Neuromuscular Characteristics of Unilateral and Bilateral Maximal Voluntary Isometric Contractions following ACL Reconstruction
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
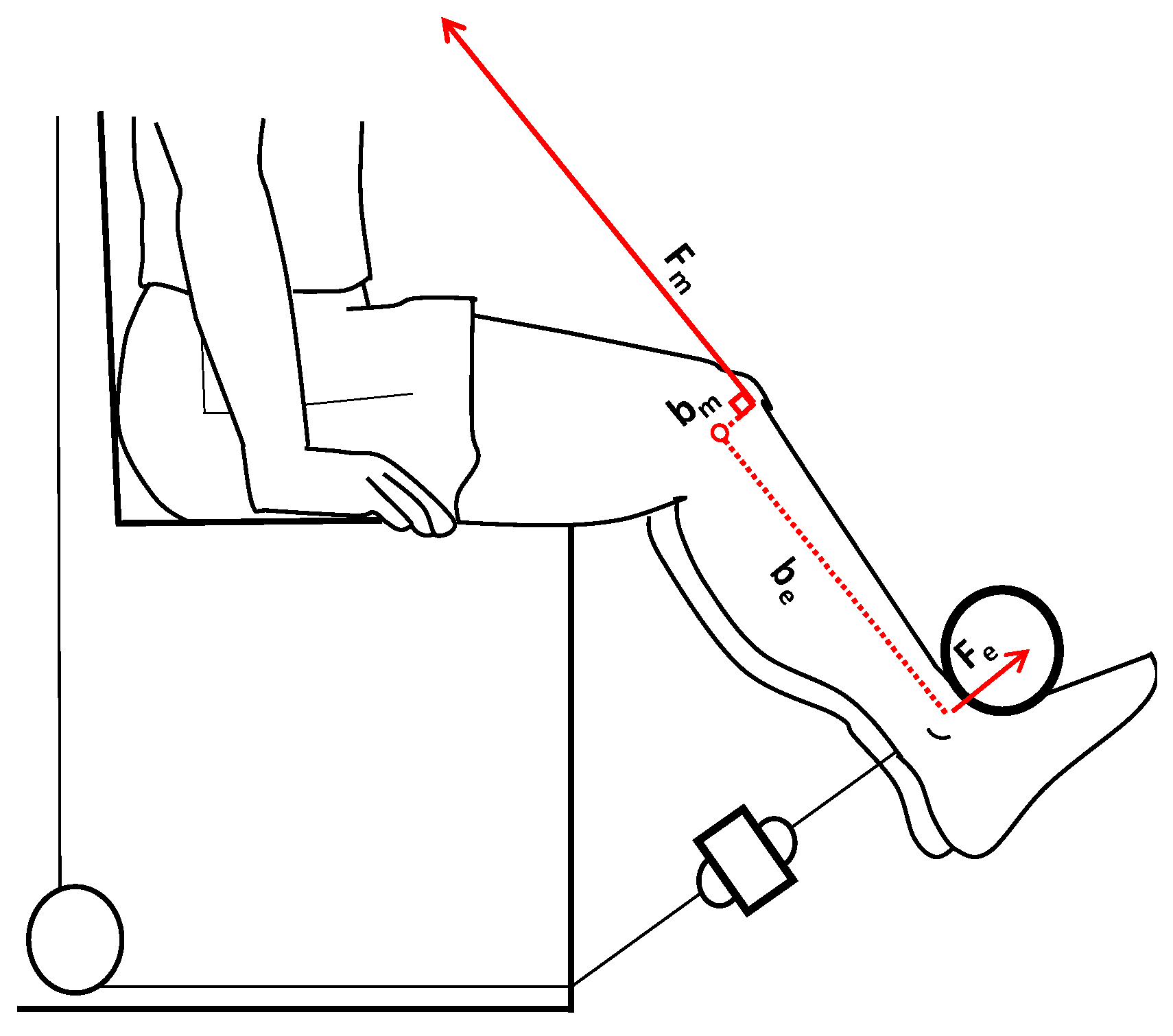
2.1. Experimental Procedures and Participants
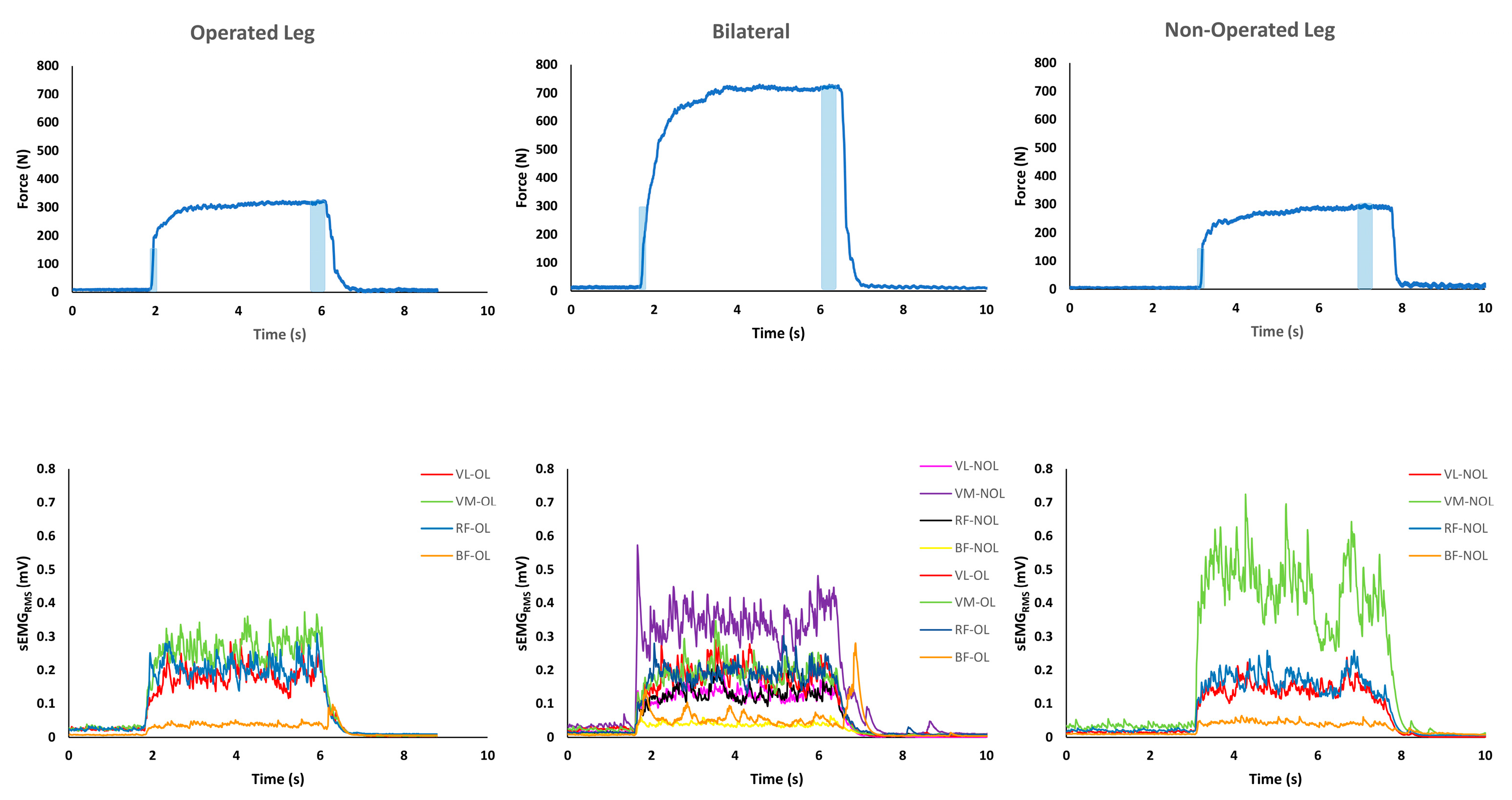
2.2. MVICs and sEMG Activity Measurements
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Reliability of the Measurements
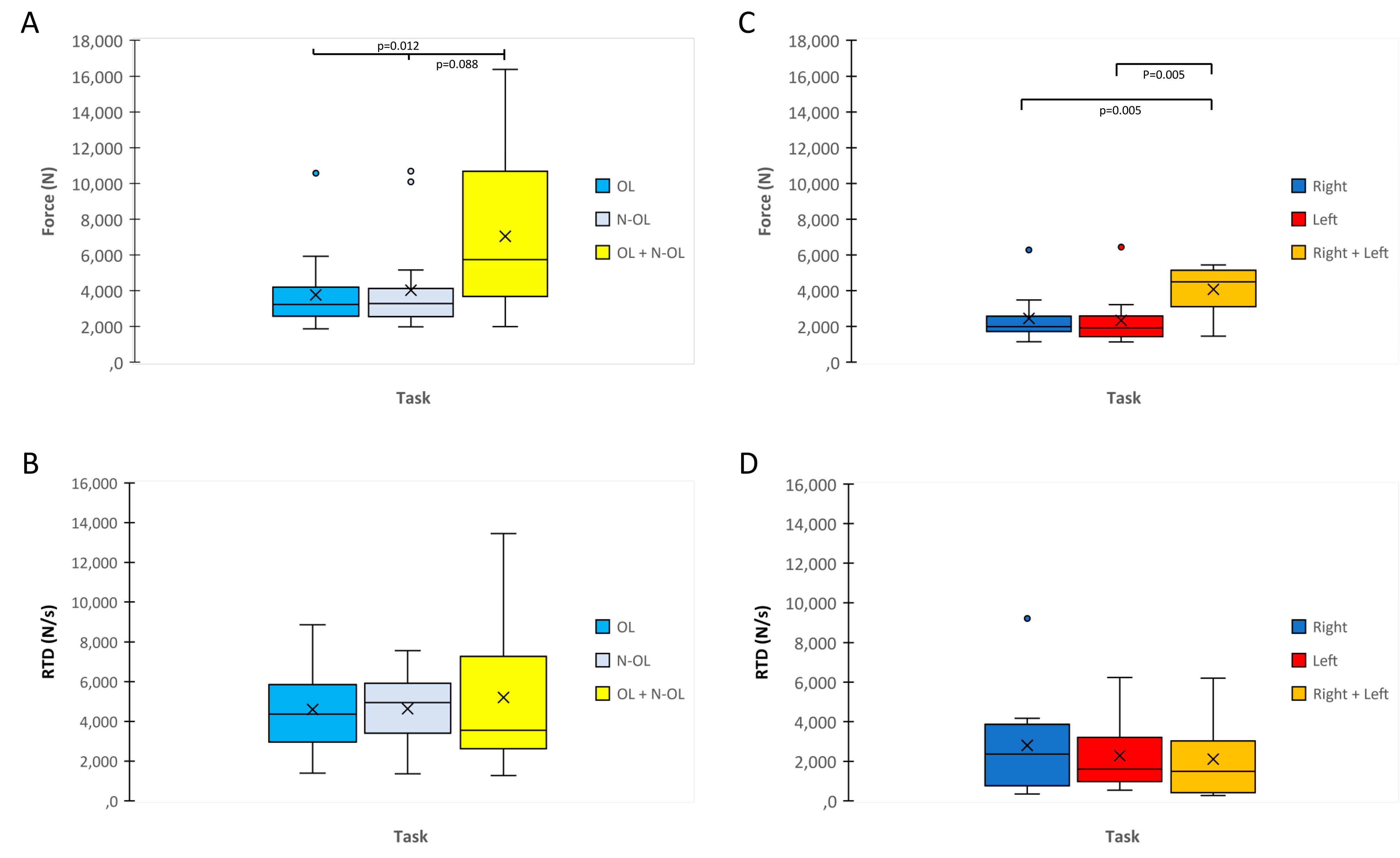
3.2. MVIC and RFD
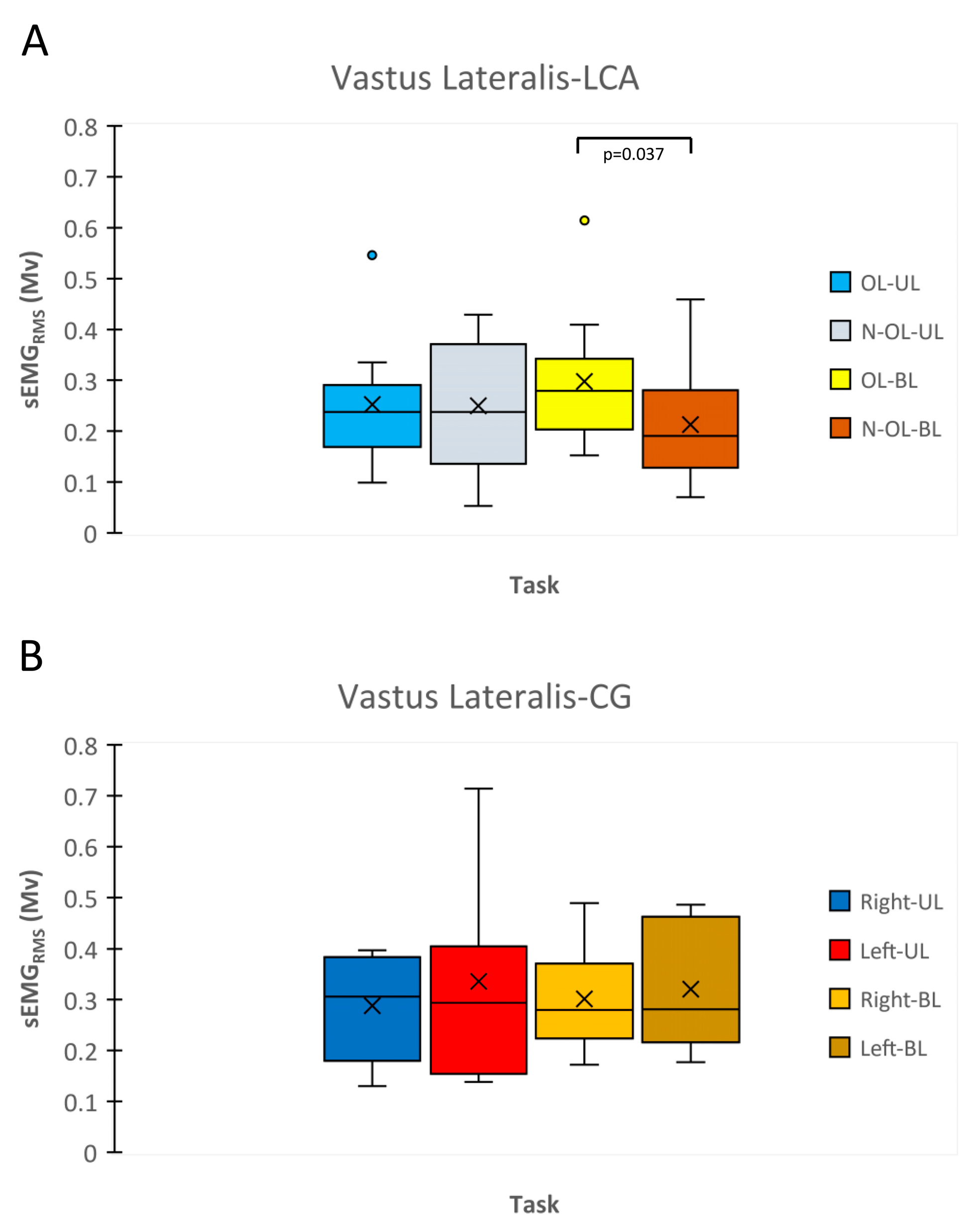
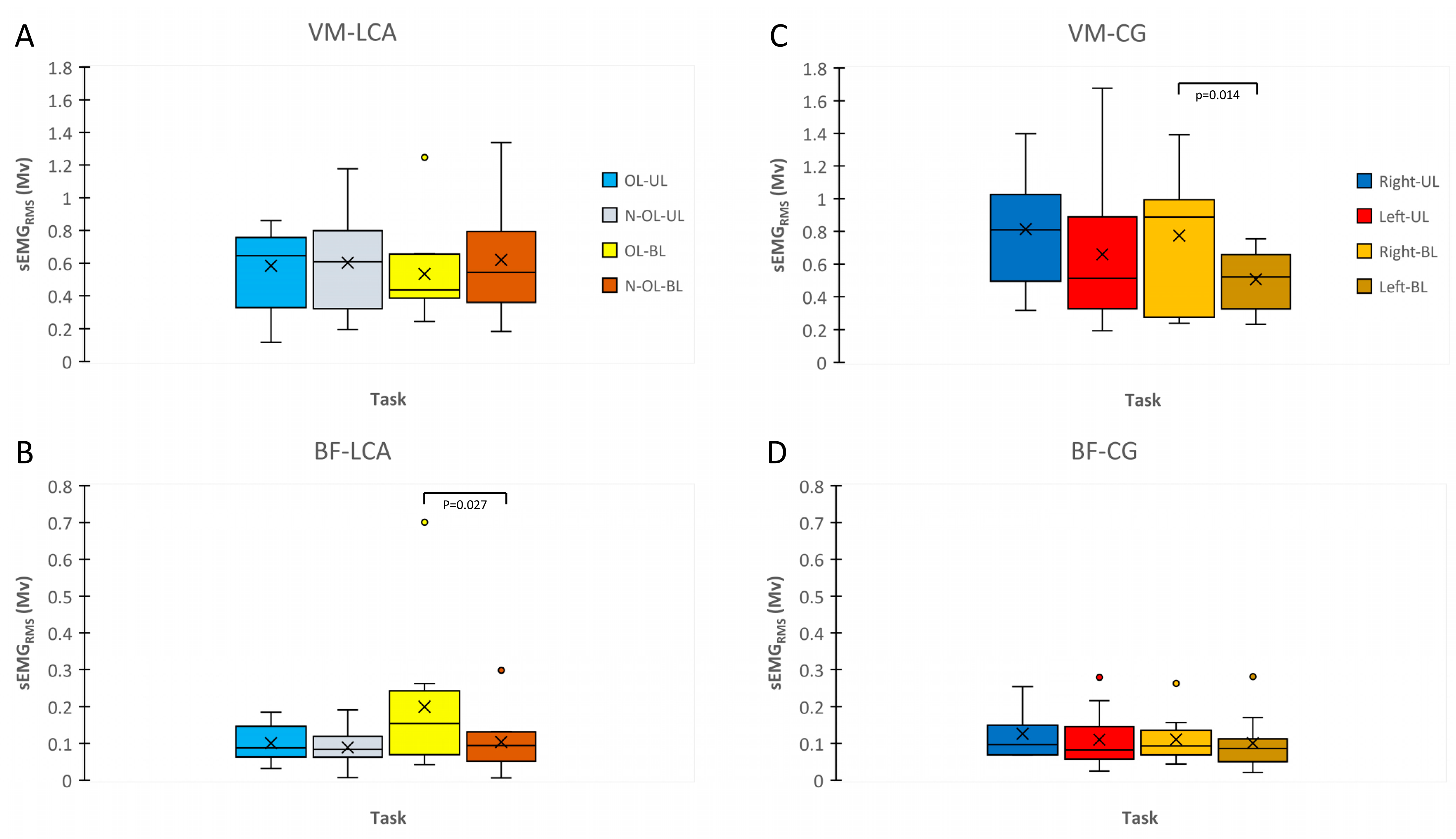
3.3. sEMGRMS during MVIC and RFD
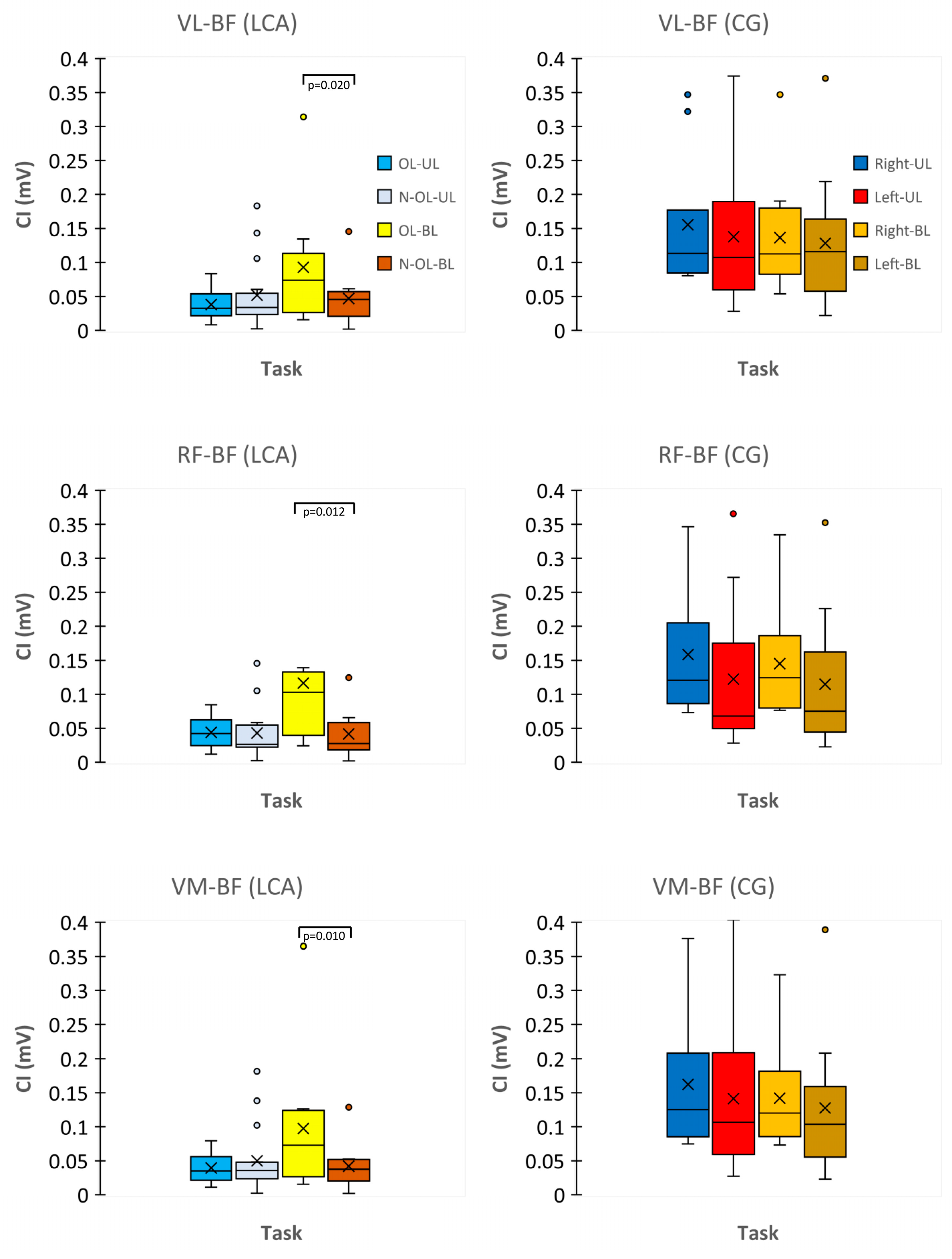
3.4. CI Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knezevic, O.M.; Mirkov, D.M.; Kadija, M.; Milovanovic, D.; Jaric, S. Evaluation of Isokinetic and Isometric Strength Measures for Monitoring Muscle Function Recovery after Anterior Cruciate Ligament Reconstruction. J. Strength Cond. Res. 2014, 28, 1722–1731. [Google Scholar] [CrossRef]
- Ardern, C.L.; Webster, K.E.; Taylor, N.F.; Feller, J.A. Return to Sport Following Anterior Cruciate Ligament Reconstruction Surgery: A Systematic Review and Meta-Analysis of the State of Play. Br. J. Sports Med. 2011, 45, 596–606. [Google Scholar] [CrossRef]
- Wiggins, A.J.; Grandhi, R.K.; Schneider, D.K.; Stanfield, D.; Webster, K.E.; Myer, G.D. Risk of Secondary Injury in Younger Athletes after Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Am. J. Sports Med. 2016, 44, 1861–1876. [Google Scholar] [CrossRef]
- Reid, A.; Birmingham, T.B.; Stratford, P.W.; Alcock, G.K.; Giffin, J.R. Hop Testing Provides a Reliable and Valid Outcome Measure During Rehabilitation after Anterior Cruciate Ligament Reconstruction. Phys. Ther. 2007, 87, 337–349. [Google Scholar] [CrossRef]
- Knezevic, O.M.; Mirkov, D.M.; Kadija, M.; Milovanovic, D.; Jaric, S. Alternating Consecutive Maximum Contraction as a Test of Muscle Function in Athletes Following ACL Reconstruction. J. Hum. Kinet. 2012, 35, 5–13. [Google Scholar] [CrossRef]
- Fleming, B.C.; Oksendahl, H.; Beynnon, B.D. Open- or Closed-Kinetic Chain Exercises after Anterior Cruciate Ligament Reconstruction? Exerc. Sport Sci. Rev. 2005, 33, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Pietrosimone, B.; Lepley, A.S.; Harkey, M.S.; Luc-Harkey, B.A.; Blackburn, J.T.; Gribble, P.A.; Spang, J.T.; Sohn, D.H. Quadriceps Strength Predicts Self-Reported Function Post-ACL Reconstruction. Med. Sci. Sports Exerc. 2016, 48, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.J.; Murphy, A.J. The Use of Isometric Tests of Muscular Function in Athletic Assessment. Sports Med. 1996, 22, 19–37. [Google Scholar] [CrossRef]
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, P.; Dyhre-Poulsen, P. Increased Rate of Force Development and Neural Drive of Human Skeletal Muscle Following Resistance Training. J. Appl. Physiol. 2002, 93, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Wojtys, E.M.; Huston, L.J. Neuromuscular Performance in Normal and Anterior Cruciate Ligament-Deficient Lower Extremities. Am. J. Sports Med. 1994, 22, 89–104. [Google Scholar] [CrossRef]
- Beard, D.J.; Dodd, C.A.; Simpson, H.A. Sensorimotor Changes after Anterior Cruciate Ligament Reconstruction. Clin. Orthop. Relat. Res. 2000, 372, 205–216. [Google Scholar] [CrossRef]
- Angelozzi, M.; Madama, M.; Corsica, C.; Calvisi, V.; Properzi, G.; McCaw, S.T.; Cacchio, A. Rate of Force Development as an Adjunctive Outcome Measure for Return-to-Sport Decisions after Anterior Cruciate Ligament Reconstruction. J. Orthop. Sports Phys. Ther. 2012, 42, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Beynnon, B.D.; Johnson, R.J.; Fleming, B.C. The Science of Anterior Cruciate Ligament Rehabilitation. Clin. Orthop. Relat. Res. 2002, 402, 9–20. [Google Scholar] [CrossRef]
- Beynnon, B.D.; Fleming, B.C. Anterior Cruciate Ligament Strain In-Vivo: A Review of Previous Work. J. Biomech. 1998, 31, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Solomonow, M.; Baratta, R.; Zhou, B.H.; Shoji, H.; Bose, W.; Beck, C.; D’Ambrosia, R. The Synergistic Action of the Anterior Cruciate Ligament and Thigh Muscles in Maintaining Joint Stability. Am. J. Sports Med. 1987, 15, 207–213. [Google Scholar] [CrossRef]
- Grabiner, M.D.; Weiker, G.G. Anterior Cruciate Ligament Injury and Hamstrings Coactivation. Clin. Biomech. 1993, 8, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Urbach, D.; Nebelung, W.; Weiler, H.T.; Awiszus, F. Bilateral Deficit of Voluntary Quadriceps Muscle Activation after Unilateral ACL Tear. Med. Sci. Sports Exerc. 1999, 31, 1691–1696. [Google Scholar] [CrossRef]
- Rice, D.A.; McNair, P.J. Quadriceps Arthrogenic Muscle Inhibition: Neural Mechanisms and Treatment Perspectives. Semin. Arthritis Rheum. 2010, 40, 250–266. [Google Scholar] [CrossRef]
- Kuenze, C.; Hertel, J.; Saliba, S.; Diduch, D.R.; Weltman, A.; Hart, J.M. Clinical Thresholds for Quadriceps Assessment after Anterior Cruciate Ligament Reconstruction. J. Sport Rehabil. 2015, 24, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Pamukoff, D.N.; Pietrosimone, B.G.; Ryan, E.D.; Lee, D.R.; Blackburn, J.T. Quadriceps Function and Hamstrings Co-Activation after Anterior Cruciate Ligament Reconstruction. J. Athl. Train. 2017, 52, 422–428. [Google Scholar] [CrossRef]
- Lepley, A.S.; Grooms, D.R.; Burland, J.P.; Davi, S.M.; Kinsella-Shaw, J.M.; Lepley, L.K. Quadriceps Muscle Function Following Anterior Cruciate Ligament Reconstruction: Systemic Differences in Neural and Morphological Characteristics. Exp. Brain Res. 2019, 237, 1267–1278. [Google Scholar] [CrossRef]
- Kapreli, E.; Athanasopoulos, S.; Gliatis, J.; Papathanasiou, M.; Peeters, R.; Strimpakos, N.; Van Hecke, P.; Gouliamos, A.; Sunaert, S. Anterior Cruciate Ligament Deficiency Causes Brain Plasticity: A Functional MRI Study. Am. J. Sports Med. 2009, 37, 2419–2426. [Google Scholar] [CrossRef]
- Grooms, D.R.; Page, S.J.; Nichols-Larsen, D.S.; Chaudhari, A.M.W.; White, S.E.; Onate, J.A. Neuroplasticity Associated With Anterior Cruciate Ligament Reconstruction. J. Orthop. Sports Phys. Ther. 2017, 47, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Luft, A.R.; Smith, G.V.; Forrester, L.; Whitall, J.; Macko, R.F.; Hauser, T.-K.; Goldberg, A.P.; Hanley, D.F. Comparing Brain Activation Associated with Isolated Upper and Lower Limb Movement across Corresponding Joints. Hum. Brain Mapp. 2002, 17, 131–140. [Google Scholar] [CrossRef]
- Noble, J.W.; Eng, J.J.; Boyd, L.A. Bilateral Motor Tasks Involve More Brain Regions and Higher Neural Activation than Unilateral Tasks: An FMRI Study. Exp. Brain Res. 2014, 232, 2785–2795. [Google Scholar] [CrossRef]
- Mirkov, D.M.; Knezevic, O.M.; Maffiuletti, N.A.; Kadija, M.; Nedeljkovic, A.; Jaric, S. Contralateral Limb Deficit after ACL-Reconstruction: An Analysis of Early and Late Phase of Rate of Force Development. J. Sports Sci. 2017, 35, 435–440. [Google Scholar] [CrossRef]
- Kish, L. Multipopulation Survey Designs: Five Types with Seven Shared Aspects. Int. Stat. Rev. 1994, 62, 167–186. [Google Scholar]
- Thobane, K.F.; Mulaudzi, F.M.; Moagi, M.M. Improvement of the Psychosocial Support for Frontline Nurses in Public Hospitals during COVID-19 Pandemic. J. Nurs. Manag. 2022, 30, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Beynnon, B.D.; Uh, B.S.; Johnson, R.J.; Abate, J.A.; Nichols, C.E.; Fleming, B.C.; Poole, A.R.; Roos, H. Rehabilitation after Anterior Cruciate Ligament Reconstruction: A Prospective, Randomized, Double-Blind Comparison of Programs Administered over 2 Different Time Intervals. Am. J. Sports Med. 2005, 33, 347–359. [Google Scholar] [CrossRef]
- Padulo, J.; Trajković, N.; Cular, D.; Grgantov, Z.; Madić, D.M.; Di Vico, R.; Traficante, A.; Alin, L.; Ardigò, L.P.; Russo, L. Validity and Reliability of Isometric-Bench for Knee Isometric Assessment. Int. J. Environ. Res. Public Health 2020, 17, 4326. [Google Scholar] [CrossRef] [PubMed]
- Courel-Ibáñez, J.; Hernández-Belmonte, A.; Cava-Martínez, A.; Pallarés, J.G. Familiarization and Reliability of the Isometric Knee Extension Test for Rapid Force Production Assessment. Appl. Sci. 2020, 10, 4499. [Google Scholar] [CrossRef]
- Ushiyama, N.; Kurobe, Y.; Momose, K. Validity of Maximal Isometric Knee Extension Strength Measurements Obtained via Belt-Stabilized Hand-Held Dynamometry in Healthy Adults. J. Phys. Ther. Sci. 2017, 29, 1987–1992. [Google Scholar] [CrossRef] [PubMed]
- Kraska, J.M.; Ramsey, M.W.; Haff, G.G.; Fethke, N.; Sands, W.A.; Stone, M.E.; Stone, M.H. Relationship between Strength Characteristics and Unweighted and Weighted Vertical Jump Height. Int. J. Sports Physiol. Perform. 2009, 4, 461–473. [Google Scholar] [CrossRef]
- Gyulai, G.; Rácz, L.; Di Giminiani, R.; Tihanyi, J. Effect of Whole Body Vibration Applied on Upper Extremity Muscles. Acta Physiol. Hung. 2013, 100, 37–47. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Tihanyi, J.; Di Giminiani, R.; Tihanyi, T.; Gyulai, G.; Trzaskoma, L.; Horváth, M. Low Resonance Frequency Vibration Affects Strength of Paretic and Non-Paretic Leg Differently in Patients with Stroke. Acta Physiol. Hung. 2010, 97, 172–182. [Google Scholar] [CrossRef]
- Finni, T.; Komi, P.V.; Lepola, V. In Vivo Human Triceps Surae and Quadriceps Femoris Muscle Function in a Squat Jump and Counter Movement Jump. Eur. J. Appl. Physiol. 2000, 83, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.A.; Álvarez-Caldas, C.; San Román, J.L.; Gutiérrez-Moizant, R. New Procedure for the Kinematic and Power Analysis of Cyclists in Indoor Training. Sensors 2020, 20, 6135. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Barzegari, A.; Taghipour, M.; Mehr Aein, R.; Gholinia, H. Changes of the Patellar Tendon Moment Arm Length in Different Knee Angles: A Biomechanical in Vivo Study. Arch. Bone Jt. Surg. 2020, 8, 641. [Google Scholar] [CrossRef]
- Schmitt, L.C.; Rudolph, K.S. Muscle Stabilization Strategies in People with Medial Knee Osteoarthritis: The Effect of Instability. J. Orthop. Res. 2008, 26, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Lyle, M.A.; Valero-Cuevas, F.J.; Gregor, R.J.; Powers, C.M. Control of Dynamic Foot-Ground Interactions in Male and Female Soccer Athletes: Females Exhibit Reduced Dexterity and Higher Limb Stiffness during Landing. J. Biomech. 2014, 47, 512–517. [Google Scholar] [CrossRef]
- Di Giminiani, R.; Giovannelli, A.; Capuano, L.; Izzicupo, P.; Di Blasio, A.; Masedu, F. Neuromuscular Strategies in Stretch–Shortening Exercises with Increasing Drop Heights: The Role of Muscle Coactivation in Leg Stiffness and Power Propulsion. Int. J. Environ. Res. Public Health 2020, 17, 8647. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Kålund, S.; Sinkjær, T.; Arendt-Nielsen, L.; Simonsen, O. Altered Timing of Hamstring Muscle Action in Anterior Cruciate Ligament Deficient Patients. Am. J. Sports Med. 1990, 18, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Palmieri-Smith, R.M.; Strickland, M.; Lepley, L.K. Hamstring Muscle Activity after Primary Anterior Cruciate Ligament Reconstruction—A Protective Mechanism in Those Who Do Not Sustain a Secondary Injury? A Preliminary Study. Sports Health 2019, 11, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Friemert, B.; Franke, S.; Gollhofer, A.; Claes, L.; Faist, M. Group I Afferent Pathway Contributes to Functional Knee Stability. J. Neurophysiol. 2010, 103, 616–622. [Google Scholar] [CrossRef]
- Konishi, Y.; Aihara, Y.; Sakai, M.; Ogawa, G.; Fukubayashi, T. Gamma Loop Dysfunction in the Quadriceps Femoris of Patients Who Underwent Anterior Cruciate Ligament Reconstruction Remains Bilaterally. Scand. J. Med. Sci. Sports 2007, 17, 393–399. [Google Scholar] [CrossRef]
- Nuccio, S.; Del Vecchio, A.; Casolo, A.; Labanca, L.; Rocchi, J.E.; Felici, F.; Macaluso, A.; Mariani, P.P.; Falla, D.; Farina, D.; et al. Deficit in Knee Extension Strength Following Anterior Cruciate Ligament Reconstruction Is Explained by a Reduced Neural Drive to the Vasti Muscles. J. Physiol. 2021, 599, 5103–5120. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.A.; Glaviano, N.R.; Norte, G.E. Hamstrings Neuromuscular Function after Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Sports Med. 2021, 51, 1751–1769. [Google Scholar] [CrossRef]
- McPherson, A.L.; Schilaty, N.D.; Anderson, S.; Nagai, T.; Bates, N.A. Arthrogenic Muscle Inhibition after Anterior Cruciate Ligament Injury: Injured and Uninjured Limb Recovery over Time. Front. Sports Act. Living 2023, 5, 1143376. [Google Scholar] [CrossRef]
- Schilaty, N.D.; McPherson, A.L.; Nagai, T.; Bates, N.A. Arthrogenic Muscle Inhibition Manifests in Thigh Musculature Motor Unit Characteristics after Anterior Cruciate Ligament Injury. Eur. J. Sport Sci. 2023, 23, 840–850. [Google Scholar] [CrossRef]
- Ito, N.; Capin, J.J.; Khandha, A.; Buchanan, T.S.; Snyder-Mackler, L. Identifying Gait Pathology after ACL Reconstruction Using Temporal Characteristics of Kinetics and Electromyography. Med. Sci. Sports Exerc. 2022, 54, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Capin, J.J.; Zarzycki, R.; Snyder-Mackler, L. Athletes With Bone-Patellar Tendon-Bone Autograft for Anterior Cruciate Ligament Reconstruction Were Slower to Meet Rehabilitation Milestones and Return-to-Sport Criteria Than Athletes With Hamstring Tendon Autograft or Soft Tissue Allograft: Secondary Analysis From the ACL-SPORTS Trial. J. Orthop. Sports Phys. Ther. 2020, 50, 259–266. [Google Scholar] [CrossRef]
- Rivera-Brown, A.M.; Frontera, W.R.; Fontánez, R.; Micheo, W.F. Evidence for Isokinetic and Functional Testing in Return to Sport Decisions Following ACL Surgery. PMR 2022, 14, 678–690. [Google Scholar] [CrossRef]
- West, T.J.; Bruder, A.M.; Crossley, K.M.; Culvenor, A.G. Unilateral Tests of Lower-Limb Function as Prognostic Indicators of Future Knee-Related Outcomes Following Anterior Cruciate Ligament Injury: A Systematic Review and Meta-Analysis of 13 150 Adolescents and Adults. Br. J. Sports Med. 2023, 57, 855–863. [Google Scholar] [CrossRef]
- Needle, A.R.; Lepley, A.S.; Grooms, D.R. Central Nervous System Adaptation after Ligamentous Injury: A Summary of Theories, Evidence, and Clinical Interpretation. Sports Med. 2017, 47, 1271–1288. [Google Scholar] [CrossRef]
- Pérez-Castilla, A.; García-Ramos, A.; Janicijevic, D.; Delgado-García, G.; De la Cruz, J.C.; Rojas, F.J.; Cepero, M. Between-Session Reliability of Performance and Asymmetry Variables Obtained during Unilateral and Bilateral Countermovement Jumps in Basketball Players. PLoS ONE 2021, 16, e0255458. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, M.; Comfort, P.; Ripley, N.; McMahon, J.J.; Evans, M.; Bishop, C. Unilateral vs. Bilateral Hamstring Strength Assessments: Comparing Reliability and Inter-Limb Asymmetries in Female Soccer Players. J. Sports Sci. 2021, 39, 1481–1488. [Google Scholar] [CrossRef]
- Kelso, J.A.; Jeka, J.J. Symmetry Breaking Dynamics of Human Multilimb Coordination. J. Exp. Psychol. Hum. Percept. Perform. 1992, 18, 645–668. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.-C.; Morton, S.M. Impaired Interlimb Coordination of Voluntary Leg Movements in Poststroke Hemiparesis. J. Neurophysiol. 2010, 104, 248–257. [Google Scholar] [CrossRef]
- De Souza, L.M.L.; Cabral, H.V.; De Oliveira, L.F.; Vieira, T.M. Motor Units in Vastus Lateralis and in Different Vastus Medialis Regions Show Different Firing Properties during Low-Level, Isometric Knee Extension Contraction. Hum. Mov. Sci. 2018, 58, 307–314. [Google Scholar] [CrossRef]
- Venturelli, M.; Tarperi, C.; Milanese, C.; Festa, L.; Toniolo, L.; Reggiani, C.; Schena, F. The Effect of Leg Preference on Mechanical Efficiency during Single-Leg Extension Exercise. J. Appl. Physiol. 2021, 131, 553–565. [Google Scholar] [CrossRef]
- Guette, M.; Gondin, J.; Martin, A. Time-of-Day Effect on the Torque and Neuromuscular Properties of Dominant and Non-Dominant Quadriceps Femoris. Chronobiol. Int. 2005, 22, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.K.; Thompson, M.W.; Adams, R.D. Relationships among Age-Associated Strength Changes and Physical Activity Level, Limb Dominance, and Muscle Group in Women. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, B264–B273. [Google Scholar] [CrossRef] [PubMed]
- Rahnama, N.; Lees, A.; Bambaecichi, E. A Comparison of Muscle Strength and Flexibility between the Preferred and Non-Preferred Leg in English Soccer Players. Ergonomics 2005, 48, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, J.A.; Lynn, S.K.; Bagley, J.R.; Brown, L.E.; Costa, P.B.; Galpin, A.J. Lower-Limb Dominance, Performance, and Fiber Type in Resistance-Trained Men. Med. Sci. Sports Exerc. 2018, 50, 1054–1060. [Google Scholar] [CrossRef]
| Variable | Group | |
|---|---|---|
| ACL Group (n = 10) | Control Group (n = 10) | |
| Age (Years) | 23.4 ± 2.1 | 24.0 ± 3.4 |
| Stature (cm) | 182 ± 9.9 | 180.3 ± 10.7 |
| Body Mass (Kg) | 78.6 ± 9.9 | 74.9 ± 13.5 |
| BMI (Kg/m2) | 23.7 ± 1.9 | 22.8 ± 2.7 |
| Sports level | Competitive | Competitive |
| Leg Dominance | Right (n = 9)/Left (n = 1) | Right (n = 9)/Left (n = 1) |
| Operated Leg | Right (n = 6)/Left (n = 4) | NA |
| Graft | SGT (n = 10) | NA |
| Event Distribution | No-Contact Mechanism | NA |
| Post Operative Period | 6 Months–2 Years | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giminiani, R.; Marinelli, S.; La Greca, S.; Di Blasio, A.; Angelozzi, M.; Cacchio, A. Neuromuscular Characteristics of Unilateral and Bilateral Maximal Voluntary Isometric Contractions following ACL Reconstruction. Biology 2023, 12, 1173. https://doi.org/10.3390/biology12091173
Di Giminiani R, Marinelli S, La Greca S, Di Blasio A, Angelozzi M, Cacchio A. Neuromuscular Characteristics of Unilateral and Bilateral Maximal Voluntary Isometric Contractions following ACL Reconstruction. Biology. 2023; 12(9):1173. https://doi.org/10.3390/biology12091173
Chicago/Turabian StyleDi Giminiani, Riccardo, Stefano Marinelli, Stefano La Greca, Andrea Di Blasio, Massimo Angelozzi, and Angelo Cacchio. 2023. "Neuromuscular Characteristics of Unilateral and Bilateral Maximal Voluntary Isometric Contractions following ACL Reconstruction" Biology 12, no. 9: 1173. https://doi.org/10.3390/biology12091173
APA StyleDi Giminiani, R., Marinelli, S., La Greca, S., Di Blasio, A., Angelozzi, M., & Cacchio, A. (2023). Neuromuscular Characteristics of Unilateral and Bilateral Maximal Voluntary Isometric Contractions following ACL Reconstruction. Biology, 12(9), 1173. https://doi.org/10.3390/biology12091173








