Comparative Effects of Calcium, Boron, and Zinc Inhibiting Physiological Disorders, Improving Yield and Quality of Solanum lycopersicum
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reproductive Parameters
2.1.1. Number of Flower Cluster−1
2.1.2. Number of Fruit Cluster−1
2.1.3. Number of Flower Clusters Plant−1
2.1.4. Flower Drop (%)
2.1.5. Total Yield (tonnes ha−1)
2.2. Physiological Disorders
2.3. Nutrient Content of the Fruit
2.3.1. Fruit Calcium Content (%)
2.3.2. Fruit Boron Content (mg 100 g−1 DW)
2.3.3. Fruit Zinc Content (mg 100 g−1 DW)
2.4. Quality or Biochemical Attributes
2.5. Statistical Analysis
3. Results
3.1. Number of Flowers Cluster−1
| Calcium Levels (%) | No. of Flowers Cluster−1 | No. of Fruit Cluster−1 | No. of Flower Cluster Plant−1 |
|---|---|---|---|
| 0 | 4.82 c | 3.74 c | 13.19 c |
| 0.3 | 5.52 b | 4.29 b | 14.82 b |
| 0.6 | 6.33 a | 4.82 a | 15.07 b |
| 0.9 | 5.37 b | 4.19 b | 16.78 a |
| LSD at α 0.05 | 0.45 | 0.33 | 0.68 |
| Boron (%) | |||
| 0 | 4.97 c | 3.75 c | 12.19 c |
| 0.25 | 6.05 a | 4.97 a | 15.28 b |
| 0.5 | 5.50 b | 4.06 b | 17.42 a |
| LSD at α 0.05 | 0.39 | 0.29 | 0.59 |
| Zinc (%) | |||
| 0 | 5.03 c | 4.00 b | 13.86 b |
| 0.25 | 5.44 b | 4.14 b | 14.39 b |
| 0.5 | 6.06 a | 4.65 a | 16.64 a |
| LSD at α 0.05 | 0.39 | 0.29 | 0.59 |
| Interactions | |||
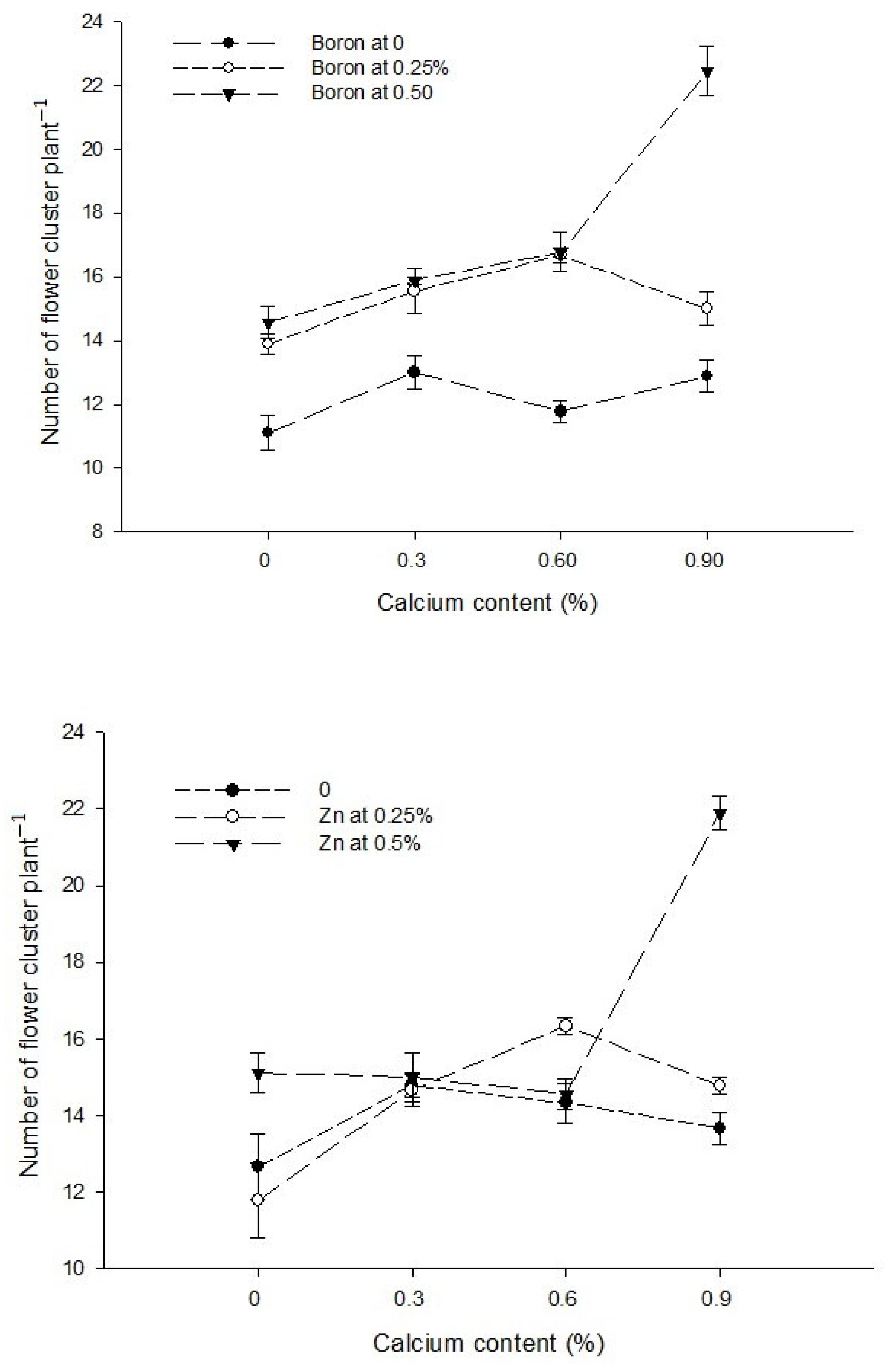
| Ca × B | Figure 1a | Figure 1b | Figure 2 |
| Level of Significance | ** | ** | ** |
| Ca × Zn | --- | --- | Figure 2 |
| Level of Significance | Ns | NS | * |
| B × Zn | Figure 1a | Figure 1b | Figure 2 |
| Level of Significance | * | ** | ** |
| Ca × B × Zn | --- | --- | --- |
| Level of Significance | NS | NS | NS |
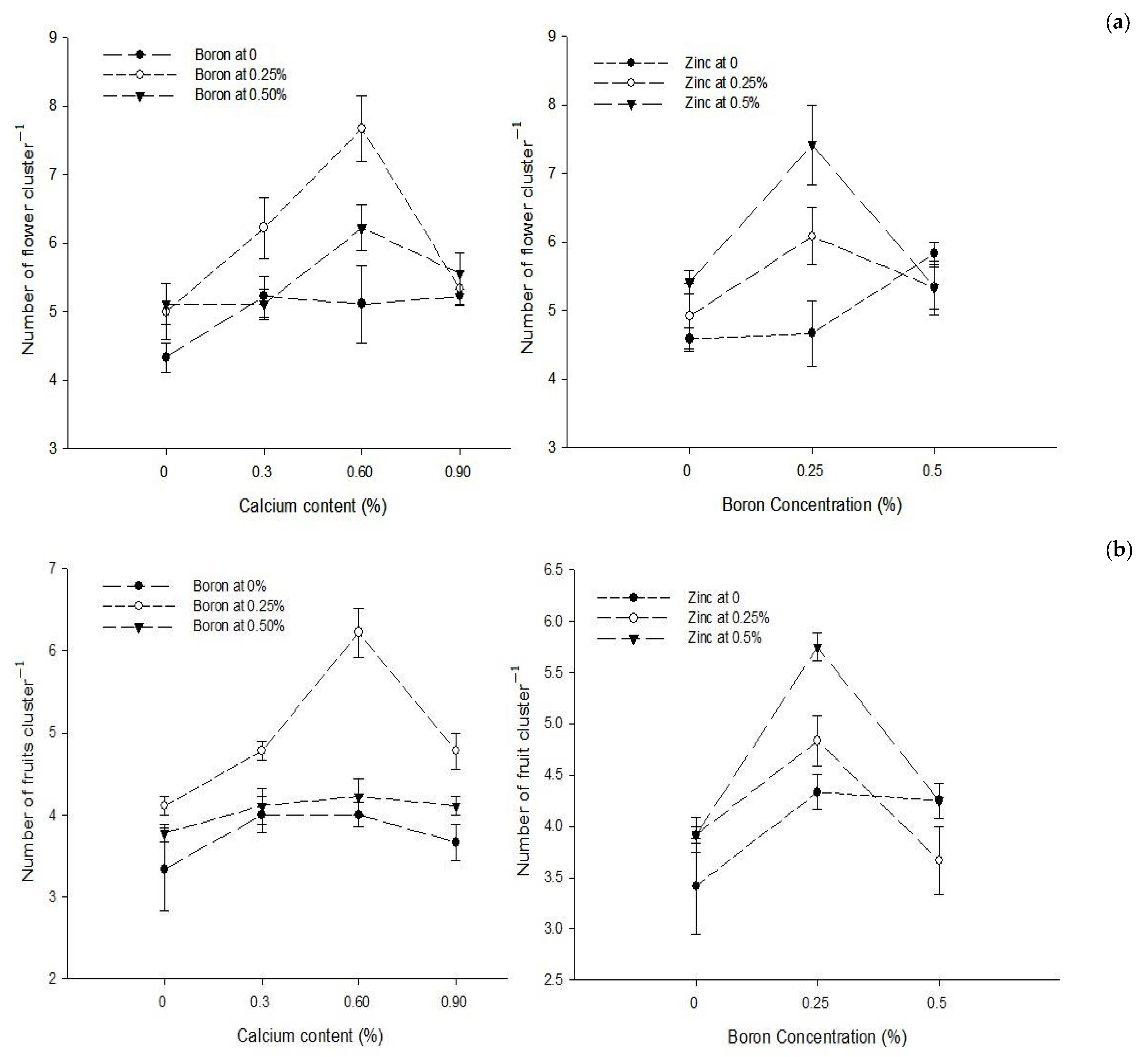

3.2. Number of Fruit Cluster−1
3.3. Number of Flower Clusters Plant−1
3.4. Flower Drop (%)
| Calcium Levels (%) | Flower Drop (%) | Yield (t ha−1) | Blossom End Rot (%) | Fruit Cracking (%) |
|---|---|---|---|---|
| 0 | 33.74 a | 19.96 c | 16.93 a | 6.74 a |
| 0.3 | 22.85 bc | 23.89 b | 15.00 b | 5.85 b |
| 0.6 | 18.85 c | 28.11 a | 11.85 c | 5.26 c |
| 0.9 | 25.69 b | 23.04 bc | 6.70 d | 3.63 d |
| LSD at α 0.05 | 4.52 | 3.76 | 0.72 | 0.52 |
| Boron (%) | ||||
| 0 | 31.50 a | 19.17 c | 14.00 a | 6.50 a |
| 0.25 | 17.86 c | 28.30 a | 12.41 b | 4.44 c |
| 0.5 | 26.50 b | 23.78 b | 11.44 c | 5.17 b |
| LSD at α 0.05 | 3.92 | 3.25 | 0.623 | 0.45 |
| Zinc (%) | ||||
| 0 | 27.81 | 19.14 c | 15.19 a | 5.97 a |
| 0.25 | 24.33 | 23.30 b | 11.67 b | 5.31 b |
| 0.5 | 23.72 | 28.80 a | 11.00 c | 4.83 c |
| LSD at α 0.05 | NS | 3.25 | 0.62 | 0.45 |
| Interactions | ||||
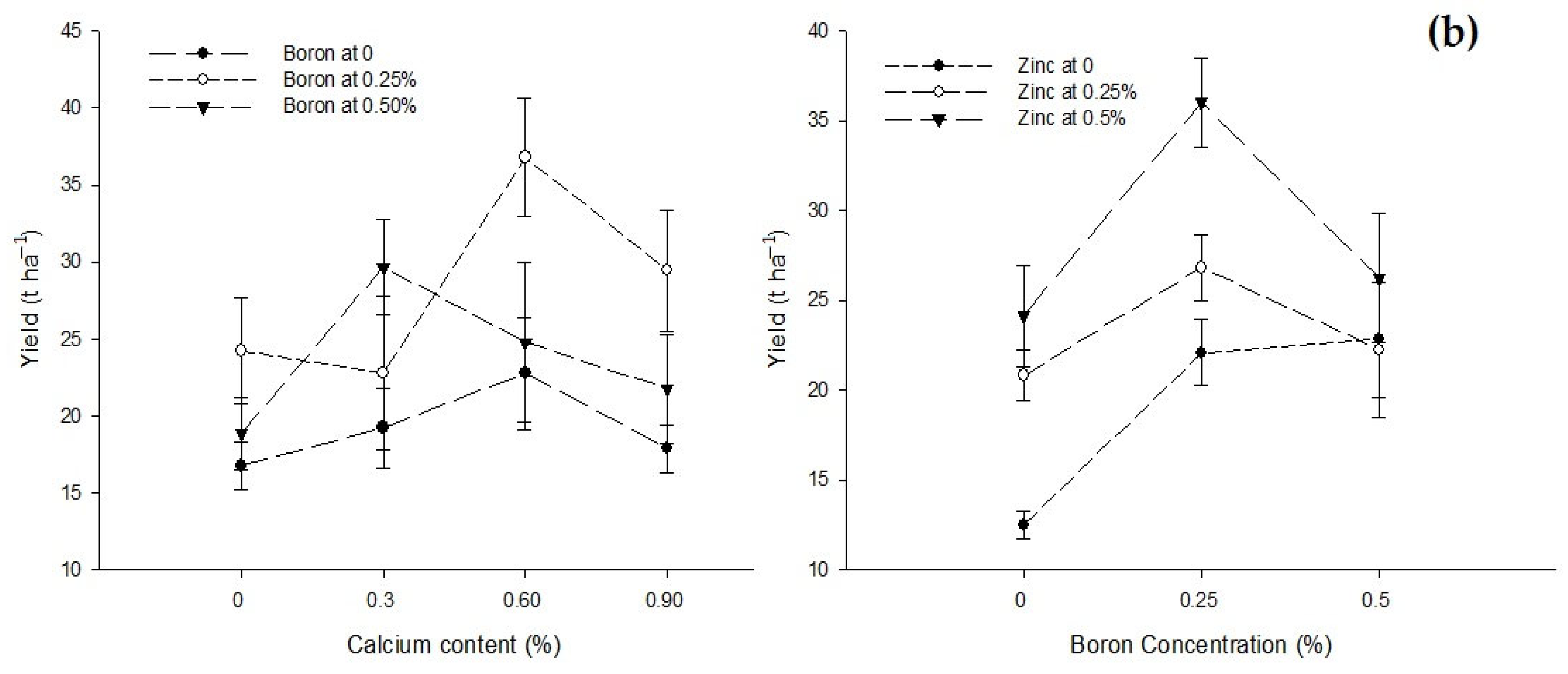
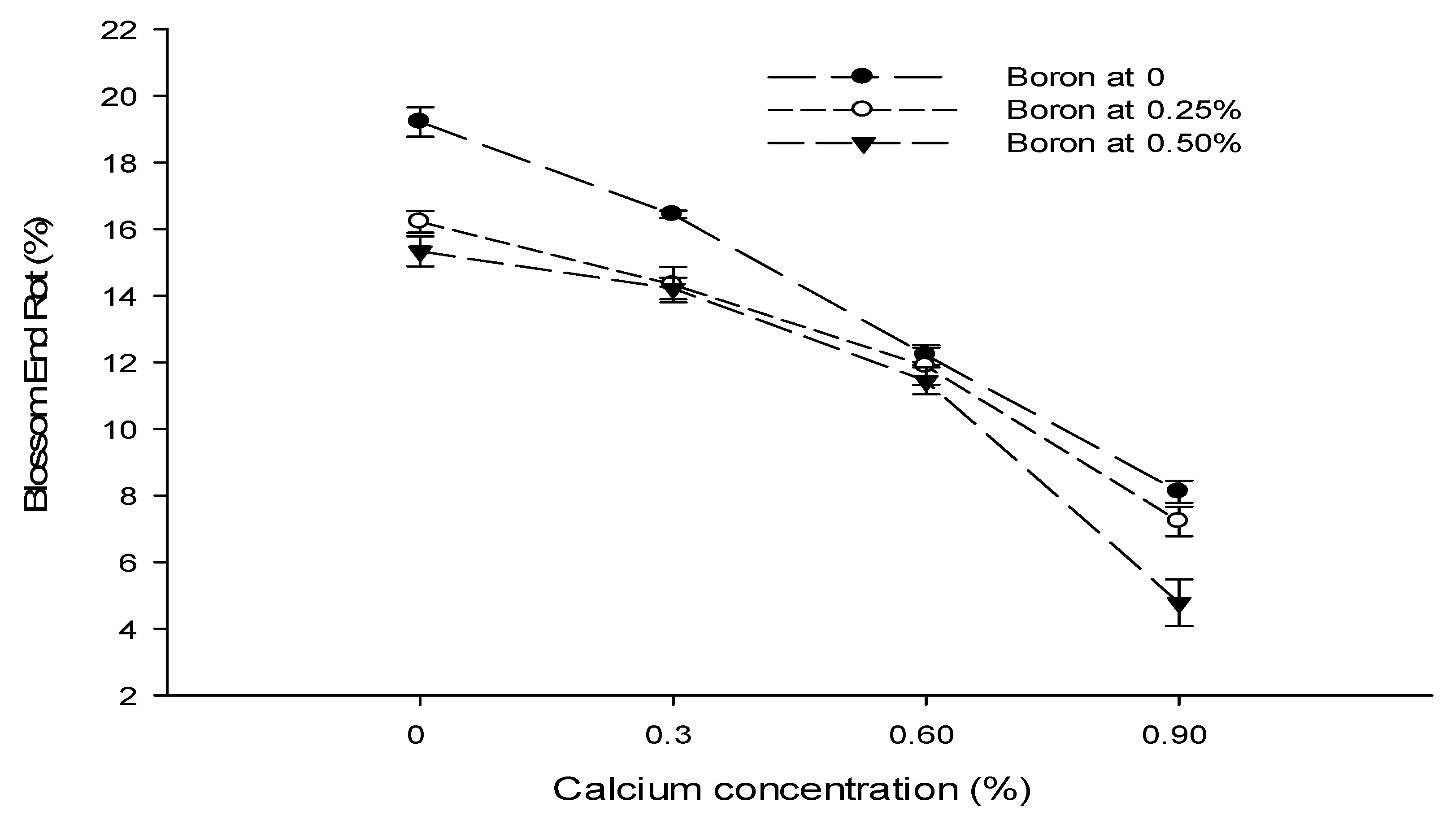
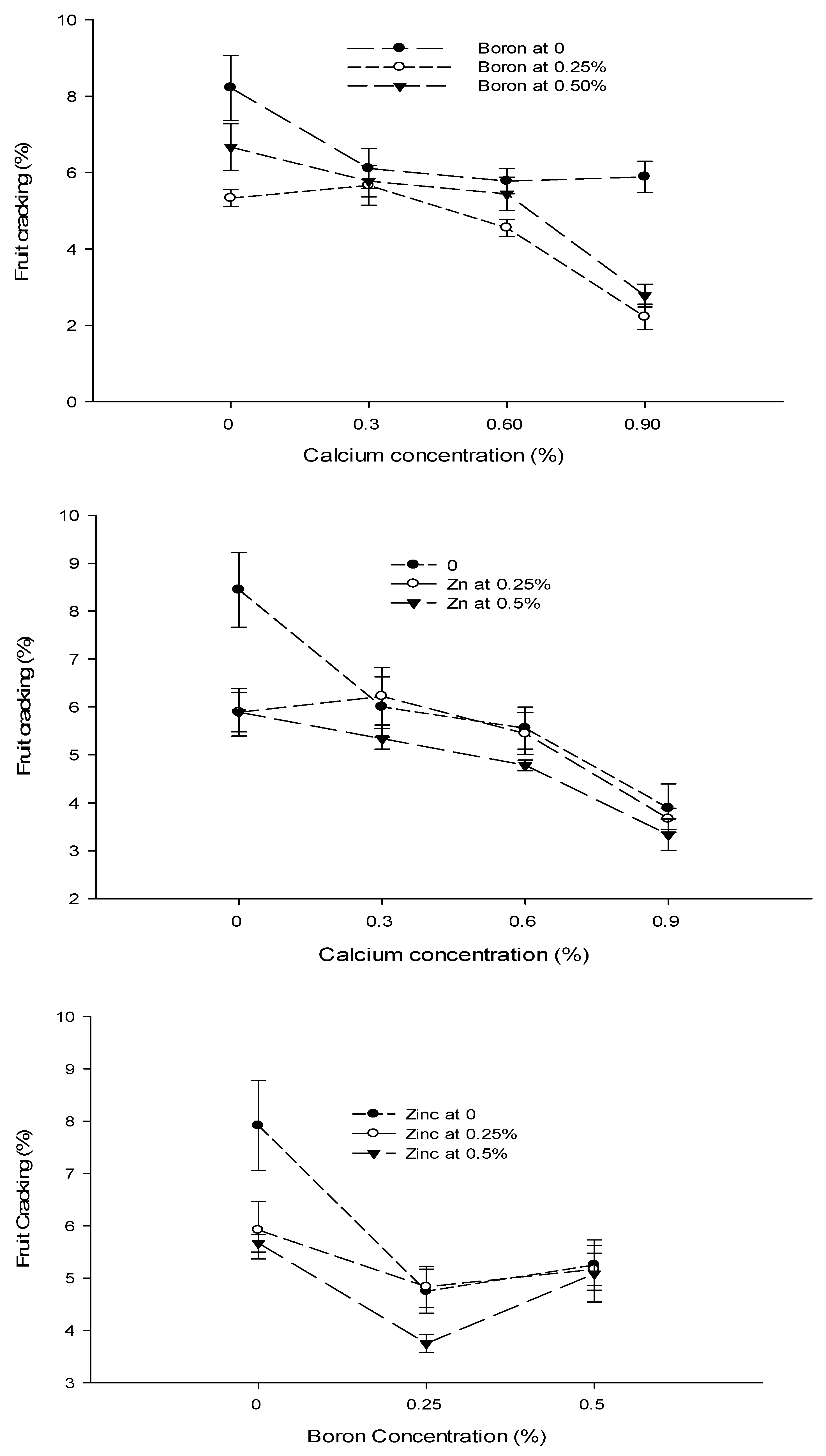
| Ca × B | Figure 3a | Figure 3b | Figure 4 | Figure 5 |
| Level of Significance | ** | ** | ** | ** |
| Ca × Zn | --- | --- | Figure 4 | Figure 5 |
| Level of Significance | NS | NS | ** | * |
| B × Zn | Figure 3a | Figure 3b | Figure 4 | Figure 5 |
| Level of Significance | ** | * | * | ** |
| Ca × B × Zn | --- | --- | --- | --- |
| Level of Significance | NS | NS | NS | NS |
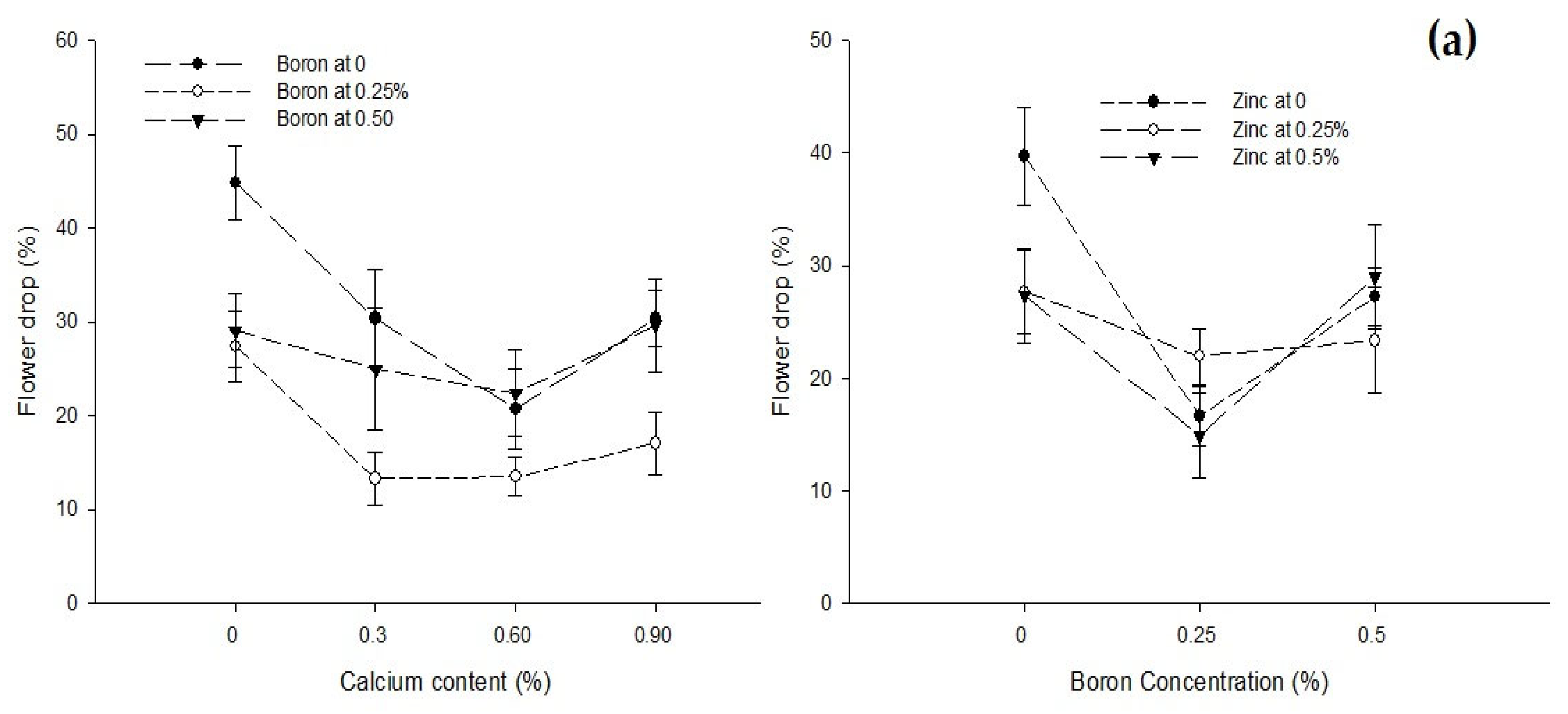

3.5. Yield (t ha−1)
3.6. Blossom End Rot (BER) (%)
3.7. Fruit Cracking (%)
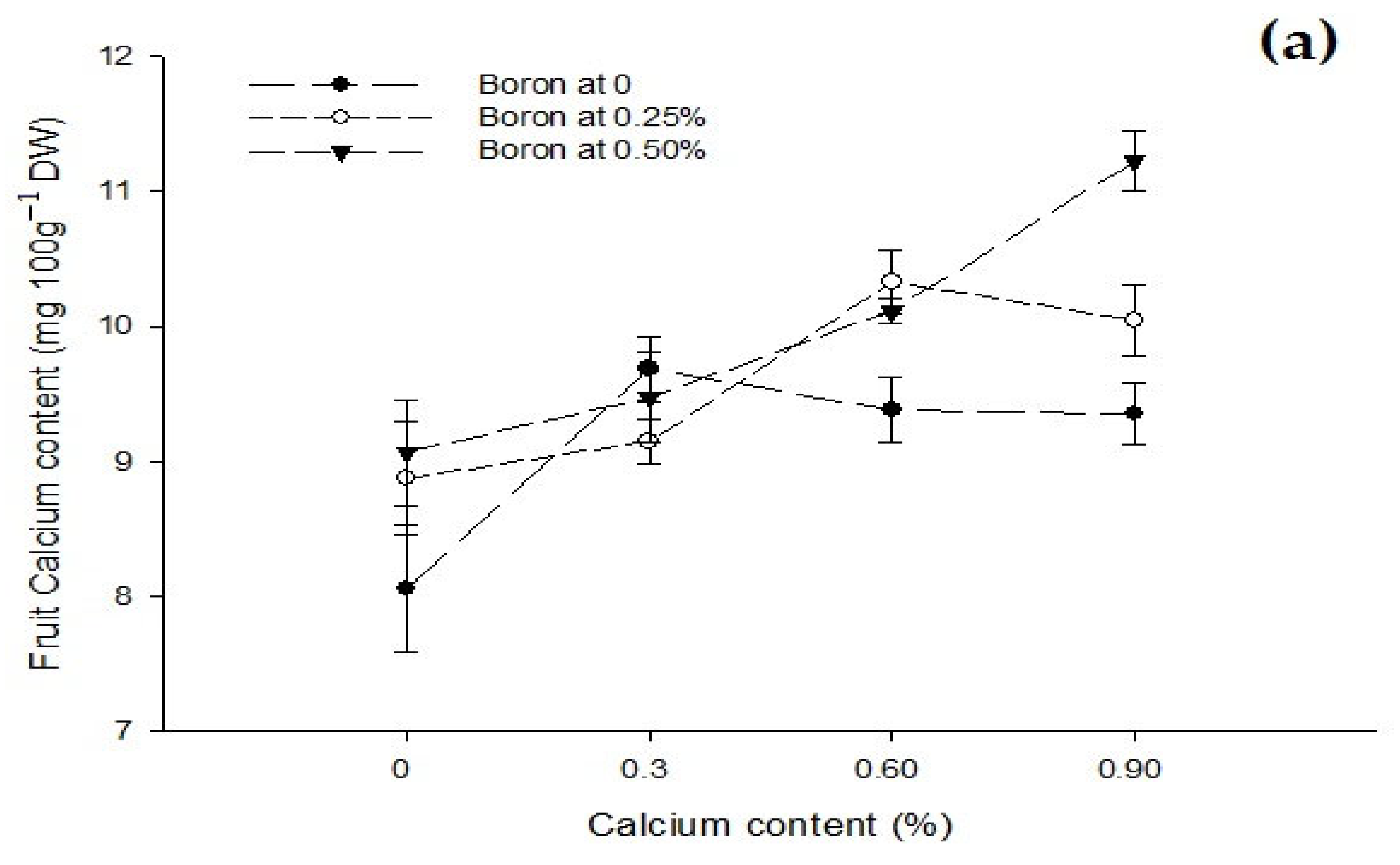
3.8. Fruit Calcium Content (mg 100 g−11 DW)
| Calcium Levels (%) | Fruit Calcium Content (mg 100 g−1 DW) | Boron Content (mg 100 g−1 DW) | Zinc Content (mg 100 g−1 DW) |
|---|---|---|---|
| 0 | 8.66 c | 2.83 | 2.43 a |
| 0.3 | 9.43 b | 2.99 | 2.34 a |
| 0.6 | 9.94 a | 2.97 | 2.28 ab |
| 0.9 | 10.21 a | 3.19 | 2.08 b |
| LSD at α 0.05 | 0.316 | NS | 0.22 |
| Boron (%) | |||
| 0 | 9.12 c | 2.64 b | 2.41 a |
| 0.25 | 9.59 b | 2.99 a | 2.26 ab |
| 0.5 | 9.97 a | 3.24 a | 2.18 b |
| LSD at α 0.05 | 0.27 | 0.31 | 0.19 |
| Zinc (%) | |||
| 0 | 8.70 a | 2.78 b | 1.81 b |
| 0.25 | 7.10 b | 2.93 b | 2.45 a |
| 0.5 | 6.88 b | 3.27 a | 2.59 a |
| LSD at α 0.05 | 0.27 | 0.31 | 0.191 |
| Interactions | |||
| Ca × B | Figure 6a | Figure 6b | Figure 7 |
| Level of Significance | ** | * | ** |
| Ca × Zn | --- | --- | Figure 7 |
| Level of Significance | NS | NS | ** |
| B × Zn | --- | --- | Figure 7 |
| Level of Significance | NS | NS | * |
| Ca × B × Zn | --- | --- | --- |
| Level of Significance | NS | NS | NS |
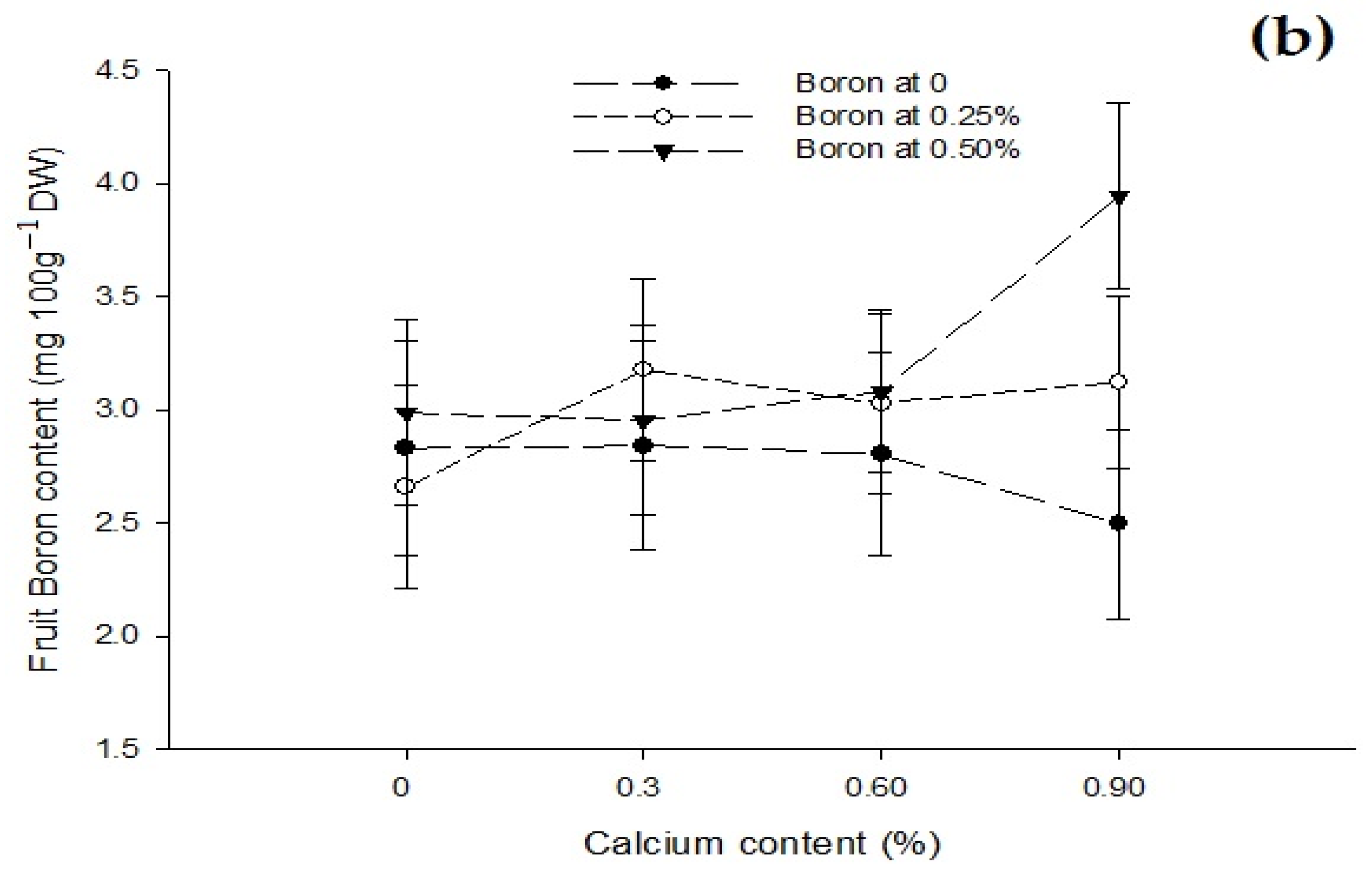
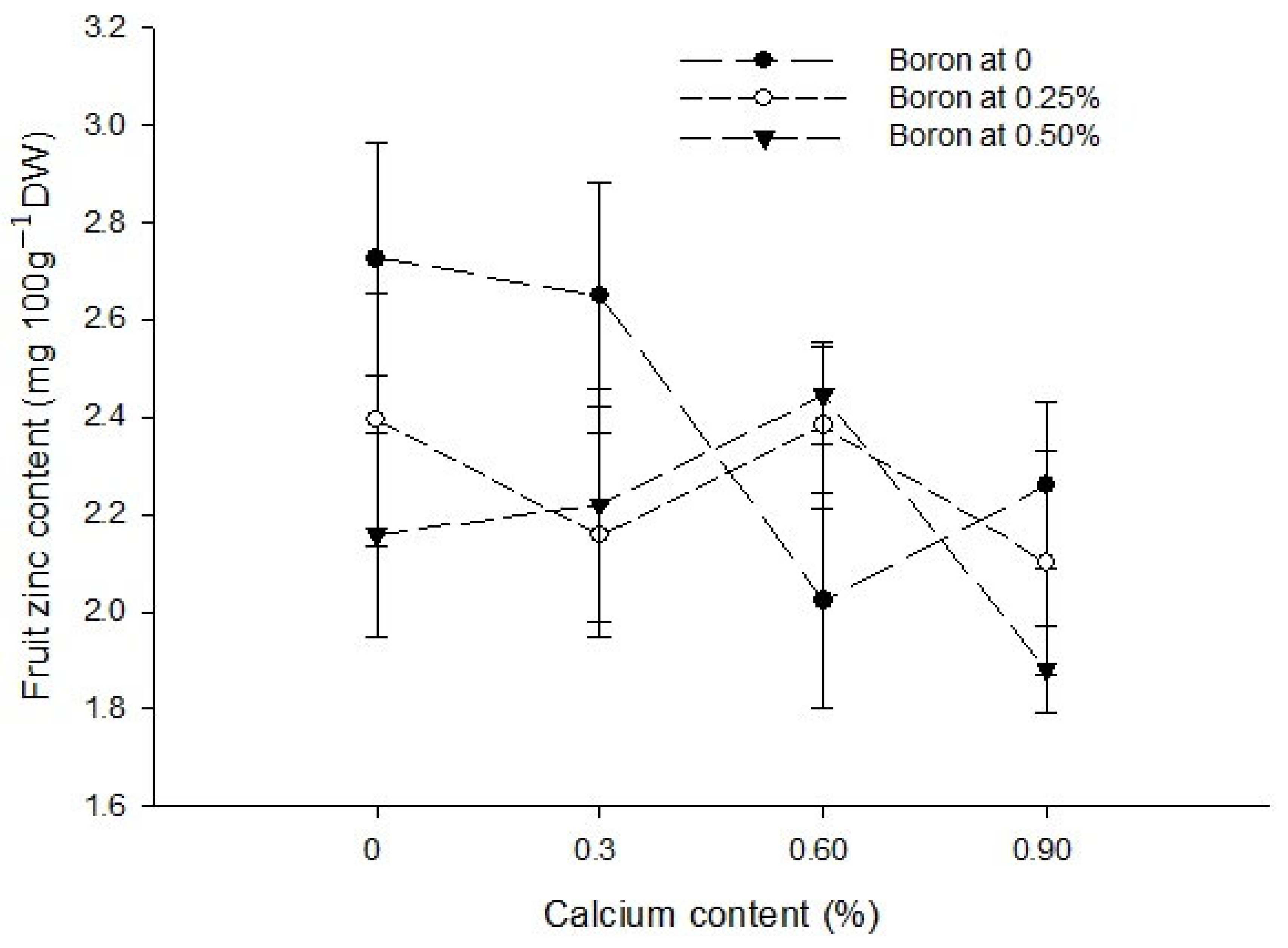
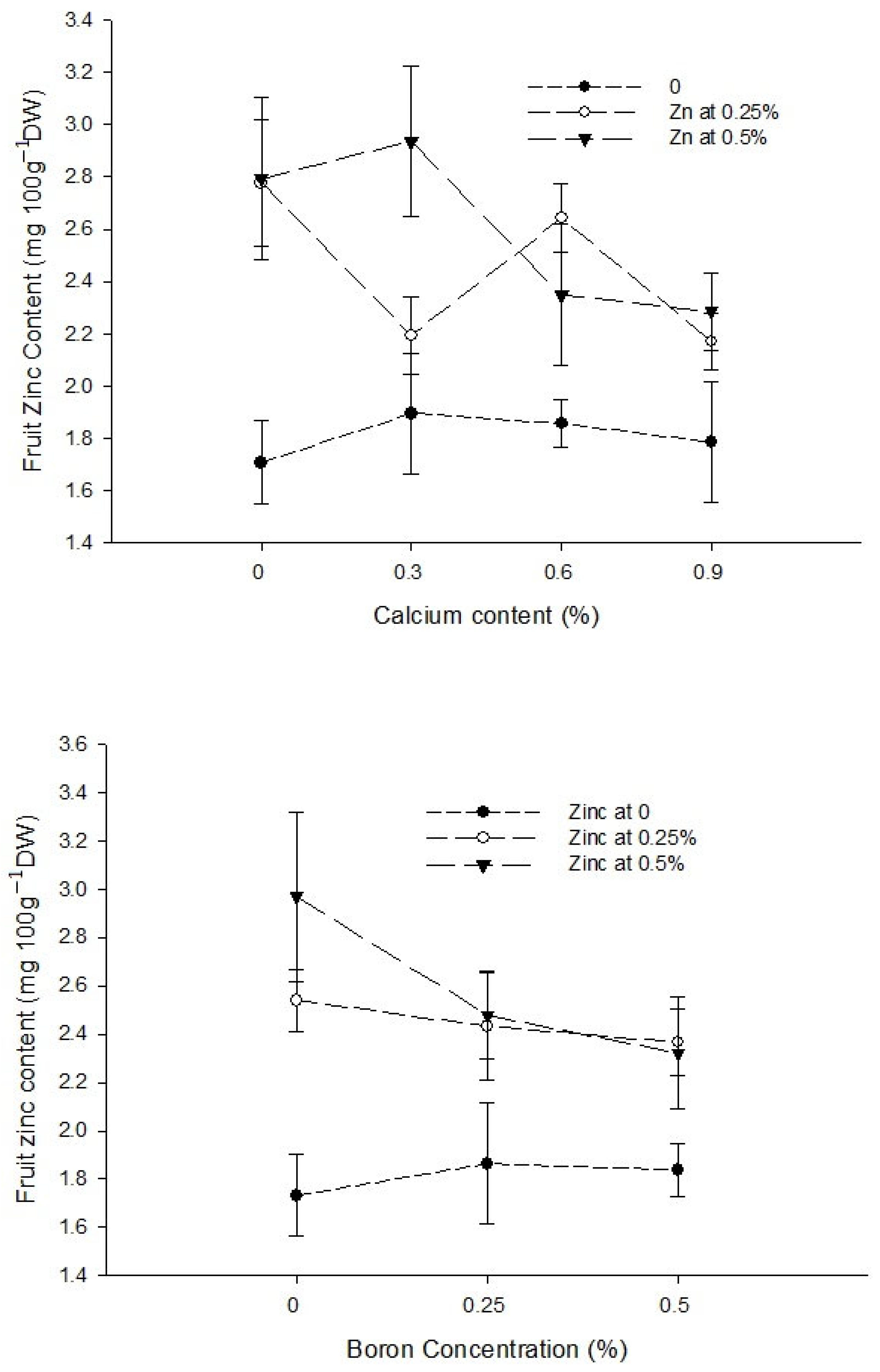
3.9. Fruit Boron Content (mg 100 g−1 DW)
3.10. Fruit Zinc Content (mg 100 g−1 DW)
3.11. Fruit Firmness (kg cm−2)
| Calcium Levels (%) | Fruit Firmness (kg cm−2) | Total Soluble Solids (°brix) | Percent Acidity (%) | TSS Acid Ratio |
|---|---|---|---|---|
| 0 | 2.37 c | 4.45 a | 0.27 | 18.27 |
| 0.3 | 2.67 b | 4.17 b | 0.31 | 15.80 |
| 0.6 | 2.80 ab | 3.82 c | 0.29 | 16.84 |
| 0.9 | 2.99 a | 3.38 d | 0.37 | 17.29 |
| LSD at α 0.05 | 0.24 | 0.24 | NS | NS |
| Boron (%) | ||||
| 0 | 2.57 b | 4.37 a | 0.27 | 17.95 |
| 0.25 | 2.70 ab | 3.93 b | 0.32 | 15.98 |
| 0.5 | 2.86 a | 3.56 c | 0.34 | 17.22 |
| LSD at α 0.05 | 0.21 | 0.21 | NS | NS |
| Zinc (%) | ||||
| 0 | 2.82 a | 3.36 c | 0.28 | 16.65 |
| 0.25 | 2.75 ab | 3.89 b | 0.27 | 18.28 |
| 0.5 | 2.56 b | 4.62 a | 0.39 | 16.22 |
| LSD at α 0.05 | 0.21 | 0.21 | NS | NS |
| Interactions | ||||
| Ca × B | Figure 8 | Figure 9 | --- | Figure 10 |
| Level of Significance | ** | NS | NS | ** |
| Ca × Zn | --- | ---- | --- | --- |
| Level of Significance | NS | ** | NS | NS |
| B × Zn | --- | Figure 9 | --- | --- |
| Level of Significance | NS | ** | NS | NS |
| Ca × B × Zn | --- | --- | --- | --- |
| Level of Significance | NS | NS | NS | NS |
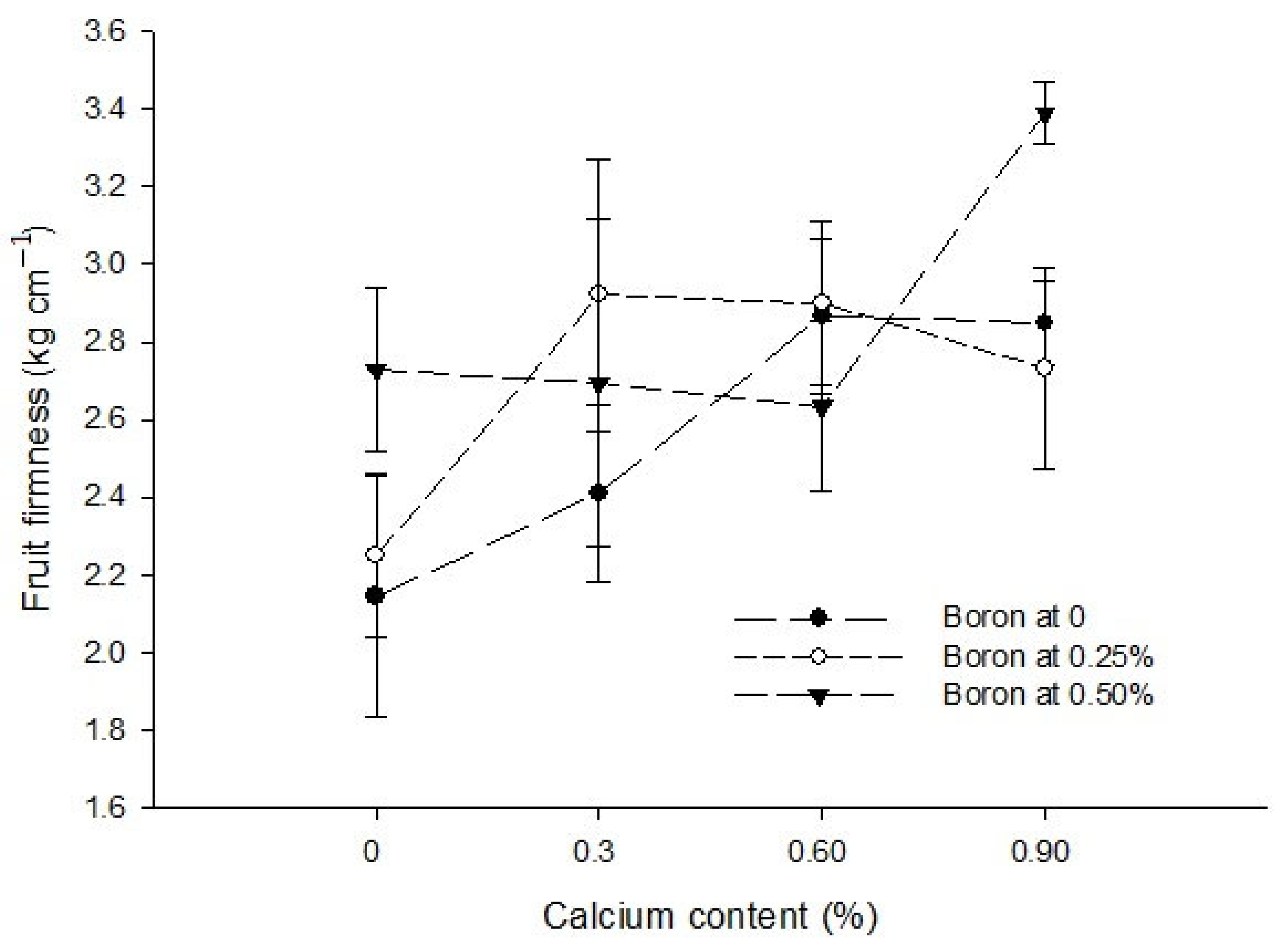
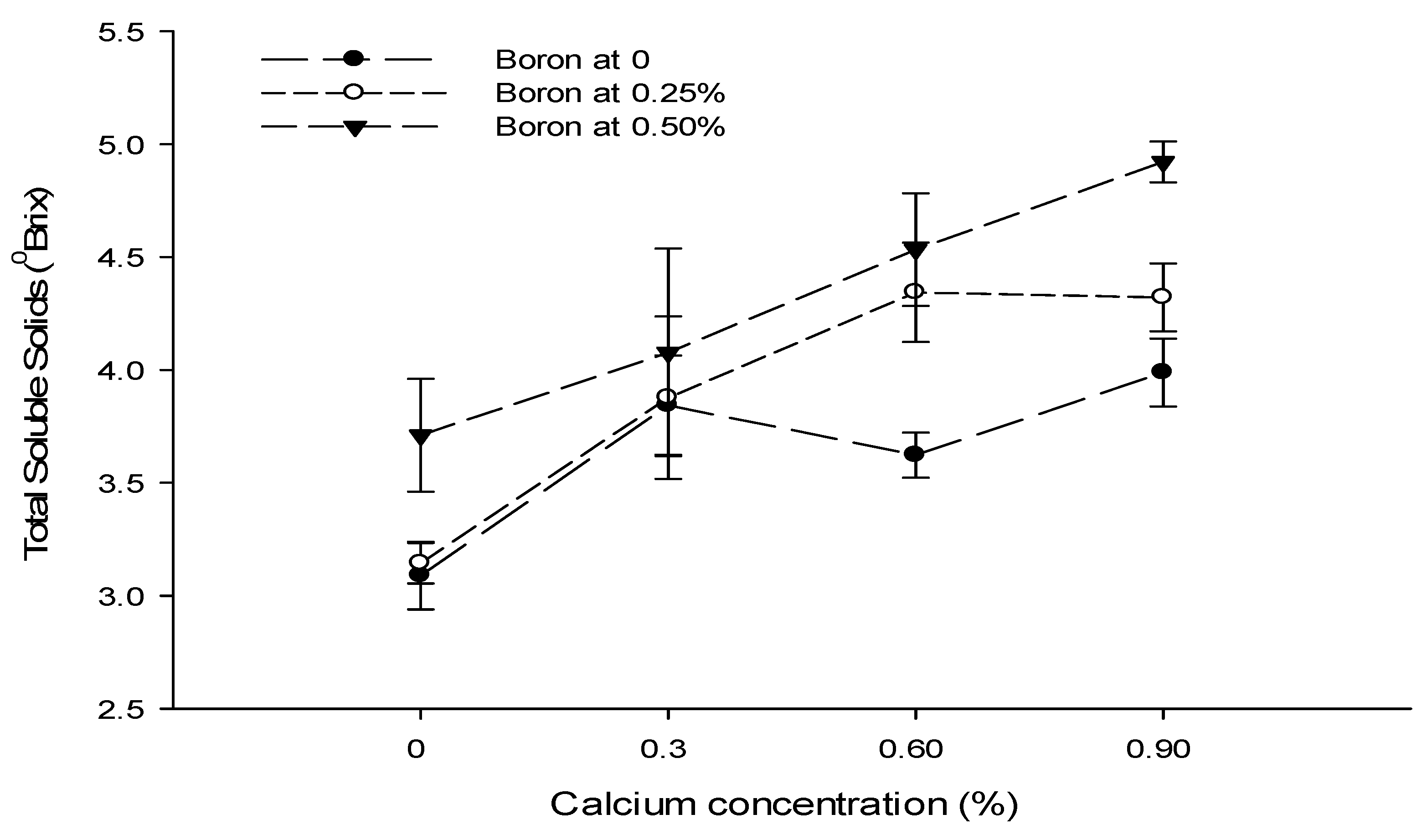
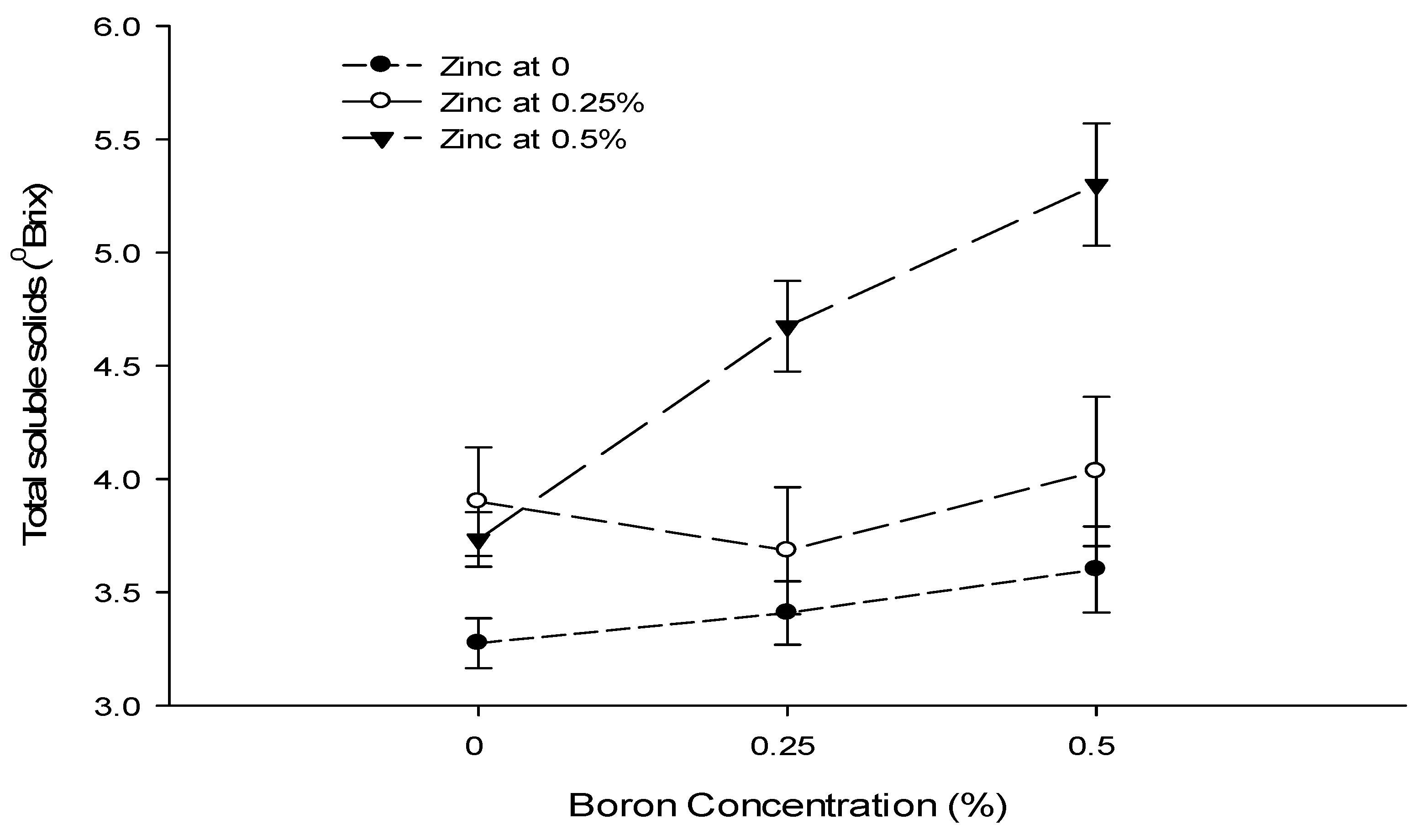
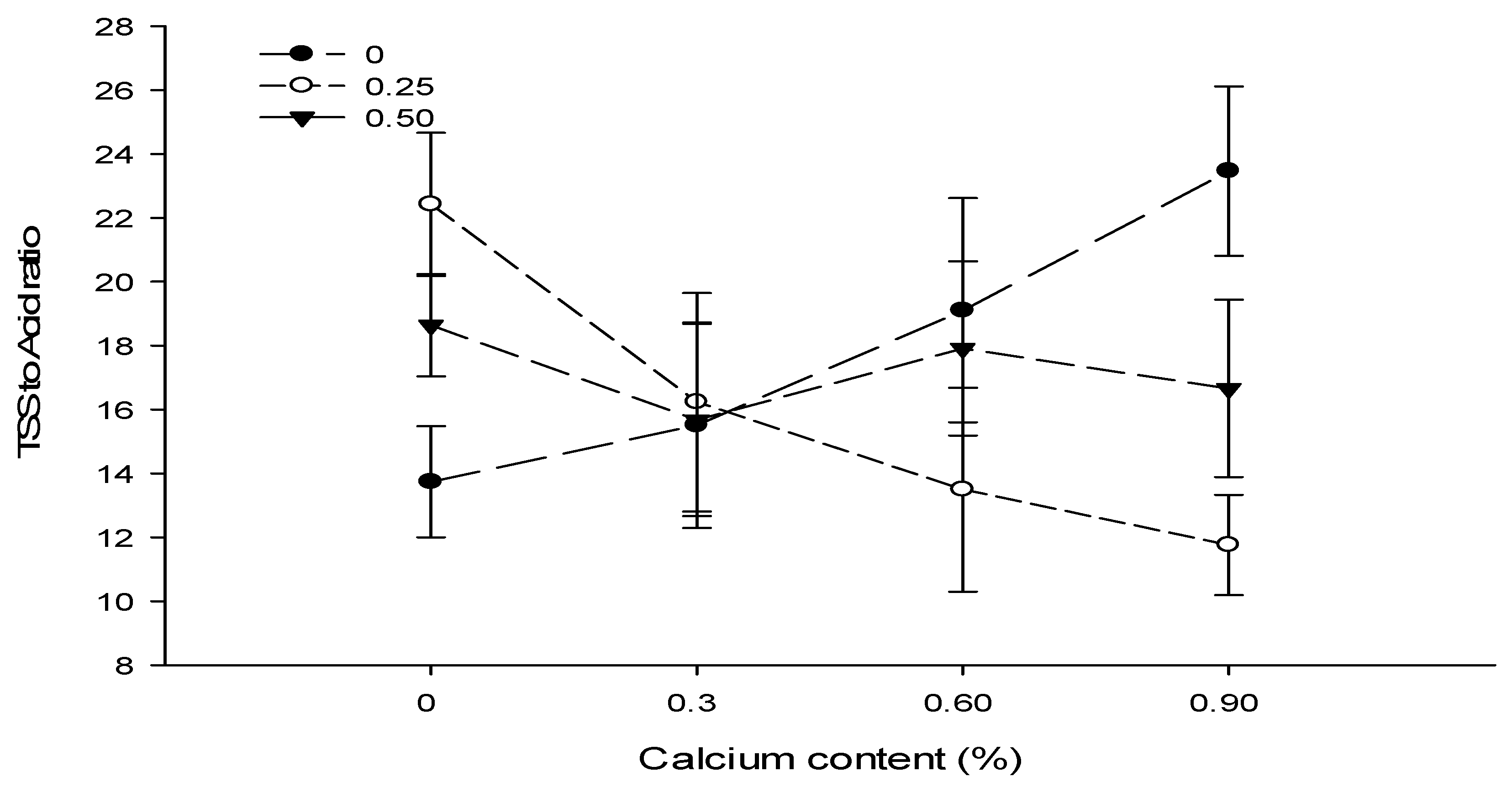
3.12. Total Soluble Solids (°brix)
3.13. Percent Acidity (%)
3.14. TSS to Acid Ratio
3.15. Ascorbic Acid Content (mg 100 g−1)
| Calcium Levels (%) | Ascorbic Acid Content (mg 100 g−1) | Reducing Sugars (%) | Non-Reducing Sugars (%) |
|---|---|---|---|
| 0 | 10.19 | 2.80 | 1.13 |
| 0.3 | 10.56 | 2.81 | 1.19 |
| 0.6 | 12.83 | 2.73 | 1.23 |
| 0.9 | 12.07 | 2.75 | 1.39 |
| LSD at α 0.05 | NS | NS | NS |
| Boron (%) | |||
| 0 | 10.68 | 2.96 | 1.09 |
| 0.25 | 12.64 | 2.71 | 1.29 |
| 0.5 | 10.92 | 2.65 | 1.33 |
| LSD at α 0.05 | NS | NS | NS |
| Zinc (%) | |||
| 0 | 8.29 c | 2.66 b | 1.45 a |
| 0.25 | 11.42 b | 2.58 b | 1.15 b |
| 0.5 | 14.52 a | 3.07 a | 1.11 b |
| LSD at α 0.05 | 2.70 | 0.26 | 0.25 |
| Interactions | |||
| Ca × B | --- | Figure 11 | --- |
| Level of Significance | NS | * | NS |
| Ca × Zn | --- | Figure 11 | Figure 12 |
| Level of Significance | NS | ** | ** |
| B × Zn | --- | --- | --- |
| Level of Significance | NS | NS | NS |
| Ca × B × Zn | --- | --- | --- |
| Level of Significance | NS | NS | NS |
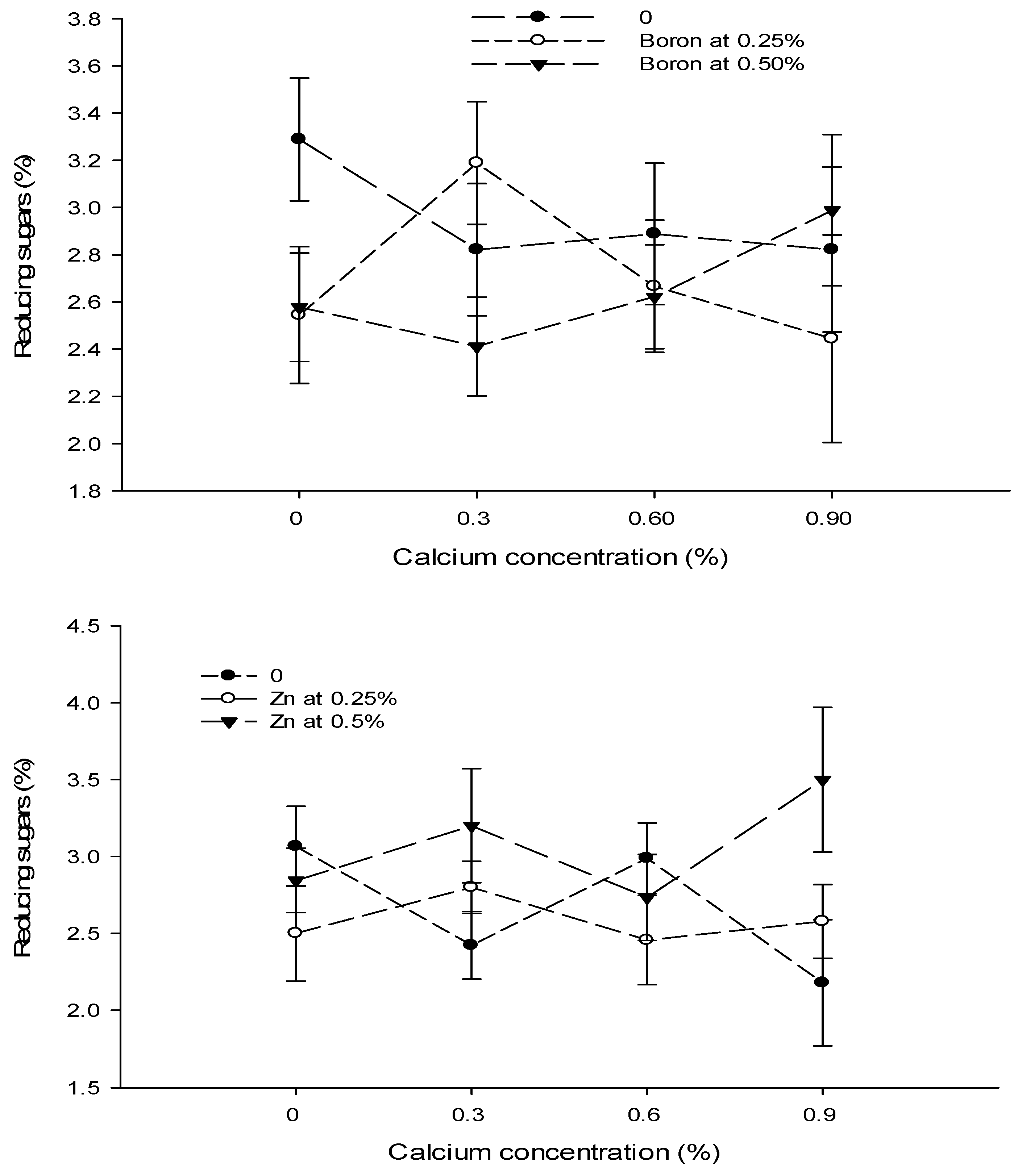

3.16. Reducing Sugars (%)
3.17. Non Reducing Sugars (%)
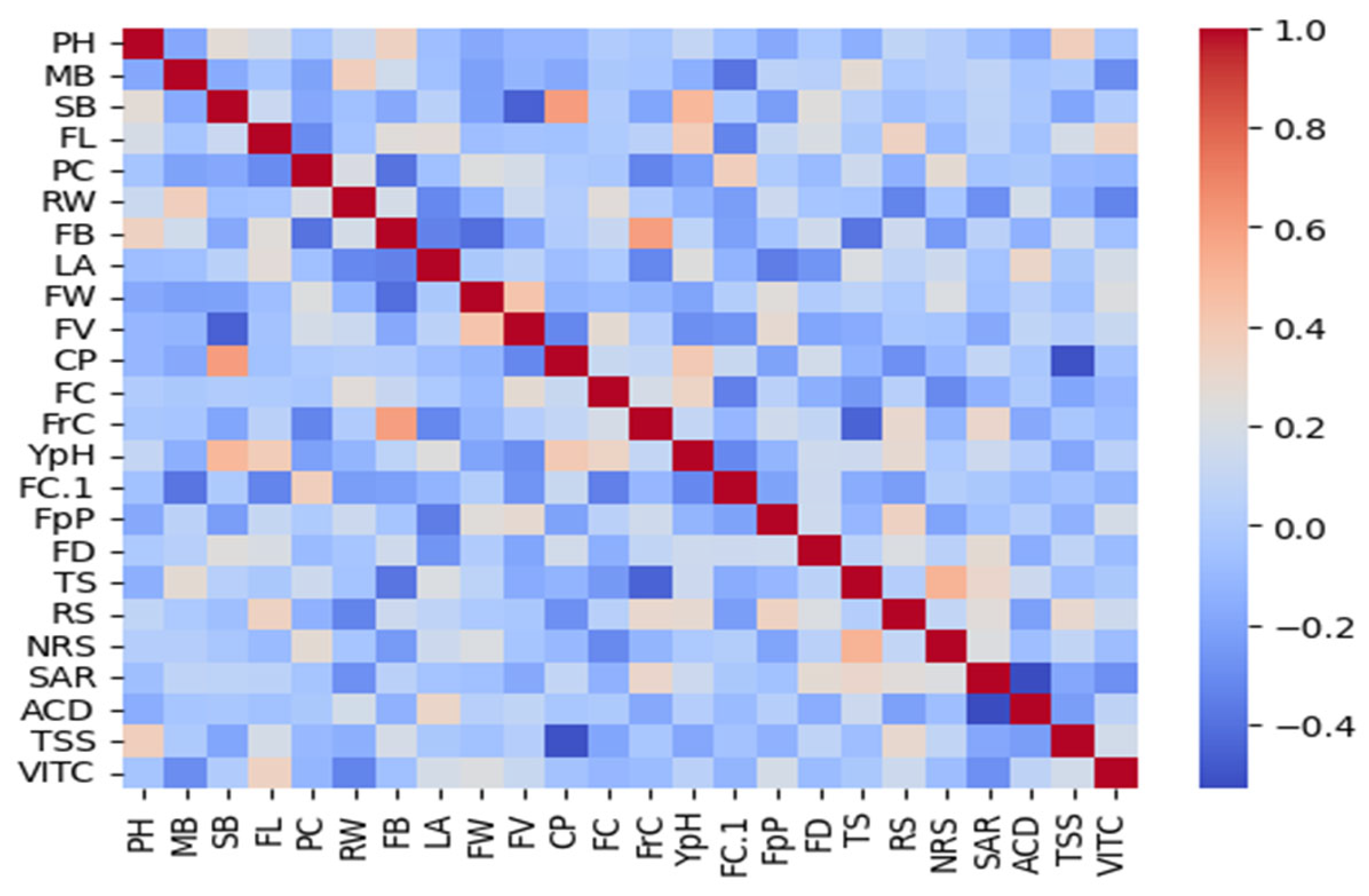
3.18. Correlation
3.19. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, W.; Wang, P.; Yuan, L.; Chen, X.; Hu, X. Effects of application methods of boron on tomato growth, fruit quality and flavor. Horticulturae 2021, 7, 223. [Google Scholar] [CrossRef]
- Terada, N.; Dissanayake, K.; Okada, C.; Sanada, A.; Koshio, K. Micro-tom tomato response to fertilization rates and the effect of cultivation systems on fruit yield and quality. Horticulturae 2023, 9, 367. [Google Scholar] [CrossRef]
- Lisboa, L.A.M.; Galindo, F.S.; Pagliari, P.H.; Goncalves, J.I.U.P.; Okazuka, M.H.; Cunha, M.L.O.; de Figueiredo, P.A.M. Morpho-physiological assessment of tomato and bell pepper in response to nutrient restriction. Stresses 2024, 4, 172–184. [Google Scholar] [CrossRef]
- Food and Agriculture Organization Corporate Statistical Database. 2024. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 18 September 2023).
- Ahmed, R.; Yusoff Abd Samad, M.; Uddin, M.K.; Quddus, M.A.; Hossain, M.A.M. Recent trends in the foliar spraying of zinc nutrient and zinc oxide nanoparticles in tomato production. Agronomy 2021, 11, 2074. [Google Scholar] [CrossRef]
- Trdan, S.; Vucajnk, F.; Bohinc, T.; Vidrih, M. The effect of a mixture of two plant growth-promoting bacteria from Argentina on the yield of potato, and occurrence of primary potato diseases and pest–short communication. Acta Agric. Scand. 2019, 69, 89–94. [Google Scholar] [CrossRef]
- Gabor, B.; Wieba, W. Tomato Disease guide: A Practical Guide for Seedsmen, Growers and Agricultural Advisors; Senminis Vegetable Seeds: Woodland, CA, USA, 1997; pp. 1–62. [Google Scholar]
- Karlsons, A.; Osvalde, A.; Cekstere, G.; Abolin, A.L. Effects of Ca sprays on fruit Ca content and yield of tomato variety susceptible to blossom-End Rot. Plants 2023, 12, 1640. [Google Scholar] [CrossRef]
- Horimoto, S.; Fukuda, K.; Yoshimura, J.; Ishida, A. Fresh-marketable tomato yields enhanced by moderate weed control and suppressed fruit dehiscence with woodchip mulching. Sci. Rep. 2022, 12, 13283. [Google Scholar] [CrossRef]
- Topcu, Y.; Nambeesan, S.U.; van der Knaap, E. Blossom-end rot: A century-old problem in tomato (Solanum lycopersicum L.) and other vegetables. Mol. Hortic. 2022, 2, 1. [Google Scholar] [CrossRef]
- Vanderlinden, C. Identification and Controlling Blossom End Rot. 2009. Available online: http://organicgardening.about.com/od/disease/p/blossomendrot.htm (accessed on 19 August 2023).
- La Spada, P.; Dominguez, E.; Continella, A.; Heredia, A.; Gentile, A. Factors influencing fruit cracking: An environmental and agronomic perspective. Front. Plant Sci. 2024, 15, 1343452. [Google Scholar] [CrossRef]
- Sano, O.; Hikawa, M.; Imanishi, S. Reduction of radial fruit cracking by single spraying of forchlorfenuron (1-(2-chloro-4-pyridyl)-3-phenylurea) of fruit clusters in tomato production under rain shelter. Hortic. Res. 2018, 17, 87–93. [Google Scholar] [CrossRef]
- Davarpanah, S.; Tehranifar, A.; Abadía, J.; Val, J.; Davarynejad, G.; Aran, M.; Khorassani, R. Foliar calcium fertilization reduces fruit cracking in pomegranate (Punica granatum cv. Ardestani). Sci. Hortic. 2018, 230, 86–91. [Google Scholar] [CrossRef]
- Lin, H.; Wu, Z.; Zhou, R.; Chen, B.; Zhong, Z.; Jiang, F. SlGH9-15 regulates tomato fruit cracking with hormonal and abiotic stress responsiveness cis-elements. J. Integr. Agric. 2023, 22, 447–463. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Kumar, P.J. Revitalitaion of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.M. Developing an integrated pest management program for tomatoes in The Red River Delta of Vietnam: A mini review. Aceh Int. J. Sci. Technol. 2015, 4, 41–53. [Google Scholar] [CrossRef]
- Brutti, L.; Alvarado, P.; Rojas, T.; Martensoon, A. Tomato seedling development is improved by a substrate inoculated with a combination of rhizobacteria and fungi. Acta Agric. Scand. Sect. B. 2015, 65, 170–176. [Google Scholar] [CrossRef]
- Zhong, W.L.; Yuan, W.Q.; Huang, X.M.; Wang, H.C.; Li, J.G.; Zhang, C.L. A study on the absorption of exogenous calcium and sucrose and their deposit onto the cell walls in litchi pericarp. J. Fruit. Sci. 2006, 23, 350–354. [Google Scholar]
- Santos, E.; Montanha, G.S.; Agostinho, L.F.; Polezi, S.; Marques, J.P.R.; de Carvalho, H.W.P. Foliar calcium absorption by tomato plants: Comparing the effects of calcium sources and adjuvant usage. Plants 2023, 12, 2587. [Google Scholar] [CrossRef]
- Zhang, X.P.; Ma, C.X.; Sun, L.R.; Hao, F.S. Roles and mechanisms of Ca2+ in regulating primary root growth of plants. Plant Signal. Behav. 2020, 15, 1748283. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- de Freitas, S.T.; do Amarante, C.V.T.; Mitcham, E.J. Calcium deficiency disorders in plants. In Postharvest Ripening Physiology of Crops; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781498703819. [Google Scholar]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gillham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef]
- Nissen, R.; Bound, S.; Adhikari, R.; Cover, I. Factors Affecting Postharvest Management of Apples: A Guide to Optimizing Quality; National Library of Australia: Canberra, Australia, 2018. [Google Scholar]
- Gupta, U.; Solanki, H. Impact of boron deficiency on plant growth. Int. J. Bioassays 2013, 2, 1048–1050. [Google Scholar]
- Yamauchi, T.; Hara, T.; Sonoda, Y. Distribution of calcium and boron in the pectin fraction of tomato leaf cell wall. Plant Cell Physiol. 1986, 27, 729–732. [Google Scholar]
- Imtiaz, M.; Rashid, A.; Khan, P.; Memon, M.Y.; Aslam, M. The role of micronutrients in crop production and human health. Pak. J. Bot. 2010, 42, 2565–2578. [Google Scholar]
- Singh, P.; Singh, J.; Ray, S.; Rajput, R.; Vaishnav, A.; Singh, R.; Singh, H. Seed biopriming with antagonistic microbes and ascorbic acid induce resistance in tomato against Fusarium wilt. J. Microbiol. Res. 2020, 237, 126482. [Google Scholar] [CrossRef] [PubMed]
- Gunes, A.; Bagci, A.; Pilbeam, D. Silicon-mediated changes of some physiological and enzymatic parameters symptomatic for oxidative stress in spinach and tomato grown in sodic-B toxic soil. Plant Soil. 2007, 290, 103–114. [Google Scholar] [CrossRef]
- Gupta, P.; Usmani, V.Z.; Rani, R.; Chandra, A.; Gupta, V. Implications of plant growth promoting Klebsiella sp. CPSB4 and Enterobacter sp. CPSB49 in luxuriant growth of tomato plants under chromium stress. Chemosphere 2020, 240, 124944. [Google Scholar] [CrossRef]
- Adrian, W.T.; Stevens, M.L. Effect of different sample preparation methods on the atomic absorption spectrophotometric determination of calcium in plant material. Analyst 1977, 102, 446–452. [Google Scholar] [CrossRef]
- Dey, S.C. Tomato Crop. In Vegetable Growing; Agrobios: New Delhi, India, 2000. [Google Scholar]
- Iqtidar, A.K. ; Saleemullah. Text Book of Chemistry One. (Bioanalytical Chemistry); National Book Foundation: Islamabad, Pakistan, 2004; pp. 39–40. [Google Scholar]
- Statistix_8 Analytical Software, Statistix_8 User’s Manual; Analytical Software: Tallahassee, FL, USA, 2003.
- Jan, M.T.; Shah, P.; Hollington, P.A.; Khan, M.J.; Sohail, Q. Agriculture Research: Design and Analysis, A Monograph; NWFP Agricultural University: Peshawar, Pakistan, 2009. [Google Scholar]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Greenacre, M.; Groenen, P.J.F.; Hastie, T.; D’Enza, A.I.; Markos, A.; Tuzhilina, E. Principal component analysis. Nat. Rev. Meth. Primers 2022, 2, 100. [Google Scholar] [CrossRef]
- Galeriani, T.M.; Neves, G.O.; Santos Ferreira, J.H.; Oliveira, R.N.; Oliveira, S.L.; Calonego, J.C.; Crusciol, C.A.C. Calcium and boron fertilization improves soybean photosynthetic efficiency and grain yield. Plants 2022, 11, 2937. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hossain, M.R.; Hossain, A.; Khan, M.I. Impact of foliar boron application on the growth and yield of summer tomato. J. Agrofor. Environ. 2023, 16, 58–63. [Google Scholar] [CrossRef]
- Michailidis, M.; Bazakos, C.; Kollaros, M.; Adamakis, I.S.; Ganopoulos, I.; Molassiotis, A.; Georgia Tanou, G. Boron stimulates fruit formation and reprograms developmental metabolism in sweet cherry. Physiol. Plant. 2023, 175, e13946. [Google Scholar] [CrossRef] [PubMed]
- Khatri, D.; Bhattarai, K.; Thapa, P.; Wagle, N.; Yadav, P.K. Effect of foliar spray of zinc and boron on performance of tomato (Solanum lycopersicum) CV. manisha under net house condition. Russ. J. Agric. Socio-Econ. Sci. 2022, 125, 252–261. [Google Scholar]
- Hapuarachchi, N.S.; Kämper, W.; Wallace, H.M.; Hosseini Bai, S.; Ogbourne, S.M.; Nichols, J.; Trueman, S.J. Boron effects on fruit set, yield, quality and paternity of hass avocado. Agronomy 2022, 12, 1479. [Google Scholar] [CrossRef]
- De Silva, A.L.; Kämper, W.; Wallace, H.M.; Ogbourne, S.M.; Hosseini Bai, S.; Nichols, J.; Trueman, S.J. Boron effects on fruit set, yield, quality and paternity of macadamia. Agronomy 2022, 12, 684. [Google Scholar] [CrossRef]
- Arakawa, R.; Fujimoto, H.; Kameoka, H.; Toriyama, S.; Yoshida, Y.; Watanabe, T.; Maruyama, H. Effects of different potassium nitrate concentrations and temperature conditions on the elemental composition and blossom-end rot in paprika (Capsicum annuum L.). Hort. J. 2021, 90, 401–409. [Google Scholar] [CrossRef]
- Indeche, A.K.; Yoshida, Y.; Goto, T.; Yasuba, K.; Tanaka, Y. Effect of defoliation on blossom-end rot incidence and calcium transport into fruit of tomato cultivars under moderate water stress. Hort. J. 2020, 89, 22–29. [Google Scholar] [CrossRef]
- Reitz, N.F.; Shackel, K.A.; Mitcham, E.J. Differential effects of excess calcium applied to whole plants vs. excised fruit tissue on blossom-end rot in tomato. Sci. Hortic. 2021, 290, 110514. [Google Scholar] [CrossRef]
- Vera-Maldonado, P.; Aquea, F.; Reyes-Díaz, M.; Cárcamo-Fincheira, P.; Soto-Cerda, B.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Role of boron and its interaction with other elements in plants. Front. Plant Sci. 2024, 15, 1332459. [Google Scholar] [CrossRef]
- Katsumi, S.; Suzuki, D.G.; Numajiri, M.; Matsushiro, J.; Yamane, M.; Kiriiwa, Y. Anatomical analysis of internally Brown tomato Fruit. Hort. J. 2019, 88, 263–269. [Google Scholar]
- Zamban, D.T.; Prochnow, D.; Caron, B.O.; Turchetto, M.; Fontana, D.C.; Schmidt, D. Applications of calcium and boron increase yields of Italian tomato hybrids (Solanum lycopersicum) in two growing seasons. Rev. Colomb. Cienc. Hortic. 2018, 12, 82–93. [Google Scholar] [CrossRef]
- Davis, J.M.; Sanders, D.C.; Nelson, P.V.; Lengnick, L.; Sperry, W.J. Boron improves growth, yield, quality, and nutrient content of tomato. J. Am. Soc. Hortic. Sci. 2003, 128, 441–446. [Google Scholar] [CrossRef]
- Gate, T.; Hill, L.; Miller, A.J.; Sanders, D. AtIAR1 is a Zn transporter that regulates auxin metabolism in Arabidopsis thaliana. J. Exp. Bot. 2024, 7528, 1437–1450. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.; Mu, J.; Tian, H.; Gao, D.; Zhou, H.; Guo, L.; Shao, X.; Geng, Y.; Zhang, Q. Zinc regulation of chlorophyll fluorescence and carbohydrate metabolism in saline-sodic stressed rice seedlings. BMC Plant Biol. 2024, 24, 464. [Google Scholar] [CrossRef]
- Haleema, B.; Rab, A.; Hussain, S.A. Effect of calcium, boron and zinc foliar application on growth and fruit production of tomato. Sarhad J. Agric. 2018, 34, 19–30. [Google Scholar] [CrossRef]
- Bastakoti, S. Role of zinc in management of plant diseases: A review. Cogent Food Agric. 2023, 9, 2194483. [Google Scholar] [CrossRef]
- Schumann, C.; Winkler, A.; Knoche, M. Calcium decreases cell wall swelling in sweet cherry fruit. Sci. Rep. 2022, 12, 16496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghanbarpour, E.; Rezaei, M.; Lawson, S. Reduction of cracking in pomegranate fruit after foliar application of humic acid, calcium-boron and Kaolin during water stress. Erwerbs-Obstbau 2019, 61, 29–37. [Google Scholar] [CrossRef]
- Seo, H.J.; Sawant, S.S.; Song, J. Fruit cracking in pears: Its cause and management—A review. Agronomy 2022, 12, 2437. [Google Scholar] [CrossRef]
- Yan, F.; Gong, Z.; Hu, G.; Ma, X.; Bai, R.; Yu, R.; Zhang, Q.; Deng, W.; Li, Z.; Wuriyanghan, H. Tomato SlBL4 plays an important role in fruit pedicel organogenesis and abscission. Hortic Res. 2021, 8, 78. [Google Scholar] [CrossRef]
- Singh, A.; Shukla, A.K.; Meghwal, P.R. Fruit cracking in pomegranate: Extent, cause, and management—A review. Int. J. Fruit Sci. 2020, 20, S1234–S1253. [Google Scholar] [CrossRef]
- Draie, E.; Aboras, A. Effectiveness of zinc foliar spraying on the cracking of pomegranate fruits. Int. Res. J. Innov. Eng. Technol. 2021, 5, 147–155. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Almendros, P.; Gonzalez, D.; Fernandez, M.D.; García-Gomez, C.; Obrador, A. Both Zn biofortification and nutrient distribution pattern in cherry tomato plants are influenced by the application of ZnO nanofertilizer. Heliyon 2022, 8, e09130. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.F.; Mohamed, M.M.; Abou El-Khashab, A.M.; Aeed, S.H. Controlling fruit splitting and improving productivity of manfalouty pomegranate trees by using salicylic acid and some nutrients. World Rural Observ. 2014, 6, 87–93. [Google Scholar]
- Serrano, M.; Amoros, A.; Pretel, M.T.; Madrid, M.C.M.; Madrid, R.; Romojaro, F. Effect of calcium deficiency on water melon. Food Sci. Technol. Int. 2002, 8, 147–154. [Google Scholar] [CrossRef]
- Cronje, R.B.; Sivakumar, D.; Mostert, P.G.; Korsten, L. Effect of different preharvest treatment regimes on fruit quality of litchi Cv. Maritius. J. Plant Nutr. 2009, 32, 19–29. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Smit, J.N.; Combrink, N.J.J. Pollination and yield of winter-grown greenhouse tomatoes as affected by boron nutrition, cluster vibration and relative humidity. S. Afr. J. Plant Soil. 2005, 22, 110–115. [Google Scholar] [CrossRef]
- Khalifa, R.K.M.; Omaima, M.H.; Abd-El-Khair, H. Influence of foliar spraying with boron and calcium on productivity, fruit quality, nutritional status and controlling of blossom end rot disease of Anna apple trees. World J. Agric. Sci. 2009, 5, 237–249. [Google Scholar]
- Hacisalihoglu, G.; Hart, J.J.; Wang, Y.H.; Cakmak, I.; Kochian, L.V. Zinc efficiency is correlated with enhanced expression and activity of zinc-requiring enzymes in wheat. Plant Physiol. 2003, 131, 602. [Google Scholar] [CrossRef]
- Abedy, A. Effects of Zinc Sulfate and Citric Acid Spray on Fruit Characteristics of Tomato Cultivar ‘Urbana’. Master’s Thesis, Shiraz University, Shiraz, Iran, 2001. [Google Scholar]
- Naik, D.M.; Mulekar, V.G.; Chandel, C.G.; Kapse, B.M. Effect of prepackaging on physico-chemical changes in tomato (Lycopersicon esculentum Mill.) during storage. Indian Food Pack. 1993, 12, 9–13. [Google Scholar]
- Rohani, A.; Nazni, W.A.; Ngo, L.V.; Ibrahim, J.; Lee, H.L. Adulticidal properties of the essential extracts of some Malaysian plants on vector mosquitoes. Trop. Biomed. 1997, 14, 5–9. [Google Scholar]
- Cheour, F.; Willemot, C.J.; Arul, Y.; Desjardins, J.; Makhlouf, P.M.; Gosselin, A. Effects of foliar application of CaCl2 on postharvest strawberry ripening. J. Amer. Soc. Hort. Sci. 1990, 115, 789–792. [Google Scholar] [CrossRef]
- Sathya, S.; Mani, S.; Mahedran, P.P.; Arulmozhiselvan, K. Effect of application of boron on growth, quality and fruit yield of PKM 1 tomato. Indian. J. Agric. Res. 2010, 44, 274–280. [Google Scholar]
- Kazemi, M. Effects of Zn, Fe and their Combination Treatments on the growth and yield of tomato. Bull. Environ. Pharmacol. Life Sci. 2013, 3, 109–114. [Google Scholar]
- Rab, A.; Haq, I. Foliar application of calcium chloride and borax influences plant growth, yield, and quality of tomato (Lycopersicon esculentum Mill.) fruit. Turk. J. Agric. For. 2012, 36, 695–701. [Google Scholar] [CrossRef]
- Brahmachari, V.S.; Rani, R. Effect of growth substances on productivity cracking, ripening and quality of fruits of litchi. Orissa J. Hort. 2001, 29, 44–45. [Google Scholar]
- Mishra, L.N.; Singh, S.K.; Sharma, H.C.; Goswami, A.M.; Bhana, P. Effect of micronutrients and rootstocks on fruit yield and quality of kinnow under high density planting. Ind. J. Hort. 2003, 60, 131–134. [Google Scholar]
- Haq, I. Evaluation of Fruit Quality and Amelioration of Fruit Cracking in Litchi (litchi chinensis sonn.) Cultivars through Water and Nutrient Management. Ph.D. Thesis, The University of Agriculture Peshawar, Peshawar, Pakistan, 2011. [Google Scholar]
- Gharezi, M.; Joshi, N.; Sadeghian, E. Effect of post-harvest treatment on stored cherry tomatoes. J. Nutr. Food Sci. 2012, 2, 157. [Google Scholar] [CrossRef]
- Dubey, B.K.; Sinha, P.; Chatterjee, C. Effect of zinc on the yield and quality of tomato. Indian J. Hort. 2003, 60, 59–63. [Google Scholar]
- Kumari, A.; Singh, R.K.; Parmar, A.S. Effect of foliar application of micronutrients on yield characters and yield of tomato (Lycopersicon esculentum Mill). Technofame. J. Multidiscip. Adv. Res. 2012, 1, 10–14. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haleema, B.; Shah, S.T.; Basit, A.; Hikal, W.M.; Arif, M.; Khan, W.; Said-Al Ahl, H.A.H.; Fhatuwani, M. Comparative Effects of Calcium, Boron, and Zinc Inhibiting Physiological Disorders, Improving Yield and Quality of Solanum lycopersicum. Biology 2024, 13, 766. https://doi.org/10.3390/biology13100766
Haleema B, Shah ST, Basit A, Hikal WM, Arif M, Khan W, Said-Al Ahl HAH, Fhatuwani M. Comparative Effects of Calcium, Boron, and Zinc Inhibiting Physiological Disorders, Improving Yield and Quality of Solanum lycopersicum. Biology. 2024; 13(10):766. https://doi.org/10.3390/biology13100766
Chicago/Turabian StyleHaleema, Bibi, Syed Tanveer Shah, Abdul Basit, Wafaa M. Hikal, Muhammad Arif, Waleed Khan, Hussein A. H. Said-Al Ahl, and Mudau Fhatuwani. 2024. "Comparative Effects of Calcium, Boron, and Zinc Inhibiting Physiological Disorders, Improving Yield and Quality of Solanum lycopersicum" Biology 13, no. 10: 766. https://doi.org/10.3390/biology13100766
APA StyleHaleema, B., Shah, S. T., Basit, A., Hikal, W. M., Arif, M., Khan, W., Said-Al Ahl, H. A. H., & Fhatuwani, M. (2024). Comparative Effects of Calcium, Boron, and Zinc Inhibiting Physiological Disorders, Improving Yield and Quality of Solanum lycopersicum. Biology, 13(10), 766. https://doi.org/10.3390/biology13100766










