Clutching at Guidance Cues: The Integrin–FAK Axis Steers Axon Outgrowth
Abstract
Simple Summary
Abstract
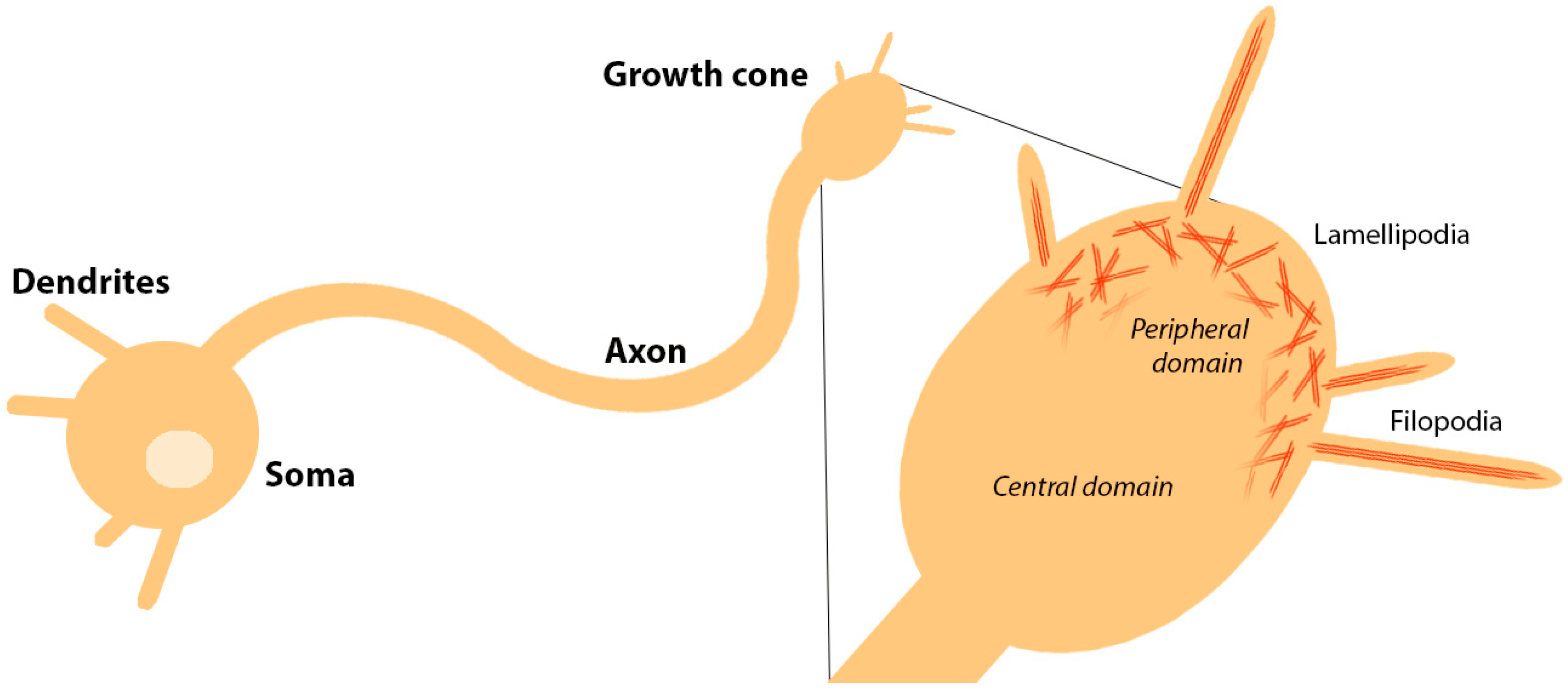
1. Introduction
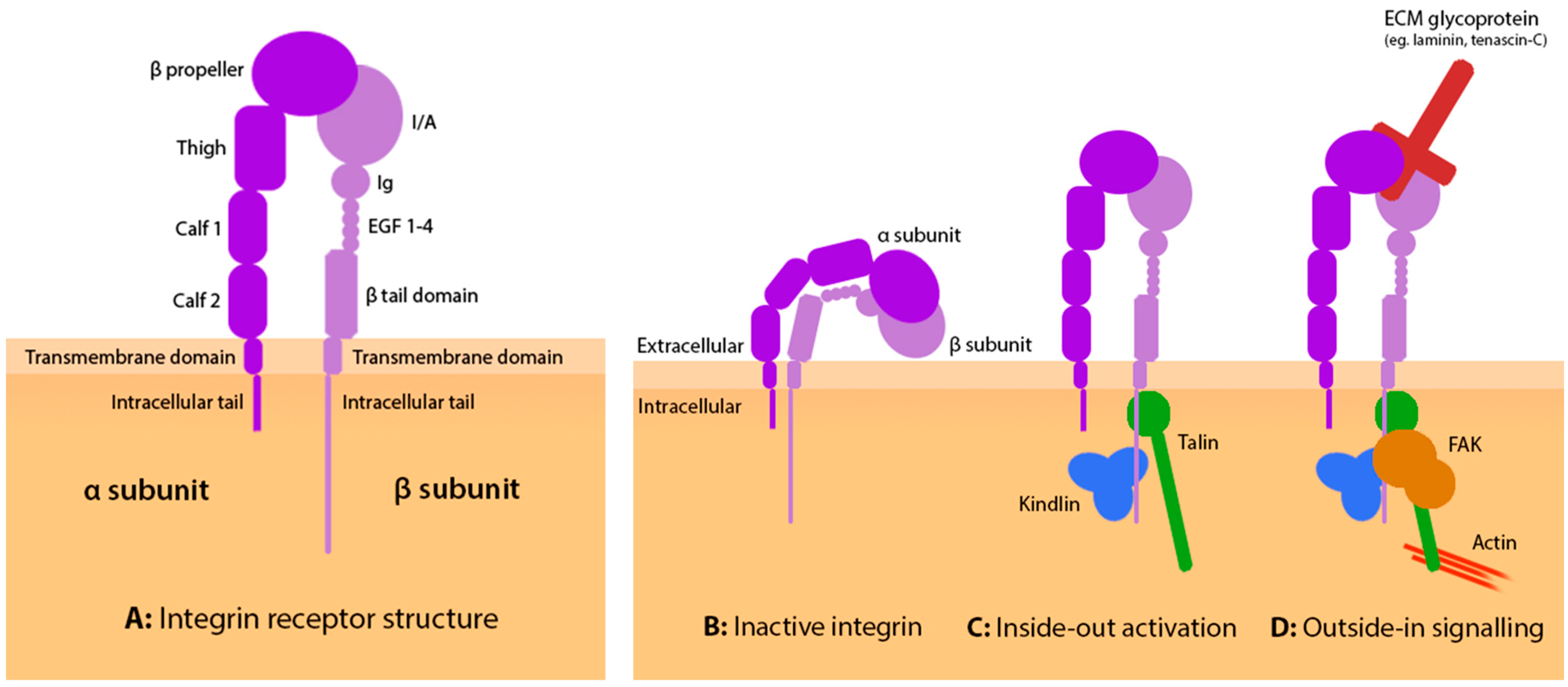
2. Integrin Structure and Activation
3. Outside-In Integrin Engagement
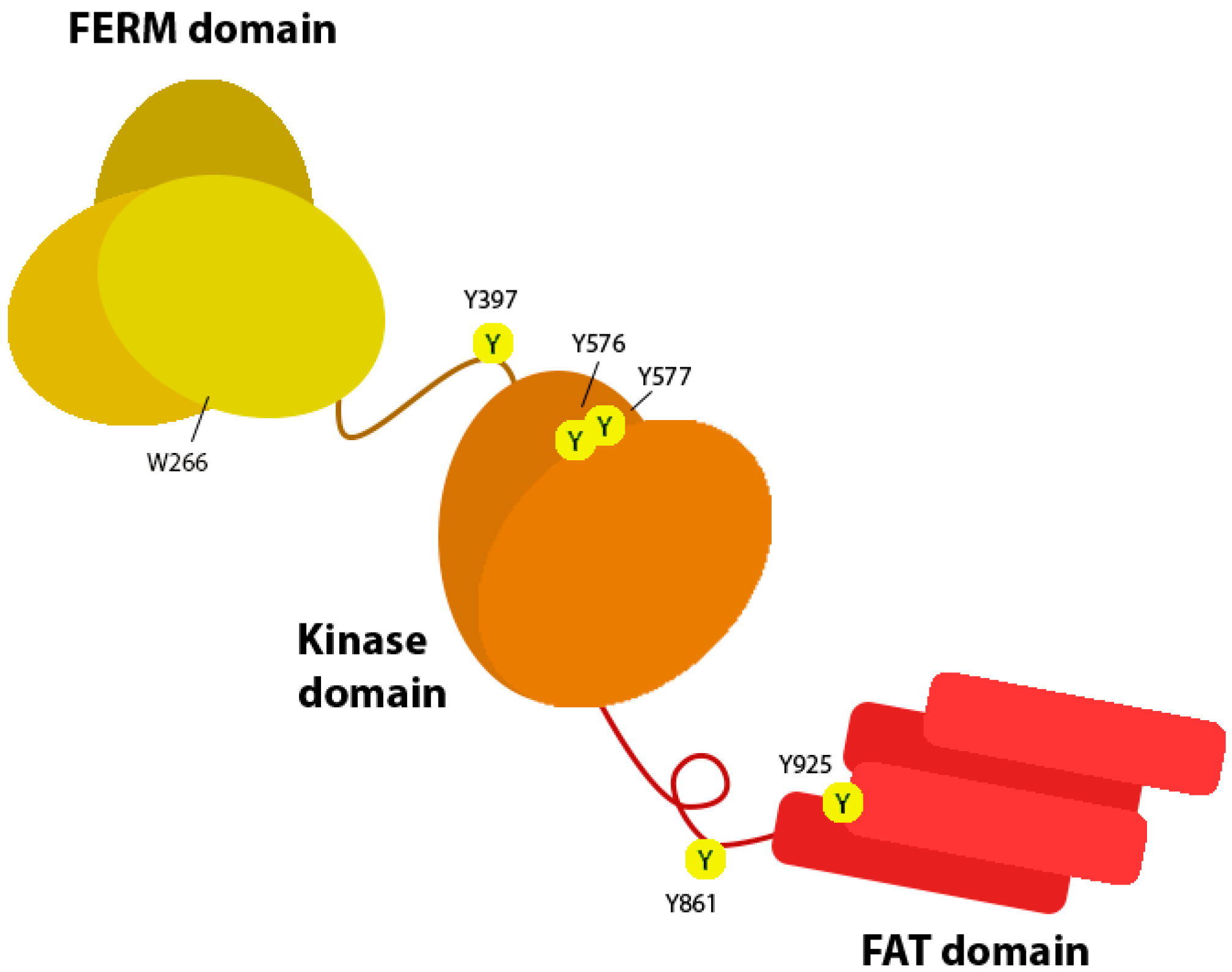
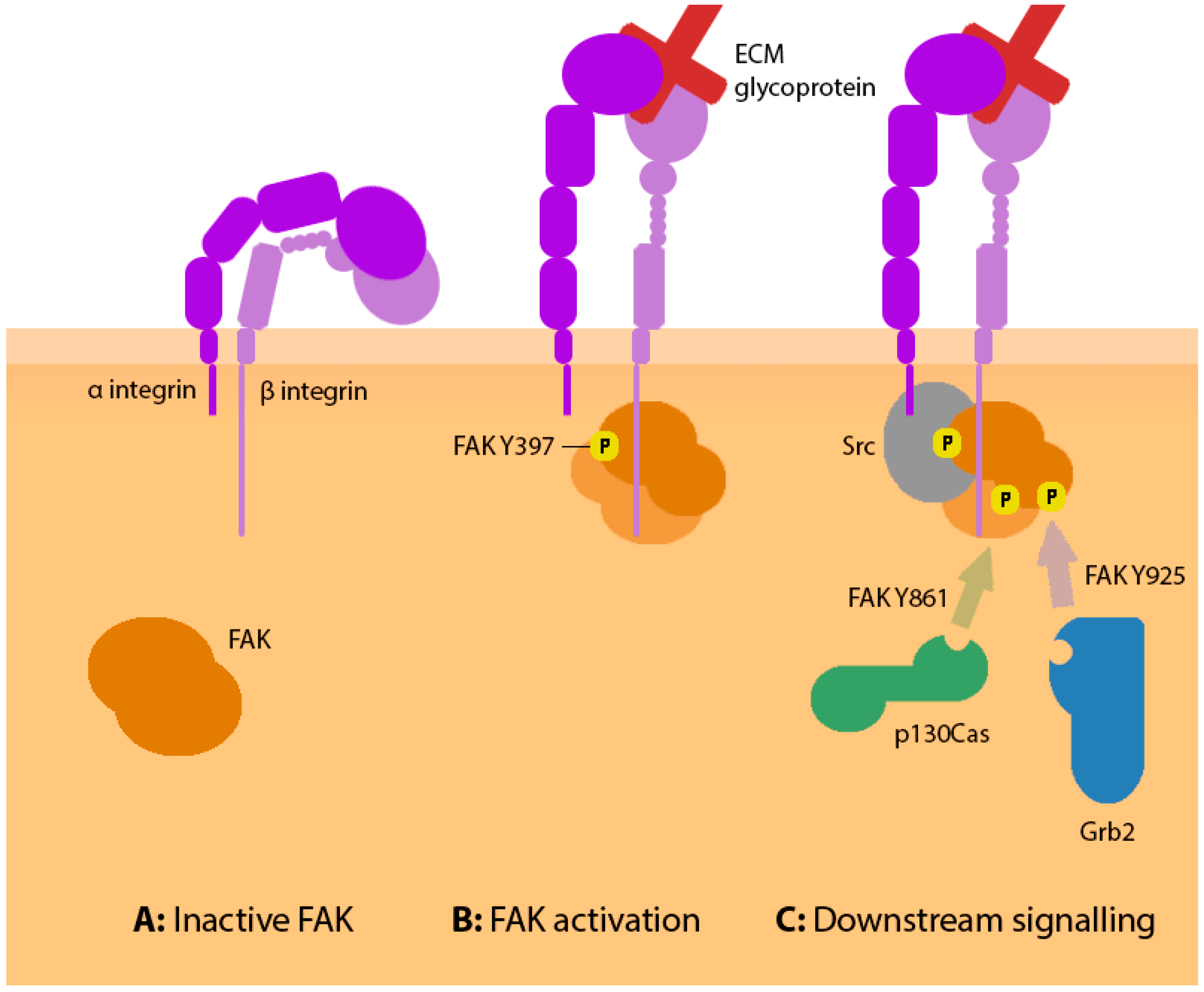
4. FAK Signalling
5. Regulation of Neurite Outgrowth by FAK
6. Targeting the Integrin–FAK Axis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mortimer, D.; Fothergill, T.; Pujic, Z.; Richards, L.J.; Goodhill, G.J. Growth cone chemotaxis. Trends Neurosci. 2008, 31, 90–98. [Google Scholar] [CrossRef]
- Fischer, R.S.; Lam, P.Y.; Huttenlocher, A.; Waterman, C.M. Filopodia and focal adhesions: An integrated system driving branching morphogenesis in neuronal pathfinding and angiogenesis. Dev. Biol. 2019, 451, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Renaudin, A.; Lehmann, M.; Girault, J.A.; McKerracher, L. Organization of point contacts in neuronal growth cones. J. Neurosci. Res. 1999, 55, 458–471. [Google Scholar] [CrossRef]
- Lin, C.-H.; Forscher, P. Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron 1995, 14, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Nichol, R.H., IV; Hagen, K.M.; Lumbard, D.C.; Dent, E.W.; Gomez, T.M. Guidance of axons by local coupling of retrograde flow to point contact adhesions. J. Neurosci. 2016, 36, 2267–2282. [Google Scholar] [CrossRef] [PubMed]
- Oria, R.; Wiegand, T.; Escribano, J.; Elosegui-Artola, A.; Uriarte, J.J.; Moreno-Pulido, C.; Platzman, I.; Delcanale, P.; Albertazzi, L.; Navajas, D.; et al. Force loading explains spatial sensing of ligands by cells. Nature 2017, 552, 219–224. [Google Scholar] [CrossRef]
- Gomez, T.M.; Letourneau, P.C. Actin dynamics in growth cone motility and navigation. J. Neurochem. 2014, 129, 221–234. [Google Scholar] [CrossRef]
- Chia, J.X.; Efimova, N.; Svitkina, T.M. Neurite outgrowth is driven by actin polymerization even in the presence of actin polymerization inhibitors. Mol. Biol. Cell 2016, 27, 3695–3704. [Google Scholar] [CrossRef]
- Dent, E.W.; Kwiatkowski, A.V.; Mebane, L.M.; Philippar, U.; Barzik, M.; Rubinson, D.A.; Gupton, S.; Van Veen, J.E.; Furman, C.; Zhang, J.; et al. Filopodia are required for cortical neurite initiation. Nat. Cell Biol. 2017, 9, 1347–1359. [Google Scholar] [CrossRef]
- Woo, S.; Gomez, T.M. Rac1 and RhoA promote neurite outgrowth through formation and stabilization of growth cone point contacts. J. Neurosci. 2006, 26, 1418–1428. [Google Scholar] [CrossRef]
- Kuo, J.C.; Han, X.; Hsiao, C.T.; Yates, J.R.; Waterman, C.M. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 2011, 13, 383–395. [Google Scholar] [CrossRef]
- Legerstee, K.; Houtsmuller, A. A Layered View on Focal Adhesions. Biology 2021, 10, 1189. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, M.; Wehrle-Haller, B.; de Curtis, I. Paxillin: A Hub for Mechano-Transduction from the β3 Integrin-Talin-Kindlin Axis. Front. Cell Dev. Biol. 2022, 10, 852016. [Google Scholar] [CrossRef]
- Bialkowska, K.; Qin, J.; Plow, E.F. Phosphorylation of kindlins and the control of integrin function. Cells 2021, 10, 825. [Google Scholar] [CrossRef] [PubMed]
- Goult, B.T.; Brown, N.H.; Schwartz, M.A. Talin in mechanotransduction and mechanomemory at a glance. J. Cell Sci. 2021, 134, jcs258749. [Google Scholar] [CrossRef]
- Carisey, A.; Ballestrem, C. Vinculin, an adapter protein in control of cell adhesion signalling. Eur. J. Cell Biol. 2011, 90, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, D.D.; Hauck, C.R.; Sieg, D.J. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 1999, 71, 435–478. [Google Scholar] [CrossRef]
- Tan, C.L.; Kwok, J.C.F.; Patani, R.; Ffrench-Constant, C.; Chandran, S.; Fawcett, J.W. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J. Neurosci. 2011, 31, 6289–6295. [Google Scholar] [CrossRef]
- Tan, C.L.; Andrews, M.R.; Kwok, J.C.F.; Heintz, T.G.P.; Gumy, L.F.; Fässler, R.; Fawcett, J.W. Kindlin-1 enhances axon growth on inhibitory chondroitin sulfate proteoglycans and promotes sensory axon regeneration. J. Neurosci. 2012, 32, 7325–7335. [Google Scholar] [CrossRef] [PubMed]
- Cheah, M.; Andrews, M.R.; Chew, D.J.; Moloney, E.B.; Verhaagen, J.; Fässler, R.; Fawcett, J.W. Expression of an activated integrin promotes long-distance sensory axon regeneration in the spinal cord. J. Neurosci. 2016, 36, 7283–7297. [Google Scholar] [CrossRef]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Lyu, Q.; Ley, K.; Goult, B.T. Structural basis of β2 integrin inside—Out activation. Cells 2022, 11, 3039. [Google Scholar] [CrossRef]
- Le Coq, J.; Acebrón, I.; Rodrigo Martin, B.; López Navajas, P.; Lietha, D. New insights into FAK structure and function in focal adhesions. J. Cell Sci. 2022, 135, jcs259089. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef]
- Hynes, R. Integrins: Bidirectional, Allosteric Signaling Machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Xiong, J.P.; Stehle, T.; Diefenbach, B.; Zhang, R.; Dunker, R.; Scott, D.L.; Joachimiak, A.; Goodman, S.L.; Arnaout, M.A. Crystal structure of the extracellular segment of integrin αVβ3. Science 2001, 294, 339–345. [Google Scholar] [CrossRef]
- Arnaout, M.A.; Mahalingam, B.; Xiong, J.P. Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. 2005, 21, 381–410. [Google Scholar] [CrossRef] [PubMed]
- Beglova, N.; Blacklow, S.C.; Takagi, J.; Springer, T.A. Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat. Struct. Biol. 2002, 9, 282–287. [Google Scholar] [CrossRef]
- Vinogradova, O.; Velyvis, A.; Velyviene, A.; Hu, B.; Haas, T.A.; Plow, E.F.; Qin, J. A structural mechanism of integrin alpha(IIb)beta(3) “inside-out” activation as regulated by its cytoplasmic face. Cell 2002, 110, 587–597. [Google Scholar] [CrossRef]
- Goult, B.T.; Zacharchenko, T.; Bate, N.; Tsang, R.; Hey, F.; Gingras, A.R.; Elliott, P.R.; Roberts, G.C.K.; Ballestrem, C.; Critchley, D.R.; et al. RIAM and vinculin binding to talin are mutually exclusive and regulate adhesion assembly and turnover. J. Biol. Chem. 2013, 288, 8238–8249. [Google Scholar] [CrossRef]
- Calderwood, D.A.; Zent, R.; Grant, R.; Rees, D.J.G.; Hynes, R.O.; Ginsberg, M.H. The talin head domain binds to integrin β subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 1999, 274, 28071–28074. [Google Scholar] [CrossRef] [PubMed]
- Anthis, N.J.; Wegener, K.L.; Ye, F.; Kim, C.; Goult, B.T.; Lowe, E.D.; Vakonakis, I.; Bate, N.; Critchley, D.R.; Ginsberg, M.H.; et al. The structure of an integrin/talin complex reveals the basis of inside-out signal transduction. EMBO J. 2009, 28, 3623–3632. [Google Scholar] [CrossRef]
- Tan, C.L.; Kwok, J.C.F.; Heller, J.P.D.; Zhao, R.; Eva, R.; Fawcett, J.W. Full length talin stimulates integrin activation and axon regeneration. Mol. Cell. Neurosci. 2015, 68, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Goult, B.T.; Yan, J.; Schwartz, M.A. Talin as a mechanosensitive signaling hub. J. Cell Biol. 2018, 217, 3776–3784. [Google Scholar] [CrossRef]
- Li, H.; Deng, Y.; Sun, K.; Yang, H.; Liu, J.; Wang, M.; Zhang, Z.; Lin, J.; Wu, C.; Wei, Z.; et al. Structural basis of kindlin-mediated integrin recognition and activation. Proc. Natl. Acad. Sci. USA 2017, 114, 9349–9354. [Google Scholar] [CrossRef] [PubMed]
- Cheah, M.; Andrews, M. Integrin Activation: Implications for Axon Regeneration. Cells 2018, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, J.; Springer, T.A. Low affinity integrin states have faster ligand binding kinetics than the high affinity state. eLife 2021, 10, e73359. [Google Scholar] [CrossRef]
- Smith, C.; Estavillo, D.; Emsley, J.; Bankston, L.A.; Liddington, R.C.; Cruz, M.A. Mapping the collagen-binding site in the I domain of the glycoprotein Ia/IIa (Integrin α2β1). J. Biol. Chem. 2000, 275, 4205–4209. [Google Scholar] [CrossRef]
- Komoriya, A.; Green, L.J.; Mervic, M.; Yamada, S.S.; Yamada, K.M.; Humphries, M.J. The Minimal Essential Sequence for a Major Cell Type-specific Adhesion Site (CS1) within the Alternatively Spliced Type I11 Connecting Segment Domain of Fibronectin Is Leucine-Aspartic Acid-Valine. J. Biol. Chem. 1991, 266, 15075–15079. [Google Scholar] [CrossRef] [PubMed]
- Pesho, M.M.; Bledzka, K.; Michalec, L.; Cierniewski, C.S.; Plow, E.F. The specificity and function of the metal-binding sites in the integrin β3 A-domain. J. Biol. Chem. 2006, 281, 23034–23041. [Google Scholar] [CrossRef]
- Lee, H.S.; Lim, C.J.; Puzon-McLaughlin, W.; Shattil, S.J.; Ginsberg, M.H. RIAM activates integrins by linking talin to Ras GTPase membrane-targeting sequences. J. Biol. Chem. 2009, 284, 5119–5122. [Google Scholar] [CrossRef]
- Kleinschmidt, E.G.; Schlaepfer, D.D. Focal adhesion kinase signaling in unexpected places. Curr. Opin. Cell Biol. 2017, 45, 24–30. [Google Scholar] [CrossRef]
- Lawson, C.; Lim, S.T.; Uryu, S.; Chen, X.L.; Calderwood, D.A.; Schlaepfer, D.D. FAK promotes recruitment of talin to nascent adhesions to control cell motility. J. Cell Biol. 2012, 196, 223–232. [Google Scholar] [CrossRef]
- Hu, Y.L.; Lu, S.; Szeto, K.W.; Sun, J.; Wang, Y.; Lasheras, J.C.; Chien, S. FAK and paxillin dynamics at focal adhesions in the protrusions of migrating cells. Sci. Rep. 2014, 4, 6024. [Google Scholar] [CrossRef] [PubMed]
- Frame, M.C.; Patel, H.; Serrels, B.; Lietha, D.; Eck, M.J. The FERM domain: Organizing the structure and function of FAK. Nat. Rev. Mol. Cell Biol. 2010, 11, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Hoellerer, M.K.; Noble, M.E.M.; Labesse, G.; Campbell, I.D.; Werner, J.M.; Arold, S.T. Molecular recognition of paxillin LD motifs by the focal adhesion targeting domain. Structure 2003, 11, 1207–1217. [Google Scholar] [CrossRef]
- Contestabile, A.; Bonanomi, D.; Burgaya, F.; Girault, J.A.; Valtorta, F. Localization of focal adhesion kinase isoforms in cells of the central nervous system. Int. J. Dev. Neurosci. 2003, 21, 83–93. [Google Scholar] [CrossRef]
- Burgaya, F.; Toutant, M.; Studler, J.-M.; Costa, A.; Le Bert, M.; le Gelman, M.; Girault, J.-A. Alternatively spliced focal adhesion kinase in rat brain with increased autophosphorylation activity. Cell Biol. Metab. 1997, 272, 28720–28725. [Google Scholar] [CrossRef] [PubMed]
- Toutant, M.; Studler, J.M.; Burgaya, F.; Costa, A.; Ezan, P.; Gelman, M.; Girault, J.A. Autophosphorylation of Tyr397 and its phosphorylation by Src-family kinases are altered in focal-adhesion-kinase neuronal isoforms. Biochem. J. 2000, 348, 119–128. [Google Scholar] [CrossRef] [PubMed]
- de Pins, B.; Mendes, T.; Giralt, A.; Girault, J.A. The Non-receptor Tyrosine Kinase Pyk2 in brain function and neurological and psychiatric diseases. Front. Synapt. Neurosci. 2021, 13, 749001. [Google Scholar] [CrossRef]
- Menegon, A.; Burgaya, F.; Baudot, P.; Dunlap, D.D.; Girault, J.A.; Valtorta, F. FAK+ and PYK2/CAKβ, two related tyrosine kinases highly expressed in the central nervous system: Similarities and differences in the expression pattern. Eur. J. Neurosci. 1999, 11, 3777–3788. [Google Scholar] [CrossRef] [PubMed]
- Giralt, A.; Coura, R.; Girault, J.A. Pyk2 is essential for astrocytes mobility following brain lesion. GLIA 2016, 64, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Lietha, D.; Cai, X.; Ceccarelli, D.F.J.; Li, Y.; Schaller, M.D. and Eck, M.J. Structural basis for the autoinhibition of focal adhesion kinase. Cell 2007, 129, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Brami-Cherrier, K.; Gervasi, N.; Arsenieva, D.; Walkiewicz, K.; Boutterin, M.C.; Ortega, A.; Leonard, P.G.; Seantier, B.; Gasmi, L.; Bouceba, T.; et al. FAK dimerization controls its kinase-dependent functions at focal adhesions. EMBO J. 2014, 33, 356–370. [Google Scholar] [CrossRef]
- Toutant, M.; Costa, A.; Studler, J.-M.; Kadaré, G.; Carnaud, M.; Girault, J.-A. Alternative splicing controls the mechanisms of FAK autophosphorylation. Mol. Cell. Biol. 2002, 22, 7731–7743. [Google Scholar] [CrossRef] [PubMed]
- Calalb, M.B.; Polte, T.R.; Hanks, S.K. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: A role for Src family kinases. Mol. Cell. Biol. 1995, 15, 954–963. [Google Scholar] [CrossRef]
- Gabarra-Niecko, V.; Keely, P.J.; Schaller, M.D. Characterization of an activated mutant of focal adhesion kinase: “SuperFAK”. Biochem. J. 2002, 365, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Han, I.; Jeon, J.; Park, H.; Bahk, Y.Y.; Oh, E.S. Phosphorylation of focal adhesion kinase at tyrosine 861 is crucial for ras transformation of fibroblasts. J. Biol. Chem. 2004, 279, 29060–29065. [Google Scholar] [CrossRef]
- Sanders, M.A.; Basson, M.D. Collagen IV regulates Caco-2 cell spreading and p130Cas phosphorylation by FAK-dependent and FAK-independent pathways. Biol. Chem. 2008, 389, 47–55. [Google Scholar] [CrossRef]
- Cheresh, D.A.; Leng, J.; Klemke, R.L. Regulation of cell contraction and membrane ruffling by distinct signals in migratory cells. J. Cell Biol. 1999, 146, 1107–1116. [Google Scholar] [CrossRef]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef]
- Webb, D.J.; Donais, K.; Whitmore, L.A.; Thomas, S.M.; Turner, C.E.; Parsons, J.T.; Horwitz, A.F. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004, 6, 154–161. [Google Scholar] [CrossRef]
- Myers, J.P.; Gomez, T.M. Focal adhesion kinase promotes integrin adhesion dynamics necessary for chemotropic turning of nerve growth cones. J. Neurosci. 2011, 31, 13585–13595. [Google Scholar] [CrossRef]
- Deramaudt, T.B.; Dujardin, D.; Hamadi, A.; Noulet, F.; Kolli, K.; De Mey, J.; Takeda, K.; Rondé, P. FAK phosphorylation at Tyr-925 regulates cross-talk between focal adhesion turnover and cell protrusion. Mol. Biol. Cell 2011, 22, 964–975. [Google Scholar] [CrossRef]
- Swaminathan, V.; Fischer, R.S.; Waterman, C.M. The FAK-Arp2/3 interaction promotes leading edge advance and haptosensing by coupling nascent adhesions to lamellipodia actin. Mol. Biol. Cell 2016, 27, 1085–1100. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, H.; Chen, J.; McNiven, M.A. A direct interaction between the large GTPase dynamin-2 and FAK regulates focal adhesion dynamics in response to active Src. Mol. Biol. Cell 2011, 22, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Nader, G.P.F.; Ezratty, E.J.; Gundersen, G.G. FAK, talin and PIPKI 3 regulate endocytosed integrin activation to polarize focal adhesion assembly. Nat. Cell Biol. 2016, 18, 491–503. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.Y.; Kim, H.W. Adhesive proteins linked with focal adhesion kinase regulate neurite outgrowth of PC12 cells. Acta Biomater. 2012, 8, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Monje, F.J.; Kim, E.J.; Pollak, D.D.; Cabatic, M.; Li, L.; Baston, A.; Lubec, G. Focal adhesion kinase regulates neuronal growth, synaptic plasticity and hippocampus-dependent spatial learning and memory. NeuroSignals 2012, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.A.; Rahimtula, M.; Mearow, K.M. Src and FAK are key early signalling intermediates required for neurite growth in NGF-responsive adult DRG neurons. Cell. Signal. 2008, 20, 241–257. [Google Scholar] [CrossRef]
- Robles, E.; Gomez, T.M. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat. Neurosci. 2006, 9, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Rico, B.; Beggs, H.E.; Schahin-Reed, D.; Kimes, N.; Schmidt, A.; Reichardt, L.F. Control of axonal branching and synapse formation by focal adhesion kinase. Nat. Neurosci. 2004, 7, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Nawabi, H.; Moret, F.; Yaron, A.; Weaver, E.; Bozon, M.; Abouzid, K.; Guan, J.L.; Tessier-Lavigne, M.; Lemmon, V.; et al. FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. EMBO J. 2008, 27, 1549–1562. [Google Scholar] [CrossRef] [PubMed]
- Chacon, M.R.; Fernández, G.; Rico, B. Focal adhesion kinase functions downstream of Sema3A signaling during axonal remodeling. Mol. Cell. Neurosci. 2010, 44, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Schlomann, U.; Schwamborn, J.C.; Müller, M.; Fässler, R.; Püschel, A.W. The stimulation of dendrite growth by Sema3A requires integrin engagement and focal adhesion kinase. J. Cell Sci. 2009, 122, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pontrello, C.G.; DeFea, K.A.; Reichardt, L.F.; Ethell, I.M. Focal adhesion kinase acts downstream of EphB receptors to maintain mature dendritic spines by regulating cofilin activity. J. Neurosci. 2009, 29, 8129–8142. [Google Scholar] [CrossRef]
- Wang, J.T.; Song, L.Z.; Li, L.L.; Zhang, W.; Chai, X.J.; An, L.; Chen, S.L.; Frotscher, M.; Zhao, S.T. Src controls neuronal migration by regulating the activity of FAK and cofilin. Neuroscience 2015, 292, 90–100. [Google Scholar] [CrossRef]
- Myers, J.P.; Robles, E.; Ducharme-Smith, A.; Gomez, T.M. Focal adhesion kinase modulates Cdc42 activity downstream of positive and negative axon guidance cues. J. Cell Sci. 2012, 125, 2918–2929. [Google Scholar]
- Condic, M.L. Adult neuronal regeneration induced by transgenic integrin expression. J. Neurosci. 2001, 21, 4782–4788. [Google Scholar] [CrossRef]
- Gardiner, N.J.; Fernyhough, P.; Tomlinson, D.R.; Mayer, U.; Von Der Mark, H.; Streuli, C.H. A7 integrin mediates neurite outgrowth of distinct populations of adult sensory neurons. Mol. Cell. Neurosci. 2005, 28, 229–240. [Google Scholar] [CrossRef]
- Shida, M.; Mikami, T.; Tamura, J.; Kitagawa, H. Chondroitin sulfate-D promotes neurite outgrowth by acting as an extracellular ligand for neuronal integrin αVβ3. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1319–1331. [Google Scholar] [CrossRef]
- Nieuwenhuis, B.; Haenzi, B.; Andrews, M.R.; Verhaagen, J.; Fawcett, J.W. Integrins promote axonal regeneration after injury of the nervous system. Biol. Rev. 2018, 93, 1339–1362. [Google Scholar] [CrossRef]
- Andrews, M.R.; Czvitkovich, S.; Dassie, E.; Vogelaar, C.F.; Faissner, A.; Blits, B.; Gage, F.H.; ffrench-Constant, C.; Fawcett, J.W. α9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J. Neurosci. 2009, 29, 5546–5557. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Strittmatter, S.M. The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J. Neurosci. 2008, 28, 1262–1269. [Google Scholar] [CrossRef]
- Luckenbill-Edds, L. Laminin and the mechanism of neuronal outgrowth. Brain Res. Rev. 1997, 23, 1–27. [Google Scholar] [CrossRef]
- Liesi, P.; Kauppila, T. Induction of type IV collagen and other basement-membrane-associated proteins after spinal cord injury of the adult rat may participate in formation of the glial scar. Exp. Neurol. 2002, 173, 31–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Winterbottom, J.K.; Schachner, M.; Lieberman, A.R.; Andersen, P.N. Tenascin-C expression and axonal sprouting following injury to the spinal dorsal columns in the adult rat. J. Neurosci. Res. 1997, 49, 433–450. [Google Scholar] [CrossRef]
- Andrews, M.R.; Soleman, S.; Cheah, M.; Tumbarello, D.A.; Mason, M.R.J.; Moloney, E.; Verhaagen, J.; Bensadoun, J.C.; Schneider, B.; Aebischer, P.; et al. Axonal localization of integrins in the CNS is neuronal type and age dependent. eNeuro 2016, 3, 5546–5557. [Google Scholar] [CrossRef] [PubMed]
- Eva, R.; Dassie, E.; Caswell, P.T.; Dick, G.; ffrench-Constant, C.; Norman, J.C.; Fawcett, J.W. Rab11 and its effector rab coupling protein contribute to the trafficking of β1 integrins during axon growth in adult dorsal root ganglion neurons and PC12 cells. J. Neurosci. 2010, 30, 11654–11669. [Google Scholar] [CrossRef]
- Sekine, Y.; Kannan, R.; Wang, X.; Strittmatter, S.M. Rabphilin3A reduces integrin-dependent growth cone signaling to restrict axon regeneration after trauma. Exp. Neurol. 2022, 353, 114070. [Google Scholar] [CrossRef] [PubMed]
- Eva, R.; Fawcett, J. Integrin signalling and traffic during axon growth and regeneration. Curr. Opin. Neurobiol. 2014, 27, 179–185. [Google Scholar] [CrossRef]
- Sango, K.; Oohira, A.; Ajiki, K.; Tokashiki, A.; Horie, M.; Kawano, H. Phosphacan and neurocan are repulsive substrata for adhesion and neurite extension of adult rat dorsal root ganglion neurons in vitro. Exp. Neurol. 2003, 182, 1–11. [Google Scholar] [CrossRef]
- Inatani, M.; Honjo, M.; Otori, Y.; Oohira, A.; Kido, N.; Tano, Y.; Honda, Y.; Tanihara, H. Inhibitory effects of neurocan and phosphacan on neurite outgrowth from retinal ganglion cells in culture. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1930–1938. [Google Scholar]
- Schmalfeldt, M.; Bandtlow, C.; Dours-Zimmermann, M.; Winterhalter, K.; Zimmermann, D. Brain derived versican V2 is a potent inhibitor of axonal growth. J. Cell Sci. 2000, 113, 807–816. [Google Scholar] [CrossRef]
- Shen, Y.; Tenney, A.; Busch, S.; Horn, K.; Cuascut, F.; Liu, K.; He, Z.; Silver, J.; Flanagan, J. PTPs is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 2009, 326, 592–596. [Google Scholar] [CrossRef]
- Aicher, B.; Lerch, M.M.; Müller, T.; Schilling, J.; Ullrich, A. Cellular redistribution of protein tyrosine phosphatases LAR and PTP by inducible proteolytic processing. J. Cell Biol. 1997, 138, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.T.; Cregg, J.M.; Depaul, M.A.; Tran, A.P.; Xu, K.; Dyck, S.M.; Madalena, K.M.; Brown, B.P.; Weng, Y.L.; Li, S.; et al. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature 2015, 518, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Day, P.; Alves, N.; Daniell, E.; Dasgupta, D.; Ogborne, R.; Steeper, A.; Raza, M.; Ellis, C.; Fawcett, J.; Keynes, R.; et al. Targeting chondroitinase ABC to axons enhances the ability of chondroitinase to promote neurite outgrowth and sprouting. PLoS ONE 2020, 15, e0221851. [Google Scholar] [CrossRef]
- McGee, A.W.; Strittmatter, S.M. The Nogo-66 receptor: Focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003, 26, 193–198. [Google Scholar] [CrossRef]
- Goh, E.L.K.; Young, J.K.; Kuwako, K.; Tessier-Lavigne, M.; He, Z.; Griffin, J.W.; Ming, G.L. beta1-integrin mediates myelin-associated glycoprotein signaling in neuronal growth cones. Mol. Brain 2008, 1, 10. [Google Scholar] [CrossRef]
| Integrin Receptor | Cell Model | Function | Reference |
|---|---|---|---|
| α1β1 α5β1 | Adult rat DRG neurons | Viral expression of α-subunit promotes neurite outgrowth on laminin or fibronectin | [79] |
| α7β1 | Adult mouse DRG neurons | α7-blocking antibody inhibits neurite outgrowth on laminin | [80] |
| α9β1 | PC12s, adult rat DRG neurons | Viral expression of α-subunit promotes neurite outgrowth on tenascin-C | [20,83] |
| α5β1 αVβ3 | COS7 cells | Inactivated by amino-Nogo | [84] |
| αVβ3 | E16 mouse hippocampals | Mediates neurite outgrowth on chondroitin sulphate-D polysaccharides | [81] |
| αIIβ3 | CHO cells | Activated by co-expression of kindlin-2 and talin | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis-Lunn, M.; Goult, B.T.; Andrews, M.R. Clutching at Guidance Cues: The Integrin–FAK Axis Steers Axon Outgrowth. Biology 2023, 12, 954. https://doi.org/10.3390/biology12070954
Davis-Lunn M, Goult BT, Andrews MR. Clutching at Guidance Cues: The Integrin–FAK Axis Steers Axon Outgrowth. Biology. 2023; 12(7):954. https://doi.org/10.3390/biology12070954
Chicago/Turabian StyleDavis-Lunn, Mathew, Benjamin T. Goult, and Melissa R. Andrews. 2023. "Clutching at Guidance Cues: The Integrin–FAK Axis Steers Axon Outgrowth" Biology 12, no. 7: 954. https://doi.org/10.3390/biology12070954
APA StyleDavis-Lunn, M., Goult, B. T., & Andrews, M. R. (2023). Clutching at Guidance Cues: The Integrin–FAK Axis Steers Axon Outgrowth. Biology, 12(7), 954. https://doi.org/10.3390/biology12070954








