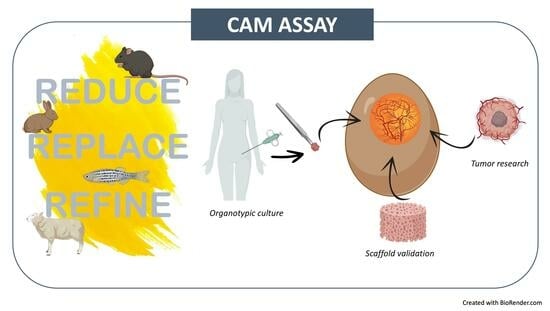
CAM Model: Intriguing Natural Bioreactor for Sustainable Research and Reliable/Versatile Testing
Abstract
:Simple Summary
Abstract
1. Introduction
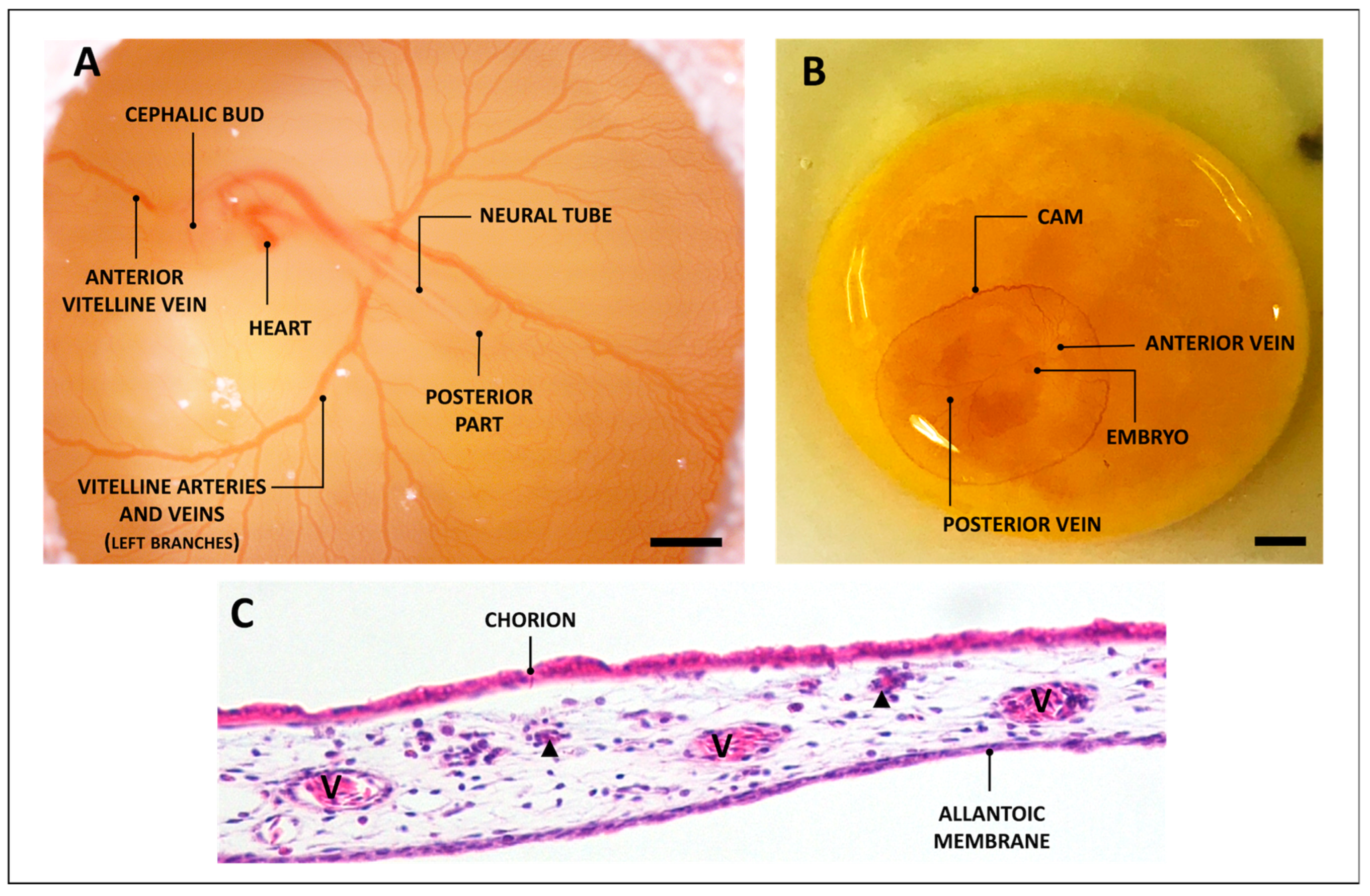
2. The CAM
3. The Application of CAM up to Now
3.1. Use of the Cam Assay for Cancer Studies
3.2. Cancer Hallmarks Studied in CAM: Angiogenesis
3.3. Cancer Hallmarks Studied in CAM: Metastatic Potential
3.4. Tumor Therapy Test in CAM
4. Use of the CAM Assay to Validate Scaffolds for Regenerative Purposes
5. Use of CAM to Set-Up Organotypic Culture
6. Discussion and Conclusions
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hippenstiel, S.; Thöne-Reineke, C.; Kurreck, J. Animal Experiments: EU Is Pushing to Find Substitutes Fast. Nature 2021, 600, 37. [Google Scholar] [CrossRef] [PubMed]
- Fentem, J.; Malcomber, I.; Maxwell, G.; Westmoreland, C. Upholding the EU’s Commitment to ‘Animal Testing as a Last Resort’ Under REACH Requires a Paradigm Shift in How We Assess Chemical Safety to Close the Gap Between Regulatory Testing and Modern Safety Science. ATLA Altern. Lab. Anim. 2021, 49, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A.H.; Smith, L.; Owens, D.; Quelch, R.; Przyborski, S. Recreating Tissue Structures Representative of Teratomas In Vitro Using a Combination of 3D Cell Culture Technology and Human Embryonic Stem Cells. Bioengineering 2022, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Bioengineering Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef]
- Darling, N.J.; Mobbs, C.L.; González-Hau, A.L.; Freer, M.; Przyborski, S. Bioengineering Novel in Vitro Co-Culture Models That Represent the Human Intestinal Mucosa With Improved Caco-2 Structure and Barrier Function. Front. Bioeng. Biotechnol. 2020, 8, 992. [Google Scholar] [CrossRef]
- Knight, E.; Murray, B.; Carnachan, R.; Przyborski, S. Alvetex®: Polystyrene Scaffold Technology for Routine Three Dimensional Cell Culture. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2011; Volume 695, pp. 323–340. [Google Scholar]
- Golinelli, G.; Talami, R.; Frabetti, S.; Candini, O.; Grisendi, G.; Spano, C.; Chiavelli, C.; Arnaud, G.F.; Mari, G.; Dominici, M. A 3D Platform to Investigate Dynamic Cell-to-Cell Interactions Between Tumor Cells and Mesenchymal Progenitors. Front. Cell Dev. Biol. 2022, 9, 767253. [Google Scholar] [CrossRef]
- Flagelli, A.; Candini, O.; Frabetti, S.; Dominici, M.; Giardino, L.; Calzà, L.; Baldassarro, V.A. A Novel Three-Dimensional Culture Device Favors a Myelinating Morphology of Neural Stem Cell-Derived Oligodendrocytes. Front. Cell Dev. Biol. 2021, 9, 759982. [Google Scholar] [CrossRef]
- Shahin-Shamsabadi, A.; Selvaganapathy, P.R. Tissue-in-a-Tube: Three-Dimensional In Vitro Tissue Constructs with Integrated Multimodal Environmental Stimulation. Mater. Today Bio 2020, 7, 100070. [Google Scholar] [CrossRef]
- Mukhopadhyay, C.; Paul, M.K. Organoid-Based 3D In Vitro Microphysiological Systems as Alternatives to Animal Experimentation for Preclinical and Clinical Research. Arch. Toxicol. 2023, 97, 1429–1431. [Google Scholar] [CrossRef]
- Puschhof, J.; Pleguezuelos-Manzano, C.; Clevers, H. Organoids and Organs-on-Chips: Insights into Human Gut-Microbe Interactions. Cell Host Microbe 2021, 29, 867–878. [Google Scholar] [CrossRef]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-Chips: Into the next Decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Wang, Y.; Chen, W.; Li, Z.; Su, W.; Guo, Y.; Deng, P.; Qin, J. Engineering Human Islet Organoids from IPSCs Using an Organ-on-Chip Platform. Lab Chip 2019, 19, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Manafi, N.; Shokri, F.; Achberger, K.; Hirayama, M.; Mohammadi, M.H.; Noorizadeh, F.; Hong, J.; Liebau, S.; Tsuji, T.; Quinn, P.M.J.; et al. Organoids and Organ Chips in Ophthalmology. Ocul. Surf. 2021, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, S.; Feng, Y.; Zhang, J.; Du, Y.; Zhang, J.; Van Ongeval, C.; Ni, Y.; Li, Y. Cells Utilisation of Chick Embryo Chorioallantoic Membrane as a Model Platform for Imaging-Navigated Biomedical Research. Cells 2021, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. Two New Applications in the Study of Angiogenesis the CAM Assay: Acellular Scaffolds and Organoids. Microvasc. Res. 2022, 140, 104304. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.B.; da Silva, M.V.; de Morais Ribeiro, L.N. The Chicken Embryo as an In Vivo Experimental Model for Drug Testing: Advantages and Limitations. Lab. Anim. 2021, 50, 138–139. [Google Scholar] [CrossRef]
- Dadhich, P.; Das, B.; Pal, P.; Srivas, P.K.; Dutta, J.; Ray, S.; Dhara, S. A Simple Approach for an Eggshell-Based 3D-Printed Osteoinductive Multiphasic Calcium Phosphate Scaffold. ACS Appl. Mater. Interfaces 2016, 8, 11910–11924. [Google Scholar] [CrossRef]
- Burgio, F.; Rimmer, N.; Pieles, U.; Buschmann, J.; Beaufils-Hugot, M. Characterization and in Ovo Vascularization of a 3D-Printed Hydroxyapatite Scaffold with Different Extracellular Matrix Coatings under Perfusion Culture. Biol. Open 2018, 7, bio034488. [Google Scholar] [CrossRef]
- Baiguera, S.; Macchiarini, P.; Ribatti, D. Chorioallantoic Membrane for In Vivo Investigation of Tissue-Engineered Construct Biocompatibility. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1425–1434. [Google Scholar] [CrossRef]
- Yalcin, H.C.; Shekhar, A.; Rane, A.A.; Butcher, J.T. An Ex-Ovo Chicken Embryo Culture System Suitable for Imaging and Microsurgery Applications. J. Vis. Exp. 2010, 44, e2154. [Google Scholar] [CrossRef]
- Moreno-Jiménez, I.; Kanczler, J.M.; Hulsart-Billstrom, G.; Inglis, S.; Oreffo, R.O.C. The Chorioallantoic Membrane Assay for Biomaterial Testing in Tissue Engineering: A Short-Term In Vivo Preclinical Model. Tissue Eng. Part. C Methods 2017, 23, 938–952. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Vacca, A.; Roncali, L.; Burri, P.H.; Djonov, V. Chorioallantoic Membrane Capillary Bed: A Useful Target for Studying Angiogenesis and Anti-Angiogenesis In Vivo. Anat. Rec. 2001, 264, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Isachenko, V.; Mallmann, P.; Petrunkina, A.M.; Rahimi, G.; Nawroth, F.; Hancke, K.; Felberbaum, R.; Genze, F.; Damjanoski, I.; Isachenko, E. Comparison of In Vitro- and Chorioallantoic Membrane (CAM)-Culture Systems for Cryopreserved Medulla-Contained Human Ovarian Tissue. PLoS ONE 2012, 7, e32549. [Google Scholar] [CrossRef]
- Moreno-Jiménez, I.; Lanham, S.A.; Kanczler, J.M.; Hulsart-Billstrom, G.; Evans, N.D.; Oreffo, R.O.C. Remodelling of Human Bone on the Chorioallantoic Membrane of the Chicken Egg: De Novo Bone Formation and Resorption. J. Tissue Eng. Regen. Med. 2018, 12, 1877–1890. [Google Scholar] [CrossRef]
- Fazely, F.; Moses, D.C.; Ledinko, N. Effects of Retinoids on Invasion of Organ Cultures of Chick Chorioallantoic Membrane by Adenovirus Transformed Cells. In Vitro Cell. Dev. Biol. 1985, 21, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Madrid, B.; Donnez, J.; Van Eyck, A.S.; Veiga-Lopez, A.; Dolmans, M.M.; Van Langendonckt, A. Chick Embryo Chorioallantoic Membrane (CAM) Model: A Useful Tool to Study Short-Term Transplantation of Cryopreserved Human Ovarian Tissue. Fertil. Steril. 2009, 91, 285–292. [Google Scholar] [CrossRef]
- Leene, W.; Duyzings, M.J.M.; Van Steeg, C. Lymphoid Stem Cell Identification in the Developing Thymus and Bursa of Fabricius of the Chick. Z. Zellforsch. 1973, 136, 521–533. [Google Scholar] [CrossRef]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane in the Study of Angiogenesis and Metastasis; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-90-481-3843-2. [Google Scholar]
- Jankovic, B.D.; Isakovic, K.; Lukic, M.L.; Vujanovic, N.L.; Petrovic, S.; Markovic, B.M. Immunological Capacity of the Chicken Embryo. I. Relationship between the Maturation of Lymphoid Tissues and the Occurrence of Cell-Mediated Immunity in the Developing Chicken Embryo. Immunology 1975, 29, 497–508. [Google Scholar]
- Genova, T.; Petrillo, S.; Zicola, E.; Roato, I.; Ferracini, R.; Tolosano, E.; Altruda, F.; Carossa, S.; Mussano, F.; Munaron, L. The Crosstalk between Osteodifferentiating Stem Cells and Endothelial Cells Promotes Angiogenesis and Bone Formation. Front. Physiol. 2019, 10, 1291. [Google Scholar] [CrossRef]
- Kanczler, J.M.; Oreffo, R.O.C. Osteogenesis and Angiogenesis: The Potential for Engineering Bone. Eur. Cell Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef]
- Portal-Núñez, S.; Lozano, D.; Esbrit, P. Role of Angiogenesis on Bone Formation. Histol. Histopathol. 2012, 27, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Checchi, M.; Stanzani, V.; Truocchio, S.; Corradini, M.; Ferretti, M.; Palumbo, C. From Morphological Basic Research to Proposals for Regenerative Medicine through a Translational Perspective. Ital. J. Anat. Embryol. 2022, 126, 139–145. [Google Scholar] [CrossRef]
- Palumbo, C.; Cavani, F.; Sena, P.; Benincasa, M.; Ferretti, M. Osteocyte Apoptosis and Absence of Bone Remodeling in Human Auditory Ossicles and Scleral Ossicles of Lower Vertebrates: A Mere Coincidence or Linked Processes? Calcif. Tissue Int. 2012, 90, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.; Palumbo, C. Static Osteogenesis versus Dynamic Osteogenesis: A Comparison between Two Different Types of Bone Formation. Appl. Sci. 2021, 11, 2025. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- National Institutes of Health. The Public Health Service Responds to Commonly Asked Questions. ILAR J. 1991, 33, 68–70. [Google Scholar] [CrossRef]
- Institutional Animal Care and Use Committee (IACUC). Policy for Use of Avian Embryos; Brown University: Schaumburg, IL, USA, 2019. [Google Scholar]
- Elaroussi, M.A.; DeLuca, H.F. Calcium Uptake by Chorioallantoic Membrane: Effects of Vitamins D and K. Endocrinol. Metab. 1994, 267, E837–E841. [Google Scholar] [CrossRef]
- Tuan, R.; Ono, T. Regulation of Extraembryonic Calcium Mobilization by the Developing Chick Embryo. J. Embryol. Exp. Morphol. 1986, 97, 63–74. [Google Scholar] [CrossRef]
- Packard, M.J. Mobilization of Shell Calcium by Chick Chorioallantoic Membrane In Vitro. J. Exp. Biol. 1994, 190, 141–153. [Google Scholar] [CrossRef]
- Maibier, M.; Reglin, B.; Nitzsche, B.; Xiang, W.; Rong, W.W.; Hoffmann, B.; Djonov, V.; Secomb, T.W.; Pries, A.R. Structure and Hemodynamics of Vascular Networks in the Chorioallantoic Membrane of the Chicken. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H913–H926. [Google Scholar] [CrossRef]
- Li, Y.; Qu, H.; Ji, J.; Wang, Y.; Liu, T.; He, J.; Wang, J.; Shu, D.; Luo, C. Characterization of the Exosomes in the Allantoic Fluid of the Chicken Embryo. Can. J. Anim. Sci. 2021, 101, 307–317. [Google Scholar] [CrossRef]
- Da Silva, M.; Labas, V.; Nys, Y.; Rehault-Godbert, S. Investigating Proteins and Proteases Composing Amniotic and Allantoic Fluids during Chicken Embryonic Development. Poult. Sci. 2017, 96, 2931–2941. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A Series of Normal Stages in the Development of the Chick Embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef]
- Crespo, P.; Casar, B. The Chick Embryo Chorioallantoic Membrane as an In Vivo Model to Study Metastasis. Bio. Protoc. 2016, 6, e1962. [Google Scholar] [CrossRef]
- Lazarovici, P.; Lahiani, A.; Gincberg, G.; Haham, D.; Marcinkiewicz, C.; Lelkes, P.I. Nerve Growth Factor-Induced Angiogenesis: 2. The Quail Chorioallantoic Membrane Assay. In Neurotrophic Factors: Methods and Protocols; Skaper, S.D., Ed.; Springer: New York, NY, USA, 2018; Volume 1727, pp. 251–259. ISBN 978-1-4939-7571-6. [Google Scholar]
- Parsons-Wingerter, P.; Lwai, B.; Che Yang, M.; Elliott, K.E.; Milaninia, A.; Redlitz, A.; Clark, J.I.; Helene Sage, E. A Novel Assay of Angiogenesis in the Quail Chorioallantoic Membrane: Stimulation by BFGF and Inhibition by Angiostatin According to Fractal Dimension and Grid Intersection. Microvasc. Res. 1998, 55, 201–214. [Google Scholar] [CrossRef]
- Kundeková, B.; Máčajová, M.; Meta, M.; Čavarga, I.; Bilčík, B. Chorioallantoic Membrane Models of Various Avian Species: Differences and Applications. Biology 2021, 10, 301. [Google Scholar] [CrossRef]
- Rasmussen, S.V.; Berlow, N.E.; Price, L.H.; Mansoor, A.; Cairo, S.; Rugonyi, S.; Keller, C. Preclinical Therapeutics Ex Ovo Quail Eggs as a Biomimetic Automation-Ready Xenograft Platform. Sci. Rep. 2021, 11, 23302. [Google Scholar] [CrossRef]
- Lusimbo, W.S.; Leighton, F.A.; Wobeser, G.A. Histology and Ultrastructure of the Chorioallantoic Membrane of the Mallard Duck (Anas Platyrhynchos). Anat. Rec. 2000, 259, 25–34. [Google Scholar] [CrossRef]
- Buhr, C.R.; Wiesmann, N.; Tanner, R.C.; Brieger, J.; Eckrich, J. The Chorioallantoic Membrane Assay in Nanotoxicological Research—An Alternative for In Vivo Experimentation. Nanomaterials 2020, 10, 2328. [Google Scholar] [CrossRef]
- Longenecker, B.M.; Pazderka, F.; Stone, H.S.; Gavora, J.S.; Ruth, R.F. In Ovo Assay for Marek’s Disease Virus and Turkey Herpesvirus. Infect. Immun. 1975, 11, 922–931. [Google Scholar] [CrossRef]
- Janser, F.; Ney, P.; Pinto, M.; Langer, R.; Tschan, M. The Chick Chorioallantoic Membrane (CAM) Assay as a Three-Dimensional Model to Study Autophagy in Cancer Cells. Bio. Protoc. 2019, 9, e3290. [Google Scholar] [CrossRef]
- Huang, W.; Arai, F.; Kawahara, T. Egg-in-Cube: Design and Fabrication of a Novel Artificial Eggshell with Functionalized Surface. PLoS ONE 2015, 10, e0118624. [Google Scholar] [CrossRef]
- Dohle, D.S.; Pasa, S.D.; Gustmann, S.; Laub, M.; Wissler, J.H.; Jennissen, H.P.; Dünker, N. Chick Ex Ovo Culture and Ex Ovo CAM Assay: How It Really Works. J. Vis. Exp. 2010, 33, e1620. [Google Scholar] [CrossRef]
- García-Gareta, E.; Binkowska, J.; Kohli, N.; Sharma, V. Towards the Development of a Novel Ex Ovo Model of Infection to Pre-Screen Biomaterials Intended for Treating Chronic Wounds. J. Funct. Biomater. 2020, 11, 37. [Google Scholar] [CrossRef]
- Winter, R.; Dungel, P.; Reischies, F.M.J.; Rohringer, S.; Slezak, P.; Smolle, C.; Spendel, S.; Kamolz, L.P.; Ghaffari-Tabrizi-Wizsy, N.; Schicho, K. Photobiomodulation (PBM) Promotes Angiogenesis in-Vitro and in Chick Embryo Chorioallantoic Membrane Model. Sci. Rep. 2018, 8, 17080. [Google Scholar] [CrossRef]
- Gómez Del Moral, M.; Fonfría, J.; Varas, A.; Jiménez, E.; Moreno, J.; Zapata, A.G. Appearance and Development of Lymphoid Cells in the Chicken (Gallus Gallus) Caecal Tonsil. Anat. Rec. 1998, 250, 182–189. [Google Scholar] [CrossRef]
- Kunzi-Rapp, K.; Rück, A.; Kaufmann, R. Characterization of the Chick Chorioallantoic Membrane Model as a Short-Term In Vivo System for Human Skin. Arch. Dermatol. Res. 1999, 291, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Vacca, A.; Presta, M. The Gelatin Sponge-Chorioallantoic Membrane Assay. Nat. Protoc. 2006, 1, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The Chicken Chorioallantoic Membrane Model in Biology, Medicine and Bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef]
- Moreno-Jiménez, I.; Hulsart-Billstrom, G.; Lanham, S.A.; Janeczek, A.A.; Kontouli, N.; Kanczler, J.M.; Evans, N.D.; Oreffo, R.O.C. The Chorioallantoic Membrane (CAM) Assay for the Study of Human Bone Regeneration: A Refinement Animal Model for Tissue Engineering. Sci. Rep. 2016, 6, 32168. [Google Scholar] [CrossRef]
- DeBord, L.C.; Pathak, R.R.; Villaneuva, M.; Liu, H.-C.; Harrington, D.A.; Yu, W.; Lewis, M.T.; Sikora, A.G. The Chick Chorioallantoic Membrane (CAM) as a Versatile Patient-Derived Xenograft (PDX) Platform for Precision Medicine and Preclinical Research. Am. J. Cancer Res. 2018, 8, 1642–1660. [Google Scholar]
- Checchi, M.; Bertacchini, J.; Cavani, F.; Magarò, M.S.; Reggiani Bonetti, L.; Pugliese, G.R.; Tamma, R.; Ribatti, D.; Maurel, D.B.; Palumbo, C. Scleral Ossicles: Angiogenic Scaffolds, a Novel Biomaterial for Regenerative Medicine Applications. Biomater. Sci. 2020, 8, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Stanzani, V.; Giubilini, A.; Checchi, M.; Bondioli, F.; Messori, M.; Palumbo, C. Eco-Sustainable Approaches in Bone Tissue Engineering: Evaluating the Angiogenic Potential of Different Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate)–Nanocellulose Composites with the Chorioallantoic Membrane Assay. Adv. Eng. Mater. 2023, 25, 2200934. [Google Scholar] [CrossRef]
- Ranjan, R.A.; Muenzner, J.K.; Kunze, P.; Geppert, C.I.; Ruebner, M.; Huebner, H.; Fasching, P.A.; Beckmann, M.W.; Bäuerle, T.; Hartmann, A.; et al. The Chorioallantoic Membrane Xenograft Assay as a Reliable Model for Investigating the Biology of Breast Cancer. Cancers 2023, 15, 1704. [Google Scholar] [CrossRef] [PubMed]
- Miebach, L.; Berner, J.; Bekeschus, S. In Ovo Model in Cancer Research and Tumor Immunology. Front. Immunol. 2022, 13, 1006064. [Google Scholar] [CrossRef]
- Schneider-Stock, R.; Ribatti, D. The CAM Assay as an Alternative In Vivo Model for Drug Testing. In Handbook of Experimental Pharmacology; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2020; Volume 265, pp. 303–323. [Google Scholar]
- Kunz, P.; Schenker, A.; Sähr, H.; Lehner, B.; Fellenberg, J. Optimization of the Chicken Chorioallantoic Membrane Assay as Reliable In Vivo Model for the Analysis of Osteosarcoma. PLoS ONE 2019, 14, e0215312. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Chu, P.Y.; Koh, A.P.F.; Antony, J.; Huang, R.Y.J. Applications of the Chick Chorioallantoic Membrane as an Alternative Model for Cancer Studies. Cells Tissues Organs 2022, 211, 222–237. [Google Scholar] [CrossRef]
- Doege, A.; Steens, R.; Dünker, N.; Busch, M.A. Retinoblastoma Cell Growth In Vitro and Tumor Formation In Ovo—Influence of Different Culture Conditions. Methods Protoc. 2022, 5, 21. [Google Scholar] [CrossRef]
- Thelen, M.; Wennhold, K.; Lehmann, J.; Garcia-Marquez, M.; Klein, S.; Kochen, E.; Lohneis, P.; Lechner, A.; Wagener-Ryczek, S.; Plum, P.S.; et al. Cancer-Specific Immune Evasion and Substantial Heterogeneity within Cancer Types Provide Evidence for Personalized Immunotherapy. NPJ Precis. Oncol. 2021, 5, 52. [Google Scholar] [CrossRef]
- Fischer, D.; Fluegen, G.; Garcia, P.; Ghaffari-Tabrizi-Wizsy, N.; Gribaldo, L.; Huang, R.Y.J.; Rasche, V.; Ribatti, D.; Rousset, X.; Pinto, M.T.; et al. The CAM Model—Q&A with Experts. Cancers 2023, 15, 191. [Google Scholar]
- Pizon, M.; Schott, D.; Pachmann, U.; Schobert, R.; Pizon, M.; Wozniak, M.; Bobinski, R.; Pachmann, K. Chick Chorioallantoic Membrane (CAM) Assays as a Model of Patient-Derived Xenografts from Circulating Cancer Stem Cells (CCSCs) in Breast Cancer Patients. Cancers 2022, 14, 1476. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ishihara, M.; Chin, A.I.; Wu, L. Establishment of Xenografts of Urological Cancers on Chicken Chorioallantoic Membrane (CAM) to Study Metastasis. Precis. Clin. Med. 2019, 2, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhou, X.; Ming, H.; Zhang, J.; Huang, G.; Zhang, Z.; Li, P. Chick Chorioallantoic Membrane Assay: A 3D Animal Model for Study of Human Nasopharyngeal Carcinoma. PLoS ONE 2015, 10, e0130935. [Google Scholar] [CrossRef] [PubMed]
- Balčiūnienė, N.; Tamašauskas, A.; Valančiūtė, A.; Deltuva, V.; Vaitiekaitis, G.; Gudinavičienė, I.; Weis, J.; Graf Von Keyserlingk, D.; Balčiūnienė, N. Histology of Human Glioblastoma Transplanted on Chicken Chorioallantoic Membrane. Medicina 2009, 45, 123. [Google Scholar] [CrossRef] [PubMed]
- Vézina-Dawod, S.; Perreault, M.; Guay, L.D.; Gerber, N.; Gobeil, S.; Biron, E. Synthesis and Biological Evaluation of Novel 1,4-Benzodiazepin-3-One Derivatives as Potential Antitumor Agents against Prostate Cancer. Bioorg. Med. Chem. 2021, 45, 116314. [Google Scholar] [CrossRef]
- Goehringer, N.; Biersack, B.; Peng, Y.; Schobert, R.; Herling, M.; Ma, A.; Nitzsche, B.; Höpfner, M. Anticancer Activity and Mechanisms of Action of New Chimeric EGFR/HDAC-Inhibitors. Int. J. Mol. Sci. 2021, 22, 8432. [Google Scholar] [CrossRef]
- Miebach, L.; Freund, E.; Horn, S.; Niessner, F.; Sagwal, S.K.; von Woedtke, T.; Emmert, S.; Weltmann, K.D.; Clemen, R.; Schmidt, A.; et al. Tumor Cytotoxicity and Immunogenicity of a Novel V-Jet Neon Plasma Source Compared to the KINPen. Sci. Rep. 2021, 11, 136. [Google Scholar] [CrossRef]
- Liedtke, K.R.; Freund, E.; Hermes, M.; Oswald, S.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Gas Plasma-Conditioned Ringer’s Lactate Enhances the Cytotoxic Activity of Cisplatin and Gemcitabine in Pancreatic Cancer In Vitro and In Ovo. Cancers 2020, 12, 123. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Verloy, R.; Cardenas Delahoz, E.; Lin, A.; Vanlanduit, S.; Smits, E.; Bogaerts, A. Cold Atmospheric Plasma Does Not Affect Stellate Cells Phenotype in Pancreatic Cancer Tissue in Ovo. Int. J. Mol. Sci. 2022, 23, 1954. [Google Scholar] [CrossRef]
- Busch, M.; Papior, D.; Stephan, H.; Dönker, N. Characterization of Etoposide- and Cisplatin-Chemoresistant Retinoblastoma Cell Lines. Oncol. Rep. 2018, 39, 160–172. [Google Scholar] [CrossRef]
- Khabipov, A.; Freund, E.; Liedtke, K.R.; Käding, A.; Riese, J.; van der Linde, J.; Kersting, S.; Partecke, L.I.; Bekeschus, S. Murine Macrophages Modulate Their Inflammatory Profile in Response to Gas Plasma-Inactivated Pancreatic Cancer Cells. Cancers 2021, 13, 2525. [Google Scholar] [CrossRef]
- Achkar, I.W.; Kader, S.; Dib, S.S.; Junejo, K.; Al-Bader, S.B.; Hayat, S.; Bhagwat, A.M.; Rousset, X.; Wang, Y.; Viallet, J.; et al. Metabolic Signatures of Tumor Responses to Doxorubicin Elucidated by Metabolic Profiling in Ovo. Metabolites 2020, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.; Koch, A.B.F.; Löffler, J.; Jelezko, F.; Lindén, M.; Li, H.; Abaei, A.; Zuo, Z.; Beer, A.J.; Rasche, V. In Vivo PET/MRI Imaging of the Chorioallantoic Membrane. Front. Phys. 2020, 8, 151. [Google Scholar] [CrossRef]
- Miura, K.; Koyanagi-Aoi, M.; Maniwa, Y.; Aoi, T. Chorioallantoic Membrane Assay Revealed the Role of TIPARP (2,3,7,8-Tetrachlorodibenzo-p-Dioxin-Inducible Poly (ADP-Ribose) Polymerase) in Lung Adenocarcinoma-Induced Angiogenesis. Cancer Cell Int. 2023, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane as an Experimental Model to Study In Vivo Angiogenesis in Glioblastoma Multiforme. Brain Res. Bull. 2022, 182, 26–29. [Google Scholar] [CrossRef]
- Damanskienė, E.; Balnytė, I.; Valančiūtė, A.; Alonso, M.M.; Preikšaitis, A.; Stakišaitis, D. The Different Temozolomide Effects on Tumorigenesis Mechanisms of Pediatric Glioblastoma PBT24 and SF8628 Cell Tumor in CAM Model and on Cells In Vitro. Int. J. Mol. Sci. 2022, 23, 2001. [Google Scholar] [CrossRef]
- Kerkhoff, M.; Grunewald, S.; Schaefer, C.; Zöllner, S.K.; Plaumann, P.; Busch, M.; Dünker, N.; Ketzer, J.; Kersting, J.; Bauer, S.; et al. Evaluation of the Effect of Photodynamic Therapy on CAM-Grown Sarcomas. Bioengineering 2023, 10, 464. [Google Scholar] [CrossRef]
- Guder, W.K.; Hartmann, W.; Buhles, C.; Burdack, M.; Busch, M.; Dünker, N.; Hardes, J.; Dirksen, U.; Bauer, S.; Streitbürger, A. 5-ALA-Mediated Fluorescence of Musculoskeletal Tumors in a Chick Chorio-Allantoic Membrane Model: Preclinical In Vivo Qualification Analysis as a Fluorescence-Guided Surgery Agent in Orthopedic Oncology. J. Orthop. Surg. Res. 2022, 17, 34. [Google Scholar] [CrossRef]
- Hu, L.; Li, K.; Lin, L.; Qian, F.; Li, P.; Zhu, L.; Cai, H.; You, L.; Song, J.; Kok, S.H.L.; et al. Reversine Suppresses Osteosarcoma Cell Growth through Targeting BMP-Smad1/5/8-Mediated Angiogenesis. Microvasc. Res. 2021, 135, 104136. [Google Scholar] [CrossRef]
- Fialho, S.L.; Silvestrini, B.R.; Vieira, J.; Paiva, M.R.B.; Silva, L.M.; Chahud, F.; Silva-Cunha, A.; Correa, Z.M.; Jorge, R. Successful Growth of Fresh Retinoblastoma Cells in Chorioallantoic Membrane. Int. J. Retina Vitreous 2020, 6, 33. [Google Scholar] [CrossRef]
- Merlos Rodrigo, M.A.; Casar, B.; Michalkova, H.; Jimenez Jimenez, A.M.; Heger, Z.; Adam, V. Extending the Applicability of In Ovo and Ex Ovo Chicken Chorioallantoic Membrane Assays to Study Cytostatic Activity in Neuroblastoma Cells. Front. Oncol. 2021, 11, 707366. [Google Scholar] [CrossRef] [PubMed]
- Barnett, S.E.; Herrmann, A.; Shaw, L.; Gash, E.N.; Poptani, H.; Sacco, J.J.; Coulson, J.M. The Chick Embryo Xenograft Model for Malignant Pleural Mesothelioma: A Cost and Time Efficient 3Rs Model for Drug Target Evaluation. Cancers 2022, 14, 5836. [Google Scholar] [CrossRef] [PubMed]
- Vu, B.T.; Shahin, S.A.; Croissant, J.; Fatieiev, Y.; Matsumoto, K.; Le-Hoang Doan, T.; Yik, T.; Simargi, S.; Conteras, A.; Ratliff, L.; et al. Chick Chorioallantoic Membrane Assay as an In Vivo Model to Study the Effect of Nanoparticle-Based Anticancer Drugs in Ovarian Cancer. Sci. Rep. 2018, 8, 8524. [Google Scholar] [CrossRef]
- Schneider-Stock, R.; Flügen, G. Editorial for Special Issue: The Chorioallantoic Membrane (CAM) Model—Traditional and State-of-the Art Applications: The 1st International CAM Conference. Cancers 2023, 15, 772. [Google Scholar] [CrossRef] [PubMed]
- Mangieri, D.; Nico, B.; Benagiano, V.; De Giorgis, M.; Vacca, A.; Ribatti, D. Angiogenic Activity of Multiple Myeloma Endothelial Cells In Vivo in the Chick Embryo Chorioallantoic Membrane Assayis Associated to a Down-Regulation in the Expression of Endogenous Endostatin. J. Cell. Mol. Med. 2008, 12, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.M.; Cheresh, D.A. Tumor Angiogenesis: Molecular Pathways and Therapeutic Targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer Review Evolve Progressively from Normalcy via a Series of Pre. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Demcisakova, Z.; Luptakova, L.; Tirpakova, Z.; Kvasilova, A.; Medvecky, L.; De Spiegelaere, W.; Petrovova, E. Evaluation of Angiogenesis in an Acellular Porous Biomaterial Based on Polyhydroxybutyrate and Chitosan Using the Chicken Ex Ovo Chorioallantoic Membrane Model. Cancers 2022, 14, 4194. [Google Scholar] [CrossRef]
- Jilani, S.M.; Murphy, T.J.; Thai, S.N.M.; Eichmann, A.; Alva, J.A.; Luisa Iruela-Arispe, M. Selective Binding of Lectins to Embryonic Chicken Vasculature. J. Histochem. Cytochem. 2003, 51, 597–604. [Google Scholar] [CrossRef]
- Hagedorn, M.; Balke, M.; Schmidt, A.; Bloch, W.; Kurz, H.; Javerzat, S.; Rousseau, B.; Wilting, J.; Bikfalvi, A. VEGF Coordinates Interaction of Pericytes and Endothelial Cells During Vasculogenesis and Experimental Angiogenesis. Dev. Dyn. 2004, 230, 23–33. [Google Scholar] [CrossRef]
- Marinaccio, C.; Nico, B.; Ribatti, D. Differential Expression of Angiogenic and Anti-Angiogenic Molecules in the Chick Embryo Chorioallantoic Membrane and Selected Organs during Embryonic Development. Int. J. Dev. Biol. 2013, 57, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Alessandri, G.; Baronio, M.; Raffaghello, L.; Cosimo, E.; Marimpietri, D.; Montaldo, P.G.; De Falco, G.; Caruso, A.; Vacca, A.; et al. Inhibition of Neuroblastoma-Induced Angiogenesis by Fenretinide. Int. J. Cancer 2001, 94, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Javanmardi, S.; Aghamaali, M.; Abolmaali, S.; Mohammadi, S.; Tamaddon, A.M. MiR-21, An Oncogenic Target MiRNA for Cancer Therapy: Molecular Mechanisms and Recent Advancements in Chemo and Radio-Resistance. Curr. Gene Ther. 2017, 16, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S.; Subramanian, R.; Saravanan, S.; Arumugam, B.; Anuradha, D. MicroRNA-432-5p Regulates Sprouting and Intussusceptive Angiogenesis in Osteosarcoma Microenvironment by Targeting PDGFB. Lab. Investig. 2021, 101, 1011–1025. [Google Scholar] [CrossRef]
- Ganesh, S.; Iyer, A.K.; Weiler, J.; Morrissey, D.V.; Amiji, M.M. Combination of SiRNA-Directed Gene Silencing with Cisplatin Reverses Drug Resistance in Human Non-Small Cell Lung Cancer. Mol. Ther. Nucleic Acids 2013, 2, e110. [Google Scholar] [CrossRef]
- Chan, J.K.; Blansit, K.; Kiet, T.; Sherman, A.; Wong, G.; Earle, C.; Bourguignon, L.Y.W. The Inhibition of MiR-21 Promotes Apoptosis and Chemosensitivity in Ovarian Cancer. Gynecol. Oncol. 2014, 132, 739–744. [Google Scholar] [CrossRef]
- Javanmardi, S.; Abolmaali, S.S.; Mehrabanpour, M.J.; Aghamaali, M.R.; Tamaddon, A.M. PEGylated Nanohydrogels Delivering Anti-MicroRNA-21 Suppress Ovarian Tumor-Associated Angiogenesis in Matrigel and Chicken Chorioallantoic Membrane Models. BioImpacts 2022, 12, 449–461. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, F.; Wang, B.; Li, H.; Xu, Y.; Liu, X.; Shi, L.; Lu, X.; Xu, W.; Lu, L.; et al. STAT3-Regulated Exosomal MiR-21 Promotes Angiogenesis and Is Involved in Neoplastic Processes of Transformed Human Bronchial Epithelial Cells. Cancer Lett. 2016, 370, 125–135. [Google Scholar] [CrossRef]
- Tome, Y.; Kimura, H.; Kiyuna, T.; Sugimoto, N.; Tsuchiya, H.; Kanaya, F.; Bouvet, M.; Hoffman, R.M. Disintegrin Targeting of an α v β 3 Integrin-over-Expressing High-Metastatic Human Osteosarcoma with Echistatin Inhibits Cell Proliferation, Migration, Invasion and Adhesion in Vitro. Oncotarget 2016, 7, 46315–46320. [Google Scholar] [CrossRef]
- Maacha, S.; Saule, S. Evaluation of Tumor Cell Invasiveness in Vivo: The Chick Chorioallantoic Membrane Assay. In Methods in Molecular Biology—Chapter 8; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1749, pp. 71–77. [Google Scholar]
- Shioda, T.; Munn, L.L.; Fenner, M.H.; Jain, R.K.; Isselbacher, K.J. Early Events of Metastasis in the Microcirculation Involve Changes in Gene Expression of Cancer Cells Tracking MRNA Levels of Metastasizing Cancer Cells in the Chick Embryo Chorioallantoic Membrane. Am. J. Pathol. 1997, 150, 2099. [Google Scholar]
- Cecilia Subauste, M.; Kupriyanova, T.A.; Conn, E.M.; Ardi, V.C.; Quigley, J.P.; Deryugina, E.I. Evaluation of Metastatic and Angiogenic Potentials of Human Colon Carcinoma Cells in Chick Embryo Model Systems. Clin. Exp. Metastasis 2009, 26, 1033–1047. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Zijlstra, A.; Partridge, J.J.; Kupriyanova, T.A.; Madsen, M.A.; Papagiannakopoulos, T.; Quigley, J.P. Unexpected Effect of Matrix Metalloproteinase Down-Regulation on Vascular Intravasation and Metastasis of Human Fibrosarcoma Cells Selected In Vivo for High Rates of Dissemination. Cancer Res. 2005, 65, 10959–10969. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Chick Embryo Chorioallantoic Membrane Model Systems to Study and Visualize Human Tumor Cell Metastasis. Histochem. Cell Biol. 2008, 130, 1119–1130. [Google Scholar] [CrossRef]
- Mira, E.; Ana Lacalle, R.; Gómez-Moutón, C.; Leonardo, E.; Mañes, S. Quantitative Determination of Tumor Cell Intravasation in a Real-Time Polymerase Chain Reaction-Based Assay. Clin. Exp. Metastasis 2002, 19, 313–318. [Google Scholar] [CrossRef]
- Zijlstra, A.; Mellor, R.; Panzarella, G.; Aimes, R.; Hooper, J.; Marchenko, N.; Quigley, J. A Quantitative Analysis of Rate-Limiting Steps in the Metastatic Cascade Using Human-Specific Real-Time Polymerase Chain Reaction. Cancer Res. 2002, 62, 7083–7092. [Google Scholar]
- Augustine, R.; Alhussain, H.; Hasan, A.; Ahmed, M.B.; Yalcin, H.C.; Al Moustafa, A.E. A Novel in Ovo Model to Study Cancer Metastasis Using Chicken Embryos and GFP Expressing Cancer Cells. Bosn J. Basic Med. Sci. 2020, 20, 140–148. [Google Scholar] [CrossRef]
- Schneider, T.; Osl, F.; Friess, T.; Stockinger, H.; Scheuer, W. V Quantification of Human Alu Sequences by Real-Time PCR-an Improved Method to Measure Therapeutic Efficacy of Anti-Metastatic Drugs in Human Xenotransplants. Clin. Exp. Metastasis 2002, 19, 571–582. [Google Scholar] [CrossRef]
- Kim, J.; Yu, W.; Kovalski, K.; Ossowski, L. Requirement for Specific Proteases in Cancer Cell Intravasation as Revealed by a Novel Semiquantitative PCR-Based Assay. Cell 1998, 94, 353–362. [Google Scholar] [CrossRef]
- Kobayashi, T.; Koshida, K.; Endo, Y.; Imao, T.; Uchibayashi, T.; Sasaki, T.; Namiki, M. Basic Science A Chick Embryo Model for Metastatic Human Prostate Cancer. Eur. Urol. 1998, 34, 154–160. [Google Scholar] [CrossRef]
- Komatsu, A.; Matsumoto, K.; Saito, T.; Muto, M.; Tamanoi, F. Patient Derived Chicken Egg Tumor Model (PDcE Model): Current Status and Critical Issues. Cells 2019, 8, 440. [Google Scholar] [CrossRef]
- Marcion, G.; Hermetet, F.; Neiers, F.; Uyanik, B.; Dondaine, L.; Dias, A.M.M.; Da Costa, L.; Moreau, M.; Bellaye, P.S.; Collin, B.; et al. Nanofitins Targeting Heat Shock Protein 110: An Innovative Immunotherapeutic Modality in Cancer. Int. J. Cancer 2021, 148, 3019–3031. [Google Scholar] [CrossRef]
- Skowron, M.A.; Sathe, A.; Romano, A.; Hoffmann, M.J.; Schulz, W.A.; van Koeveringe, G.A.; Albers, P.; Nawroth, R.; Niegisch, G. Applying the Chicken Embryo Chorioallantoic Membrane Assay to Study Treatment Approaches in Urothelial Carcinoma. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 544.e11–544.e23. [Google Scholar] [CrossRef] [PubMed]
- Swadi, R.; Mather, G.; Pizer, B.L.; Losty, P.D.; See, V.; Moss, D. Optimising the Chick Chorioallantoic Membrane Xenograft Model of Neuroblastoma for Drug Delivery. BMC Cancer 2018, 18, 28. [Google Scholar] [CrossRef]
- Eckrich, J.; Kugler, P.; Buhr, C.R.; Ernst, B.P.; Mendler, S.; Baumgart, J.; Brieger, J.; Wiesmann, N. Monitoring of Tumor Growth and Vascularization with Repetitive Ultrasonography in the Chicken Chorioallantoic-Membrane-Assay. Sci. Rep. 2020, 10, 18585. [Google Scholar] [CrossRef]
- Gilson, P.; Couvet, M.; Vanwonterghem, L.; Henry, M.; Vollaire, J.; Baulin, V.; Werner, M.; Orlowska, A.; Josserand, V.; Mahuteau-Betzer, F. The Pyrrolopyrimidine Colchicine-Binding Site Agent PP-13 Reduces the Metastatic Dissemination of Invasive Cancer Cells In Vitro and In Vivo. Biochem. Pharmacol. 2019, 160, 1–13. [Google Scholar] [CrossRef]
- Kleibeuker, E.A.; ten Hooven, M.A.; Castricum, K.C.; Honeywell, R.; Griffioen, A.W.; Verheul, H.M.; Slotman, B.J.; Thijssen, V.L. Optimal Treatment Scheduling of Ionizing Radiation and Sunitinib Improves the Antitumor Activity and Allows Dose Reduction. Cancer Med. 2015, 4, 1003–1015. [Google Scholar] [CrossRef]
- Marimpietri, D.; Brignole, C.; Nico, B.; Pastorino, F.; Pezzolo, A.; Piccardi, F.; Cilli, M.; Di Paolo, D.; Pagnan, G.; Longo, L.; et al. Combined Therapeutic Effects of Vinblastine and Rapamycin on Human Neuroblastoma Growth, Apoptosis, and Angiogenesis. Clin. Cancer Res. 2007, 13, 3977–3988. [Google Scholar] [CrossRef]
- Marimpietri, D.; Nico, B.; Vacca, A.; Mangieri, D.; Catarsi, P.; Ponzoni, M.; Ribatti, D. Synergistic Inhibition of Human Neuroblastoma-Related Angiogenesis by Vinblastine and Rapamycin. Oncogene 2005, 24, 6785–6795. [Google Scholar] [CrossRef]
- Ademii, H.; Shinde, D.A.; Gassmann, M.; Gerst, D.; Chaachouay, H.; Vogel, J.; Gorr, T.A. Targeting Neovascularization and Respiration of Tumor Grafts Grown on Chick Embryo Chorioallantoic Membranes. PLoS ONE 2021, 16, e0251765. [Google Scholar] [CrossRef]
- Katrancioglu, N.; Karahan, O.; Kilic, A.T.; Altun, A.; Katrancioglu, O.; Polat, Z.A. The Antiangiogenic Effects of Levosimendan in a CAM Assay. Microvasc. Res. 2012, 83, 263–266. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R. A Decade of Progress in Tissue Engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef]
- Donderwinkel, I.; Tuan, R.S.; Cameron, N.R.; Frith, J.E. Tendon Tissue Engineering: Current Progress towards an Optimized Tenogenic Differentiation Protocol for Human Stem Cells. Acta Biomater. 2022, 145, 25–42. [Google Scholar] [CrossRef]
- Gao, J.; Yu, X.; Wang, X.; He, Y.; Ding, J. Biomaterial–Related Cell Microenvironment in Tissue Engineering and Regenerative Medicine. Engineering 2022, 13, 31–45. [Google Scholar] [CrossRef]
- Wang, J.; Huang, D.; Yu, H.; Cheng, Y.; Ren, H.; Zhao, Y. Developing Tissue Engineering Strategies for Liver Regeneration. Eng. Regen. 2022, 3, 80–91. [Google Scholar] [CrossRef]
- Sainsbury, E.; Amaral, R.d.; Blayney, A.W.; Walsh, R.M.C.; O’Brien, F.J.; O’Leary, C. Tissue Engineering and Regenerative Medicine Strategies for the Repair of Tympanic Membrane Perforations. Biomater. Biosyst. 2022, 6, 100046. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, Y.; Hu, Y.; Zou, L.; Chen, J. New Perspectives: In-Situ Tissue Engineering for Bone Repair Scaffold. Compos. B Eng. 2020, 202, 108445. [Google Scholar] [CrossRef]
- Arjunan, A.; Baroutaji, A.; Robinson, J.; Wang, C. Tissue Engineering Concept. In Encyclopedia of Smart Materials; Olabi, A.-G., Ed.; Elsevier: Oxford, UK, 2022; pp. 103–112. ISBN 978-0-12-815733-6. [Google Scholar]
- Blume, C.; Kraus, X.; Heene, S.; Loewner, S.; Stanislawski, N.; Cholewa, F.; Blume, H. Vascular Implants—New Aspects for in Situ Tissue Engineering. Eng. Life Sci. 2022, 22, 344–360. [Google Scholar] [CrossRef]
- Ding, T.; Kang, W.; Li, J.; Yu, L.; Ge, S. An In Situ Tissue Engineering Scaffold with Growth Factors Combining Angiogenesis and Osteoimmunomodulatory Functions for Advanced Periodontal Bone Regeneration. J. Nanobiotechnol. 2021, 19, 247. [Google Scholar] [CrossRef]
- Fu, L.; Li, P.; Li, H.; Gao, C.; Yang, Z.; Zhao, T.; Chen, W.; Liao, Z.; Peng, Y.; Cao, F.; et al. The Application of Bioreactors for Cartilage Tissue Engineering: Advances, Limitations, and Future Perspectives. Stem Cells Int. 2021, 2021, 6621806. [Google Scholar] [CrossRef]
- Radisic, M.; Marsano, A.; Maidhof, R.; Wang, Y.; Vunjak-Novakovic, G. Cardiac Tissue Engineering Using Perfusion Bioreactor Systems. Nat. Protoc. 2008, 3, 719–738. [Google Scholar] [CrossRef]
- Todros, S.; Spadoni, S.; Maghin, E.; Piccoli, M.; Pavan, P.G. A Novel Bioreactor for the Mechanical Stimulation of Clinically Relevant Scaffolds for Muscle Tissue Engineering Purposes. Processes 2021, 9, 474. [Google Scholar] [CrossRef]
- Montorsi, M.; Genchi, G.G.; De Pasquale, D.; De Simoni, G.; Sinibaldi, E.; Ciofani, G. Design, Fabrication, and Characterization of a Multimodal Reconfigurable Bioreactor for Bone Tissue Engineering. Biotechnol. Bioeng. 2022, 119, 1965–1979. [Google Scholar] [CrossRef]
- Sun, T.; Meng, C.; Ding, Q.; Yu, K.; Zhang, X.; Zhang, W.; Tian, W.; Zhang, Q.; Guo, X.; Wu, B.; et al. In Situ Bone Regeneration with Sequential Delivery of Aptamer and BMP2 from an ECM-Based Scaffold Fabricated by Cryogenic Free-Form Extrusion. Bioact. Mater. 2021, 6, 4163–4175. [Google Scholar] [CrossRef]
- Poudel, B.K.; Robert, M.C.; Simpson, F.C.; Malhotra, K.; Jacques, L.; Labarre, P.; Griffith, M. In Situ Tissue Regeneration in the Cornea from Bench to Bedside. Cells Tissues Organs 2021, 211, 506–526. [Google Scholar] [CrossRef]
- Periayah, M.H.; Halim, A.S.; Saad, A.Z.M. Chitosan: A Promising Marine Polysaccharide for Biomedical Research. Pharmacogn. Rev. 2016, 10, 39–42. [Google Scholar] [CrossRef]
- Pavlovic, M. Bioengineering—A Conceptual Approach; Springer: Berlin/Heidelberg, Germany; Florida Atlantic University: Boca Raton, FL, USA, 2015. [Google Scholar]
- Kohli, N.; Sharma, V.; Orera, A.; Sawadkar, P.; Owji, N.; Frost, O.G.; Bailey, R.J.; Snow, M.; Knowles, J.C.; Blunn, G.W.; et al. Pro-Angiogenic and Osteogenic Composite Scaffolds of Fibrin, Alginate and Calcium Phosphate for Bone Tissue Engineering. J. Tissue Eng. 2021, 12, 20417314211005610. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef]
- AL-Hamoudi, F.; Rehman, H.U.; Almoshawah, Y.A.; Talari, A.C.S.; Chaudhry, A.A.; Reilly, G.C.; Rehman, I.U. Bioactive Composite for Orbital Floor Repair and Regeneration. Int. J. Mol. Sci. 2022, 23, 333. [Google Scholar] [CrossRef]
- Ribatti, D.; Annese, T.; Tamma, R. The Use of the Chick Embryo CAM Assay in the Study of Angiogenic Activiy of Biomaterials. Microvasc. Res. 2020, 131, 104026. [Google Scholar] [CrossRef]
- Mishra, R.; Roux, B.M.; Posukonis, M.; Bodamer, E.; Brey, E.M.; Fisher, J.P.; Dean, D. Effect of Prevascularization on In Vivo Vascularization of Poly(Propylene Fumarate)/Fibrin Scaffolds. Biomaterials 2016, 77, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Ignjatovic, N.; Ajdukovic, Z.; Uskokovic, D. New Biocomposite [Biphasic Calcium Phosphate/Poly-DL-Lactide-Co-Glycolide/Biostimulative Agent] Filler for Reconstruction of Bone Tissue Changed by Osteoporosis. J. Mater. Sci. Mater. Med. 2005, 16, 621–626. [Google Scholar] [CrossRef]
- Wittmann, K.; Storck, K.; Muhr, C.; Mayer, H.; Regn, S.; Staudenmaier, R.; Wiese, H.; Maier, G.; Bauer-Kreisel, P.; Blunk, T. Development of Volume-Stable Adipose Tissue Constructs Using Polycaprolactone-Based Polyurethane Scaffolds and Fibrin Hydrogels. J. Tissue Eng. Regen. Med. 2016, 10, E409–E418. [Google Scholar] [CrossRef]
- Schagemann, J.C.; Chung, H.W.; Mrosek, E.H.; Stone, J.J.; Fitzsimmons, J.S.; O’Driscoll, S.W.; Reinholz, G.G. Poly-ε-Caprolactone/Gel Hybrid Scaffolds for Cartilage Tissue Engineering. J. Biomed. Mater. Res. A 2010, 93, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Panadero, J.A.; Vikingsson, L.; Gomez Ribelles, J.L.; Sencadas, V.; Lanceros-Mendez, S. Fatigue Prediction in Fibrin Poly-ε-Caprolactone Macroporous Scaffolds. J. Mech. Behav. Biomed. Mater. 2013, 28, 55–61. [Google Scholar] [CrossRef]
- Fu, S.Z.; Ni, P.Y.; Wang, B.Y.; Chu, B.Y.; Zheng, L.; Luo, F.; Luo, J.C.; Qian, Z.Y. Injectable and Thermo-Sensitive PEG-PCL-PEG Copolymer/Collagen/n-HA Hydrogel Composite for Guided Bone Regeneration. Biomaterials 2012, 33, 4801–4809. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Kocak, F.Z.; Talari, A.C.S.; Yar, M.; Rehman, I.U. In-Situ Forming Ph and Thermosensitive Injectable Hydrogels to Stimulate Angiogenesis: Potential Candidates for Fast Bone Regeneration Applications. Int. J. Mol. Sci. 2020, 21, 1633. [Google Scholar] [CrossRef]
- Conde-González, A.; Glinka, M.; Dutta, D.; Wallace, R.; Callanan, A.; Oreffo, R.O.C.; Bradley, M. Rapid Fabrication and Screening of Tailored Functional 3D Biomaterials: Validation in Bone Tissue Repair—Part II. Biomater. Adv. 2023, 145, 213250. [Google Scholar] [CrossRef] [PubMed]
- Okesola, B.O.; Mendoza-Martinez, A.K.; Cidonio, G.; Derkus, B.; Boccorh, D.K.; Osuna De La Peña, D.; Elsharkawy, S.; Wu, Y.; Dawson, J.I.; Wark, A.W.; et al. De Novo Design of Functional Coassembling Organic-Inorganic Hydrogels for Hierarchical Mineralization and Neovascularization. ACS Nano 2021, 15, 11202–11217. [Google Scholar] [CrossRef] [PubMed]
- Müller-Heupt, L.K.; Wiesmann-Imilowski, N.; Schröder, S.; Groß, J.; Ziskoven, P.C.; Bani, P.; Kämmerer, P.W.; Schiegnitz, E.; Eckelt, A.; Eckelt, J.; et al. Oxygen-Releasing Hyaluronic Acid-Based Dispersion with Controlled Oxygen Delivery for Enhanced Periodontal Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 5936. [Google Scholar] [CrossRef] [PubMed]
- Mesa, F.L.; Aneiros, J.; Cabrera, A.; Bravo, M.; Caballero, T.; Revelles, F.; Del Moral, R.G.; O’Valle, F.J. Antiproliferative Effect of Topic Hyaluronic Acid Gel. Study in Gingival Biopsies of Patients with Periodontal Disease. Histol. Histopathol. 2002, 17, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.; Renatus, A.; Heinicke, M.; Pfister, W.; Stratul, S.-I.; Jentsch, H. Hyaluronic Acid as an Adjunct After Scaling and Root Planing: A Prospective Randomized Clinical Trial. J. Periodontol. 2013, 84, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Decker, S.; Arango-Ospina, M.; Rehder, F.; Moghaddam, A.; Simon, R.; Merle, C.; Renkawitz, T.; Boccaccini, A.R.; Westhauser, F. In Vitro and in Ovo Impact of the Ionic Dissolution Products of Boron-Doped Bioactive Silicate Glasses on Cell Viability, Osteogenesis and Angiogenesis. Sci. Rep. 2022, 12, 8510. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.X.; Li, Y.M.; Han, D. Co-Substitution of Carbonate and Fluoride in Hydroxyapatite: Effect on Substitution Type and Content. Front. Mater. Sci. 2015, 9, 192–198. [Google Scholar] [CrossRef]
- Barry, A.B.; Zhuang, H.; Baig, A.A.; Higuchi, W.I. Effect of Fluoride Pretreatment on the Solubility of Synthetic Carbonated Apatite. Calcif. Tissue Int. 2003, 72, 236–242. [Google Scholar] [CrossRef]
- Verma, G.; Barick, K.C.; Shetake, N.G.; Pandey, B.N.; Hassan, P.A. Citrate-Functionalized Hydroxyapatite Nanoparticles for PH-Responsive Drug Delivery. RSC Adv. 2016, 6, 77968–77976. [Google Scholar] [CrossRef]
- Khan, A.S.; Aamer, S.; Chaudhry, A.A.; Wong, F.S.L.; Rehman, I.U. Synthesis and Characterizations of a Fluoride-Releasing Dental Restorative Material. Mater. Sci. Eng. C 2013, 33, 3458–3464. [Google Scholar] [CrossRef]
- Da Mota, M.; De, V.; Branco, A. Polyurethane-Based Scaffolds for Bone Tissue Regeneration; Instituto Superior Técnico: Lisbon, Portugal, 2015. [Google Scholar]
- Bongio, M.; Lopa, S.; Gilardi, M.; Bersini, S.; Moretti, M. A 3D Vascularized Bone Remodeling Model Combining Osteoblasts and Osteoclasts in a CaP Nanoparticle-Enriched Matrix. Nanomedicine 2016, 11, 1073–1091. [Google Scholar] [CrossRef]
- Tadic, D.; Epple, M. A Thorough Physicochemical Characterisation of 14 Calcium Phosphate-Based Bone Substitution Materials in Comparison to Natural Bone. Biomaterials 2004, 25, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Vishnubalaji, R.; Atteya, M.; Al-Nbaheen, M.; Oreffo, R.O.C.; Aldahmash, A.; Alajez, N.M. Angio-genic Potential of Human Neonatal Foreskin Stromal Cells in the Chick Embryo Chorioallantoic Membrane Model. Stem Cells Int. 2015, 2015, 257019. [Google Scholar] [CrossRef]
- Black, C.; Kanczler, J.M.; de Andrés, M.C.; White, L.J.; Savi, F.M.; Bas, O.; Saifzadeh, S.; Henkel, J.; Zannettino, A.; Gronthos, S.; et al. Characterisation and Evaluation of the Regenerative Capacity of Stro-4+ Enriched Bone Marrow Mesenchymal Stromal Cells Using Bovine Extracellular Matrix Hydrogel and a Novel Biocompatible Melt Electro-Written Medical-Grade Polycaprolactone Scaf-fold. Biomaterials 2020, 247, 119998. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M.M.; Simmerman, L.A.; Reed, G.L.; Sharkey, N.A.; Taylor, A.F. Biomimetic Bone Mechanotransduction Modeling in Neonatal Rat Femur Organ Cultures: Structural Verification of Proof of Concept. Biomech. Model. Mechanobiol. 2010, 9, 539–550. [Google Scholar] [CrossRef]
- Aldahmash, A.; Vishnubalaji, R. Transplantation of Human Neonatal Foreskin Stromal Cells in Ex Vivo Organotypic Cultures of Embryonic Chick Femurs. Saudi J. Biol. Sci. 2017, 24, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.M.; Kanczler, J.M.; Oreffo, R.O.C. Evolving Applications of the Egg: Chorioallantoic Membrane Assay and Ex Vivo Organotypic Culture of Materials for Bone Tissue Engineering. J. Tissue Eng. 2020, 11, 2041731420942734. [Google Scholar] [CrossRef]
- Feder, A.-L.; Pion, E.; Troebs, J.; Lenze, U.; Prantl, L.; Htwe, M.M.; Phyo, A.; Haerteis, S.; Aung, T. Extended Analysis of Intratumoral Heterogeneity of Primary Osteosarcoma Tissue Using 3D- in-Vivo-Tumor-Model. Clin. Hemorheol. Microcirc. 2020, 76, 133–141. [Google Scholar] [CrossRef]
- Kanczler, J.M.; Smith, E.L.; Roberts, C.A.; Oreffo, R.O.C. A Novel Approach for Studying the Temporal Modulation of Embryonic Skeletal Development Using Organotypic Bone Cultures and Microcomputed Tomography. Tissue Eng. Part C Methods 2012, 18, 747–760. [Google Scholar] [CrossRef]
- Smith, E.; Kanczler, J.; Oreffo, R.O. A New Take on an Old Story: Chick Limb Organ Culture for Skeletal Niche Development and Regenerative Medicine Evaluation. Eur. Cell Mater. 2013, 11, 91–106. [Google Scholar] [CrossRef]
- Smith, E.L.; Rashidi, H.; Kanczler, J.M.; Shakesheff, K.M.; Oreffo, R.O.C. The Effects of 1α, 25-Dihydroxyvitamin D3 and Transforming Growth Factor-Β3 on Bone Development in an Ex Vivo Organotypic Culture System of Embryonic Chick Femora. PLoS ONE 2015, 10, e0121653. [Google Scholar] [CrossRef] [PubMed]
- Kaisto, S.; Saarela, U.; Dönges, L.; Raykhel, I.; Skovorodkin, I.; Vainio, S.J. Optimization of Renal Organoid and Organotypic Culture for Vascularization, Extended Development, and Improved Microscopy Imaging. J. Vis. Exp. 2020, 2020, e60995. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, C.; Sisi, F.; Checchi, M. CAM Model: Intriguing Natural Bioreactor for Sustainable Research and Reliable/Versatile Testing. Biology 2023, 12, 1219. https://doi.org/10.3390/biology12091219
Palumbo C, Sisi F, Checchi M. CAM Model: Intriguing Natural Bioreactor for Sustainable Research and Reliable/Versatile Testing. Biology. 2023; 12(9):1219. https://doi.org/10.3390/biology12091219
Chicago/Turabian StylePalumbo, Carla, Federica Sisi, and Marta Checchi. 2023. "CAM Model: Intriguing Natural Bioreactor for Sustainable Research and Reliable/Versatile Testing" Biology 12, no. 9: 1219. https://doi.org/10.3390/biology12091219
APA StylePalumbo, C., Sisi, F., & Checchi, M. (2023). CAM Model: Intriguing Natural Bioreactor for Sustainable Research and Reliable/Versatile Testing. Biology, 12(9), 1219. https://doi.org/10.3390/biology12091219