Intense Locomotion Enhances Oviposition in the Freshwater Mollusc Lymnaea stagnalis: Cellular and Molecular Correlates
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Animals
2.3. Procedure for Crawling in Shallow Water
2.4. Analysis of Oviposition
2.5. RT-PCR Assessment of the Expression Level of Ovulation Prohormone and Tryptophan Hydroxylase Genes
2.5.1. Total RNA Extraction
2.5.2. Reverse Transcription PCR
- EF1a:
- 2.
- GAPDH:
- 3.
- Ovulation prohormone L. stagnalis:
- 4.
- Tryptophan hydroxylase:
2.6. Electrophysiology
2.7. Statistical Analysis
2.7.1. Behavioural Experiments
2.7.2. RT-PCR Data
2.7.3. Electrophysiological Data
3. Results
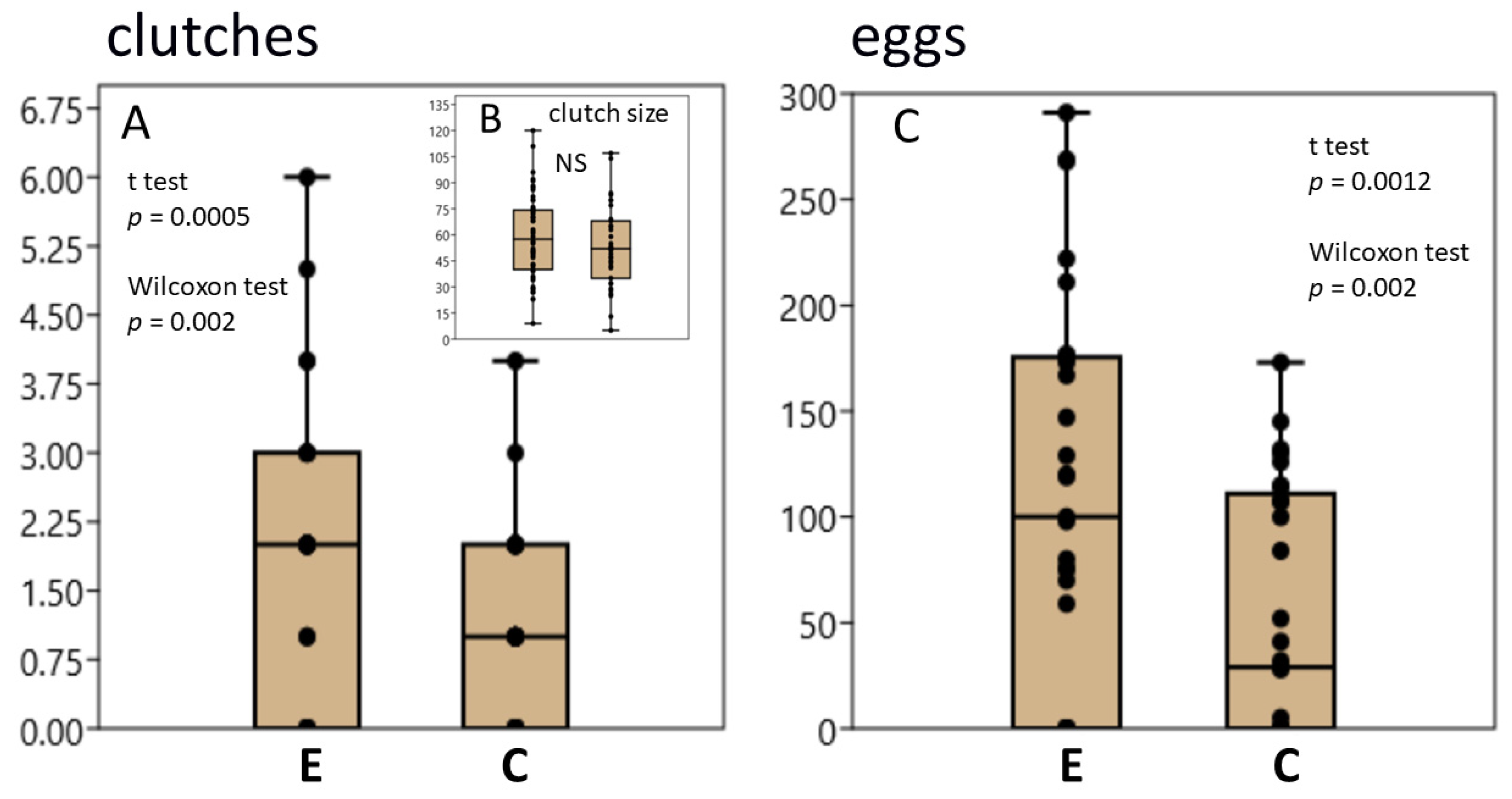
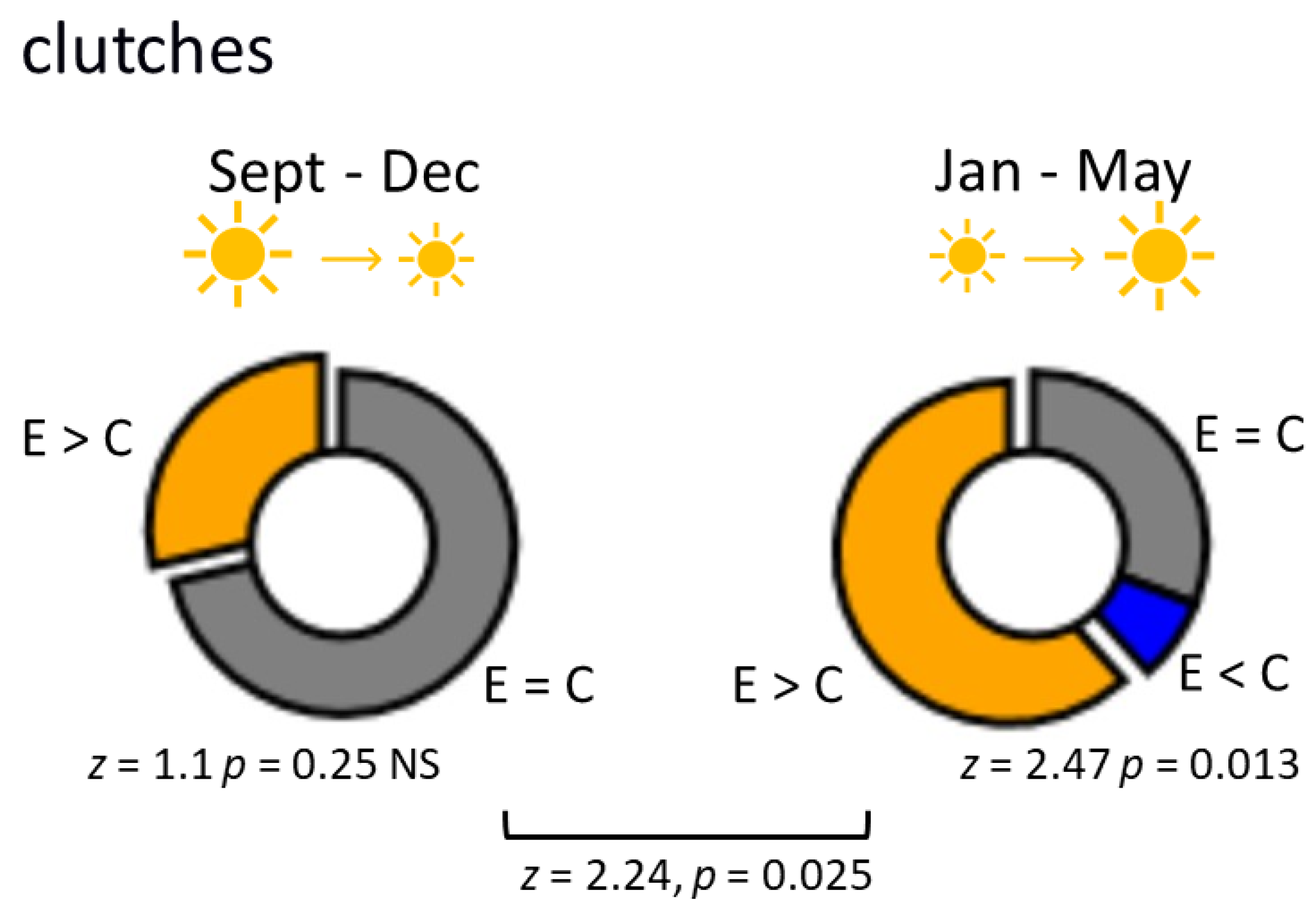
3.1. L. stagnalis Oviposits More Clutches and Eggs after Crawling in Shallow Water and the Influence Is Season-Dependent
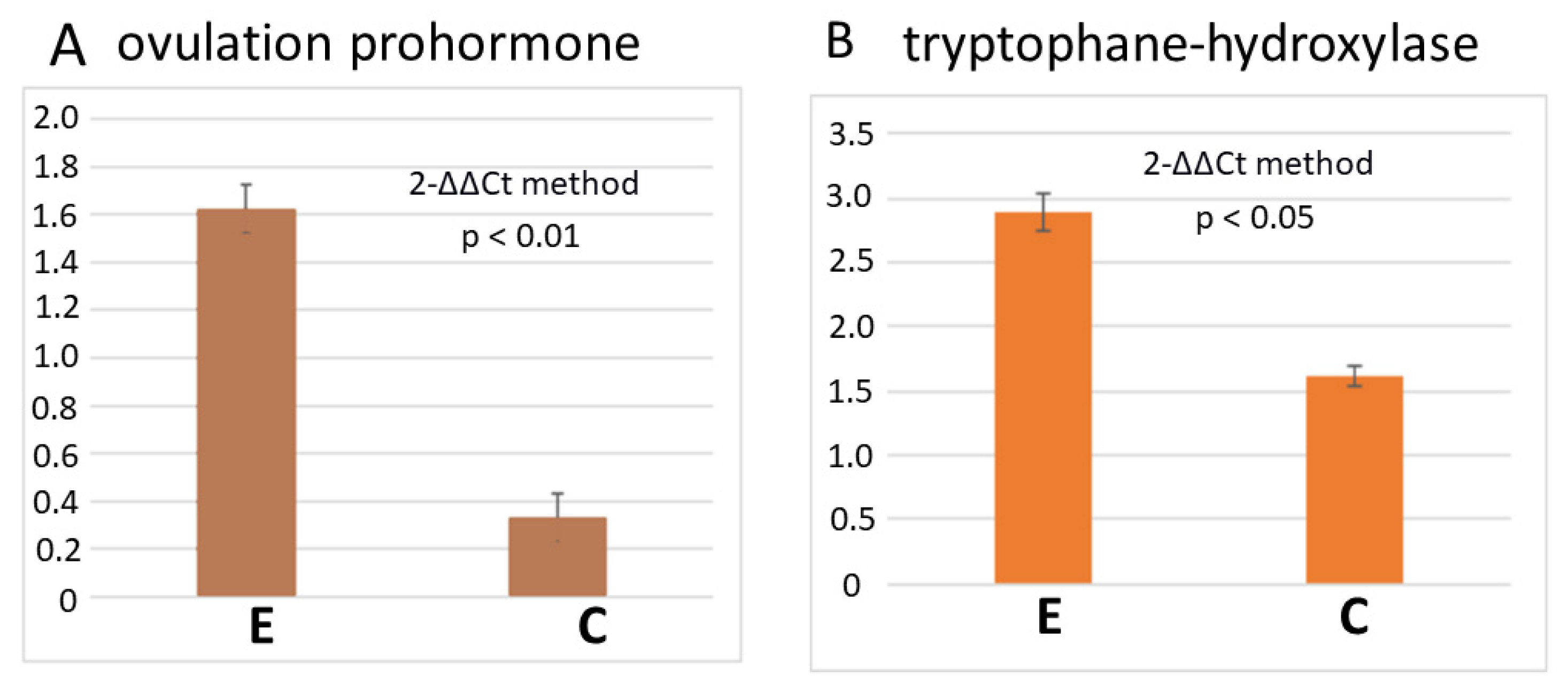
3.2. RT-PCR Reveals a Higher Number of Transcripts of Egg-Laying Prohormone and Tryptophan Hydroxylase Genes
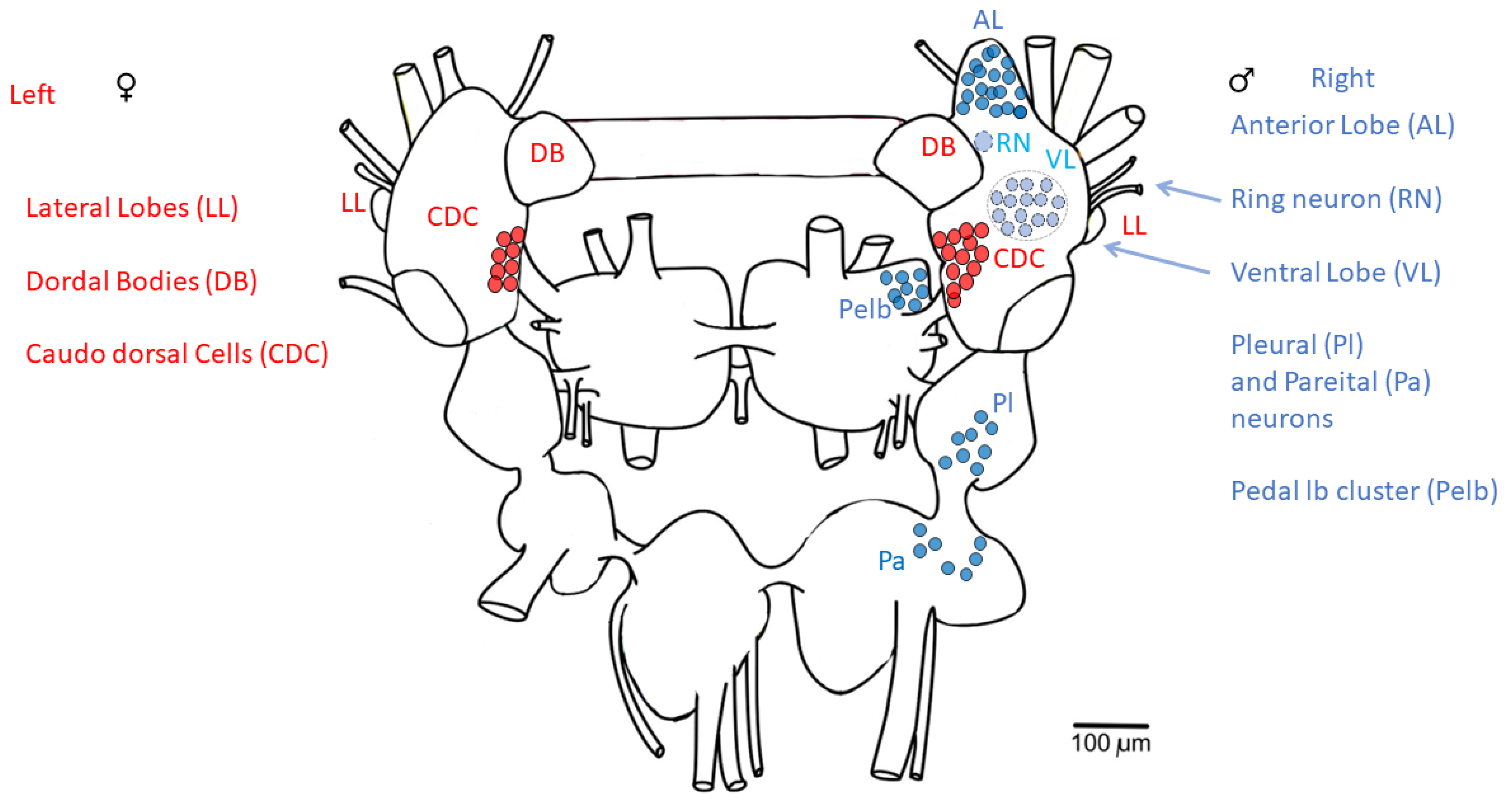
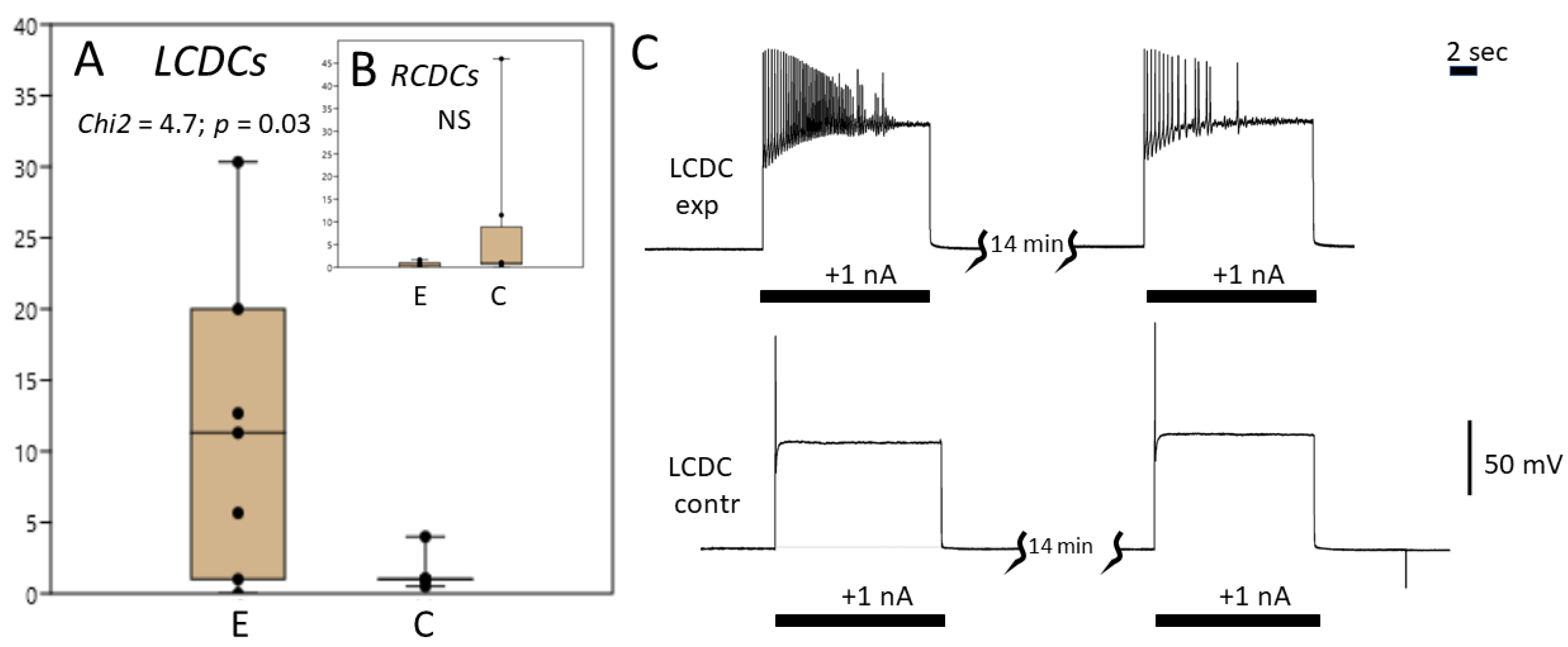
3.3. Caudo-Dorsal Cells Producing Egg-Laying Hormones Are More Responsive to Electrical Stimulation in Snails Exercised in Shallow Water
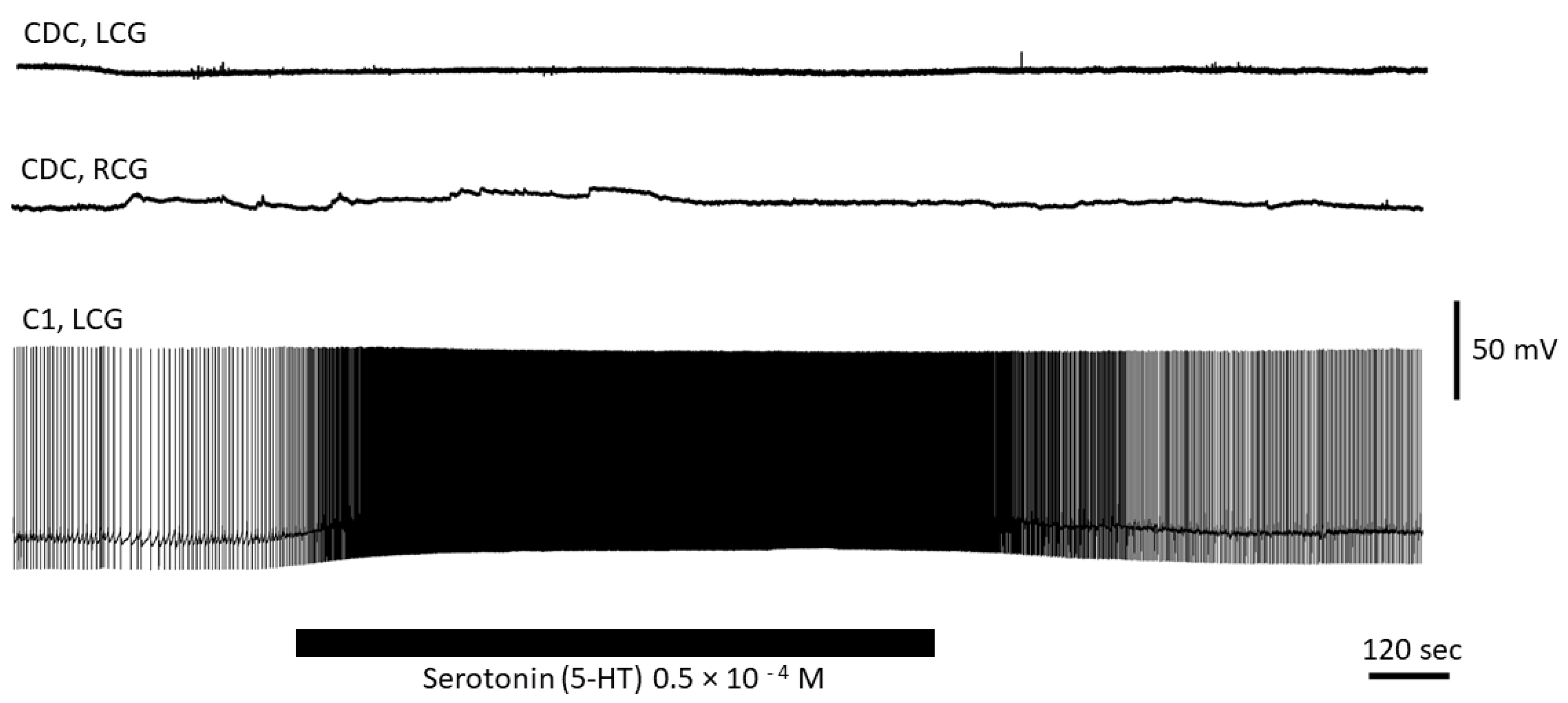
3.4. Serotonin Has No Direct Effect on CDC Neurons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belladelli, F.; Basran, S.; Eisenberg, M.L. Male fertility and physical exercise. World J. Men’s Health 2023, 41, e22. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, O.; Cameron, L.C. Effect of exercise on ovulation: A systematic review. Sport. Med. 2017, 47, 1555. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.A.; Son, J.S.; Du, M. Prenatal exercise in fetal development: A placental perspective. FEBS J. 2022, 289, 3058. [Google Scholar] [CrossRef]
- Davenport, M.H.; Meah, V.L.; Ruchat, S.M.; Davies, G.A.; Skow, R.J.; Barrowman, N.; Mottola, M.F. Impact of prenatal exercise on neonatal and childhood outcomes: A systematic review and meta-analysis. Br. J. Sport. Med. 2018, 52, 1386–1396. [Google Scholar] [CrossRef]
- Dingena, C.F.; Arofikina, D.; Campbell, M.D.; Holmes, M.J.; Scott, E.M.; Zulyniak, M.A. Nutritional and exercise-focused lifestyle interventions and glycemic control in women with diabetes in pregnancy: A systematic review and meta-analysis of randomized clinical trials. Nutrients 2023, 15, 323. [Google Scholar] [CrossRef] [PubMed]
- Sadhir, S.; Pontzer, H. Impact of energy availability and physical activity on variation in fertility across human populations. J. Physiol. Anthropol. 2023, 42, 1. [Google Scholar] [CrossRef]
- Korshunova, T.; Dyakonova, V.; Vorontsov, D. Previous motor activity affects transition from uncertainty to decision-making in snails. J. Exp. Biol. 2016, 219, 3635–3641. [Google Scholar] [CrossRef] [PubMed]
- Aonuma, H.; Mezheritskiy, M.; Boldyshev, B.; Totani, Y.; Vorontsov, D.; Zakharov, I.; Ito, E.; Dyakonova, V. The role of serotonin in the influence of intense locomotion on the behavior under uncertainty in the mollusk Lymnaea stagnalis. Front. Physiol. 2020, 11, 221. [Google Scholar] [CrossRef]
- Mezheritskiy, M.; Vorontsov, D.; Lapshin, D.; Dyakonova, V. Previous flight facilitates partner finding in female crickets. Sci. Rep. 2020, 10, 22328. [Google Scholar] [CrossRef]
- Dyakonova, V.; Mezheritskiy, M.; Boguslavsky, D.; Dyakonova, T.; Chistopolsky, I.; Ito, E.; Zakharov, I. Exercise and the brain: Lessons from invertebrate studies. Front. Behav. Neurosci. 2022, 16, 928093. [Google Scholar] [CrossRef]
- Mezheritskiy, M.I.; Dyakonova, V.E. Direct and inherited epigenetic changes in the nervous system caused by intensive locomotion: Possible adaptive significance. Russ. J. Dev. Biol. 2022, 53, 317. [Google Scholar] [CrossRef]
- Salmon, P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: A unifying theory. Clin. Psychol. Rev. 2001, 21, 33–61. [Google Scholar] [CrossRef] [PubMed]
- Basso, J.C.; Suzuki, W.A. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: A review. Brain Plast. 2017, 2, 127–152. [Google Scholar] [CrossRef] [PubMed]
- Fodor, I.; Hussein, A.A.; Benjamin, P.R.; Koene, J.M.; Pirger, Z. The unlimited potential of the great pond snail, Lymnaea stagnalis. Elife 2020, 9, e56962. [Google Scholar] [CrossRef]
- Kuroda, R.; Abe, M. The pond snail Lymnaea stagnalis. Evodevo 2020, 11, 24. [Google Scholar] [CrossRef]
- Rivi, V.; Benatti, C.; Colliva, C.; Radighieri, G.; Brunello, N.; Tascedda, F.; Blom, J.M.C. Lymnaea stagnalis as model for translational neuroscience research: From pond to bench. Neurosci. Biobehav. Rev. 2020, 108, 602. [Google Scholar] [CrossRef]
- Rivi, V.; Benatti, C.; Lukowiak, K.; Colliva, C.; Alboni, S.; Tascedda, F.; Blom, J.M.C. What can we teach Lymnaea and what can Lymnaea teach us? Biol. Rev. Camb. Philos. Soc. 2021, 96, 1590. [Google Scholar] [CrossRef]
- Kemenes, G.; Benjamin, P.R.; Kemenes, I. The role of non-coding RNAs in the formation of long-term associative memory after single-trial learning in Lymnaea. Front. Behav. Neurosci. 2022, 16, 1005867. [Google Scholar] [CrossRef]
- Nakai, J.; Chikamoto, N.; Fujimoto, K.; Totani, Y.; Hatakeyama, D.; Dyakonova, V.E.; Ito, E. Insulin and memory in invertebrates. Front. Behav. Neurosci. 2022, 16, 882932. [Google Scholar] [CrossRef]
- Dyakonova, T.L.; Sultanakhmetov, G.S.; Mezheritskiy, M.I.; Sakharov, D.A.; Dyakonova, V.E. Storage and erasure of behavioural experiences at the single neuron level. Sci. Rep. 2019, 9, 14733. [Google Scholar] [CrossRef]
- Voronezhskaya, E.E. Maternal serotonin: Shaping developmental patterns and behavioral strategy on progeny in molluscs. Front. Ecol. Evol. 2021, 9, 739787. [Google Scholar] [CrossRef]
- Geraerts, W.P.M.; Bohlken, S. The control of ovulation in the hermaphrodite freshwater snail Lymnaea stagnalis by the neurohormone of the caudo-dorsal cells. Gen. Comp. Endocrinol. 1976, 28, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, E.; Jackson, J.F.; Bouwmeester, T.; Smit, A.B.; Van Minnen, J.; Van Heerikhuizen, H.; Klootwijk, J.; Joosse, J. Isolation, characterization, and evolutionary aspects of a cDNA clone encoding multiple neuropeptides involved in the stereotyped egg-laying behavior of the freshwater snail Lymnaea stagnalis. J. Neurosci. 1988, 8, 4184. [Google Scholar] [CrossRef] [PubMed]
- Koene, J. Neuro-endocrine control of reproduction in hermaphroditic freshwater snails: Mechanisms and evolution. Front. Behav. Neurosci. 2010, 4, 167. [Google Scholar] [CrossRef]
- Wood, E.A.; Stopka, S.A.; Zhang, L.; Mattson, S.; Maasz, G.; Pirger, Z.; Vertes, A. Neuropeptide localization in Lymnaea stagnalis: From the central nervous system to subcellular compartments. Front. Mol. Neurosci. 2021, 14, 670303. [Google Scholar] [CrossRef]
- Hermann, P.M.; de Lange, R.P.; Pieneman, A.W.; ter Maat, A.; Jansen, R.F. Role of neuropeptides encoded on CDCH-1 gene in the organization of egg-laying behavior in the pond snail, Lymnaea stagnalis. J. Neurophysiol. 1997, 78, 2859. [Google Scholar] [CrossRef]
- de Vlieger, T.A.; Kits, K.S.; ter Maat, A.; Lodder, J.C. Morphology and electrophysiology of the ovulation hormone producing neuro-endocrine cells of the freshwater snail Lymnaea stagnalis (L.). J. Exp. Biol. 1980, 84, 259. [Google Scholar] [CrossRef]
- Kits, K.S. States of excitability in ovulation hormone producing neuroendocrine cells of Lymnaea stagnalis (Gastropoda) and their relation to the-egg-laying cycle. J. Neurobiol. 1980, 11, 397–410. [Google Scholar] [CrossRef]
- Ter Maat, A.; Roubos, E.W.; Lodder, J.C.; Buma, P. Integration of synaptic input modulating pacemaker activity of electrotonically coupled neuroendocrine Caudo-Dorsal Cells in the pond snail. J. Neurophysiol. 1983, 49, 1392–1409. [Google Scholar] [CrossRef]
- Ter Maat, A.; Geraerts, W.P.M.; Jansen, R.F.; Bos, N.P.A. Chemically mediated positive feedback generates long-lasting discharge in the molluscan neuroendocrine system. Brain Res. 1988, 438, 77–82. [Google Scholar] [CrossRef]
- Lockyer, A.E.; Spinks, J.N.; Walker, A.J.; Kane, R.A.; Noble, L.R.; Rollinson, D.; Dias-Neto, E.; Jones, C.S. Biomphalaria glabrata transcriptome: Identification of cell-signalling, transcriptional control and immune-related genes from open reading frame expressed sequence tags (ORESTES). Dev. Comp. Immunol. 2007, 31, 763. [Google Scholar] [CrossRef] [PubMed]
- Young, A.P.; Landry, C.F.; Jackson, D.J.; Wyeth, R.C. Tissue-specific evaluation of suitable reference genes for RT-qPCR in the pond snail, Lymnaea stagnalis. PeerJ 2019, 7, e7888. [Google Scholar] [CrossRef] [PubMed]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Ter Maat, A.; Lodder, J.C.; Wilbrink, M. Induction of egg-laying in the pond snail Lymnaea stagnalis by environmental stimulation of the release of ovulation hormone from the Caudo-Dorsal Cells. Int. J. Inv. Repr. 1983, 6, 239–247. [Google Scholar] [CrossRef]
- Hernádi, L.; Hiripi, L.; Dyakonova, V.; Gyori, J.; Vehovszky, A. Thee effect of food intake on the central monoaminergic system in the snail, Lymnaea stagnalis. Acta Biol. Hung. 2004, 55, 185. [Google Scholar] [CrossRef]
- Dogterom, G.E.; van Loenhout, H.; Koomen, W.; Roubos, E.W.; Geraerts, W.P. Ovulation hormone, nutritive state, and female reproductive activity in Lymnaea stagnalis. Gen. Comp. Endocrinol. 1984, 55, 29–35. [Google Scholar] [CrossRef]
- Ter Maat, A.; Lodder, J.C.; Veenstra, J.; Goldschmeding, J.T. Suppression of egg-laying during starvation in the snail Lymnaea stagnalis by inhibition of the ovulation hormone producing caudo-dorsal cells. Brain Res. 1982, 239, 535–542. [Google Scholar] [CrossRef]
- Baz, E.S.; Hussein, A.A.A.; Vreeker, E.M.T.; Soliman, M.F.M.; Tadros, M.M.; El-Shenawy, N.S.; Koene, J.M. Consequences of artificial light at night on behavior, reproduction, and development of Lymnaea stagnalis. Environ. Pollut. 2022, 307, 119507. [Google Scholar] [CrossRef]
- Kitai, H.; Kakuda, U.; Goto, S.G.; Shiga, S. Photoperiod controls egg laying and caudodorsal cell hormone expression but not gonadal development in the pond snail Lymnaea stagnalis. J. Comp. Physiol. A 2021, 207, 523. [Google Scholar] [CrossRef]
- Ivashkin, E.; Khabarova, M.; Melnikova, V.; Nezlin, L.; Kharchenko, O.; Voronezhskaya, E.; Adameyko, I. Serotonin mediates maternal effects and directs developmental and behavioral changes in the progeny of snails. Cell Rep. 2015, 12, 1144–1158. [Google Scholar] [CrossRef]
- Dogterom, G.E.; Hofs, H.P.; Wapenaar, P.; Roubos, E.W.; Geraerts, W.P. The effect of temperature on spontaneous, and ovulation hormone-induced female reproduction in Lymnaea stagnalis. Gen. Comp. Endocrinol. 1984, 56, 204. [Google Scholar] [CrossRef] [PubMed]
- Brussaard, A.B.; Kits, K.S.; Ter Maat, A.; Van Minnen, J.; Moed, P.J. Dual inhibitory action of FMRFamide on neurosecretory cells controlling egg laying behavior in the pond snail. Brain Res. 1988, 447, 35–51. [Google Scholar] [CrossRef]
- Croll, R.P.; Van Minnen, J.; Kits, K.S.; Smit, A.B. APGWamide: Molecular, histological and physiological examination of the novel neuropeptide involved with reproduction in the snail Lymnaea stagnalis. In Molluscan Neurobiology; Kits, K.S., Boer, H.H., Joosse, J., Eds.; Free University of Amsterdam: Amsterdam, The Netherlands, 1991; pp. 248–254. [Google Scholar]
- Brussaard, A.B.; Schluter, N.C.M.; Ebberink, R.H.M.; Kits, K.S.; Ter Maat, A. Discharge induction in molluscan peptidergic cells requires a specific set of four autoexcitatory neuropeptides. Neuroscience 1990, 39, 479–491. [Google Scholar] [CrossRef]
- Ter Maat, A.; Jansen, R.F. The egg-laying behaviour of the pond snail: Electrophysiological aspects. In Biosynthesis, Metabolism and Mode of Action of Invertebrate Hormones; Hoffmann, J.A., Porchet, M., Eds.; Springer: Heidelberg, Germany, 1984; pp. 57–62. [Google Scholar]
- Jansen, R.F.; Ter Maat, A. Ring neuron control of columellar motor neurons during egg-laying behavior in the pond snail Lymnaea stagnalis. J. Neurobiol. 1985, 16, 1–14. [Google Scholar] [CrossRef]
- Croll, R.P.; Van Minnen, J. Distribution of the peptide Ala-ProGly-Trp-NH2 (APGWamide) in the nervous system and periphery of the snail Lymnaea stagnalis as revealed by immunocytochemistry and in situ hybridization. J. Comp. Neurol. 1992, 324, 567–574. [Google Scholar] [CrossRef]
- Schmidt, E.D.; Veenstra, E.; Broers-Vendrig, C.M.; van de Ven, A.M.; Roubos, E.W. Developmental and comparative aspects of nonsynaptic release by the egg-laying controlling caudodorsal cells of basommatophoran snails. Gen. Comp. Endocrinol. 1989, 75, 17. [Google Scholar] [CrossRef] [PubMed]
- Chistopol’skii, I.A.; Sakharov, D.A. Non-synaptic integration of the cell bodies of neurons into the central nervous system of the snail. Neurosci. Behav. Physiol. 2003, 33, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Chistopol’skii, I.A.; Sakharov, D.A. Isolated neurons as biosensors responding to the release of neuroactive substances. Neurosci. Behav. Physiol. 2008, 38, 703–705. [Google Scholar] [CrossRef]
- Dyakonova, V.E.; Dyakonova, T.L. Coordination of rhythm-generating units via NO and extrasynaptic neurotransmitter release. J. Comp. Physiol. A 2010, 196, 529. [Google Scholar] [CrossRef]
- Dyakonova, V.E.; Hernádi, L.; Ito, E.; Dyakonova, T.L.; Chistopolsky, I.A.; Zakharov, I.S.; Sakharov, D.A. The activity of isolated neurons and the modulatory state of an isolated nervous system represent a recent behavioural state. J. Exp. Biol. 2015, 218, 1151. [Google Scholar] [CrossRef]
- Ter Maat, A. Neuronal input on the ovulation hormone producing neuro-endocrine caudo-dorsal cells of the freshwater snail Lymnaea stagnalis. Proc. Kon Ned. Akad. Wetensch. 1979, 82C, 333–342. [Google Scholar]
- Ter Maat, A.; Lodder, J.C. A biphasic cholinergic input on the ovulation hormone producing caudodorsal cells of the freshwater snail Lymnaea stagnalis. Comp. Biochem. Physiol. 1980, 66, 115–119. [Google Scholar]
- Buznikov, G.A.; Nikitina, L.A.; Ayu, G.; Malchenko, L.A.; Trubnikova, O.B. The control of oocyte maturation in the starfish and amphibians by serotonin and its antagonists. Int. J. Dev. Biol. 1993, 37, 363–364. [Google Scholar] [PubMed]
- Stricker, S.A.; Smythe, T.L. Multiple triggers of oocyte maturation in nemertean worms: The roles of calcium and serotonin. J. Exp. Zool. 2000, 287, 243. [Google Scholar] [CrossRef] [PubMed]
- Alyoshina, N.M.; Tkachenko, M.D.; Malchenko, L.A.; Shmukler, Y.B.; Nikishin, D.A. Uptake and metabolization of serotonin by granulosa cells form a functional barrier in the mouse ovary. Int. J. Mol. Sci. 2022, 23, 14828. [Google Scholar] [CrossRef]
- Buznikov, G.; Chudakova, I.; Zvezdina, N. The role of neurohumours in early embryogenesis. I. Serotonin content of developing embryos of sea urchin and loach. J. Embr. Exp. Morph. 1964, 4, 563–573. [Google Scholar] [CrossRef]
- Emanuelsson, H. Localization of serotonin in cleavage embryos of Ophryotrocha labronica. Wilhelm Roux’Archiv Entwickl. Org. 1974, 175, 253–271. [Google Scholar] [CrossRef]
- Candiani, S.; Augello, A.; Oliveri, D.; Passalacqua, M.; Pennati, R.; De Bernardi, F.; Pestarino, M. Immunocytochemical localization of serotonin in embryos, larvae and adults of the lancelet, Branchiostoma floridae. J. Mol. Histol. 2001, 33, 413–420. [Google Scholar]
- Ivashkin, E.; Khabarova, M.; Voronezhskaya, E. Serotonin transport and synthesis systems during early development of invertebrates: Functional analysis on a bivalve model. Acta Biol. Hung. 2012, 2, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Dubé, F.; Amireault, P. Local serotonergic signaling in mammalian follicles, oocytes and early embryos. Life Sci. 2007, 81, 1627–1637. [Google Scholar] [CrossRef]
- Nikishin, D.A.; Khramova, Y.V.; Bagayeva, T.S.; Semenova, M.L.; Shmukler, Y.B. Expression of components of the serotonergic system in folliculogenesis and preimplantation development in mice. Russ. J. Dev. Biol. 2018, 49, 184–192. [Google Scholar] [CrossRef]
- Nikishin, D.A.; Alyoshina, N.M.; Semenova, M.L.; Shmukler, Y.B. Analysis of expression and functional activity of aromatic l-amino acid decarboxylase (ddc) and serotonin transporter (sert) as potential sources of serotonin in mouse ovary. Int. J. Mol. Sci. 2019, 20, 3070. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chistopolsky, I.; Leonova, A.; Mezheritskiy, M.; Boguslavsky, D.; Kristinina, A.; Zakharov, I.; Sorminskiy, A.; Vorontsov, D.; Dyakonova, V. Intense Locomotion Enhances Oviposition in the Freshwater Mollusc Lymnaea stagnalis: Cellular and Molecular Correlates. Biology 2023, 12, 764. https://doi.org/10.3390/biology12060764
Chistopolsky I, Leonova A, Mezheritskiy M, Boguslavsky D, Kristinina A, Zakharov I, Sorminskiy A, Vorontsov D, Dyakonova V. Intense Locomotion Enhances Oviposition in the Freshwater Mollusc Lymnaea stagnalis: Cellular and Molecular Correlates. Biology. 2023; 12(6):764. https://doi.org/10.3390/biology12060764
Chicago/Turabian StyleChistopolsky, Ilya, Alexandra Leonova, Maxim Mezheritskiy, Dmitri Boguslavsky, Angelina Kristinina, Igor Zakharov, Andrey Sorminskiy, Dmitri Vorontsov, and Varvara Dyakonova. 2023. "Intense Locomotion Enhances Oviposition in the Freshwater Mollusc Lymnaea stagnalis: Cellular and Molecular Correlates" Biology 12, no. 6: 764. https://doi.org/10.3390/biology12060764
APA StyleChistopolsky, I., Leonova, A., Mezheritskiy, M., Boguslavsky, D., Kristinina, A., Zakharov, I., Sorminskiy, A., Vorontsov, D., & Dyakonova, V. (2023). Intense Locomotion Enhances Oviposition in the Freshwater Mollusc Lymnaea stagnalis: Cellular and Molecular Correlates. Biology, 12(6), 764. https://doi.org/10.3390/biology12060764







