Integrating Omics Technologies for a Comprehensive Understanding of the Microbiome and Its Impact on Cattle Production
Abstract
:Simple Summary
Abstract
1. Introduction
2. Multi-Omics Approaches for Understanding Microbe-Host Interactions: Implications for Animal Health and Nutrition
3. The Complex and Vital Role of Rumen Microbiota in Ruminant Nutrition and Health
4. Factors Affecting Microbiome Establishment in Rumens
4.1. Age-Dependent Changes in Microbial Population
4.2. Stress-Related Changes in the Composition of the Microbiota
5. Role of Lower-Gut Microbiome in Host Gut Health
6. Lower-Gut Microbiome Influence the Immune System of the Host
7. Gut-Microbiota-Generated Metabolites in Animal Health
8. Future Prospective
- Understanding the impact of the microbiome on production traits: Research can focus on understanding how the microbiome of the rumen and other digestive organs influences production traits such as feed efficiency, weight gain, and milk yield. By better understanding these relationships, it may be possible to develop management strategies to optimize microbiome function and improve production efficiency.
- Developing microbial interventions for improved animal health: Similar to human medicine, microbial interventions could be developed to promote health and prevent disease in cattle. For example, probiotics or microbial supplements could be used to promote beneficial microorganisms or microbial therapies could be used to treat infections or other health conditions.
- Exploring the role of the microbiome in animal behavior: Emerging research suggests that the microbiome may play a role in regulating animal behavior, potentially influencing stress response, social behavior, and other traits. Investigating these relationships in cattle could provide new insights into how the microbiome influences animal welfare and production.
- Investigating the impact of management practices on the microbiome: Management practices such as feed composition, housing conditions, and antibiotic use may all influence the composition and function of the cattle microbiome. Understanding these relationships can help identify best practices for managing the microbiome and improving animal health and productivity.
- Developing precision management tools for the microbiome: Advances in technology are enabling increasingly precise analysis of the microbiome which could, in turn, enable more targeted and personalized management strategies. For example, microbial profiling could be used to identify individual animals or herds with particular microbiome profiles, which could be managed in a more targeted manner to optimize production and health outcomes.
9. Limitation of the Study Topic
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cammack, K.M.; Austin, K.J.; Lamberson, W.R.; Conant, G.C.; Cunningham, H.C. Ruminnat Nutrition Symposium: Tiny but mighty: The role of the rumen microbes in livestock production. J. Anim. Sci. 2018, 96, 752–770. [Google Scholar] [CrossRef]
- Shashikumar, N.G.; Baithalu, R.K.; Bathla, S.; Ali, S.A.; Rawat, P.; Kumaresan, A.; Kumar, S.; Maharana, B.R.; Singh, G.; Kumar, D.P.; et al. Global proteomic analysis of water buffalo (Bubalus bubalis) saliva at different stages of estrous cycle using high throughput mass spectrometry. Theriogenology 2018, 110, 52–60. [Google Scholar] [CrossRef]
- Chopra, A.; Ali, S.A.; Bathla, S.; Rawat, P.; Vohra, V.; Kumar, S.; Mohanty, A.K. High-Resolution Mass Spectrometer–Based Ultra-Deep Profile of Milk Whey Proteome in Indian Zebu (Sahiwal) Cattle. Front. Nutr. 2020, 7, 150. [Google Scholar] [CrossRef]
- Pragya, P.; Kaur, G.; Ali, S.A.; Bhatla, S.; Rawat, P.; Lule, V.; Kumar, S.; Mohanty, A.K.; Behare, P. High-resolution mass spectrometry-based global proteomic analysis of probiotic strains Lactobacillus fermentum NCDC 400 and RS2. J. Proteom. 2017, 152, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Ali, S.A.; Kumar, S.; Mohanty, A.K.; Behare, P. Label-free quantitative proteomic analysis of Lactobacillus fermentum NCDC 400 during bile salt exposure. J. Proteom. 2017, 167, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Singh, P.; Tomar, S.K.; Mohanty, A.K.; Behare, P. Proteomics fingerprints of systemic mechanisms of adaptation to bile in Lactobacillus fermentum. J. Proteom. 2020, 213, 103600. [Google Scholar] [CrossRef] [PubMed]
- Azmal Ali, S.; Kumar, S.; Mohanty, A.K.; Behare, P. Draft genome sequence of Lactobacillus fermentum NCDC 400, isolated from a traditional Indian dairy product. Genome Announc. 2018, 6, 01492-17. [Google Scholar] [CrossRef]
- Behare, P.V.; Ali, S.A.; McAuliffe, O. Draft genome sequences of Fructobacillus fructosus DPC 7238 and Leuconostoc mesenteroides DPC 7261, mannitol-producing organisms isolated from fructose-rich honeybee-resident flowers on an Irish farm. Microbiol. Resour. Announc. 2020, 9, e01297-20. [Google Scholar] [CrossRef] [PubMed]
- Sanjorjo, R.A.; Tseten, T.; Kang, M.-K.; Kwon, M.; Kim, S.-W. In Pursuit of Understanding the Rumen Microbiome. Fermentation 2023, 9, 114. [Google Scholar] [CrossRef]
- Kumar, R.; Ali, S.; Singh, S.; Bhushan, V.; Kaushik, J.; Mohanty, A.; Kumar, S. Comprehensive profiling of urinary peptides in cow reveals physiology specific signatures and several bioactive properties. Sci. Rep. 2021, 1, 12427. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Factories 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Malmuthuge, N.; Guan, L.L. Gut microbiome and omics: A new definition to ruminant production and health. Anim. Front. 2016, 6, 8–12. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Y.; Yu, Z.; Xu, Q.; Zheng, N.; Zhao, S.; Wang, J. Ruminal microbiota–host interaction and its effect on nutrient metabolism. Anim. Nut. 2021, 7, 49–55. [Google Scholar] [CrossRef]
- Thapa, S.; Mishra, J.; Arora, N.; Mishra, P.; Li, H.; Bhatti, S.; Zhou, S. Microbial cellulolytic enzymes: Diversity and biotechnology with reference to lignocellulosic biomass degradation. Rev. Environ. Sci. 2020, 19, 621–648. [Google Scholar]
- Hassan, F.U.; Arshad, M.A.; Ebeid, H.M.; Rehman, M.S.U.; Khan, M.S.; Shahid, S.; Yang, C. Phytogenic additives can modulate rumen microbiome to mediate fermentation kinetics and methanogenesis through exploiting diet–microbe interaction. Front. Vet. Sci. 2020, 7, 575801. [Google Scholar] [CrossRef]
- Hoedt, E.C.; Cuív, P.Ó.; Evans, P.N.; Smith, W.J.; McSweeney, C.S.; Denman, S.E.; Morrison, M. Differences down-under: Alcohol-fueled methanogenesis by archaea present in Australian macropodids. J. ISME 2016, 10, 2376–2388. [Google Scholar] [CrossRef]
- Black, J.L.; Davison, T.M.; Box, I. Methane emissions from ruminants in Australia: Mitigation potential and applicability of mitigation strategies. Animals 2021, 11, 951. [Google Scholar] [CrossRef]
- Asselstine, V.; Lam, S.; Miglior, F.; Brito, L.F.; Sweett, H.; Guan, L.; Cánovas, A. The potential for mitigation of methane emissions in ruminants through the application of metagenomics, metabolomics, and other-OMICS technologies. J. Anim. Sci. 2021, 99, 193. [Google Scholar] [CrossRef]
- Mohan, J.; Ali, S.A.; Suvartan, R.; Kapila, S.; Sharma, R.; Tomar, S.K.; Yadav, H. Bioavailability of biotransformed zinc enriched dahi in wistar rats. Int. J. Probiotics Prebiotics 2018, 13, 45. [Google Scholar]
- Vaibhao, L.; Kanchan, M.; Ali, S.A.; Preeti, R.; Sudarshan, K.; Pradip, B.; Mohanty, A.K. Evaluation of stationary phase and bile stress related protein spots in Lactobacillus fermentum NCDC 400 by 2-DE method. Ind. J. Dairy Sci. 2016, 69, 455–459. [Google Scholar]
- Dixit, S.; Kumar, S.; Sharma, R.; Banakar, P.S.; Singh, M.; Keshri, A.; Tyagi, A.K. Rumen multi-omics addressing diet–host–microbiome interplay in farm animals: A review. Anim. Biotechnol. 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Richa Bharti, Dominik G Grimm, Current challenges and best-practice protocols for microbiome analysis. Brief. Bioinform. 2021, 22, 178–193. [CrossRef] [PubMed]
- Yen, S.; Johnson, J.S. Metagenomics: A path to understanding the gut microbiome. Mamm. Genome 2021, 32, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Pramanik, S. Structural diversity, functional aspects and future therapeutic applications of human gut microbiome. Arch Microbiol 2021, 203, 5281–5308. [Google Scholar] [CrossRef]
- Zhang, Y.; Thompson, K.N.; Branck, T.; Yan, Y.; Nguyen, L.H.; Franzosa, E.A.; Huttenhower, C. Metatranscriptomics for the human microbiome and microbial community functional profiling. Annu. Rev. Biomed. Data Sci. 2021, 4, 279–311. [Google Scholar] [CrossRef]
- Karaduta, O.; Dvanajscak, Z.; Zybailov, B. Metaproteomics—An Advantageous Option in Studies of Host-Microbiota Interaction. Microorganisms 2021, 9, 980. [Google Scholar] [CrossRef]
- Lobanov, V.; Gobet, A.; Joyce, A. Ecosystem-specific microbiota and microbiome databases in the era of big data. Environ. Microbiome 2022, 17, 37. [Google Scholar] [CrossRef]
- Xu, Q.; Qiao, Q.; Gao, Y.; Hou, J.; Hu, M.; Du, Y.; Zhao, K.; Li, X. Gut microbiota and their role in health and metabolic disease of dairy cow. Front. Nutr. 2021, 8, 701511. [Google Scholar] [CrossRef]
- Schiebenhoefer, H.; Van Den Bossche, T.; Fuchs, S.; Renard, B.Y.; Muth, T.; Martens, L. Challenges and promise at the interface of metaproteomics and genomics: An overview of recent progress in metaproteogenomic data analysis. Expert Rev. Proteom. 2019, 16, 375–390. [Google Scholar] [CrossRef]
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef]
- Li, R.W.; Connor, E.E.; Li, C.; Baldwin, V.I.R.L.; Sparks, M.E. Characterization of the rumen microbiota of pre-ruminant calves using metagenomic tools. Environ. Microbiol. 2012, 14, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Cholewińska, P.; Górniak, W.; Wojnarowski, K. Impact of selected environmental factors on microbiome of the digestive tract of ruminants. BMC Vet. Res. 2021, 17, 25. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P.; Dingkao, R.; Du, M.; Ahmad, A.A.; Liang, Z.; Ding, X. Fecal Microbiota Dynamics Reveal the Feasibility of Early Weaning of Yak Calves under Conventional Grazing System. Biology 2021, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bian, G.; Sun, D.; Zhu, W.; Mao, S. Starter feeding supplementation alters colonic mucosal bacterial communities and modulates mucosal immune homeostasis in newborn lambs. Front. Microbiol. 2017, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Shabat, S.K.B.; Sasson, G.; Doron-Faigenboim, A.; Durman, T.; Yaacoby, S.; Berg Miller, M.E.; Mizrahi, I. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. J. ISME 2016, 10, 2958–2972. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Penner, G.B.; Li, M.; Oba, M.; Guan, L.L. Changes in bacterial diversity associated with epithelial tissue in the beef cow rumen during the transition to a high-grain diet. Appl. Environ. Microbiol. 2011, 77, 5770–5781. [Google Scholar] [CrossRef]
- Xue, M.Y.; Xie, Y.Y.; Zhong, Y.; Ma, X.J.; Sun, H.Z.; Liu, J.X. Integrated meta-omics reveals new ruminal microbial features associated with feed efficiency in dairy cattle. Microbiome 2022, 10, 32. [Google Scholar] [CrossRef]
- Bharanidharan, R.; Lee, C.H.; Thirugnanasambantham, K.; Ibidhi, R.; Woo, Y.W.; Lee, H.G.; Kim, K.H. Feeding systems and host breeds influence ruminal fermentation, methane production, microbial diversity and metagenomic gene abundance. Front. Microbiol. 2021, 12, 2038. [Google Scholar] [CrossRef]
- Sasson, G.; Kruger Ben-Shabat, S.; Seroussi, E.; Doron-Faigenboim, A.; Shterzer, N.; Yaacoby, S.; Mizrahi, I. Heritable bovine rumen bacteria are phylogenetically related and correlated with the cow’s capacity to harvest energy from its feed. MBio 2017, 8, 00703–00717. [Google Scholar] [CrossRef]
- Khalil, A.; Batool, A.; Arif, S. Healthy cattle microbiome and dysbiosis in diseased phenotypes. Ruminants 2022, 2, 134–156. [Google Scholar] [CrossRef]
- Ribeiro, G.O.; Oss, D.B.; He, Z.; Gruninger, R.J.; Elekwachi, C.; Forster, R.J.; McAllister, T.A. Repeated inoculation of cattle rumen with bison rumen contents alters the rumen microbiome and improves nitrogen digestibility in cattle. Sci. Rep. 2017, 7, 1276. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.P.; Raffatellu, M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 2016, 16, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Abbas, W.; Howard, J.T.; Paz, H.A.; Hales, K.E.; Wells, J.E.; Kuehn, L.A.; Erickson, G.E.; Spangler, M.L.; Fernando, S.C. Influence of host genetics in shaping the rumen bacterial community in beef cattle. Sci. Rep. 2020, 10, 15101. [Google Scholar] [CrossRef]
- XPeters, D.L.; Wang, W.; Zhang, X.; Ning, Z.; Mayne, J.; Figeys, D. Metaproteomic and metabolomic approaches for characterizing the gut microbiome. Proteomics 2019, 19, 1800363. [Google Scholar] [CrossRef]
- Loor, J.J.; Elolimy, A.A.; McCann, J.C. Dietary impacts on rumen microbiota in beef and dairy production. Anim. Front. 2016, 6, 22–29. [Google Scholar] [CrossRef]
- Kumar, R.; Ali, S.A.; Singh, S.K.; Bhushan, V.; Mathur, M.; Jamwal, S.; Kumar, S. Antimicrobial peptides in farm animals: An updated review on its diversity, function, modes of action and therapeutic prospects. Vet. Sci. 2020, 7, 206. [Google Scholar] [CrossRef]
- O’Hara, E.; Neves, A.L.; Song, Y.; Guan, L.L. The role of the gut microbiome in cattle production and health: Driver or passenger? Annu. Rev. Anim. Biosci. 2020, 8, 199–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Chen, Y.; Wu, H.; Meng, Q.; Guan, L.L. Metatranscriptomic profiling reveals the effect of breed on active rumen eukaryotic composition in beef cattle with varied feed efficiency. Front. Microbiol. 2020, 11, 367. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Schloter, M. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Gharechahi, J.; Vahidi, M.F.; Bahram, M.; Han, J.L.; Ding, X.Z.; Salekdeh, G.H. Metagenomic analysis reveals a dynamic microbiome with diversified adaptive functions to utilize high lignocellulosic forages in the cattle rumen. ISME J. 2021, 15, 1108–1120. [Google Scholar] [CrossRef]
- Difford, G.F.; Plichta, D.R.; Løvendahl, P.; Lassen, J.; Noel, S.J.; Højberg, O.; Wright, A.D.G.; Zhu, Z.; Kristensen, L.; Nielsen, H.B.; et al. Host genetics and the rumen microbiome jointly associate with methane emissions in dairy cows. PLoS Genet. 2018, 14, e1007580. [Google Scholar] [CrossRef]
- Gu, F.; Zhu, S.; Tang, Y.; Liu, X.; Jia, M.; Malmuthuge, N.; Valencak, T.G.; McFadden, J.W.; Liu, J.X.; Sun, H.Z. Gut microbiome is linked to functions of peripheral immune cells in transition cows during excessive lipolysis. Microbiome 2023, 11, 40. [Google Scholar] [CrossRef]
- Fan, Q.; Wanapat, M.; Hou, F. Rumen bacteria influence milk protein yield of yak grazing on the Qinghai-Tibet plateau. Anim. Biosci. 2021, 34, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Arsenault, R.J. Gut health: The new paradigm in food animal production. Front. Vet. Sci. 2016, 3, 71. [Google Scholar] [CrossRef] [PubMed]
- Zeineldin, M.; Barakat, R.; Elolimy, A.; Salem, A.Z.; Elghandour, M.M.; Monroy, J.C. Synergetic action between the rumen microbiota and bovine health. Microb. Pathog. 2018, 124, 106–115. [Google Scholar] [CrossRef]
- Heinrichs, A.J.; Lesmeister, K.E. Rumen development in the dairy calf. Calf and heifer rearing: Principles of rearing the modern dairy heifer from calf to calving. In Proceedings of the 60th University of Nottingham Easter School in Agricultural Science, Nottingham, UK, 23–24 March 2005; pp. 53–65. [Google Scholar]
- Hristov, A.N.; Bannink, A.; Crompton, L.A.; Huhtanen, P.; Kreuzer, M.; McGee, M.; Nozière, P.; Reynolds, C.K.; Bayat, A.R.; Yáñez-Ruiz, D.R.; et al. Invited review: Nitrogen in ruminant nutrition: A review of measurement techniques. J. Dairy Sci. 2019, 102, 5811–5852. [Google Scholar] [CrossRef] [PubMed]
- Malmuthuge, N.; Griebel, P.J.; Guan, L.L. The gut microbiome and its potential role in the development and function of newborn calf gastrointestinal tract. Front. Vet. Sci. 2015, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M. The interplay between the gut microbiome and the immune system in the context of infectious diseases throughout life and the role of nutrition in optimizing treatment strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; Ross, R.P.; Stanton, C.; Clarke, G. The gut microbiome as a virtual endocrine organ with implications for farm and domestic animal endocrinology. Domest. Anim. Endocrinol. 2016, 56, S44–S55. [Google Scholar] [CrossRef]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef]
- Zhou, B.; Yuan, Y.; Zhang, S.; Guo, C.; Li, X.; Li, G.; Xiong, W.; Zeng, Z. Intestinal flora and disease mutually shape the regional immune system in the intestinal tract. Front. Immunol. 2020, 11, 575. [Google Scholar] [CrossRef]
- Lo, B.C.; Chen, G.Y.; Núñez, G.; Caruso, R. Gut microbiota and systemic immunity in health and disease. Int. Immunol. 2021, 33, 197–209. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, S.; Di, H.; Deng, Z.; Liu, J.; Wang, H. Gut health benefit and application of postbiotics in animal production. J. Anim. Sci. Biotechnol. 2022, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, B.A.; Voy, B.H.; Myer, P.R. Altering the gut microbiome of cattle: Considerations of host-microbiome interactions for persistent microbiome manipulation. Microb. Ecol. 2019, 77, 523–536. [Google Scholar] [CrossRef]
- Li, F.; Li, C.; Chen, Y.; Liu, J.; Zhang, C.; Irving, B.; Guan, L.L. Host genetics influence the rumen microbiota and heritable rumen microbial features associate with feed efficiency in cattle. Microbiome 2019, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Rey, M.; Enjalbert, F.; Combes, S.; Cauquil, L.; Bouchez, O.; Monteils, V. Establishment of ruminal bacterial community in dairy calves from birth to weaning is sequential. J. Appl. Microbiol. 2014, 116, 245–257. [Google Scholar] [CrossRef] [PubMed]
- De Barbieri, I.; Hegarty, R.S.; Silveira, C.; Gulino, L.M.; Oddy, V.H.; Gilbert, R.A.; Klieve, A.V.; Ouwerkerk, D. Programming rumen bacterial communities in newborn Merino lambs. Small Rumin Res. 2015, 129, 48–59. [Google Scholar] [CrossRef]
- Meale, S.J.; Popova, M.; Saro, C.; Martin, C.; Bernard, A.; Lagree, M.; Yáñez-Ruiz, D.R.; Boudra, H.; Duval, S.; Morgavi, D.P. Early life dietary intervention in dairy calves results in a long-term reduction in methane emissions. Sci. Rep. 2021, 11, 3003. [Google Scholar] [CrossRef] [PubMed]
- Abecia, L.; Waddams, K.E.; Martínez-Fernandez, G.; Martín-García, A.I.; Ramos-Morales, E.; Newbold, C.J.; Yáñez-Ruiz, D.R. An antimethanogenic nutritional intervention in early life of ruminants modifies ruminal colonization by Archaea. Archaea 2014, 2014, 841463. [Google Scholar] [CrossRef]
- Liu, S.; Yu, Z.; Zhong, H.; Zheng, N.; Huws, S.; Wang, J.; Zhao, S. Functional gene-guided enrichment plus in situ microsphere cultivation enables isolation of new crucial ureolytic bacteria from the rumen of cattle. Microbiome 2023, 11, 76. [Google Scholar] [CrossRef]
- McAllister, T.A.; Cheng, K.J.; Okine, E.K.; Mathison, G.W. Dietary, environmental and microbiological aspects of methane production in ruminants. Can. J. Anim. Sci. 1996, 76, 231–243. [Google Scholar] [CrossRef]
- Mekuriaw, S.; Tsunekawa, A.; Ichinohe, T.; Tegegne, F.; Haregeweyn, N.; Kobayashi, N.; Tassew, A.; Mekuriaw, Y.; Walie, M.; Tsubo, M.; et al. Effect of Feeding Improved Grass Hays and Eragrostis tef Straw Silage on Milk Yield, Nitrogen Utilization, and Methane Emission of Lactating Fogera Dairy Cows in Ethiopia. Animals 2020, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.; Menz, C.; Pinto, S.; Galán, E.; Janke, D.; Estellés, F.; Müschner-Siemens, T.; Wang, X.; Heinicke, J.; Zhang, G.; et al. Heat stress risk in European dairy cattle husbandry under different climate change scenarios–uncertainties and potential impacts. Earth Syst. Dyn. 2019, 10, 859–884. [Google Scholar] [CrossRef]
- Khafipour, E.; Li, S.; Tun, H.M.; Derakhshani, H.; Moossavi, S.; Plaizier, J.C. Effects of grain feeding on microbiota in the digestive tract of cattle. Anim. Front. 2016, 6, 13–19. [Google Scholar] [CrossRef]
- Deusch, S.; Camarinha-Silva, A.; Conrad, J.; Beifuss, U.; Rodehutscord, M.; Seifert, J. A structural and functional elucidation of the rumen microbiome influenced by various diets and microenvironments. Front. Microbiol. 2017, 8, 1605. [Google Scholar] [CrossRef]
- Zhou, M.; Ghoshal, B.; Stothard, P. Distinctive roles between rumen epimural and content bacterial communities on beef cattle feed efficiency: A combined analysis. Curr. Res. Microb. Sci. 2021, 2, 100085. [Google Scholar] [CrossRef]
- Arshad, M.A.; Hassan, F.U.; Rehman, M.S.; Huws, S.A.; Cheng, Y.; Din, A.U. Gut microbiome colonization and development in neonatal ruminants: Strategies, prospects, and opportunities. Anim. Nutr. 2021, 7, 883–895. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Li, S.; Danscher, A.M.; Derakshani, H.; Andersen, P.H.; Khafipour, E. Changes in microbiota in rumen digesta and feces due to a grain-based subacute ruminal acidosis (SARA) challenge. Microb. Ecol. 2017, 74, 485–495. [Google Scholar] [CrossRef]
- Huuki, H.; Ahvenjärvi, S.; Lidauer, P.; Popova, M.; Vilkki, J.; Vanhatalo, A.; Tapio, I. Fresh Rumen Liquid Inoculant Enhances the Rumen Microbial Community Establishment in Pre-weaned Dairy Calves. Front. Microbiol. 2021, 12, 758395. [Google Scholar] [CrossRef]
- Thornton, P.; Nelson, G.; Mayberry, D.; Herrero, M. Impacts of heat stress on global cattle production during the 21st century: A modelling study. Lancet Planet. Health 2022, 6, e192–e201. [Google Scholar] [CrossRef]
- He, B.; Fan, Y.; Wang, H. Lactate uptake in the rumen and its contributions to subacute rumen acidosis of goats induced by high-grain diets. Front. Vet. Sci. 2022, 9, 964027. [Google Scholar] [CrossRef]
- Jiménez, N.; Reverón, I.; Esteban-Torres, M.; López de Felipe, F.; de Las Rivas, B.; Muñoz, R. Genetic and biochemical approaches towards unravelling the degradation of gallotannins by Streptococcus gallolyticus. Microb. Cell. Fact. 2014, 13, 154. [Google Scholar] [CrossRef]
- Zhao, S.; Min, L.; Zheng, N.; Wang, J. Effect of Heat Stress on Bacterial Composition and Metabolism in the Rumen of Lactating Dairy Cows. Animals 2019, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, T.; Yu, Z. Metagenomic investigation of gastrointestinal microbiome in cattle. Asian. Australas. J. Anim. Sci. 2017, 30, 1515. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, J.; Kirs, M.; Ronimus, R.S.; Hoskin, S.O.; Janssen, P.H. Methanogen community structure in the rumens of farmed sheep, cattle and red deer fed different diets. FEMS Microbiol. 2011, 76, 311–326. [Google Scholar] [CrossRef]
- Shivani, S.; Srivastava, A.; Shandilya, U.K.; Kale, V.; Tyagi, A.K. Dietary supplementation of Butyrivibrio fibrisolvens alters fatty acids of milk and rumen fluid in lactating goats. J. Sci. Food Agric. 2016, 96, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Cotta, M.A.; Hespell, R.B. Proteolytic activity of the ruminal bacterium Butyrivibrio fibrisolvens. Appl. Environ. Microbiol. 1986, 52, 51–58. [Google Scholar] [CrossRef]
- Fuentes, M.C.; Calsamiglia, S.; Cardozo, P.W.; Vlaeminck, B. Effect of pH and level of concentrate in the diet on the production of biohydrogenation intermediates in a dual-flow continuous culture. J Dairy Sci. 2009, 92, 4456–4466. [Google Scholar] [CrossRef] [PubMed]
- Dušková, D.; Marounek, M. Fermentation of pectin and glucose, and activity of pectin-degrading enzymes in the rumen bacterium Lachnospira multiparus. Lett. Appl. Microbiol. 2001, 33, 159–163. [Google Scholar] [CrossRef]
- Danielsson, R.; Dicksved, J.; Sun, L.; Gonda, H.; Müller, B.; Schnürer, A.; Bertilsson, J. Methane production in dairy cows correlates with rumen methanogenic and bacterial community structure. Front. Microbiol. 2017, 8, 226. [Google Scholar] [CrossRef]
- Susanto, I.; Wiryawan, K.G.; Suharti, S.; Retnani, Y.; Zahera, R.; Jayanegara, A. Evaluation of Megasphaera elsdenii supplementation on rumen fermentation, production performance, carcass traits and health of ruminants: A meta-analysis. Anim. Biosci. 2023, 36, 879. [Google Scholar] [CrossRef]
- Littledike, E.T.; Young, J.W.; Beitz, D.C. Common metabolic diseases of cattle: Ketosis, milk fever, grass tetany, and downer cow complex. J. Dairy Sci. 1981, 64, 1465–1482. [Google Scholar] [CrossRef]
- Newbold, C.J.; De la Fuente, G.; Belanche, A.; Ramos-Morales, E.; McEwan, N.R. The role of ciliate protozoa in the rumen. Front. Microbiol. 2015, 6, 1313. [Google Scholar] [CrossRef]
- Firkins, J.L.; Yu, Z.; Park, T.; Plank, J.E. Extending Burk Dehority’s perspectives on the role of ciliate protozoa in the rumen. Front. Microbiol. 2020, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, X.; Dong, W.; Huang, B.; Wang, Y.; Zhu, M.; Wang, C. Plant cell wall breakdown by hindgut microorganisms: Can we get scientific insights from rumen microorganisms? J. Equine Vet. Sci. 2022, 115, 104027. [Google Scholar] [CrossRef] [PubMed]
- Myer, P.R. Bovine genome-microbiome interactions: Metagenomic frontier for the selection of efficient productivity in cattle systems. Msystems 2019, 4, e00103-19. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.C.; Purvis, H.T.; Najar, F.Z.; Sukharnikov, L.O.; Krehbiel, C.R.; Nagaraja, T.G.; Roe, B.A.; DeSilva, U. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol. 2010, 76, 7482–7490. [Google Scholar] [CrossRef]
- McCann, J.C.; Luan, S.; Cardoso, F.C.; Derakhshani, H.; Khafipour, E.; Loor, J.J. Induction of subacute ruminal acidosis affects the ruminal microbiome and epithelium. Front. Microbiol. 2016, 7, 701. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, H.; Yang, Q.; Yang, D.; Liu, S.; Cui, Z. Evaluating Starter Feeding on Ruminal Function in Yak Calves: Combined 16S rRNA Sequencing and Metabolomics. Front. Microbiol. 2022, 13, 821613. [Google Scholar] [CrossRef]
- Vasco, K.; Nohomovich, B.; Singh, P.; Venegas-Vargas, C.; Mosci, R.E.; Rust, S.; Bartlett, P.; Norby, B.; Grooms, D.; Zhang, L.; et al. Characterizing the cattle gut microbiome in farms with a high and low prevalence of shiga toxin producing Escherichia coli. Microorganisms 2021, 9, 1737. [Google Scholar] [CrossRef]
- Wu, S.; Cui, Z.; Chen, X.; Wang, P.; Yao, J. Changed caecal microbiota and fermentation contribute to the beneficial effects of early weaning with alfalfa hay, starter feed, and milk replacer on the growth and organ development of yak calves. Animals 2019, 9, 921. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, G.; Liu, Z.; Wu, P.; Yu, Z.; Wang, J. Repeated inoculation with fresh rumen fluid before or during weaning modulates the microbiota composition and co-occurrence of the rumen and colon of lambs. BMC Microbiol. 2020, 20, 29. [Google Scholar] [CrossRef] [PubMed]
- Raabis, S.; Li, W.; Cersosimo, L. Effects and immune responses of probiotic treatment in ruminants. Vet. Immunol. Immunopathol. 2019, 208, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Gunaswetha, K.; Sujatha, E.; Bramhachari, P.V. Understanding the Interplay Between the Host Immune–Microbiome Interactions: A State of the Art Review. In Microbiome in Human Health and Disease; Springer: Singapore, 2021; pp. 123–141. [Google Scholar] [CrossRef]
- Mulder, I.E.; Schmidt, B.; Lewis, M.; Delday, M.; Stokes, C.R.; Bailey, M.; Aminov, R.I.; Gill, B.P.; Pluske, J.R.; Mayer, C.D.; et al. Restricting microbial exposure in early life negates the immune benefits associated with gut colonisation in environments of high microbial diversity. PLoS ONE 2011, 6, 28279. [Google Scholar] [CrossRef]
- Malmuthuge, N. Understanding host-microbial interactions in rumen: Searching the best opportunity for microbiota manipulation. J. Anim. Sci. Biotechnol. 2017, 8, 8. [Google Scholar] [CrossRef]
- Liang, G.; Malmuthuge, N.; Bao, H.; Stothard, P.; Griebel, P.J.; Guan, L.L. Transcriptome analysis reveals regional and temporal differences in mucosal immune system development in the small intestine of neonatal calves. BMC Genom. 2016, 17, 602. [Google Scholar] [CrossRef]
- Liang, G.; Malmuthuge, N.; Griebel, P. Model systems to analyse the role of miRNAs and commensal microflora in bovine mucosal immune system development. Mol. Immunol. 2015, 66, 57–67. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Liang, G.; Griebel, P.J. Taxonomic and functional compositions of the small intestinal microbiome in neonatal calves provide a framework for understanding early life gut health. Appl. Environ. Microb. 2019, 85, e02534-18. [Google Scholar] [CrossRef]
- Spiljar, M.; Merkler, D.; Trajkovski, M. The immune system bridges the gut microbiota with systemic energy homeostasis: Focus on TLRs, mucosal barrier, and SCFAs. Front. Immunol. 2017, 8, 1353. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y. The effect of high-fat diet-induced pathophysiological changes in the gut on obesity: What should be the ideal treatment? Clin. Transl. Gastroenterol. 2013, 4, e39. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Lauterbach, M.; Latz, E. Western diet and the immune system: An inflammatory connection. Immunity 2019, 1, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Goldansaz, S.A.; Guo, A.C.; Sajed, T.; Steele, M.A.; Plastow, G.S.; Wishart, D.S. Livestock metabolomics and the livestock metabolome: A systematic review. PLoS ONE 2017, 12, e0177675. [Google Scholar] [CrossRef]
- Moran, N.A.; Ochman, H.; Hammer, T.J. Evolutionary and ecological consequences of gut microbial communities. Annu. Rev. Ecol. Evol. 2019, 50, 451. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut microbiome: Profound implications for diet and disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- Cordeiro, L.; Cabrita, A.R.; Oliveira, H.M.; Maia, M.R.; Rodrigues, J.A.; Fonseca, A.J.; Valente, I.M. A Novel Approach for Monitoring the Volatile Metabolome in Biological Samples from Ruminants through Miniaturized Liquid–Liquid Extraction and Multiclass Gas Chromatography Analysis. J. Agric. Food Chem. 2022, 70, 3886–3897. [Google Scholar] [CrossRef]
- Sun, H.Z.; Wang, D.M.; Wang, B.; Wang, J.K.; Liu, H.Y.; Guan, L.L.; Liu, J.X. Metabolomics of four biofluids from dairy cows: Potential biomarkers for milk production and quality. J. Proteome Res. 2015, 14, 1287–1298. [Google Scholar] [CrossRef]
- Artegoitia, V.M.; Foote, A.P.; Lewis, R.M.; Freetly, H.C. Rumen fluid metabolomics analysis associated with feed efficiency on crossbred steers. Sci. Rep. 2017, 7, 2864. [Google Scholar] [CrossRef] [PubMed]
- White, H.M. The role of TCA cycle anaplerosis in ketosis and fatty liver in periparturient dairy cows. Animals 2015, 5, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Arias-Islas, E.; Morales-Barrera, J.; Prado-Rebolledo, O.; García-Casillas, A. Metabolism in ruminants and its association with blood biochemical analytes. Abanico Vet. 2020, 10, 1–24. [Google Scholar] [CrossRef]
- Saleem, F.; Ametaj, B.N.; Bouatra, S.; Mandal, R.; Zebeli, Q.; Dunn, S.M.; Wishart, D.S. A metabolomics approach to uncover the effects of grain diets on rumen health in dairy cows. J. Dairy Sci. 2012, 95, 6606–6623. [Google Scholar] [CrossRef]
- Aschenbach, J.R.; Kristensen, N.B.; Donkin, S.S.; Hammon, H.M.; Penner, G.B. Gluconeogenesis in dairy cows: The secret of making sweet milk from sour dough. IUBMB Life 2010, 62, 869–877. [Google Scholar] [CrossRef]
- Carberry, C.A.; Kenny, D.A.; Han, S.; McCabe, M.S.; Waters, S.M. Effect of phenotypic residual feed intake and dietary forage content on the rumen microbial community of beef cattle. Appl. Environ. Microbiol. 2012, 78, 4949–4958. [Google Scholar] [CrossRef]
- Myer, P.R.; Wells, J.E.; Smith, T.P.L.; Kuehn, L.A.; Freetly, H.C. Microbial community profiles of the jejunum from steers differing in feed efficiency. Anim. Sci. J. 2016, 94, 327–338. [Google Scholar] [CrossRef]
- Abdela, N. Sub-acute ruminal acidosis (SARA) and its consequence in dairy cattle: A review of past and recent research at global prospective. Achiev. Life Sci. 2016, 10, 187–196. [Google Scholar] [CrossRef]
- Nagata, R.; Kim, Y.H.; Ohkubo, A.; Kushibiki, S.; Ichijo, T.; Sato, S. Effects of repeated subacute ruminal acidosis challenges on the adaptation of the rumen bacterial community in Holstein bulls. J. Dairy Sci. 2018, 101, 4424–4436. [Google Scholar] [CrossRef]
- Myer, P.R.; Wells, J.E.; Smith, T.P.; Kuehn, L.A.; Freetly, H.C. Microbial community profiles of the colon from steers differing in feed efficiency. Springerplus 2015, 4, 454. [Google Scholar] [CrossRef]
- Akhtar, M.; Naqvi, S.U.-A.-S.; Liu, Q.; Pan, H.; Ma, Z.; Kong, N.; Chen, Y.; Shi, D.; Kulyar, M.F.-e.-A.; Khan, J.A.; et al. Short Chain Fatty Acids (SCFAs) Are the Potential Immunomodulatory Metabolites in Controlling Staphylococcus aureus-Mediated Mastitis. Nutrients 2022, 14, 3687. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Ma, Y.; Yang, S.; Liu, S.; Xiao, J.; Wang, Y.; Wang, W.; Yang, H.; Li, S.; Cao, Z. Gut microbiota-derived ursodeoxycholic acid from neonatal dairy calves improves intestinal homeostasis and colitis to attenuate extended-spectrum β-lactamase-producing enteroaggregative Escherichia coli infection. Microbiome 2022, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Alrubaye, B.; Abraha, M.; Almansour, A.; Bansal, M.; Wang, H.; Kwon, Y.M.; Huang, Y.; Hargis, B.; Sun, X. Microbial metabolite deoxycholic acid shapes microbiota against Campylobacter jejuni chicken colonization. PLoS ONE 2019, 14, e0214705. [Google Scholar] [CrossRef]
- Welch, C.B.; Ryman, V.E.; Pringle, T.D.; Lourenco, J.M. Utilizing the Gastrointestinal Microbiota to Modulate Cattle Health through the Microbiome-Gut-Organ Axes. Microorganisms 2022, 10, 1391. [Google Scholar] [CrossRef]
- Xie, Z.; Li, M.; Qian, M.; Yang, Z.; Han, X. Co-Cultures of Lactobacillus acidophilus and Bacillus subtilis Enhance Mucosal Barrier by Modulating Gut Microbiota-Derived Short-Chain Fatty Acids. Nutrients 2022, 14, 4475. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Yang, S.; Luo, J.; Chen, Y.; Wu, R.; Liu, H.; Zhou, Z.; Yuncai, X. A buffalo rumen-derived probiotic (SN-6) could effectively increase Simmental growth performance by regulating fecal microbiota and metabolism. Front. Microbiol. 2022, 13, 935884. [Google Scholar]
- Vasquez, R.; Oh, J.K.; Song, J.H.; Kang, D.K. Gut microbiome-produced metabolites in pigs: A review on their biological functions and the influence of probiotics. J. Anim. Sci. Technol. 2022, 64, 671. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Xiang, K.; Zhao, C.; He, Z.; Qiu, M.; Hu, X.; Zhang, N. The Role of Rumen Microbiota and Its Metabolites in Subacute Ruminal Acidosis (SARA)-Induced Inflammatory Diseases of Ruminants. Microorganisms 2022, 10, 1495. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, M.; Wang, H.; Zhang, F.; Xue, F.; Hua, D.; Liu, J.; et al. Rumen microbiome structure and metabolites activity in dairy cows with clinical and subclinical mastitis. J. Anim. Sci. Biotechnol. 2021, 12, 36. [Google Scholar] [CrossRef]
- Chang, G.; Wang, L.; Ma, N.; Zhang, W.; Zhang, H.; Dai, H.; Shen, X. Histamine activates inflammatory response and depresses casein synthesis in mammary gland of dairy cows during SARA. BMC Vet. Res. 2018, 14, 168. [Google Scholar] [CrossRef]
- Guo, J.; Mu, R.; Li, S.; Zhang, N.; Fu, Y.; Hu, X. Characterization of the Bacterial Community of Rumen in Dairy Cows with Laminitis. Genes 2021, 12, 1996. [Google Scholar] [CrossRef] [PubMed]
- Goodlad, R.A. Some effects of diet on the mitotic index and the cell cycle of the ruminal epithelium of sheep. Q. J. Exp. Physiol. 1981, 66, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Pacífico, C.; Castillo-Lopez, E.; Rivera-Chacon, R.; Schwartz-Zimmermann, H.E.; Reisinger, N.; Berthiller, F.; Zebeli, Q.; Petri, R.M. Progressive microbial adaptation of the bovine rumen and hindgut in response to a step-wise increase in dietary starch and the influence of phytogenic supplementation. Front Microbiol. 2022, 13, 920427. [Google Scholar] [CrossRef]
- Bertin, Y.; Girardeau, J.P.; Chaucheyras-Durand, F.; Lyan, B.; Pujos-Guillot, E.; Harel, J.; Martin, C. Enterohaemorrhagic Escherichia coli gains a competitive advantage by using ethanolamine as a nitrogen source in the bovine intestinal content. Environ. Microbiol. 2011, 13, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Schären, M.; Frahm, J.; Kersten, S.; Meyer, U.; Hummel, J.; Breves, G.; Dänicke, S. Interrelations between the rumen microbiota and production, behavioral, rumen fermentation, metabolic, and immunological attributes of dairy cows. J. Dairy Sci. 2018, 101, 4615–4637. [Google Scholar] [CrossRef] [PubMed]
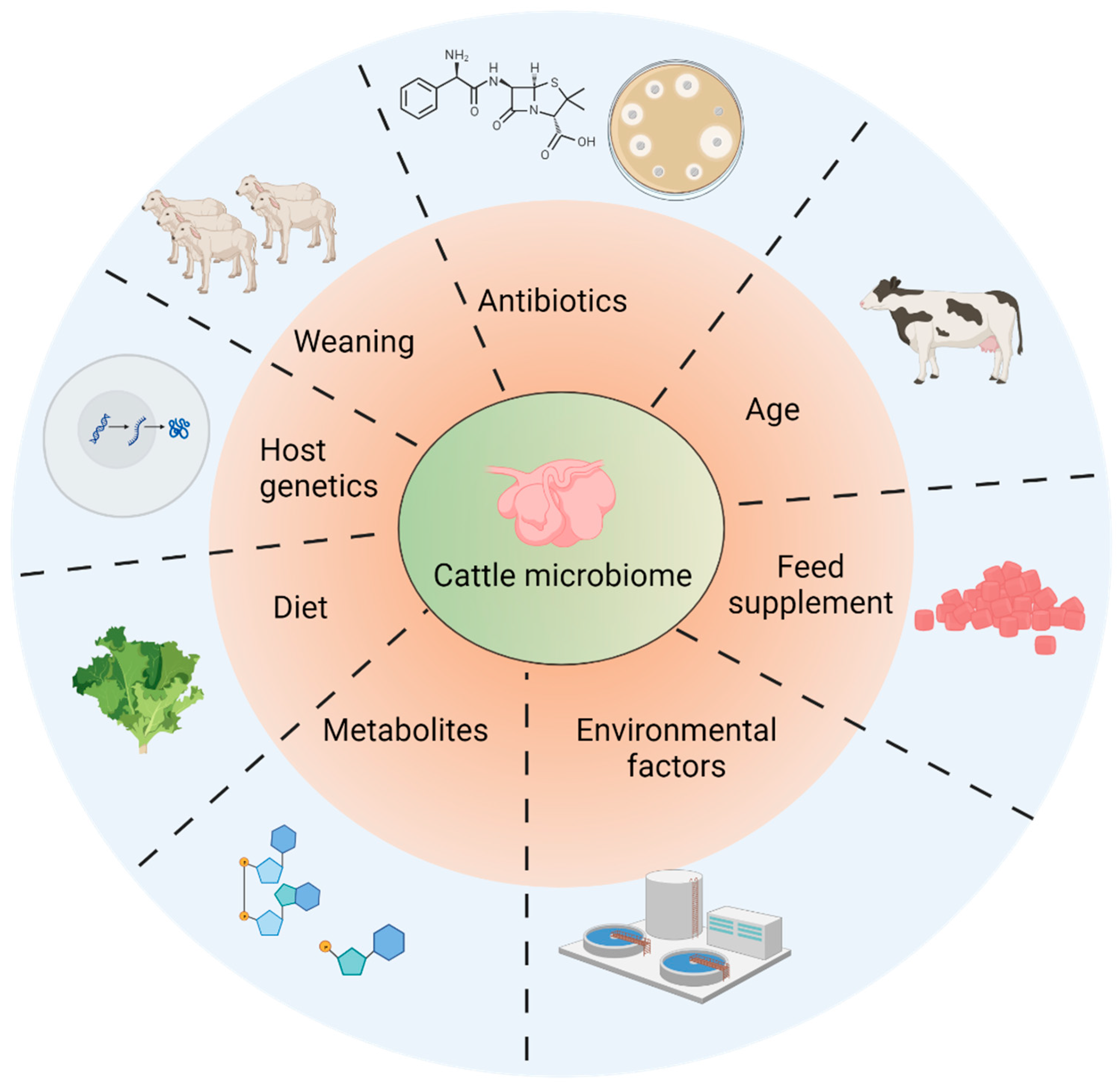
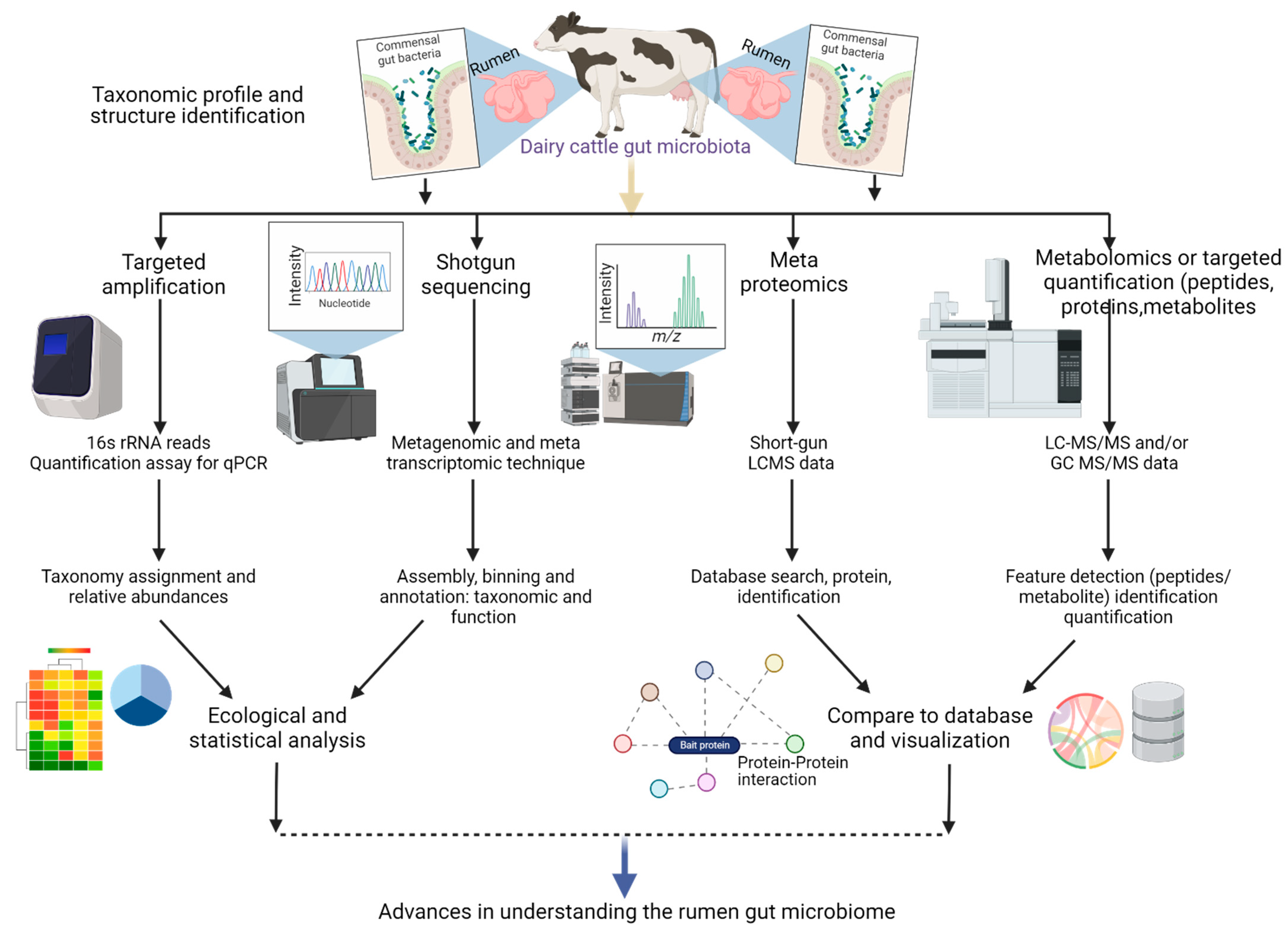
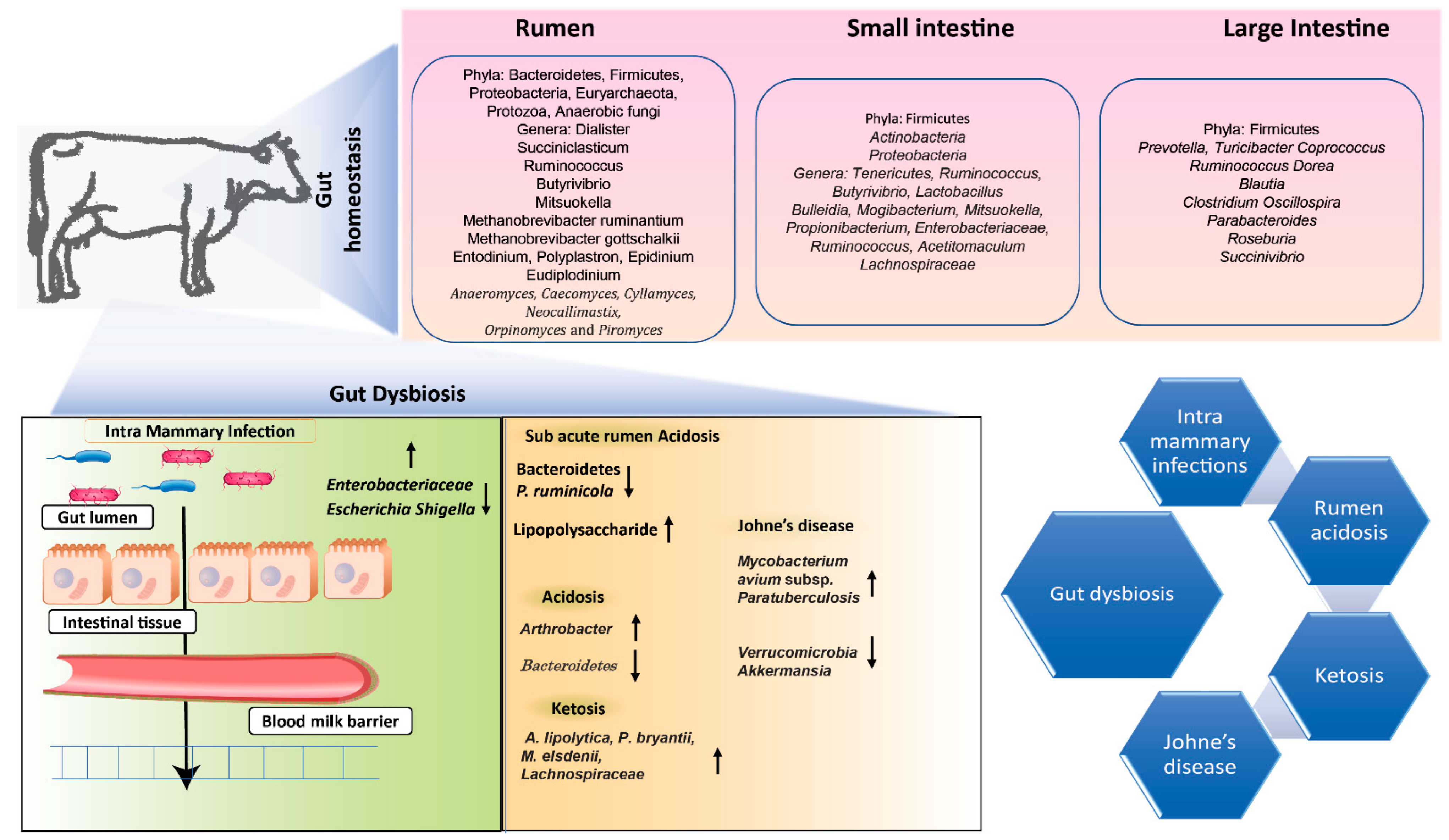
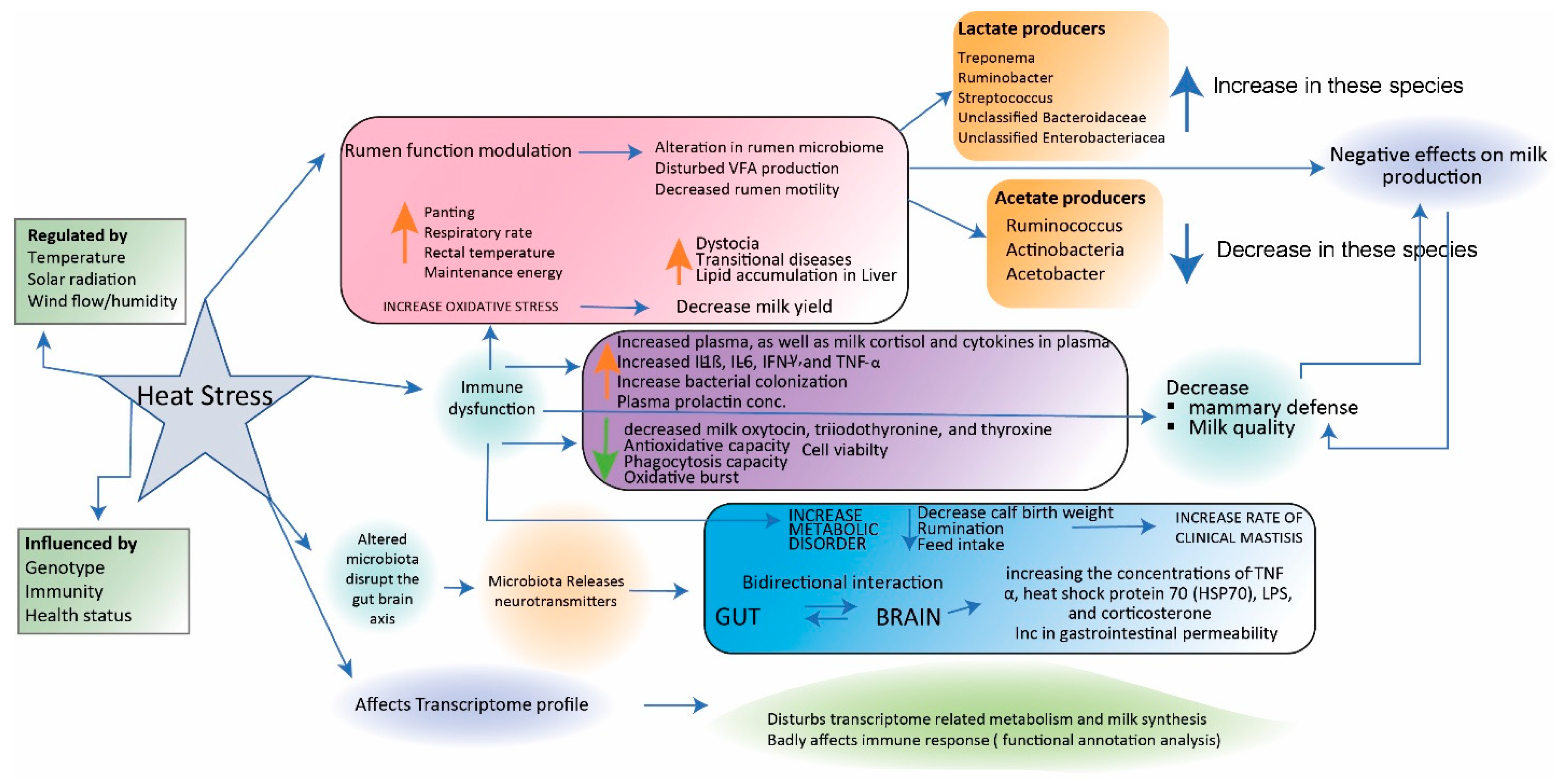
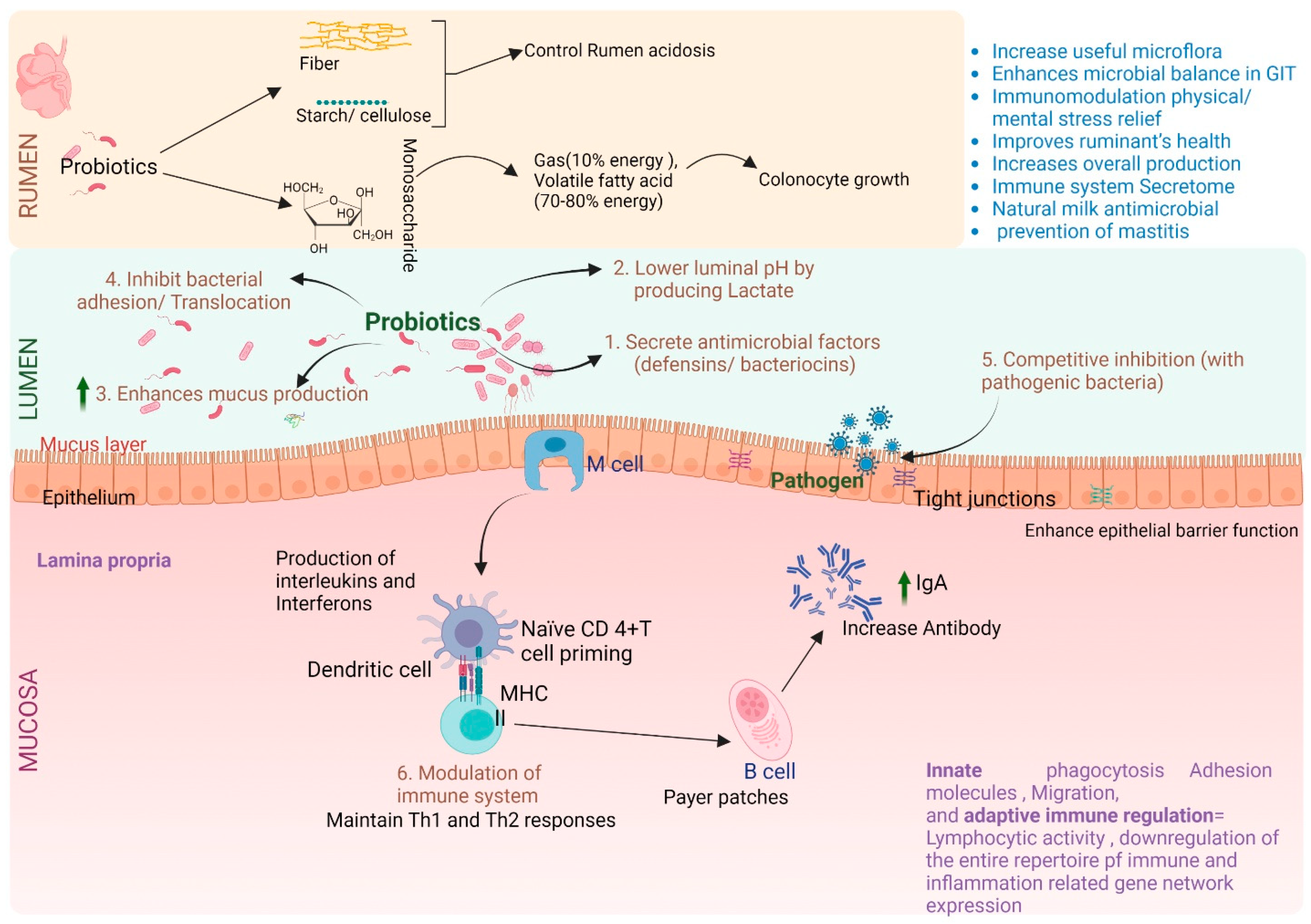
| Factors | Model | Technology | Results | References |
|---|---|---|---|---|
| Age | Bovine | 454 tag-encoded amplicon pyrosequencing | Between high residual feed intake groups and low residual feed intake groups, the diversity and within-group similarity increase with age, and each group has its own distinct microbiota | [30] |
| Calf | 16S rRNA gene sequencing, whole genome shotgun approach | Rumen microbiota of preruminant calves displays compositional heterogeneity, but functional classes between the 2 age groups (14-day-old calves and 42-day-old calves) are similar | [31] | |
| Calf | 16S rRNA gene amplicon sequencing and functional composition prediction. | The fecal microbial community has a great alteration within the time after weaning in both cattle and yak calves, but cattle showed a larger change. | [32] | |
| Bovine | 16S rRNA gene amplicon sequencing and microbial diversity analysis | Prevotella was strongly correlated with methanobrevibacter in heifers; however, in older cows (96–120 months) this association was replaced by a correlation between Succinivibrio and Methanobrevibacter | [33] | |
| Diet | Bovine | Metabolomics | Roughage type can significantly influence the ruminal microbial metabolome, especially organic acids, amines, and amino acids | [34] |
| Feed efficiency | Bovine | 16S rRNA gene sequencing, shotgun DNA sequencing | Megasphaera elsdenii is enriched in the rumen of the feed-efficient group; Methanobrevibacter was diminished | [35] |
| Bovine | Metatranscriptomics | Lachnospiraceae, Lactobacillaceae, and Veillonellaceae are more abundant in low-feed efficiency animals; Methanomassiliicoccales was diminished | [36] | |
| Bovine | metagenomics, metatranscriptomics, and metabolomics | Rumen of HiEf animals, Selenomonas and some species of Succinivibrionaceae might interact positively with each other and play an important role as keystone bacteria. | [37] | |
| Bovine | 16SrRNA Gene Sequencing | Host-associated microbial interactions differed within each breed depending on the feeding system, which indicated that breed-specific feeding systems should be taken into account for farm management | [38] | |
| Genetics | Bovine | SNP-based heritability estimates and 16S rRNA gene sequencing | Host genetic variation is associated with specific microbes | [39] |
| Bovine | Metagenomics | Host genetics shapes the microbiome Inoculation with bison rumen contents alters the cattle rumen microbiome and metabolism | [40,41] | |
| Bovine | High throughput sequencing | Rumen microbial features are heritable and could be influenced by host genetics, highlighting a potential to manipulate and obtain a desirable and efficient rumen microbiota using genetic selection and breeding. | [42] | |
| Bovine | 16S rRNA gene sequencing | Single-nucleotide polymorphisms found in cattle genomes and corresponding rumen bacterial community composition, annotated genes associated suggest the associations observed are directed toward selective absorption of volatile fatty acids from the rumen to increase energy availability to the host | [43] |
| Bacterial Species | End Product | Function | Reference |
|---|---|---|---|
| Fibrobacter succinogens, Clostridium longisporum, Clostridium cellobioparum | Acetate, Formate, Ethanol, propionate | Degrade the cellulose into smaller oligo/disaccharides | [84] |
| Butyrivibrio fibrisolvens | Saturated fatty acids and conjugated linoleic acids | Lipolysis and biodehydrogenation | [85] |
| Prevotella ruminicola, Ruminobacter amylophilus, Streptococcus Bovis | Hydrolysis of starch | [86] | |
| Anaerovibrio lipolytica | Acetate and propionate | Lipolytic activity of rumen contents of cattle | [87] |
| Lachnospira multiparus, Treponema saccharophilum | Acetate and formate | Fermentation of pectin and glucose | [88] |
| Methanobrevibacter spp. | Methane | Methane emissions | [89] |
| Succinivibrio sp. Lactobacillus sp. | Lactate, Acetate, Fumarate, Succinate | Lactate, Acetate, Fumarate, Succinate | [84,90] |
| Prevotella ruminicola, Eubacterium uniformis, Eubacterium xylanophilum | Acetate, Formate, Ethanol, propionate | Xylan consumption | [88,91] |
| Ruminococcaceae, Clostridium, Turicibacter and unclassified Peptostreptococcaceae | Volatile fatty acid | Degradation of starch and fiber | [45] |
| Ruminobacter amylophilus, Prevotella sp., Clostridium bifermentans | Amino acids, nitrogen | Starch hydrolysis | [91] |
| Entodiniomorphs, Eudiplodinium, Dasytricha, Diplodinium, Metadinium, Ophryoscolex, and Ostracodinium | Methane | Support methanogenesis, Engulf and digest a wide range of bacteria | [92,93] |
| Isotricha Dasytricha Charonina | Glucose, Fructose, Galcturonic acid | Fermentation of Starch, pectin, soluble sugars, proteins | [94] |
| Megasphaera elsdenii | Ammonia and CO2 | Acts upon lactate (end product of bacterial fermentation) | [84,90] |
| Entodinium, Diplodinium, Eudiplodinium, Ostracodinium, Metadinium, Polyplastron, Ophryoscolex, Epidinium | Glucose, Xylose | Hydrolyze structural polysaccharides | [95] |
| Eubacterium oxidoreducens, Streptococcus Caprinus | Lactate, Acetate, Fumarate, Succinate | Degradation of tannin | [83] |
| Metabolites | Model Organism | Diseases/Infection Control | Mode of Action | Reference |
|---|---|---|---|---|
| SCFA | Cow | Mastitis | Improve condition by conserving mucosal barrier integrity, restoring blood-milk barrier and prevent pathogens and their metabolites intrusion, maintain immune homeostasis | [130] |
| Ursodeoxycholic acid | Neonatal dairy calves | ESBL-EAEC-induced clinical symptoms and colitis | Show antibacterial action, inhibit proinflammatory effect, and decreased cell integrity loss | [131] |
| Deoxycholic acid | Chicken | Campylobacter jejuni colonization | Prevent infection by modulating microbiota composition | [132] |
| Butyrate | Cattle | Subacute ruminal acidosis (SARA) | Prevent SARA by increasing ruminal pH | [133] |
| SCFA | Pig | Intestinal Barrier Function | Improve gut health by regulating IL-22 production | [134] |
| Indole derivatives (3-indoleacrylic acid, kynurenic acid) | Simmental cattle | Daily weight gain | Enhance body weight by regulating intestinal homeostasis, gut barrier function and immune system | [135] |
| Putrescine | Pig | Diarrhea | Alleviate diarrhea by enhancing anti-inflammatory function and suppressing inflammatory response | [136] |
| volatile fatty acids (VFAs) | Dairy Cow | Subacute ruminal acidosis (SARA) | Cause acidosis by decreasing rumen pH below physiological range | [137] |
| Butyrate | Pig | lipopolysaccharide (LPS)-induced colitis | Protect against colitis by diminishing secretion of pro-inflammatory cytokines (IL)-1β, IL-6, tumor necrosis factor (TNF)α, IL-8, and IL-12 | [136] |
| Leukotriene B4 | Cow | Clinical mastitis | Cause mastitis by increasing inflammatory response | [138] |
| Histamine | Dairy Cow | Subacute ruminal acidosis (SARA) | Cause SARA diseases by activating NF-kb and mTOR signaling followed by mammary inflammtion | [139] |
| LPS, lactate | Dairy Cow | Laminitis | Disturb rumen environment by decreasing rumen pH | [140] |
| 5-Hydroxymethyl-2-furancarboxaldehyde | Lactating Holstein dairy cows | Clinical Mastitis | Cause mastitis by enhancing production of pro-inflammatory factors, such as TNF-α and IL-1β | [138] |
| δ-aminolevulinic acid | Dairy Calves | Diarrhea | Cause diarrhea by gut dysbiosis | [141] |
| Non-esterified fatty acid (NEFA), and β-hydroxybutyric acid (BHBA) | Dairy Cow | Left Displaced Abomasum | Cause LDA by increased Ketosis and negative energy balance (NEB) prepartum | [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, H.; Kaur, G.; Gupta, T.; Mittal, D.; Ali, S.A. Integrating Omics Technologies for a Comprehensive Understanding of the Microbiome and Its Impact on Cattle Production. Biology 2023, 12, 1200. https://doi.org/10.3390/biology12091200
Kaur H, Kaur G, Gupta T, Mittal D, Ali SA. Integrating Omics Technologies for a Comprehensive Understanding of the Microbiome and Its Impact on Cattle Production. Biology. 2023; 12(9):1200. https://doi.org/10.3390/biology12091200
Chicago/Turabian StyleKaur, Harpreet, Gurjeet Kaur, Taruna Gupta, Deepti Mittal, and Syed Azmal Ali. 2023. "Integrating Omics Technologies for a Comprehensive Understanding of the Microbiome and Its Impact on Cattle Production" Biology 12, no. 9: 1200. https://doi.org/10.3390/biology12091200
APA StyleKaur, H., Kaur, G., Gupta, T., Mittal, D., & Ali, S. A. (2023). Integrating Omics Technologies for a Comprehensive Understanding of the Microbiome and Its Impact on Cattle Production. Biology, 12(9), 1200. https://doi.org/10.3390/biology12091200





