Matrix Metalloproteinases and the Pathogenesis of Recurrent Corneal Erosions and Epithelial Basement Membrane Dystrophy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Matrix Metalloproteinases—Structure, Nomenclature, Activation, and Inhibition
2.1. MMPs—Structure and Nomenclature
2.2. MMP Activation
2.3. MMP Inhibitors
3. The Process of Corneal Healing—Involvement of MMPs
4. The Role of MMPs in the Pathogenesis of Recurrent Corneal Erosions and Epithelial Basement Membrane Dystrophy
4.1. Epithelial Basement Membrane Dystrophy—Epidemiology, Clinical Manifestations, and Molecular Background
4.2. Recurrent Corneal Erosions—Etiology, Epidemiology, and Pathogenesis
4.3. MMPs and the Pathogenesis of Recurrent Corneal Erosions
4.4. The Role of MMPs in the Pathogenesis of Epithelial Basement Membrane Dystrophy
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gross, J.; Lapiere, C.M. Collagenolytic Activity In Amphibian Tissues: A Tissue Culture Assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Birkedal-Hansen, H.; Werb, Z.; Welgus, H.G.; Van Wart, H.E. Matrix Metalloproteinase and Inhibitors. In Proceedings of the Matrix Metalloproteinase Conference, Sandestin Beach, FL, USA, 11–15 September 1989; G Fisher: Portland, OR, USA, 1992. [Google Scholar]
- Nagase, H.; Barrett, A.J.; Woessner, J.F., Jr. Nomenclature and glossary of the matrix metalloproteinases. Matrix Suppl. 1992, 1, 421–424. [Google Scholar] [PubMed]
- Kolaczkowska, E. Metaloproteinaza 9 (MMP-9) jako szczególny przedstawiciel metaloproteinaz macierzy zewnątrzkomórkowej: Rola w napływie i apoptozie neutrofili w trakcie reakcji zapalnej. Postępy Biol. Komórki 2010, 37, 471–499. [Google Scholar]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Chernov, A.V.; Sounni, N.E.; Remacle, A.G.; Strongin, A.Y. Epigenetic control of the invasionpromoting MT1-MMP/MMP-2/TIMP-2 axis in cancer cells. J. Biol. Chem. 2009, 284, 12727–12734. [Google Scholar] [CrossRef] [PubMed]
- Collazos, J.; Asensi, V.; Martin, G.; Montes, A.H.; Suárez-Zarracina, T.; Valle-Garay, E. The effect of gender and genetic polymorphisms on matrix metalloprotease (MMP) and tissue inhibitor (TIMP) plasma levels in different infectious and non-infectious conditions. Clin. Exp. Immunol. 2015, 182, 213–219. [Google Scholar] [CrossRef]
- Otero-Estévez, O.; De Chiara, L.; Rodríguez-Girondo, M.; Rodríguez-Berrocal, F.J.; Cubiella, J.; Castro, I.; Hernández, V.; Martínez-Zorzano, V.S. Serum matrix metalloproteinase-9 in colorectal cancer family-risk population screening. Sci. Rep. 2015, 5, 13030. [Google Scholar] [CrossRef]
- Chau, K.Y.; Sivaprasad, S.; Patel, N.; Donaldson, T.A.; Luthert, P.J.; Chong, N.V. Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye 2008, 22, 855–859. [Google Scholar] [CrossRef]
- Pescosolido, N.; Giannotti, R.; Buomprisco, G. Metalloproteinases and eye diseases. Biomed. Aging Pathol. 2013, 3, 97–105. [Google Scholar] [CrossRef]
- Lipka, D.; Boratyński, J.; Metaloproteinazy, M.M.P. Struktura I funkcje. Postępy Hig. Med. Dośw. 2008, 62, 328–336. [Google Scholar]
- Sakimoto, T.; Sawa, M. Metalloproteinases in Corneal Diseases: Degradation and Processing. Cornea 2012, 31, S50–S56. [Google Scholar] [CrossRef]
- Murphy, G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta BBA Mol. Cell Res. 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibi-tors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Sivak, J.M.; Fini, M. MMPs in the eye: Emerging roles for matrix metalloproteinases in ocular physiology. Prog. Retin. Eye Res. 2002, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Tyagi, S.C. Metalloproteinases as mediators of inflammation and the eyes: Molecular genetic underpinnings gov-erning ocular pathophysiology. Int. J. Ophthalmol. 2017, 10, 1308–1318. [Google Scholar] [PubMed]
- Nagase, H. Activation mechanisms of matrix metalloproteinases. Biol. Chem. 1997, 378, 151–160. [Google Scholar]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Hykin, P.G.; Foss, A.E.; Pavesio, C.; Dart, J.K.G. The natural history and management of recurrent corneal erosion: A prospective ran-domized trial. Eye 1994, 8 Pt 1, 35–40. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and bio-chemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Murphy, G.; Knäuper, V. Relating matrix metalloproteinase structure to function: Why the “hemopexin” domain? Matrix Biol. 1997, 15, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Maskos, K. Crystal structures of MMPs in complex with physiological and pharmacological inhibitors. Biochimie 2005, 87, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Tam, E.M.; Moore, T.R.; Butler, G.S.; Overall, C.M. Characterization of the distinct collagen binding and cleavage mechanisms of matrix metalloproteinase 2 and 14 (gelatinase A and MT1-MMP): The differentia roles of the MMP hemopexin C domains and the MMP-2 fibronectin type II modules in collagen triple helicase activities. J. Biol. Chem. 2004, 279, 43336–43344. [Google Scholar] [PubMed]
- Murphy, G. Tailoring TIMPs for Selective Metalloproteinase Inhibition. In The Cancer Degradome; Edwards, D., Hoyer-Hansen, G., Blasi, F., Sloane, B.F., Eds.; Springer Science: New York, NY, USA, 2008; pp. 787–810. [Google Scholar]
- Stetler-Stevenson, W.G. The tumor microenvironment: Regulation by MMP-independent effects of tissue inhibitor of metal-loproteinases-2. Cancer Metastasis Rev. 2008, 27, 57–66. [Google Scholar] [CrossRef]
- Kenney, M.; Chwa, M.; Alba, A.; Saghizadeh, M.; Huang, Z.-S.; Brown, D. Localization of TIMP-1, TIMP-2, TIMP-3, gelatinase A and gelatinase B in pathological human corneas. Curr. Eye Res. 1998, 17, 238–246. [Google Scholar] [CrossRef]
- Mobaraki, M.; Abbasi, R.; Omidian Vandchali, S.; Ghaffari, M.; Moztarzadeh, F.; Mozafari, M. Corneal Repair and Regeneration: Current Concepts and Future Directions. Front. Bioeng. Biotechnol. 2019, 7, 135. [Google Scholar] [CrossRef]
- Wilson, S.E. Corneal wound healing. Exp. Eye Res. 2020, 197, 108089. [Google Scholar] [CrossRef]
- Wilson, S.E.; Mohan, R.R.; Mohan, R.R.; Ambrosio, R., Jr.; Hong, J.; Lee, J. The Corneal Wound Healing Response: Cytokine-mediated Interaction of the Epi-thelium, Stroma, and Inflammatory Cells. Prog. Retin. Eye Res. 2001, 20, 625–637. [Google Scholar] [CrossRef]
- Dawson, D.G. Corneal Scars; Dartt, D.A., Ed.; Encyclopedia of the Eye; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; pp. 499–514. [Google Scholar]
- Wilson, S.E.; Schultz, G.S.; Chegini, N.; Weng, J.; He, Y.G. Epidermal Growth Factor, Transforming Growth Factor Alpha, Transforming Growth Factor Beta, Acidic Fibroblast Growth Factor, Basic Fibroblast Growth Factor, and Interleukin-1 Proteins in the Cornea. Exp. Eye Res. 1994, 59, 63–72. [Google Scholar] [CrossRef]
- Mohan, R.R.; Hutcheon, A.E.; Choi, R.; Hong, J.; Lee, J.; Mohan, R.R.; Ambrósio, R.; Zieske, J.D.; Wilson, S.E. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp. Eye Res. 2003, 76, 71–87. [Google Scholar] [CrossRef]
- Lassance, L.; Marino, G.K.; Medeiros, C.S.; Thangavadivel, S.; Wilson, S.E. Fibrocyte migration, differentiation and apoptosis during the corneal wound healing response to injury. Exp. Eye Res. 2018, 170, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. Everett Kinsey Lecture. Keratocyte apoptosis in refractive surgery. CLAO J. 1998, 24, 181–185. [Google Scholar] [PubMed]
- Wilson, S.E.; He, Y.G.; Weng, J.; Li, Q.; McDOWALL, A.W.; Vital, M.; Chwang, E.L. Epithelial injury induces keratocyte apoptosis: Hypothesized role for the interleukin-1 sys-tem in the modulation of corneal tissue organization. Exp. Eye Res. 1996, 62, 325–338. [Google Scholar] [CrossRef]
- Weng, J.; Mohan, R.R.; Li, Q.; E Wilson, S. IL-1 upregulates keratinocyte growth factor and hepatocyte growth factor mRNA and protein production by cultured stromal fibroblast cells: Interleukin-1 beta expression in the cornea. Cornea 1997, 16, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E.; Lloyd, S.A.; He, Y.-G. Glucocorticoid Receptor and Interleukin-1 Receptor Messenger RNA Expression in Corneal Cells. Cornea 1994, 13, 4–8. [Google Scholar] [CrossRef]
- Bureau, J.; Fabre, E.J.; Hecquet, C.; Pouliquen, Y.; Lorans, G. Modification of prostiglandin E2 and collagen synthesis in keratoconus fibroblasts asso-ciated with an increase of interleukin-1 alpha receptor number. Comptes Rendus Acad. Sci. 1993, 316, 425–430. [Google Scholar]
- Fabre, E.J.; Bureau, J.; Pouliquen, Y.; Lorans, G. Binding sites for human interleukin 1 α, gamma interferon and tumor necrosis factor on cultured fibroblasts of normal cornea and keratoconus. Curr. Eye Res. 1991, 10, 585–592. [Google Scholar] [CrossRef]
- Barbosa, F.L.; Chaurasia, S.; Cutler, A.; Asosingh, K.; Kaur, H.; de Medeiros, F.; Agrawal, V.; Wilson, S.E. Corneal myofibroblast generation from bone marrow-derived cells. Exp. Eye Res. 2010, 91, 92–96. [Google Scholar] [CrossRef]
- Strissel, K.J.; Rinehart, W.B.; Fini, M.E. Regulation of paracrine cytokine balance controlling collagenase synthesis by corneal cells. Investig. Ophthalmol. Vis. Sci. 1997, 38, 546–552. [Google Scholar]
- West-Mays, J.A.; Strissel, K.J.; Sadow, P.M.; Fini, M.E. Competence for collagenase gene expression by tissue fibroblasts requires acti-vation of an interleukin 1 alpha autocrine loop. Proc. Natl. Acad. Sci. USA 1995, 92, 6768–6772. [Google Scholar] [CrossRef]
- Dinarello, C.A. The interleukin-1 family: 10 years of discovery 1. FASEB J. 1994, 8, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E.; He, Y.G.; Weng, J.; Zieske, J.D.; Jester, J.V.; Schultz, G.S. Effect of epidermal growth factor, hepatocyte growth factor and keratinocyte growth factor on proliferation, motility, and differentiation of human corneal epithelial cells. Exp. Eye Res. 1994, 59, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Zieske, J.D.; Guimaraes, S.R.; Hutcheon, A.E.K. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp. Eye Res. 2001, 72, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.R.; Wilson, S.E. Discoidin domain receptor (DDR) 1 and 2: Collagen activated tyrosine kinase receptors in the cornea. Exp. Eye Res. 2001, 72, 87–92. [Google Scholar] [CrossRef]
- Tervo, T.; Vesaluoma, M.; Bennett, G.L.; Schwall, R.; Helena, M.; Liang, Q.; Wilson, S.E. Tear Hepatocyte Growth Factor (HGF) Availability Increases Markedly after Excimer Laser Surface Ablation. Exp. Eye Res. 1997, 64, 501–504. [Google Scholar] [CrossRef]
- Chi, C.; Trinkaus-Randall, V. New insights in wound response and repair of epithelium. J. Cell. Physiol. 2013, 228, 925–929. [Google Scholar] [CrossRef]
- Kim, W.-J.; Mohan, R.R.; Mohan, R.R.; Wilson, S.E. Effect of PDGF, IL-1 alpha, and BMP2/4 on corneal fibroblast chemotaxis: Expression of the platelet-derived growth factor system in the cornea. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1364–1372. [Google Scholar]
- Chaurasia, S.S.; Lim, R.R.; Lakshminarayanan, R.; Mohan, R.R. Nanomedicine Approaches for Corneal Diseases. J. Funct. Biomater. 2015, 6, 277–298. [Google Scholar] [CrossRef]
- Vu, T.H.; Werb, Z. Matrix metalloproteinases: Effectors of development and normal physiology. J. Bone Jt. Surg. 2000, 14, 2123–2133. [Google Scholar] [CrossRef]
- Gearing, A.J.H.; Beckett, P.; Christodoulou, M.; Churchill, M.; Clements, J.D.A.H.; Davidson, A.H.; Woolley, K. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature 1994, 370, 555–557. [Google Scholar] [CrossRef]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Noë, V.; Fingleton, B.; Jacobs, K.; Crawford, H.C.; Vermeulen, S.; Steelant, W.; Bruyneel, E.; Matrisian, L.M.; Mareel, M. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J. Cell Sci. 2001, 114, 111–118. [Google Scholar] [CrossRef]
- Ochieng, J.; Fridman, R.; Nangia-Makker, P.; Kleiner, D.E.; Liotta, L.A.; Stetler-Stevenson, W.G.; Raz, A. Galectin-3 Is a Novel Substrate for Human Matrix Metalloproteinases-2 and -9. Biochemistry 1994, 33, 14109–14114. [Google Scholar] [CrossRef]
- Preece, G.; Murphy, G.; Ager, A. Metalloproteinase-mediated Regulation of L-selectin Levels on Leucocytes. J. Biol. Chem. 1996, 271, 11634–11640. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, M.; Girard, M.T.; Kublin, C.L.; Cintron, C.; Fini, M. Differential roles for two gelatinolytic enzymes of the matrix metalloproteinase family in the remodelling cornea. Dev. Biol. 1991, 147, 425–439. [Google Scholar] [CrossRef]
- Danjo, Y.; Gipson, I.K. Actin ‘purse string’ filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J. Cell Sci. 1998, 111, 3323–3332. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E.; Marino, G.K.; Torricelli, A.A.M.; Medeiros, C.S. Corneal fibrosis: Injury and defective regeneration of the epithelial basement membrane. A paradigm for fibrosis in other organs? Matrix Biol. 2017, 64, 17–26. [Google Scholar] [CrossRef]
- Fini, M.E.; Parks, W.C.; Rinehart, W.B.; Girard, M.T.; Matsubara, M.; Cook, J.R.; West-Mays, J.A.; Sadow, P.M.; Burgeson, R.E.; Jeffrey, J.J.; et al. Role of matrix metalloproteinases in failure to re-epithelialize after corneal injury. Am. J. Pathol. 1996, 149, 1287–1302. [Google Scholar]
- Wilson, S.E.; Medeiros, C.S.; Santhiago, M.R. Pathophysiology of Corneal Scarring in Persistent Epithelial Defects After PRK and Other Corneal Injuries. J. Refract. Surg. 2018, 34, 59–64. [Google Scholar] [CrossRef]
- Girard, M.T.; Matsubara, M.; Kublin, C.; Tessier, M.J.; Cintron, C.; Fini, M.E. Stromal fibroblasts synthesize collagenase and stromelysin during long-term tissue remodeling. J. Cell Sci. 1993, 104, 1001–1011. [Google Scholar] [CrossRef]
- Jester, J.V.; Petroll, W.M.; Barry, P.A.; Cavanagh, H.D. Expression of alpha-smooth muscle (alpha-SM) actin during corneal stromal wound healing. Investig. Ophthalmol. Vis. Sci. 1995, 36, 809–819. [Google Scholar]
- Masur, S.K.; Cheung, J.K.; Antohi, S. Identification of integrins in cultured corneal fibroblasts and in isolated keratocytes. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2690–2698. [Google Scholar]
- Girard, M.T.; Matsubara, M.; Fini, M.E. Transforming growth factor-beta and interleukin-1 modulate metalloproteinase expression by corneal stromal cells. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2441–2454. [Google Scholar]
- Li, D.-Q.; Lokeshwar, B.L.; Solomon, A.; Monroy, D.; Ji, Z.; Pflugfelder, S.C. Regulation of MMP-9 Production by Human Corneal Epithelial Cells. Exp. Eye Res. 2001, 73, 449–459. [Google Scholar] [CrossRef]
- Ko, J.-A.; Yanai, R.; Chikama, T.-I.; Nishida, T. Downregulation of Matrix Metalloproteinase–2 in Corneal Fibroblasts by Interleukin-1 Receptor Antagonist Released from Corneal Epithelial Cells. Investig. Opthalmology Vis. Sci. 2010, 51, 6286–6293. [Google Scholar] [CrossRef]
- Denk, P.O.; Knorr, M. The in vitro effect of platelet derived growth factor isoforms on the proliferation of bovine corneal stromal fibroblasts depends on cel density. Graefe’s Arch. Clin. Exp. Ophthalmol. 1997, 235, 530–534. [Google Scholar] [CrossRef]
- Andresen, J.L.; Ehlers, N. Chemotaxis of human keratocytes is increased by plateletderived growth factor-BB, epidermal growth factor, transforming growth factor-alpha, acidic fibroblast growth factor, insulin-like growth factor-I, and trans-forming growth factorbeta. Curr. Eye Res. 1998, 17, 79–87. [Google Scholar] [CrossRef]
- Kamiyama, K.; Iguchi, I.; Wang, X.; Imanishi, J. Effects of PDGF on the Migration of Rabbit Corneal Fibroblasts and Epithelial Cells. Cornea 1998, 17, 315–325. [Google Scholar] [CrossRef]
- Ye, H.Q.; Azar, D.T. Expression of gelatinases A and B, and TIMPs 1 and 2 during corneal wound healing. Investig. Ophthalmol. Vis. Sci. 1998, 39, 913–921. [Google Scholar]
- Mulholland, B.; Tuft, S.J.; Khaw, P.T. Matrix metalloproteinase distribution during early corneal wound healing. Eye 2005, 19, 584–588. [Google Scholar] [CrossRef]
- Lu, P.C.; Ye, H.; Maeda, M.; Azar, D.T. Immunolocalization and gene expression of matrilysin during corneal wound healing. Investig. Ophthalmol. Vis. Sci. 1999, 40, 20–27. [Google Scholar]
- Ye, H.Q.; Maeda, M.; Yu, F.S.; Azar, D.T. Differential expression of MT1-MMP (MMP-14) and collagenase III (MMP-13) genes in normal and wounded rat corneas. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2894–2899. [Google Scholar]
- Yang, C.; Nguyen, D.D.; Lai, J. Poly(l-Histidine)-Mediated On-Demand Therapeutic Delivery of Roughened Ceria Nanocages for Treatment of Chemical Eye Injury. Adv. Sci. 2023, 10, e2302174. [Google Scholar] [CrossRef] [PubMed]
- Rigal-Sastourne, J.C.; Tixier, J.M.; Renard, J.P.; Maurin, J.F.; Pouliquen, Y.; Legeais, J.M. Corneal burns and matrix metalloproteinases (MMP-2 and -9): The effects of human amniotic membrane transplantation. J. Fr. D’ophtalmologie 2002, 25, 685–693. [Google Scholar]
- Sharma, C.; Dobson, G.P.; Davenport, L.M.; Morris, J.L.; Letson, H.L. The role of matrix metalloproteinase-9 and its inhibitor TIMP-1 in burn injury: A systematic review. Int. J. Burn. Trauma 2021, 11, 275–288. [Google Scholar]
- Mitchell, B.M.; Wu, T.G.; Chong, E.-M.; Pate, J.C.; Wilhelmus, K.R. Expression of Matrix Metalloproteinases 2 and 9 in Experimental Corneal Injury and Fungal Keratitis. Cornea 2007, 26, 589–593. [Google Scholar] [CrossRef]
- Singh, A.; Maurya, O.P.S.; Jagannadhan, M.; Patel, A. Matrix metalloproteinases (MMP-2 and MMP-9) activity in corneal ulcer and ocular surface disorders determined by gelatin zymography. J. Ocul. Biol. Dis. Informatics 2012, 5, 31–35. [Google Scholar] [CrossRef]
- García-López, C.; Rodríguez-Calvo-De-Mora, M.; Borroni, D.; Sánchez-González, J.-M.; Romano, V.; Rocha-De-Lossada, C. The role of matrix metalloproteinases in infectious corneal ulcers. Surv. Ophthalmol. 2023, 68, 929–939. [Google Scholar] [CrossRef]
- Gao, N.; Kumar, A.; Yu, F.-S.X. Matrix Metalloproteinase-13 as a Target for Suppressing Corneal Ulceration Caused by Pseudomonas aeruginosa Infection. J. Infect. Dis. 2015, 212, 116–127. [Google Scholar] [CrossRef]
- Berger, E.A.; McClellan, S.A.; Barrett, R.P.; Hazlett, L.D. Testican-1 Promotes Resistance against Pseudomonas aeruginosa–Induced Keratitis through Regulation of MMP-2 Expression and Activation. Investig. Opthalmology Vis. Sci. 2011, 52, 5339–5346. [Google Scholar] [CrossRef]
- Kimura, K.; Nomi, N.; Yan, Z.H.; Orita, T.; Nishida, T. Inhibition of poly(I:C)–induced matrix metalloproteinase expression in human corneal fibroblasts by triptolide. Mol. Vis. 2011, 17, 526–532. [Google Scholar] [PubMed]
- Lee, S.; Zheng, M.; Kim, B.; Rouse, B.T. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J. Clin. Investig. 2002, 110, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Mitchell, B.M.; Wilhelmus, K.R. Expression of Matrix Metalloproteinases during Experimental Candida albicans Keratitis. Investig. Ophthalmol. Vis. Sci. 2009, 50, 737–742. [Google Scholar] [CrossRef]
- Dushku, N.; John, M.K.; Schultz, G.S.; Reid, T.W. Pterygia pathogenesis: Corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch. Ophthalmol. 2001, 119, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.Y.; Chiang, C.C.; Yeh, K.T.; Lee, H.; Cheng, Y.W. Effect of TIMP-1 and MMP in Pterygium Invasion. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3462–3467. [Google Scholar] [CrossRef]
- Weiss, J.S.; Møller, H.U.; Lisch, W.; Kinoshita, S.; Aldave, A.J.; Belin, M.W.; Kivelä, T.; Busin, M.; Munier, F.L.; Seitz, B.; et al. The IC3D Classification of the Corneal Dystrophies. Cornea 2008, 27, S1–S42. [Google Scholar] [CrossRef]
- Buffault, J.; Zéboulon, P.; Liang, H.; Chiche, A.; Luzu, J.; Robin, M.; Rabut, G.; Labetoulle, M.; Labbé, A.; Baudouin, C. Assessment of corneal epithelial thickness mapping in epithelial basement membrane dystrophy. PLoS ONE 2020, 15, e0239124. [Google Scholar] [CrossRef]
- Laibson, P.R. Microcystic corneal dystrophy. Trans. Am. Ophthalmol. Soc. 1976, 74, 488–531. [Google Scholar]
- Weiss, J.S.; Møller, H.U.; Aldave, A.J.; Seitz, B.; Bredrup, C.; Kivelä, T.M.; Munier, F.L.; Rapuano, C.J.; Nischal, K.K.M.; Kim, E.K.; et al. IC3D Classification of Corneal Dystrophies—Edition 2. Cornea 2015, 34, 117–159. [Google Scholar] [CrossRef]
- Cogan, D.G.; Donaldson, D.D.; Kuwabara, T.; Marshall, D. Microcystic Dystrophy of the Corneal Epithelium. Trans. Am. Ophthalmol. Soc. 1964, 62, 213–225. [Google Scholar]
- Rodrigues, M.M.; Fine, B.S.; Laibson, P.R.; Zimmerman, L.E. Disorders of the corneal epithelium. A clinicopathologic study of dot, geographic, and fingerprint patterns. Arch. Ophthalmol. 1974, 92, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Werblin, T.P.; Hirst, L.W.; Stark, W.J.; Maumenee, I.H. Prevalence of map-dot-fingerprint changes in the cornea. Br. J. Ophthalmol. 1981, 65, 401–409. [Google Scholar] [CrossRef]
- Dark, A.J. Cogan’s microcystic dystrophy of the cornea: Ultrastructure and photomicroscopy. Br. J. Ophthalmol. 1978, 62, 821–830. [Google Scholar] [CrossRef]
- Wood, T.O. Recurrent erosion. Trans. Am. Ophthalmol. Soc. 1984, 82, 850–898. [Google Scholar] [CrossRef]
- Hansen, E. On den intermitterende keratitis visicularis neuralgica af traumatisk oprindelse. Hosp. Tindende 1872, 51, 201–203. [Google Scholar]
- Miller, D.D.; Hasan, S.A.; Simmons, N.L.; Stewart, M.W. Recurrent corneal erosion: A comprehensive review. Clin. Ophthalmol. 2019, 13, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.A. Recurrent Erosion of the Cornea. Am. J. Ophthalmol. 1945, 28, 355–363. [Google Scholar] [CrossRef]
- Lee, W.-S.; Lam, C.K.; Manche, E.E. Phototherapeutic keratectomy for epithelial basement membrane dystrophy. Clin. Ophthalmol. 2017, 11, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Reeves, S.W.; Kang, P.C.; Zlogar, D.F.; Gupta, P.K.; Stinnett, S.; Afshari, N.A. Recurrent Corneal Erosion Syndrome: A Study of 364 Episodes. Ophthalmic Surgery, Lasers Imaging Retin. 2010, 41, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Mrukwa-Kominek, E. Metody leczenia erozji nawrotowych rogówki. Okul. Dypl. 2012, 2, 18–22. [Google Scholar]
- Watson, S.; Leung, V. Interventions for recurrent corneal erosions. Cochrane Database Syst. Rev. 2018, 2018, CD001861. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Aldave, A.J.; Chodosh, J. Recurrent corneal erosion syndrome. Br. J. Ophthalmol. 2019, 103, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Song, S.W.; Kim, J.H.; Woo, H.M. Multifocal phototherapeutic keratectomy for the treatment of persistent epithelial defect. J. Cataract. Refract. Surg. 2000, 26, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Aitken, D.A.; Beirouty, Z.A.; Lee, W.R. Ultrastructural study of the corneal epithelium in the recurrent erosion syndrome. Br. J. Ophthalmol. 1995, 79, 282–289. [Google Scholar] [CrossRef]
- Hope-Ross, M.W.; Chell, P.B.; Kervick, G.N.; McDonnell, P.J. Recurrent corneal erosion: Clinical features. Eye 1994, 8, 373–377. [Google Scholar] [CrossRef]
- Jan, R.-L.; Tai, M.-C.; Ho, C.-H.; Chu, C.-C.; Wang, J.-J.; Tseng, S.-H.; Chang, Y.-S. Risk of recurrent corneal erosion in patients with diabetes mellitus in Taiwan: A population-based cohort study. BMJ Open 2020, 10, e035933. [Google Scholar] [CrossRef]
- Nanba, H.; Mimura, T.; Mizuno, Y. Clinical course and risk factors of recurrent corneal erosio. Medicine 2019, 98, e14964. [Google Scholar] [CrossRef]
- Das, S.; Seitz, B. Recurrent Corneal Erosion Syndrome. Surv. Ophthalmol. 2008, 53, 3–15. [Google Scholar] [CrossRef]
- Jadczyk-Sorek, K.; Garczorz, W.; Bubała-Stachowicz, B.; Francuz, T.; Mrukwa-Kominek, E. Increased Matrix Metalloproteinase-2 and Matrix Metalloproteinase-3 Concentrations in Corneal Epithelium of Patients with Recurrent Corneal Erosions. J. Ophthalmol. 2022, 2022, 5024037. [Google Scholar] [CrossRef]
- Reidy, J.J.; Paulus, M.P.; Gona, S. Recurrent erosions of the cornea: Epidemiology and treatment. Cornea 2000, 19, 767–771. [Google Scholar] [CrossRef]
- Fujikawa, L.S.; Foster, C.S.; Gipson, I.K.; Colvin, R.B. Basement membrane components in healing rabbit corneal epithelial wounds: Immunofluorescence and ultrastructural studies. J. Cell Biol. 1984, 98, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.C.; Bron, A.J. Ultrastructural study of non-traumatic recurrent corneal erosion. Br. J. Ophthalmol. 1972, 56, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Del-Castillo, J.M.; Rodríguez-Bayo, S.; Fontan-Rivas, E.; Martinez-De-La-Casa, J.M.; Garcia-Sanchez, J. Treatment of Recurrent Corneal Erosion With Substance P–Derived Peptide and Insulin-like Growth Factor, I. Arch. Ophthalmol. 2005, 123, 1445. [Google Scholar] [CrossRef] [PubMed]
- Di, G.; Qi, X.; Zhao, X.; Zhang, S.; Danielson, P.; Zhou, Q. Corneal Epithelium-Derived Neurotrophic Factors Promote Nerve Regeneration. Investig. Opthalmology Vis. Sci. 2017, 58, 4695–4702. [Google Scholar] [CrossRef]
- Dursun, D.; Kim, M.C.; Solomon, A.; Pflugfelder, S.C. Treatment of recalcitrant recurrent corneal erosions with inhibitors of matrix met-alloproteinase-9, doxycycline and corticosteroids. Am. J. Ophthalmol. 2001, 132, 8–13. [Google Scholar] [CrossRef]
- Garrana, R.M.; Zieske, J.D.; Assouline, M.; Gipson, I.K. Matrix metalloproteinases in epithelia from human recurrent corneal ero-sion. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1266–1270. [Google Scholar]
- Sakimoto, T.; Shoji, J.; Yamada, A.; Sawa, M. Upregulation of Matrix Metalloproteinase in Tear Fluid of Patients with Recurrent Corneal Erosion. Jpn. J. Ophthalmol. 2007, 51, 343–346. [Google Scholar] [CrossRef]
- Fini, M.E.; Girard, M.T. Expression of collagenolytic/gelatinolytic metalloproteinases by normal cornea. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1779–1788. [Google Scholar]
- Mohan, R.; Chintala, S.K.; Jung, J.C.; Villar, W.V.L.; McCabe, F.; Russo, L.A.; Lee, Y.; McCarthy, B.E.; Wollenberg, K.R.; Jester, J.V.; et al. Matrix Metalloproteinase Gelatinase B (MMP-9) Coordinates and Effects Epithelial Regeneration. J. Biol. Chem. 2002, 277, 2065–2072. [Google Scholar] [CrossRef]
- Hope-Rross, M.W.; Chell, P.B.; Kervick, G.N.; McDonnell, P.J.; Junes, H.S. Oral tetracycline in the treatment of recurrent corneal erosions. Eye 1994, 8, 384–388. [Google Scholar] [CrossRef]
- Cogan, D.G.; Kuwabara, T.; Donaldson, D.D.; Collins, E. Microcystic Dystrophy of the Cornea A Partial Explanation for Its Pathogen-esis. Arch. Ophthalmol. 1974, 92, 470–474. [Google Scholar] [CrossRef] [PubMed]
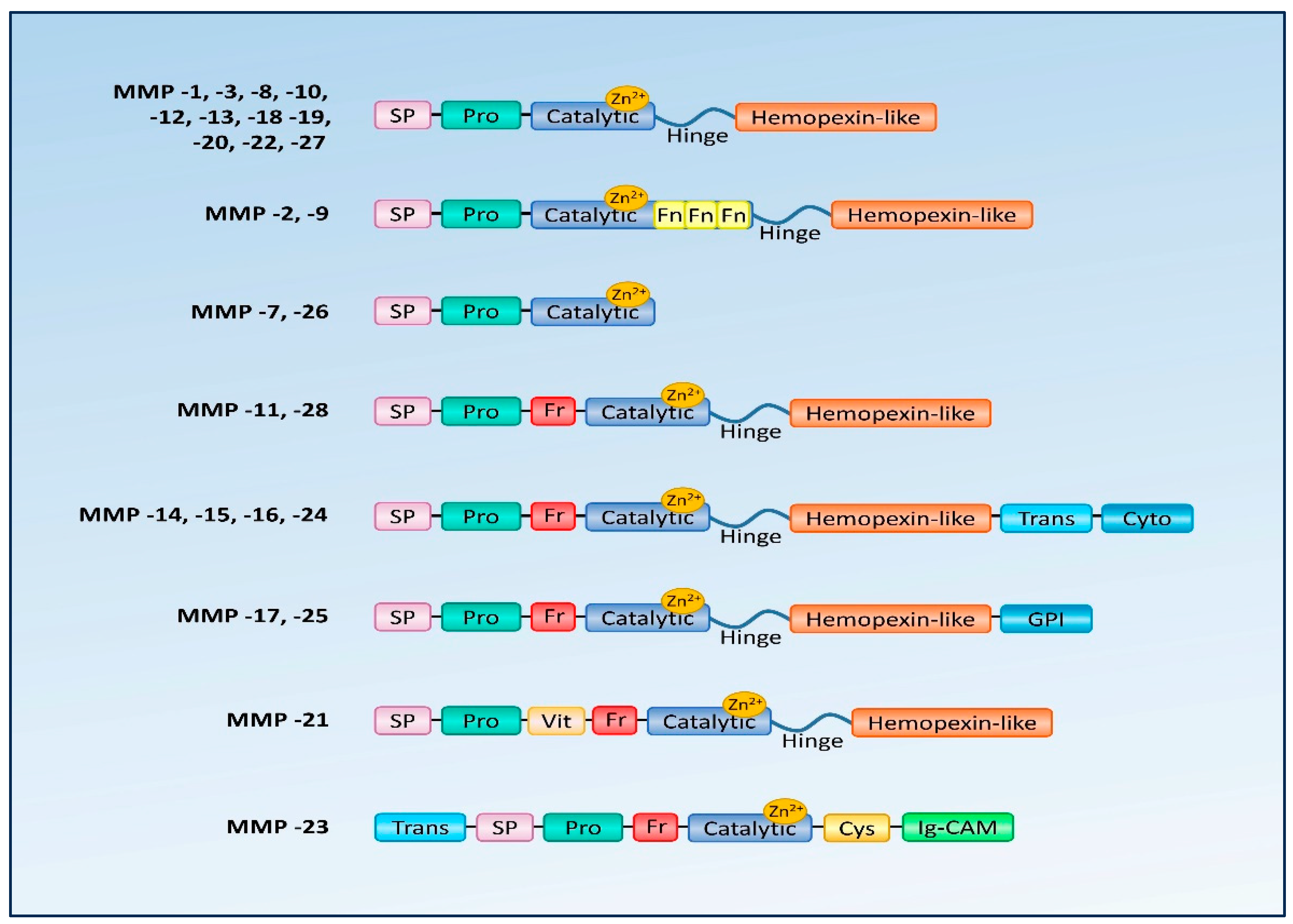

| MMP | Common Name | Analogous Structure or Substrate Affinity with Other MMPs | Selected Degradable MMP Substrates |
|---|---|---|---|
| MMP-1 | collagenase | MMP-8, -13, -18 | collagen type I, II, III, V, VII, VIII, IX, X, XI |
| MMP-2 | gelatinase A | MMP-9 | collagen type I, IV, V, VII, X, gelatin, elastin |
| MMP-3 | stromelysin 1, proteoglycanase | MMP-10, -11 | elastin, proteoglycans, aggrecans, gelatin, proMMP-1, proMMP-8, proMMP-9, collagen type III, IV, V, IX, IX |
| MMP-7 | matrilysin 1, metalloendopeptidase | MMP-26 | collagen type IV, gelatin glycoproteins |
| MMP-8 | collagenase 2 | MMP-1, -13, -18 | collagen type I, II, III, IV |
| MMP-9 | gelatinase B | MMP-2 | collagen type I, II, III, IV, XI, XVI, fibronectin, gelatin, laminin, osteopontin |
| MMP-10 | stromelysin 2 | MMP-3, -11 | collagen type I, II, III, V |
| MMP-11 | stromelysin 3 | MMP-3, -10 | laminin, antitrypsin |
| MMP-12 | elastase, MME | - | elastin |
| MMP-13 | collagenase 3 | MMP- 1, -8, -18 | collagen type I, II, III, IV, V, IX, X, XI, gelatin, laminin |
| MMP-14 | MT1-MMP | MMP-15, -16, -17, -24, -25 (membrane-type MMPs) | collagen type I, II, III, gelatin, laminin, aggrecans, proMMP-2, proMMP-13 |
| MMP-15 | MT2-MMP | MMP-14, -16, -17, -24, -25 (membrane-type MMPs) | collagen type I, II, III, gelatin, proMMP-13 |
| MMP-16 | MT3-MMP | MMP-14, -15, -17, -24, -25 (membrane-type MMPs) | collagen type I, II, laminin, proMMP-2, proMMP-13 |
| MMP-17 | MT4-MMP | MMP-14, -15, -16, -24, -25 (membrane-type MMPs) | fibronectin, fibrin, gelatin |
| MMP-18 | collagenase 4, Xenopus | MMP- 1, -8, -13 | - |
| MMP-19 | RASI 1 | - | collagen type I, IV, gelatin, fibronectin, laminin, aggrecan, entactin, tenascin |
| MMP-20 | enamelysin | - | amelogenin, aggrecans |
| MMP-21 | XMMP | - | - |
| MMP-22 | CMMP | - | gelatin |
| MMP-23 | CA-MMP | - | gelatin |
| MMP-24 | MT5-MMP | MMP-14, -15, -16, -17, -25 (membrane-type MMPs) | proMMP-2, proMMP-13 |
| MMP-25 | MT6-MMP | MMP-14, -15, -16, -17, -24 (membrane-type MMPs) | proMMP-2 |
| MMP-26 | matrilysin 2 | MMP-7 | collagen type IV, gelatin, fibrinogen, fibronectin, vitronectin, casein, pro-MMP-9 |
| MMP-27 | MMP-22, C-MMP | - | - |
| MMP-28 | epilysin | - | casein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadczyk-Sorek, K.; Garczorz, W.; Bubała-Stachowicz, B.; Francuz, T.; Mrukwa-Kominek, E. Matrix Metalloproteinases and the Pathogenesis of Recurrent Corneal Erosions and Epithelial Basement Membrane Dystrophy. Biology 2023, 12, 1263. https://doi.org/10.3390/biology12091263
Jadczyk-Sorek K, Garczorz W, Bubała-Stachowicz B, Francuz T, Mrukwa-Kominek E. Matrix Metalloproteinases and the Pathogenesis of Recurrent Corneal Erosions and Epithelial Basement Membrane Dystrophy. Biology. 2023; 12(9):1263. https://doi.org/10.3390/biology12091263
Chicago/Turabian StyleJadczyk-Sorek, Katarzyna, Wojciech Garczorz, Beata Bubała-Stachowicz, Tomasz Francuz, and Ewa Mrukwa-Kominek. 2023. "Matrix Metalloproteinases and the Pathogenesis of Recurrent Corneal Erosions and Epithelial Basement Membrane Dystrophy" Biology 12, no. 9: 1263. https://doi.org/10.3390/biology12091263
APA StyleJadczyk-Sorek, K., Garczorz, W., Bubała-Stachowicz, B., Francuz, T., & Mrukwa-Kominek, E. (2023). Matrix Metalloproteinases and the Pathogenesis of Recurrent Corneal Erosions and Epithelial Basement Membrane Dystrophy. Biology, 12(9), 1263. https://doi.org/10.3390/biology12091263






