DNA-Binding Protein Dps Protects Escherichia coli Cells against Multiple Stresses during Desiccation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria
2.2. Cultivation
2.3. Obtaining Dehydrated Cell Preparations
2.4. Watering of Cell Preparations
2.5. Cell Count Determination
2.6. Cell Reactivation
2.7. Scanning Electron Microscopy (SEM)
2.8. Transmission Electron Microscopy (TEM)
2.9. Co-Crystal Molecular Models
2.10. Molecular Dynamics (MD)
2.11. Statistical Methods
3. Results
3.1. Effect of Dps on the Viability of Desiccated of E. coli Top10 and E. coli BL21-Gold Cells
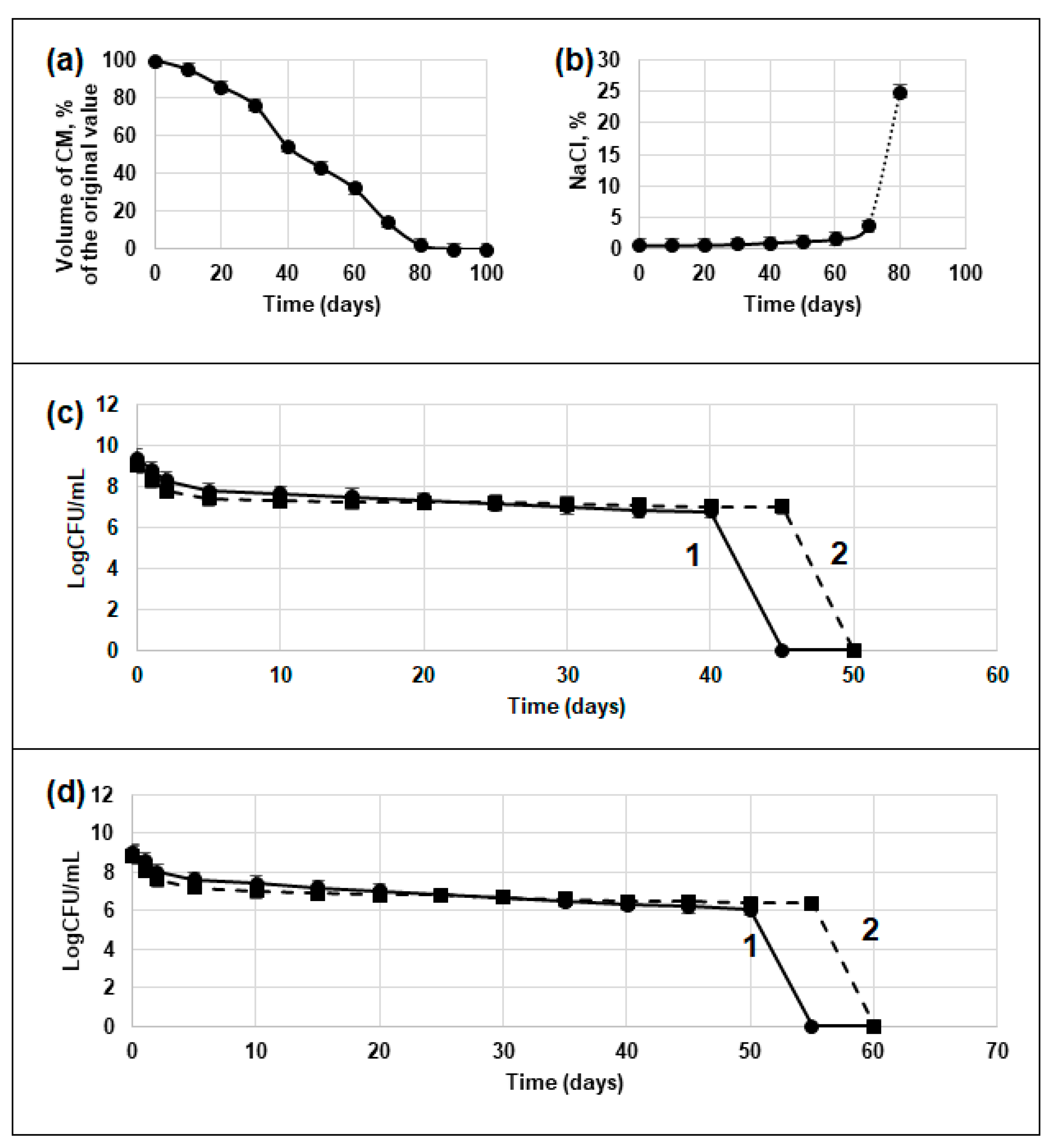
3.1.1. Changing Medium Parameters during the Desiccation Experiment
3.1.2. Decrease in Viable Cell Titer during the Desiccation Experiment
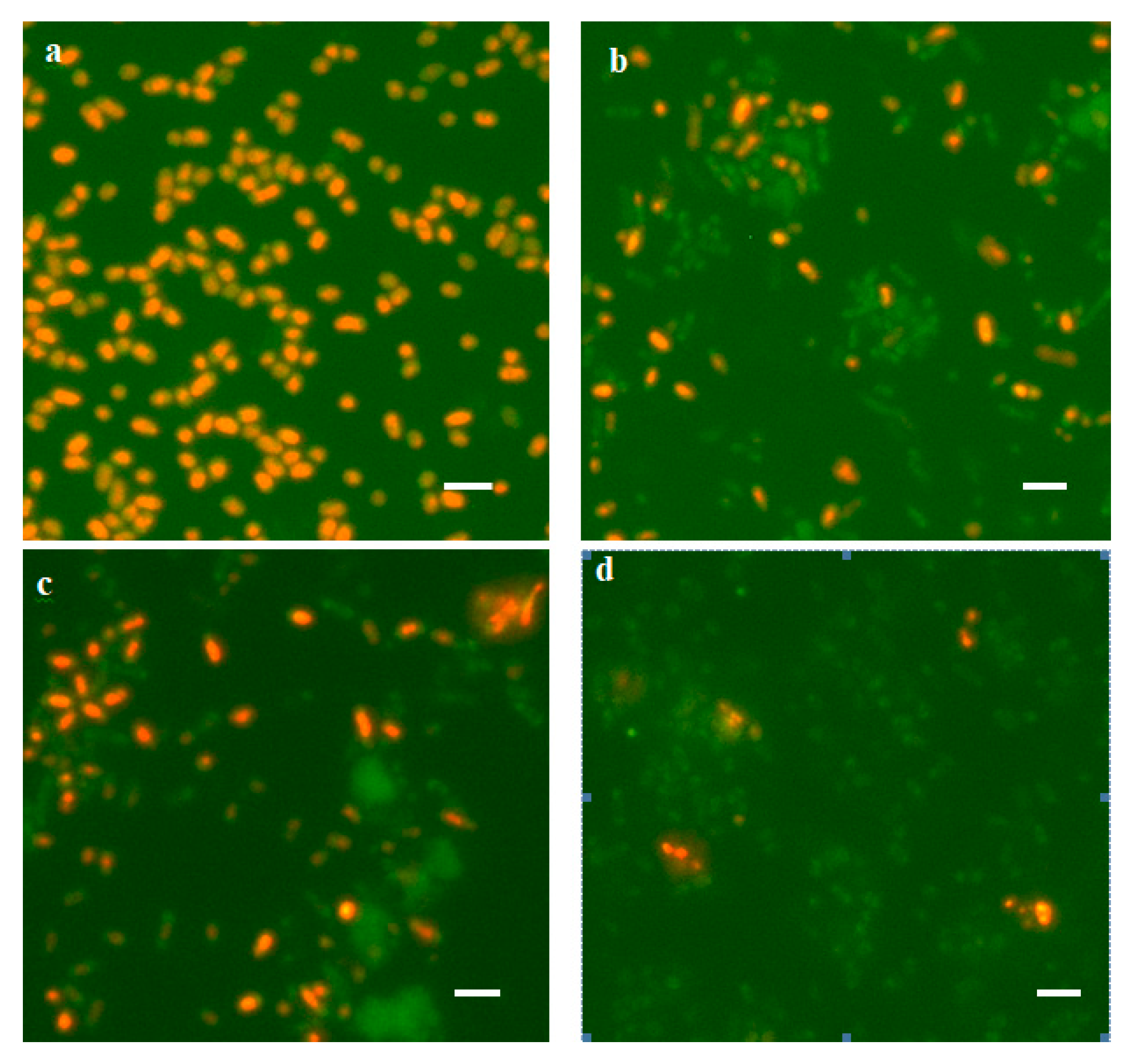
3.1.3. Viability of Rehydrated Cells
3.2. Changes in Cell Morphology and Ultrastructure during Desiccation and Subsequent Watering
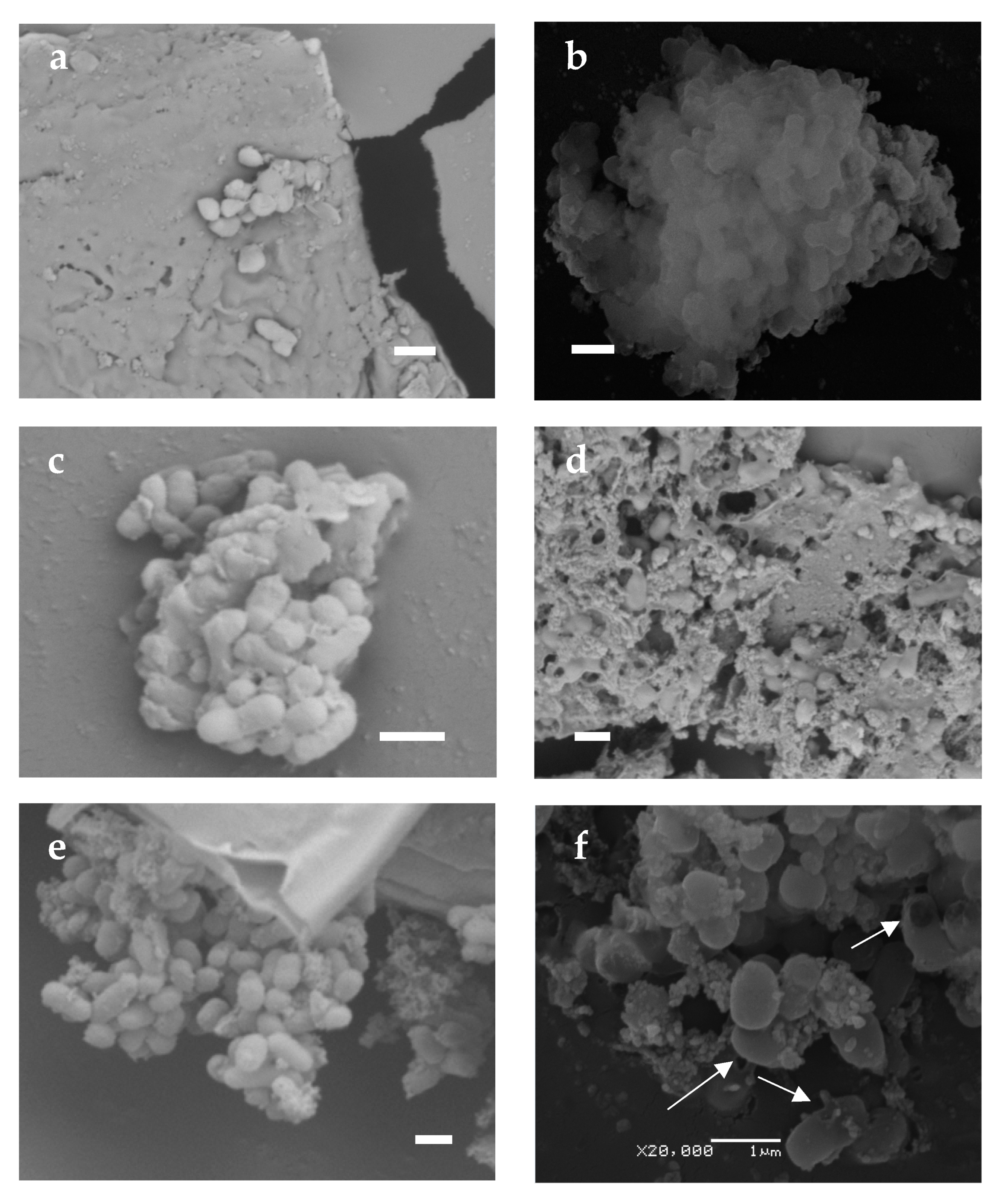
3.2.1. Changes in the Morphology of Desiccated Cells Revealed by SEM
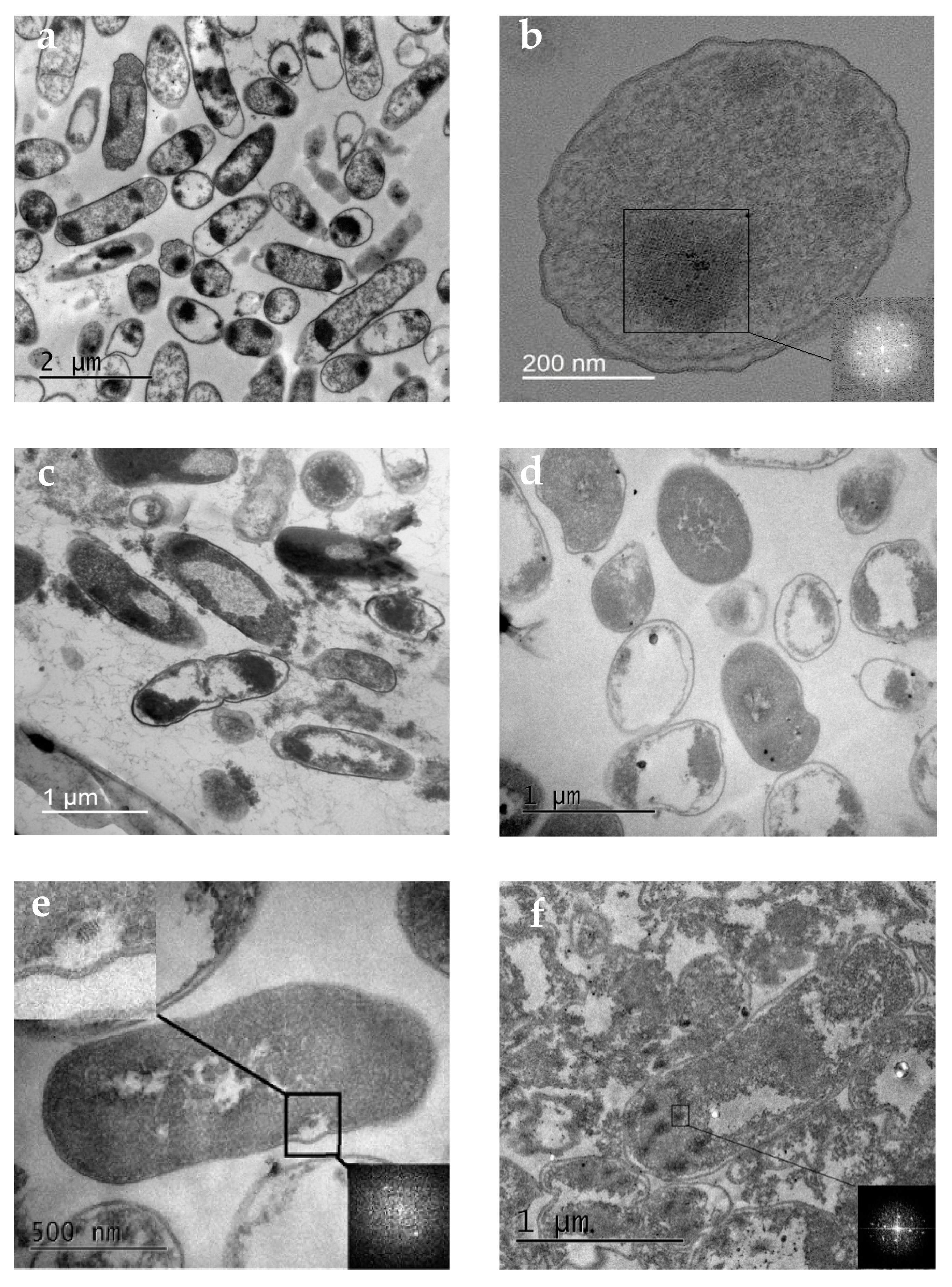
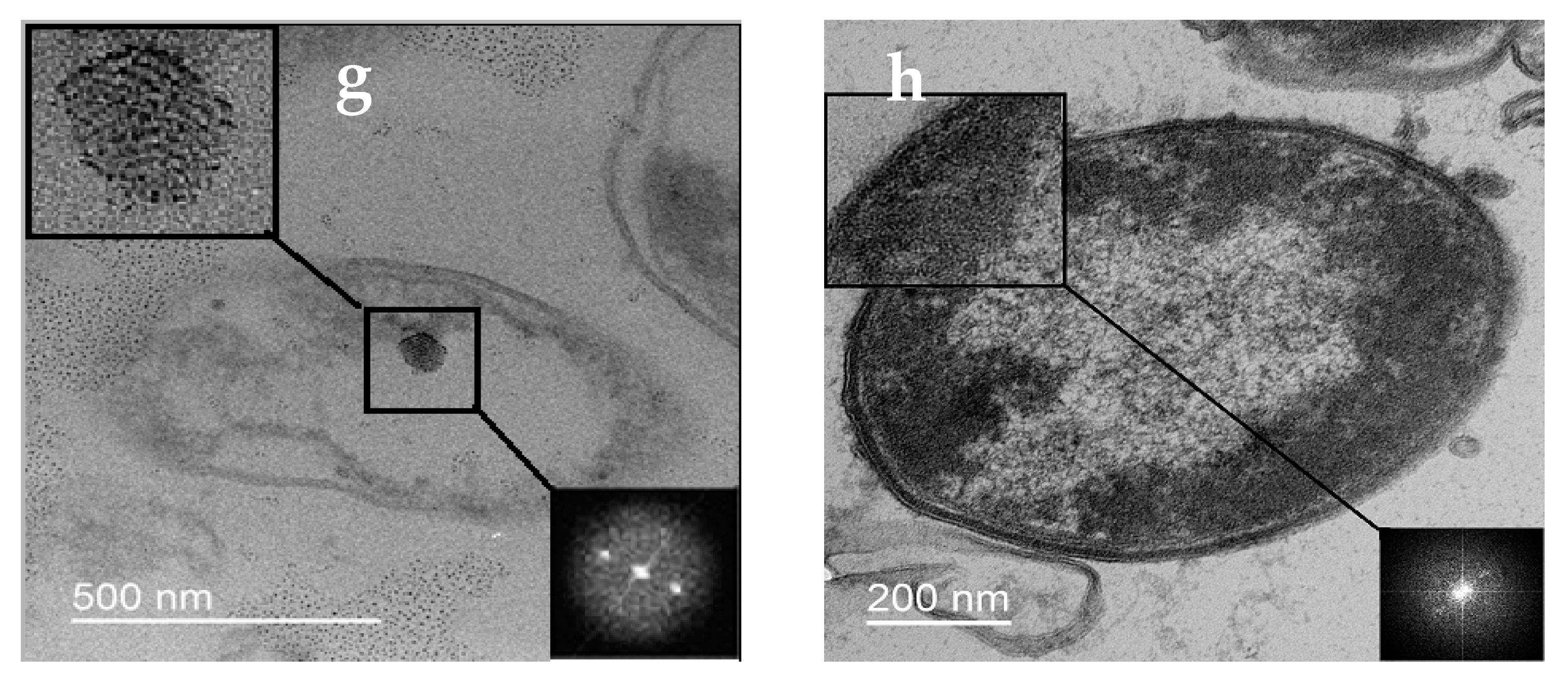
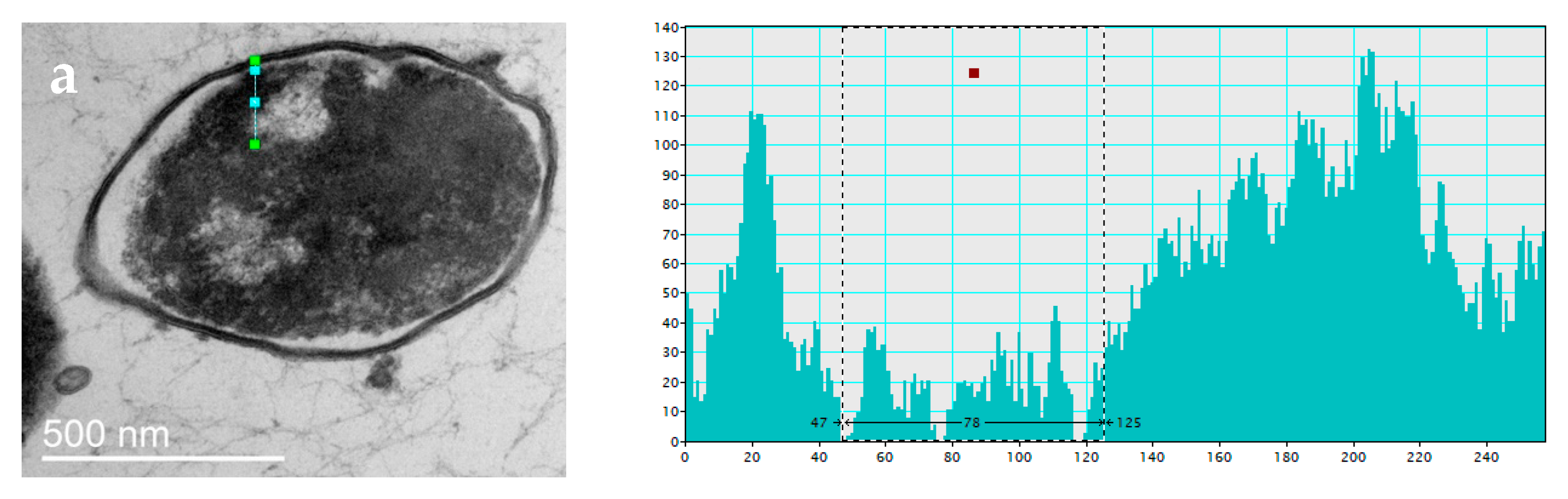
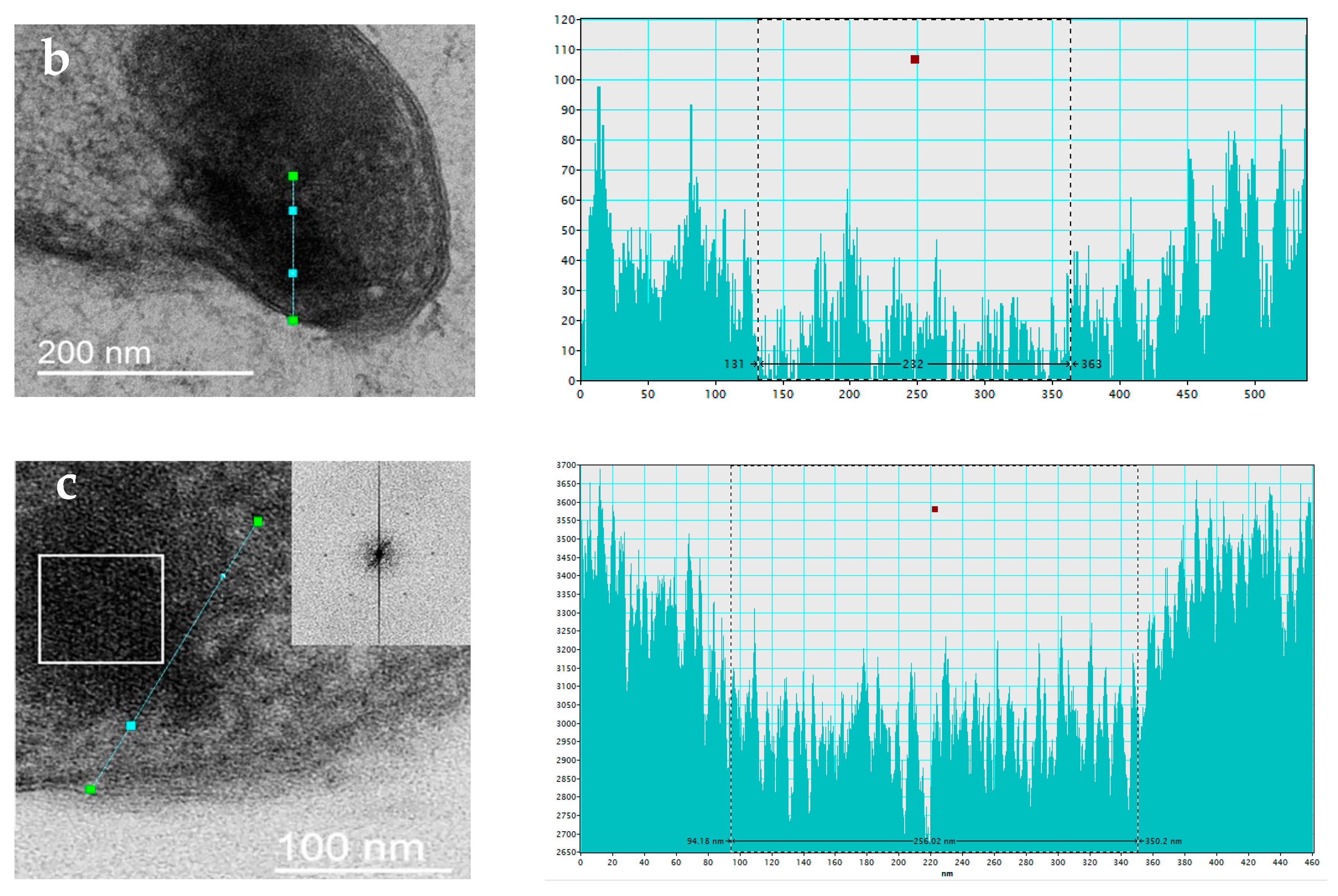
3.2.2. Changes in Cell Ultrastructure Revealed by Transmission Electron Microscopy
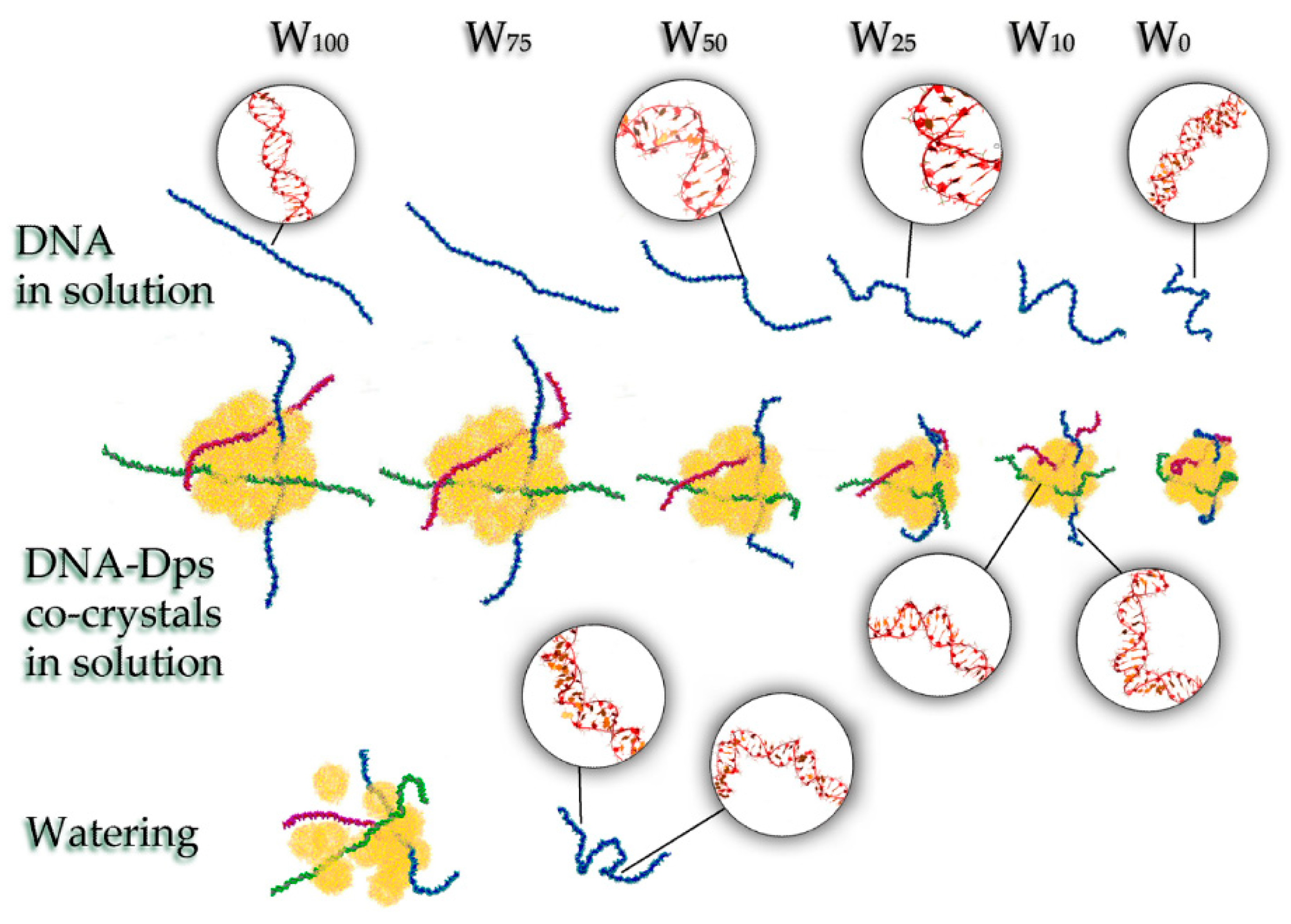
3.3. Explaining the Mechanisms of the Stress-Protective Action of Dps Protein during Cell Desiccation by Methods of Classical Molecular Dynamics (MD)
3.3.1. Systems W100 and W75
3.3.2. System W50
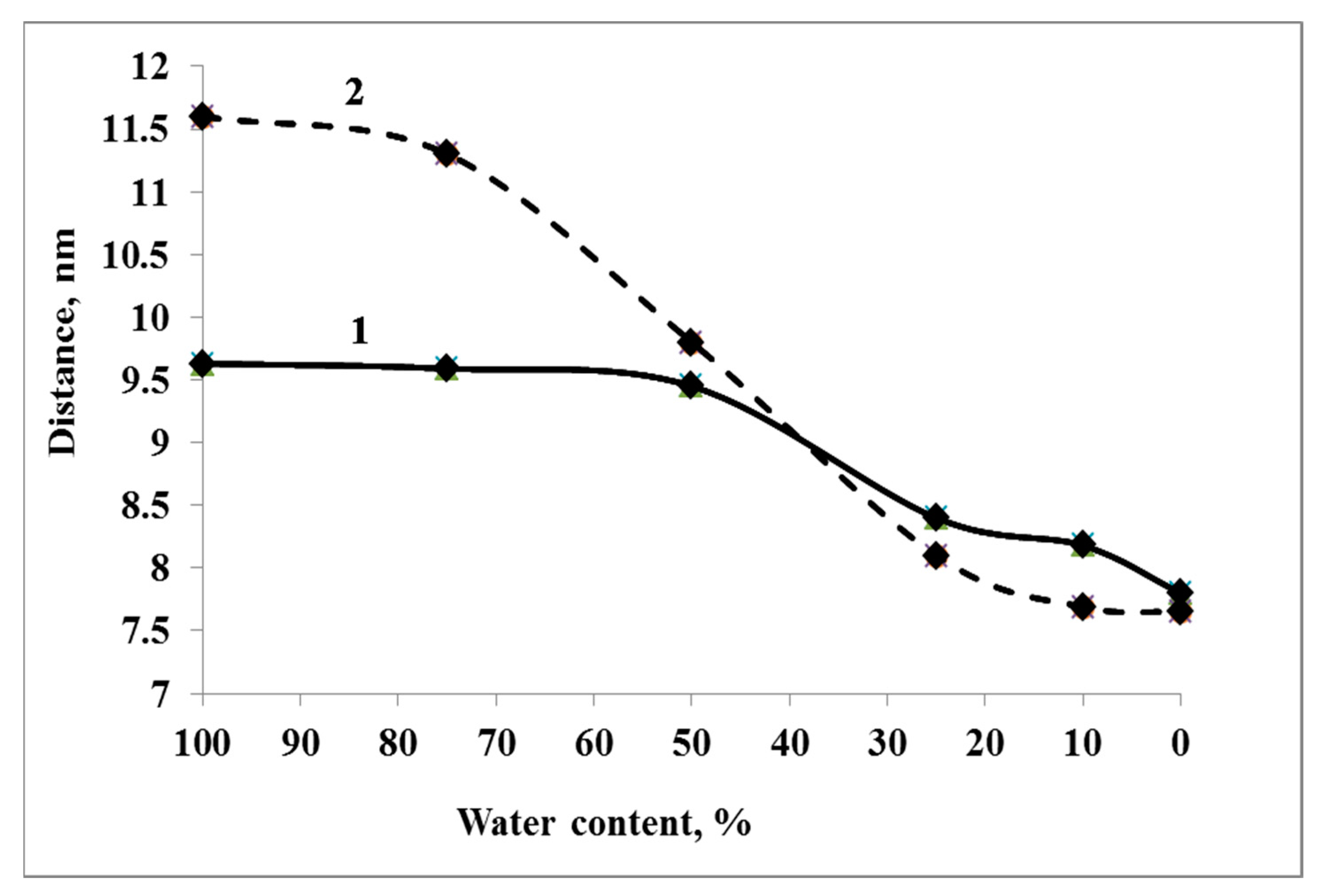
3.3.3. Systems W25 and More Desiccated
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esbelin, J.; Santos, T.; Hébraud, M. Desiccation: An environmental and food industry stress that bacteria commonly face. Food Microbiol. 2018, 69, 82–88. [Google Scholar] [CrossRef]
- Berninger, T.; López, Ó.G.; Bejarano, A.; Preininger, C.; Sessitsch, A. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb. Biotechnol. 2017, 11, 277–301. [Google Scholar] [CrossRef] [Green Version]
- Greffe, V.R.G.; Michiels, J. Desiccation-induced cell damage in bacteria and the relevance for inoculant production. Appl. Biotechnol. 2020, 104, 3757–3770. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Billi, D.; Potts, M. Life and death of dried prokaryotes. Res. Microbiol. 2002, 153, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gruzdev, N.; Pinto, R.; Sela, S. Effect of Desiccation on Tolerance of iSalmonella enterica/i to Multiple Stresses. Appl. Environ. Microbiol. 2011, 77, 1667–1673. [Google Scholar] [CrossRef] [Green Version]
- Potts, M. Desiccation tolerance of prokaryotes. Microbiol. Rev. 1994, 58, 755–805. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, X. Thermal Resistance and Gene Expression of both Desiccation-Adapted and Rehydrated Salmonella enterica Serovar Typhimurium Cells in Aged Broiler Litter. Appl. Environ. Microbiol. 2017, 83, e00367-17. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.I.; Goulian, M. A network of regulators promotes dehydration tolerance in Escherichia coli. Environ. Microbiol. 2018, 20, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Kragh, M.L.; Muchaamba, F.; Tasara, T.; Hansen, L.T. Cold-shock proteins affect desiccation tolerance, biofilm formation and motility in Listeria monocytogenes. Int. J. Food Microbiol. 2020, 329, 108662. [Google Scholar] [CrossRef] [PubMed]
- Azam, T.A.; Iwata, A.; Nishimura, A.; Ueda, S.; Ishihama, A. Growth Phase-Dependent Variation in Protein Composition of the Escherichia coli Nucleoid. J. Bacteriol. 1999, 181, 6361–6370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiancone, E. Role of Dps (DNA-binding proteins from starved cells) aggregation on DNA. Front. Biosci. 2010, 15, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calhoun, L.; Kwon, Y. Structure, function and regulation of the DNA-binding protein Dps and its role in acid and oxidative stress resistance in Escherichia coli: A review. J. Appl. Microbiol. 2010, 110, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Talukder, A.A.; Ishihama, A. Dps Is a Stationary Phase-Specific Protein of Escherichia coli Nucleoid. Adv. Microbiol. 2014, 04, 1095–1104. [Google Scholar] [CrossRef] [Green Version]
- Karas, V.O.; Westerlaken, I.; Meyer, A.S. The DNA-Binding Protein from Starved Cells (Dps) Utilizes Dual Functions To Defend Cells against Multiple Stresses. J. Bacteriol. 2015, 197, 3206–3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martino, M.D.; Ershov, D.; van den Berg, P.J.; Tans, S.J.; Meyer, A.S. Single-Cell Analysis of the Dps Response to Oxidative Stress. J. Bacteriol. 2016, 198, 1662–1674. [Google Scholar] [CrossRef] [Green Version]
- Loiko, N.; Danilova, Y.; Moiseenko, A.; Kovalenko, V.; Tereshkina, K.; Tutukina, M.; El-Registan, G.; Sokolova, O.; Krupyanskii, Y. Morphological peculiarities of the DNA-protein complexes in starved Escherichia coli cells. PLoS ONE 2020, 15, e0231562. [Google Scholar] [CrossRef]
- de Alcântara, N.R.; de Oliveira, F.M.; Garcia, W.; dos Santos, O.A.L.; Junqueira-Kipnis, A.P.; Kipnis, A. Dps protein is related to resistance of Mycobacterium abscessus subsp. massiliense against stressful conditions. Appl. Microbiol. Biotechnol. 2020, 104, 5065–5080. [Google Scholar] [CrossRef]
- Lackraj, T.; Birstonas, S.; Kacori, M.; Foster, D.B. Dps Protects Enterohemorrhagic Escherichia coli against Acid-Induced Antimicrobial Peptide Killing. J. Bacteriol. 2020, 202, e00114-20. [Google Scholar] [CrossRef]
- Suzuki, T.; Kobayashi, S.; Miyahira, K.; Sugiyama, M.; Katsuki, K.; Ishikawa, M. DNA-binding protein from starvation cells traps intracellular free-divalent iron and plays an important role in oxidative stress resistance in Acetobacter pasteurianus NBRC 3283. J. Biosci. Bioeng. 2021, 131, 256–263. [Google Scholar] [CrossRef]
- Williams, S.M.; Chatterji, D. An Overview of Dps: Dual Acting Nanovehicles in Prokaryotes with DNA Binding and Ferroxidation Properties. In Subcellular Biochemistry; Springer International Publishing: Cham, Switzerland, 2020; pp. 177–216. [Google Scholar] [CrossRef]
- Orban, K.; Finkel, S.E. Dps Is a Universally Conserved Dual-Action DNA-Binding and Ferritin Protein. J. Bacteriol. 2022, 204, e00036-22. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.G.; Frenkiel, D.; Arad, T.; Finkel, S.E.; Kolter, R.; Minsky, A. DNA protection by stress-induced biocrystallization. Nature 1999, 400, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Finkel, S.E. Dps Protects Cells against Multiple Stresses during Stationary Phase. J. Bacteriol. 2004, 186, 4192–4198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malone, A.S.; Chung, Y.K.; Yousef, A.E. Genes of Escherichia coli O157:H7 That Are Involved in High-Pressure Resistance. Appl. Environ. Microbiol. 2006, 72, 2661–2671. [Google Scholar] [CrossRef] [Green Version]
- Jeong, K.C.; Hung, K.F.; Baumler, D.J.; Byrd, J.J.; Kaspar, C.W. Acid stress damage of DNA is prevented by Dps binding in Escherichia coli O157:H7. BMC Microbiol. 2008, 8, 181. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.-J.; Ren, J.; Zeng, Y.-L.; Zhou, S.-N.; Lu, Y.-J. The iLegionella pneumophila/i Dps homolog is regulated by iron and involved in multiple stress tolerance. J. Basic Microbiol. 2009, 49, S79–S86. [Google Scholar] [CrossRef]
- Zeth, K. Dps biomineralizing proteins: Multifunctional architects of nature. Biochem. J. 2012, 445, 297–311. [Google Scholar] [CrossRef]
- Minsky, A.; Shimoni, E.; Frenkiel-Krispin, D. Stress, order and survival. Nat. Rev. Mol. Cell Biol. 2002, 3, 50–60. [Google Scholar] [CrossRef]
- Doye, J.; Poon, W. Protein crystallization in vivo. Curr. Opin. Colloid Interface Sci. 2006, 11, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Ceci, P. DNA condensation and self-aggregation of Escherichia coli Dps are coupled phenomena related to the properties of the N-terminus. Nucleic Acids Res. 2004, 32, 5935–5944. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Lim, C.J.; Dröge, P.; Yan, J. Regulation of Bacterial DNA Packaging in Early Stationary Phase by Competitive DNA Binding of Dps and IHF. Sci. Rep. 2015, 5, 18146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melekhov, V.V.; Shvyreva, U.S.; Timchenko, A.A.; Tutukina, M.N.; Preobrazhenskaya, E.V.; Burkova, D.V.; Artiukhov, V.G.; Ozoline, O.N.; Antipov, S.S. Modes of Escherichia coli Dps Interaction with DNA as Revealed by Atomic Force Microscopy. PLoS ONE 2015, 10, e0126504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Ceci, P.; Ilari, A.; Giangiacomo, L.; Laue, T.M.; Chiancone, E.; Chasteen, N.D. Iron and Hydrogen Peroxide Detoxification Properties of DNA-binding Protein from Starved Cells. J. Biol. Chem. 2002, 277, 27689–27696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellapadrona, G.; Ardini, M.; Ceci, P.; Stefanini, S.; Chiancone, E. Dps proteins prevent Fenton-mediated oxidative damage by trapping hydroxyl radicals within the protein shell. Free Radic. Biol. Med. 2010, 48, 292–297. [Google Scholar] [CrossRef]
- Chesnokov, Y.; Mozhaev, A.; Kamyshinsky, R.; Gordienko, A.; Dadinova, L. Structural Insights into Iron Ions Accumulation in Dps Nanocage. Int. J. Mol. Sci. 2022, 23, 5313. [Google Scholar] [CrossRef]
- Almirón, M.; Link, A.J.; Furlong, D.; Kolter, R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 1992, 6, 2646–2654. [Google Scholar] [CrossRef] [Green Version]
- Haikarainen, T.; Papageorgiou, A.C. Dps-like proteins: Structural and functional insights into a versatile protein family. Cell. Mol. Life Sci. 2010, 67, 341–351. [Google Scholar] [CrossRef]
- Grant, R.; Filman, D.; Finkel, S.; Kolter, R.; Hogle, J. The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nat. Struct. Biol. 1998, 5, 294–303. [Google Scholar] [CrossRef]
- Guerra, J.P.L.; Blanchet, C.E.; Vieira, B.J.C.; Almeida, A.V.; Waerenborgh, J.C.; Jones, N.C.; Hoffmann, S.V.; Tavares, P.; Pereira, A.S. The Conformation of the N-Terminal Tails of Deinococcus grandis Dps Is Modulated by the Ionic Strength. Int. J. Mol. Sci. 2022, 23, 4871. [Google Scholar] [CrossRef]
- Guerra, J.P.; Jacinto, J.P.; Tavares, P. Mini ferritins: Small multifunctional protein cages. Coord. Chem. Rev. 2021, 449, 214187. [Google Scholar] [CrossRef]
- Weber, A.; Kogl, S.A.; Jung, K. Time-Dependent Proteome Alterations under Osmotic Stress during Aerobic and Anaerobic Growth in Escherichia coli. J. Bacteriol. 2006, 188, 7165–7175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stasic, A.J.; Wong, A.C.L.; Kaspar, C.W. Osmotic and Desiccation Tolerance in Escherichia coli O157:H7 Requires rpoS (σ38). Curr. Microbiol. 2012, 65, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Sinitsyn, D.O.; Loiko, N.G.; Gularyan, S.K.; Stepanov, A.S.; Tereshkina, K.B.; Chulichkov, A.L.; Nikolaev, A.A.; El-Registan, G.I.; Popov, V.O.; Sokolova, O.S.; et al. Biocrystallization of bacterial nucleoid under stress. Russ. J. Phys. Chem. B 2017, 11, 833–838. [Google Scholar] [CrossRef]
- Loiko, N.G.; Suzina, N.E.; Soina, V.S.; Smirnova, T.A.; Zubasheva, M.V.; Azizbekyan, R.R.; Sinitsyn, D.O.; Tereshkina, K.B.; Nikolaev, Y.A.; Krupyanskii, Y.F.; et al. Biocrystalline structures in the nucleoids of the stationary and dormant prokaryotic cells. Microbiology 2017, 86, 714–727. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate at high ph as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera. A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Tereshkin, E.V.; Tereshkina, K.B.; Kovalenko, V.V.; Loiko, N.G.; Krupyanskii, Y.F. Structure of DPS Protein Complexes with DNA. Russ. J. Phys. Chem. B 2019, 13, 769–777. [Google Scholar] [CrossRef]
- Antipov, S.S.; Tutukina, M.N.; Preobrazhenskaya, E.V.; Kondrashov, F.A.; Patrushev, M.V.; Toshchakov, S.V.; Dominova, I.; Shvyreva, U.S.; Vrublevskaya, V.V.; Morenkov, O.S.; et al. The nucleoid protein Dps binds genomic DNA of Escherichia coli in a 264 non-random manner. PLoS ONE 2017, 12, e0182800. [Google Scholar] [CrossRef] [Green Version]
- Moiseenko, A.; Loiko, N.; Tereshkina, K.; Danilova, Y.; Kovalenko, V.; Chertkov, O.; Feofanov, A.V.; Krupyanskii, Y.F.; Sokolova, O.S. Projection structures reveal the position of the DNA within DNA–Dps Co-crystals. Biochem. Biophys. Res. Commun. 2019, 517, 463–469. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [Green Version]
- Marrink, S.J.; Tieleman, D.P. Perspective on the Martini model. Chem. Soc. Rev. 2013, 42, 6801. [Google Scholar] [CrossRef] [Green Version]
- Szatmári, D.; Sárkány, P.; Kocsis, B.; Nagy, T.; Miseta, A.; Barkó, S.; Longauer, B.; Robinson, R.C.; Nyitrai, M. Intracellular ion concentrations and cation-dependent remodelling of bacterial MreB assemblies. Sci. Rep. 2020, 10, 12002. [Google Scholar] [CrossRef]
- Pirozzi, B.; Napolitano, R.; Esposito, S. Contents: Macromol. Theory Simul. 8/2004. Macromol. Theory Simul. 2004, 13, 671–673. [Google Scholar] [CrossRef]
- Tereshkin, E.; Tereshkina, K.; Loiko, N.; Chulichkov, A.; Kovalenko, V.; Krupyanskii, Y. Interaction of deoxyribonucleic acid with deoxyribonucleic acid-binding protein from starved cells: Cluster formation and crystal growing as a model of initial stages of nucleoid biocrystallization. J. Biomol. Struct. Dyn. 2018, 37, 2600–2607. [Google Scholar] [CrossRef]
- Jenkins, D.E.; Chaisson, S.A.; Matin, A. Starvation-induced cross protection against osmotic challenge in Escherichia coli. J. Bacteriol. 1990, 172, 2779–2781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayrapetyan, M.; Williams, T.; Oliver, J.D. Relationship between the Viable but Nonculturable State and Antibiotic Persister Cells. J. Bacteriol. 2018, 200, e00249-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryazhevskikh, N.A.; Demkina, E.V.; Manucharova, N.A.; Soina, V.S.; Gal’chenko, V.F.; El’-Registan, G.I. Reactivation of dormant and nonculturable bacterial forms from paleosoils and subsoil permafrost. Microbiology 2012, 81, 435–445. [Google Scholar] [CrossRef]
- Dragoš, A.; Kovács, Á.T. The Peculiar Functions of the Bacterial Extracellular Matrix. Trends Microbiol. 2017, 25, 257–266. [Google Scholar] [CrossRef]
- Dillon, S.C.; Dorman, C.J. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat. Rev. Microbiol. 2010, 8, 185–195. [Google Scholar] [CrossRef]
- Amemiya, H.M.; Schroeder, J.; Freddolino, P.L. Nucleoid-associated proteins shape chromatin structure and transcriptional regulation across the bacterial kingdom. Transcription 2021, 12, 182–218. [Google Scholar] [CrossRef]
- Tereshkin, E.V.; Tereshkina, K.B.; Krupyanskii, Y.F. Predicting Binding Free Energies for DPS Protein-DNA Complexes and Crystals Using Molecular Dynamics. Supercomput. Front. Innov. 2022, 9, 33–45. [Google Scholar] [CrossRef]
- Krupyanskii, Y.F.; Kovalenko, V.V.; Loiko, N.G.; Generalova, A.A.; Moiseenko, A.V.; Tereshkin, E.V.; Sokolova, O.S.; Tereshkina, K.B.; El’-Registan, G.I.; Popov, A.N. Architecture of Condensed DNA in the Nucleoid of Escherichia coli Bacterium. Biophysics 2022, 67, 506–517. [Google Scholar] [CrossRef]
- Møretrø, T.; Langsrud, S. Residential bacteria on surfaces in the food industry and their implications for food safety and quality. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1022–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Deusenbery, C.; Wang, Y.; Shukla, A. Recent innovations in bacterial infection detection and treatment. ACS Infect. Dis. 2021, 7, 695–720. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Liu, L.; Ma, W.; Wang, X.; Li, S. Recent Progress of Surface-Enhanced Raman Spectroscopy for Bacteria Detection. Biosensors 2023, 13, 350. [Google Scholar] [CrossRef]
- Żur, J.; Wojcieszyńska, D.; Guzik, U. Metabolic Responses of Bacterial Cells to Immobilization. Molecules 2016, 21, 958. [Google Scholar] [CrossRef] [Green Version]
- Prévost, C.; Takahashi, M.; Lavery, R. Deforming DNA: From Physics to Biology. ChemPhysChem 2009, 10, 1399–1404. [Google Scholar] [CrossRef]
- Ghosh, A.; Bansal, M. A glossary of DNA structures from A to Z. Acta Crystallogr. Sect. D Biol. Crystallogr. 2003, 59, 620–626. [Google Scholar] [CrossRef] [Green Version]
- Shakked, Z.; Guerstein-Guzikevich, G.; Eisenstein, M.; Frolow, F.; Rabinovich, D. The conformation of the DNA double helix in the crystal is dependent on its environment. Nature 1989, 342, 456–460. [Google Scholar] [CrossRef] [PubMed]


| Name/Ion | Na+ | Ca++ | Cl− |
|---|---|---|---|
| W100 | 199.2 | 1.3 | 179.7 |
| W75 | 254.5 | 1.6 | 229.6 |
| W50 | 368.0 | 2.4 | 332.1 |
| W25 | 658.7 | 4.2 | 594.3 |
| W10 | 822.1 | 5.3 | 741.8 |
| W0 | 1252.4 | 8.1 | 1130.0 |
| Time of Experiments | Method | Titre of Cells, Cell/mL | |||
|---|---|---|---|---|---|
| E. coli Top10 | E. coli BL21-Gold | ||||
| Without Dps Activation | With Dps Activation | Without Dps Activation | With Dps Activation | ||
| Start of experiment | by CFU | (2.5 ± 0.5) × 109 | (1.3 ± 0.1) × 109 | (1.0 ± 0.1) × 109 | (7.6 ± 0.6) × 108 |
| 30 days | by CFU | (1.0 ± 0.1) × 107 | (1.5 ± 0.1) × 107 | (5.0 ± 0.3) × 106 | (5.1 ± 0.3) × 106 |
| total score with L/D | (5.4 ± 0.2) × 108 | (6.9 ± 0.4) × 108 | (1.9 ± 0.1) × 108 | (2.6 ± 0.2) × 108 | |
| 60 days | by CFU | 0 | 0 | 0 | 0 |
| total score with L/D | (6.1 ± 0.5) × 107 | (8.3 ± 0.6) × 107 | (3.3 ± 0.2) × 107 | (4.9 ± 0.3) × 107 | |
| Strain | Dps Activation | Titre of Cells, Cells/mL | |||
|---|---|---|---|---|---|
| By the CFU Method | Total Score with Live/Dead | ||||
| Without Reactivation | After Reactivation | Number of All Cells | Number of Living Cells (in %) | ||
| Top10 | - | 0 | (3.6 ± 0.2) × 102 | (2.1 ± 0.2) × 106 | (3.8 ± 0.2) × 105 (18.1) |
| + | (2.4 ± 0.2) × 102 | (8.2 ± 0.5) × 103 | (4.9 ± 0.3) × 106 | (4.2 ± 0.2) × 106 (85.7) | |
| BL21-Gold | - | (6.4 ± 0.3) × 102 | (3.5 ± 0.2) × 103 | (4.5 ± 0.3) × 106 | (3.2 ± 0.2) × 106 (71.1) |
| + | (2.2 ± 0.1) × 103 | (9.7 ± 0.7) × 103 | (9.7 ± 0.6) × 106 | (9.4 ± 0.2) × 106 (96.9) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loiko, N.; Tereshkina, K.; Kovalenko, V.; Moiseenko, A.; Tereshkin, E.; Sokolova, O.S.; Krupyanskii, Y. DNA-Binding Protein Dps Protects Escherichia coli Cells against Multiple Stresses during Desiccation. Biology 2023, 12, 853. https://doi.org/10.3390/biology12060853
Loiko N, Tereshkina K, Kovalenko V, Moiseenko A, Tereshkin E, Sokolova OS, Krupyanskii Y. DNA-Binding Protein Dps Protects Escherichia coli Cells against Multiple Stresses during Desiccation. Biology. 2023; 12(6):853. https://doi.org/10.3390/biology12060853
Chicago/Turabian StyleLoiko, Nataliya, Ksenia Tereshkina, Vladislav Kovalenko, Andrey Moiseenko, Eduard Tereshkin, Olga S. Sokolova, and Yurii Krupyanskii. 2023. "DNA-Binding Protein Dps Protects Escherichia coli Cells against Multiple Stresses during Desiccation" Biology 12, no. 6: 853. https://doi.org/10.3390/biology12060853
APA StyleLoiko, N., Tereshkina, K., Kovalenko, V., Moiseenko, A., Tereshkin, E., Sokolova, O. S., & Krupyanskii, Y. (2023). DNA-Binding Protein Dps Protects Escherichia coli Cells against Multiple Stresses during Desiccation. Biology, 12(6), 853. https://doi.org/10.3390/biology12060853








