wild or weedy relatives. Appl. Plant Sci. 2016, 4, 1500114. [Google Scholar] [CrossRef]
- Muller, M.H.; Latreille, M.; Tollon, C. The origin and evolution of a recent agricultural weed: Population genetic diversity of weedy populations of sunflower (Helianthus annuus L.) in Spain and France. Evol. Appl. 2011, 4, 499–514. [Google Scholar] [CrossRef]
- Valérie, L.C.; Siol, M.; Vigouroux, Y.; Tenaillon, M.I.; Délye, C. Adaptive introgression from maize has facilitated the establishment of teosinte as a noxious weed in Europe. Proc. Natl. Acad. Sci. USA 2020, 117, 25618–25627. [Google Scholar]
- Pandolfo, E.C.; Presotto, A.; Carbonell, F.T.; Ureta, S.; Poverene, M.; Cantamutto, M. Transgene escape and persistence in an agroecosystem: The case of glyphosate-resistant Brassica rapa L. in central Argentina. Environ. Sci. Pollut. Res. 2018, 25, 6251–6264. [Google Scholar] [CrossRef]








| Origin | Fj | Type 1 | Percent 2 | Introgression 3 |
|---|---|---|---|---|
| Guangdong | 0.07 (0.021) | Indica | - | Low |
| Jiangsu | 0.07 (0.001) | Indica | 91.85% | Low |
| Jiangsu | 0.38 (0.012) | Intermediate | 6.72% | Middle |
| Jiangsu | 0.86 (0.015) | Japonica | 1.43% | High |
| Northeast | 0.94 (0.058) | Japonica | - | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, X.-X.; Wang, Z.; Yuan, Y.; Pang, L.-H.; Wang, Y.; Lu, B.-R. Crop–Weed Introgression Plays Critical Roles in Genetic Differentiation and Diversity of Weedy Rice: A Case Study of Human-Influenced Weed Evolution. Biology 2023, 12, 744. https://doi.org/10.3390/biology12050744
Cai X-X, Wang Z, Yuan Y, Pang L-H, Wang Y, Lu B-R. Crop–Weed Introgression Plays Critical Roles in Genetic Differentiation and Diversity of Weedy Rice: A Case Study of Human-Influenced Weed Evolution. Biology. 2023; 12(5):744. https://doi.org/10.3390/biology12050744
Chicago/Turabian StyleCai, Xing-Xing, Zhi Wang, Ye Yuan, Li-Hao Pang, Ying Wang, and Bao-Rong Lu. 2023. "Crop–Weed Introgression Plays Critical Roles in Genetic Differentiation and Diversity of Weedy Rice: A Case Study of Human-Influenced Weed Evolution" Biology 12, no. 5: 744. https://doi.org/10.3390/biology12050744
APA StyleCai, X.-X., Wang, Z., Yuan, Y., Pang, L.-H., Wang, Y., & Lu, B.-R. (2023). Crop–Weed Introgression Plays Critical Roles in Genetic Differentiation and Diversity of Weedy Rice: A Case Study of Human-Influenced Weed Evolution. Biology, 12(5), 744. https://doi.org/10.3390/biology12050744








