Bioactive Peptides from Corn (Zea mays L.) with the Potential to Decrease the Risk of Developing Non-Communicable Chronic Diseases: In Silico Evaluation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Study Design
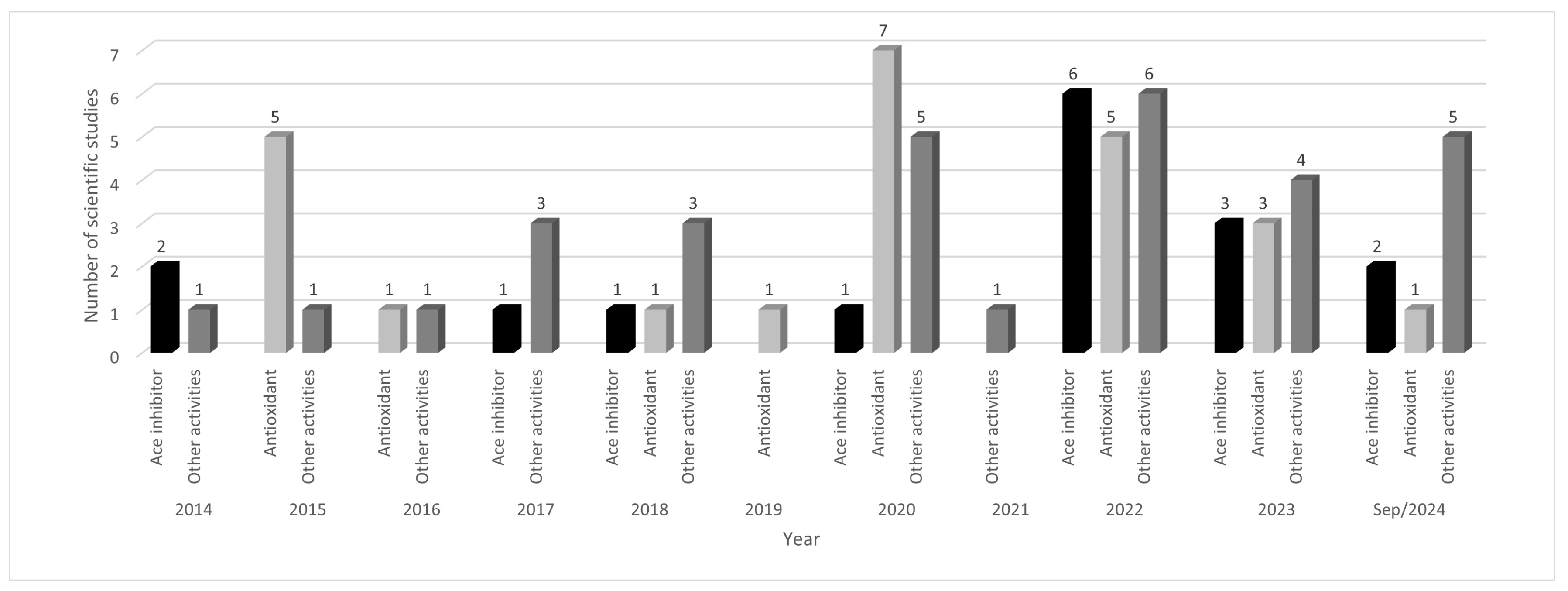
2.1. Selection of the Studies in the Literature
2.2. Use of Database to Predict Bioactive Peptides
3. Corn Production, Processing and Composition
4. Obtention of Bioactive Peptides
4.1. Production of Bioactive Peptides
4.2. Enzymatic Hydrolysis of Proteins
5. Non-Communicable Chronic Diseases
6. Studies Reported in the Literature
6.1. In Vitro Evidence of Health Benefits Associated with Corn Bioactive Peptides
- (1)
- 19ZP1 (FNQLAALNSAAYLQQQQLLPFSQLA)
- (2)
- 19ZP2 (QLADVSPAAFLTQQQLLPFYLHAM)
- (3)
- 19ZP3 (AYLQAQQLLPFNQLVRSPAA)
6.2. In Vivo Evidence of Health Benefits Associated with Corn Bioactive Peptides
6.3. In Silico Approach to Investigate Bioactive Peptides
6.4. In Silico Prediction of Bioactive Peptides from Corn Storage Proteins
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Okoye, C.O.; Ezeorba, T.P.C.; Okeke, E.S.; Okagu, I.U. Recent findings on the isolation, identification and quantification of bioactive peptides. Appl. Food Res. 2022, 2, 100065. [Google Scholar] [CrossRef]
- Trinidad-Calderón, P.A.; Acosta-Cruz, E.; Rivero-Massante, M.N.; Díaz-Gómez, J.L.; García-Lara, S.; López-Castillo, L.M. Maize bioactive peptides: From structure to human health. J. Cereal Sci. 2021, 100, 103232. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, J.; Ma, H.; Yagoub, A.E.A.; Yu, X.; Owusu, J.; Ma, H.; Qin, X. Antioxidant peptides from corn gluten meal: Orthogonal design evaluation. Food Chem. 2015, 187, 270–278. [Google Scholar] [CrossRef]
- Qu, W.; Feng, Y.; Xiong, T.; Li, Y.; Wahia, H.; Ma, H. Preparation of corn ACE inhibitory peptide-ferrous chelate by dual-frequency ultrasound and its structure and stability analyses. Ultrason. Sonochem. 2022, 83, 105937. [Google Scholar] [CrossRef]
- Wang, X.; Fu, J.; Bhullar, K.S.; Chen, B.; Liu, H.; Zhang, Y.; Wang, C.; Liu, C.; Su, D.; Ma, X.; et al. Identification, in silico selection, and mechanistic investigation of antioxidant peptides from corn gluten meal hydrolysate. Food Chem. 2024, 446, 138777. [Google Scholar] [CrossRef]
- Jing, Y.; Liu, X.; Wang, J.; Zheng, X. Corn protein hydrolysate with glutamine-rich peptides protects intestinal barrier in Caco-2 cells: Insights into structural characteristics of identified glutamine peptides. J. Od. Funct. Foods 2024, 117, 106232. [Google Scholar] [CrossRef]
- Ortiz-Martinez, M.; Gonzalez de Mejia, E.; García-Lara, S.; Aguilar, O.; Lopez-Castillo, L.M.; Otero-Pappatheodorou, J.T. Antiproliferative effect of peptide fractions isolated from a quality protein maize, a white hybrid maize, and their derived peptides on hepatocarcinoma human HepG2 cells. J. Funct. Foods 2017, 34, 36–48. [Google Scholar] [CrossRef]
- Liang, Q.; Chalamaiah, M.; Ren, X.; Ma, H.; Wu, J. Identification of new anti-inflammatory peptides from zein hydrolysate after simulated gastrointestinal digestion and transport in CaCO-2 cells. J. Agric. Food Chem. 2018, 66, 1114–1120. [Google Scholar] [CrossRef]
- Hira, T.; Mochida, T.; Mijashita, K.; Hara, H. GLP-1 secretion is enhanced directly in the ileum but indirectly in the duodenum by a newly identified potent stimulator, zein hydrolysate, in rats. Am. J. Physiol.—Gastrointest. Liver Physiol. 2009, 297, 663–671. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Non-Communicable Diseases. 2023. Geneva: WHO. Non-Communicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases#:~:text=Noncommunicable%20diseases%20(NCDs)%20kill%2041,%2D%20and%20middle%2Dincome%20countries (accessed on 22 October 2023).
- American Heart Association. 2023. Available online: https://www.heart.org/en/health-topics/high-blood-pressure (accessed on 12 December 2023).
- Yeo, J.; Shahidi, F. Bioactive peptides in health and disease: An overview. Biol. Act. Pept. 2021, 1, 1–26. [Google Scholar]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gómez, J.L.; Neundorf, I.; López-Castillo, L.; Castorena-Torres, F.; Serna-Saldívar, S.O.; García-Lara, S. In silico analysis and in vitro characterization of the bioactive profile of three novel peptides identified from 19 kDa α-zein sequences of maize. Molecules 2020, 25, 5450. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Iwaniak, P.; Darewicz, M. BIOPEP-UWM virtual—A novel database of food-derived peptides with in silico-predicted biological activity. Appl. Sci. 2022, 12, 7204. [Google Scholar] [CrossRef]
- Foreign Agricultural Service. 2024. Corn 2024. Available online: https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=0440000 (accessed on 19 May 2024).
- FAO—Food and Agriculture Organization of the United Nations. 2024. Available online: https://www.fao.org/giews/countrybrief/country.jsp?code=BRA (accessed on 25 May 2024).
- Revilla, P.; Alves, M.L.; Andelković, V.; Balconi, C.; Dinis, I.; Mendes-Moreira, P.; Redaelli, R.; Ruiz de Galarreta, J.I.; Patto, M.C.V.; Žilić, S.; et al. Traditional foods from maize (Zea mays L.) in Europe. Front. Nutr. 2021, 8, 683399. [Google Scholar] [CrossRef]
- Budak, F.; Aydemir, S.K. Grain yield and nutritional values of sweet corn (Zea mays Var. Saccharata) in produced with good agricultural implementation. Nutr. Int. J. Food Sci. 2018, 7, 555710. [Google Scholar]
- Vidal, J.K.; Simões, C.T.; Mallmann, A.O.; Tyska, D.; Pereira, H.V.; Mallmann, C.A. A three-year study on the nutritional composition and occurrence of mycotoxins of corn varieties with different transgenic events focusing on poultry nutrition. Vet. Sci. 2024, 11, 97. [Google Scholar] [CrossRef]
- Deepak, T.S.; Jayadeep, P.A. Prospects of maize (corn) wet milling by-products as a source of functional food ingredients and nutraceuticals. Food Technol. Biotechnol. 2022, 60, 109–120. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, S.; Li, L.; Zhang, M.; Tian, S.; Wang, D.; Liu, K.; Liu, H.; Zhu, W.; Wang, X. Comprehensive utilization of corn starch processing by-products: A review. Grain Oil Sci. Techn. 2021, 4, 89–107. [Google Scholar] [CrossRef]
- Hu, R.; Xu, J.; Qi, G.; Wang, W.; Sun, X.S.; Li, Y. Antioxidative hydrolysates from corn gluten meal may effectively reduce lipid oxidation and inhibit HepG2 cancer cell growth. J. Agric. Food Res. 2022, 7, 100252. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Liu, X.; Sun, Y.; Zheng, Y.; Wang, X.; Liu, Y. Effect of hydrolysis time on the physicochemical and functional properties of corn glutelin by protamex hydrolysis. Food Chem. 2015, 172, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Song, R. The regulation of zein biosynthesis in maize endosperm. Theor. Appl. Genet. 2020, 133, 1443–1453. [Google Scholar] [CrossRef]
- Giteru, S.G.; Ali, M.A.; Oey, I. Recent progress in understanding fundamental interactions and applications of zein. Food Hydrocoll. 2021, 120, 106948. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Esfandiari, Z.; Caraca, A.C.; Jafari, S.M.; Rostamabadi, H. Recent trends in the application of protein electrospun fibers for loading food bioactive compounds. Food Chem. X 2023, 20, 100922. [Google Scholar] [CrossRef]
- Liu, C.; Hayat, U.; Raza, A.; Jia, C.; Wang, J. Zein-based injectable biomaterial and angiogenic activity through peptides produced by enzymatic degradation. J. Drug Deliv. Sci. Technol. 2022, 78, 103937. [Google Scholar] [CrossRef]
- Kasaai, M.R. Zein and zein-based nano-materials for food and nutrition applications: A review. Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.; Shim, J.; El-Aty, A.M.A. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and by-products: A review. Front. Nutr. 2021, 8, 815640. [Google Scholar] [CrossRef]
- Amigo, L.; Hernández-Ledesma, B. Current evidence on the bioavailability of food bioactive peptides. Molecules 2020, 25, 4479. [Google Scholar] [CrossRef]
- Chinnadurai, R.K.; Khan, N.; Meghwanshi, G.K.; Ponne, S.; Althobiti, M.; Kumar, R. Current research status of anti-cancer peptides: Mechanism of action, production, and clinical applications. Biomed. Pharmacother. 2023, 164, 114996. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Nasri, M. Basic and recent advances in marine antihypertensive peptides: Production, structure-activity relationship and bioavailability. Trends Food Sci. Technol. 2019, 88, 543–557. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive peptides: Synthesis, sources, applications, and proposed mechanisms of action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.S.; Yu, B.; Wu, D.; Lu, Y.; Wu, W.; Wang, J.; Zhang, Y.; Fu, Y. Zein-Derived Peptides from Corn Promote the Proliferation of C2C12 Myoblasts via Crosstalk of mTORC1 and mTORC2 Signaling Pathways. Foods 2024, 13, 919. [Google Scholar] [CrossRef] [PubMed]
- Félix-Medina, J.V.; Sepúlveda-Haro, A.G.; Quintero-Soto, M.F. Stability of antioxidant and hypoglycemic activities of peptide fractions of Maize (Zea mays L.) under different processes. Food Meas. 2023, 17, 362–370. [Google Scholar] [CrossRef]
- Trinidad-Calderón, P.A.; López-Castillo, L.M.; Díaz-Gómez, J.L.; Soto, R.B.M.; Castorena-Torres, F.; García-Lara, S. Acetone-precipitated zein protein hydrolysates from blue-maize selectively target hepatocellular carcinoma and fibroblasts in a dose-dependent manner. Food Hydrocoll. Health 2023, 3, 100106. [Google Scholar] [CrossRef]
- Díaz-Gómez, J.L.; Ortíz-Martínez, M.; Aguilar, O.; García-Lara, S.; Castorena-Torres, F. Antioxidant activity of zein hydrolysates from Zea Species and their cytotoxic effects in a hepatic cell culture. Molecules 2018, 23, 312. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Chalamaiah, M.; Liao, W.; Ren, X.; Ma, H.; Wu, J. Zein hydrolysate and its peptides exert anti-inflammatory activity on endothelial cells by preventing TNF-α-induced NF-κB activation. J. Funct. Foods 2020, 64, 103598. [Google Scholar] [CrossRef]
- Ulug, S.K.; Jahandideh, F.; Wu, J. Novel technologies for the production of bioactive peptides. Trends Food Sci. Technol. 2021, 108, 27–39. [Google Scholar] [CrossRef]
- Tian, R.; Feng, J.; Huang, G.; Tian, B.; Zhang, Y.; Jiang, L.; Sui, X. Ultrasound driven conformational and physicochemical changes of soy protein hydrolysates. Ultrason. Sonochem. 2020, 68, 105202. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Zandile, M.; Luo, X.; Duan, Y.; Ma, H. Structure of the zein protein as treated with subcritical water. Int. J. Food Prop. 2018, 21, 128–138. [Google Scholar] [CrossRef]
- Saadi, S.; Saari, N.; Anwar, F.; Hamid, A.A.; Ghazali, H.M. Recent advances in food biopeptides: Production, biological functionalities and therapeutic applications. Biotechnol. Adv. 2015, 33, 80–116. [Google Scholar] [CrossRef]
- Wang, X.; Codreanu, S.G.; Wen, B.; Li, K.; Chambers, M.C.; Liebler, D.C.; Zhang, B. Detection of proteome diversity resulted from alternative splicing is limited by trypsin cleavage specificity. Mol. Cell. Proteom. 2018, 17, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A Review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar] [PubMed]
- Peterle, D.; Pontarollo, G.; Spada, S.; Brun, P.; Palazzi, L.; Sokolov, A.V.; Spolaore, B.; Polverino de Laureto, P.; Vasiyev, V.B.; Castagliuolo, I.; et al. A serine protease secreted from Bacillus subtilis cleaves human plasma transthyretin to generate an amyloidogenic fragment. Commun. Biol. 2020, 3, 764. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association (ADA). Classification and diagnosis of diabetes: Standards of medical care in diabetes. Diabetes Care 2019, 42 (Suppl. S1), S13–S28. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kamata, A.; Konishi, T. Dipeptidyl peptidase-IV inhibitory peptides derived from salmon milt and their effects on postprandial blood glucose level. Fish. Sci. 2021, 87, 619–626. [Google Scholar] [CrossRef]
- Oseguera-Toledo, M.E.E.; González de Mejía, E.; Reynoso-Camacho, R.; Cardador-Martínez, A.; Amaya-Llano, S.L. Proteins and bioative peptides: Mechanisms of action on diabetes management. Nutrafoods 2014, 13, 147–157. [Google Scholar] [CrossRef]
- Abramić, M.; Agić, D. Survey of dipeptidyl peptidase III inhibitors: From small molecules of microbial or synthetic origin to aprotinin. Molecules 2022, 27, 3006. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, C.; Sun-Waterhouse, D.; Zhao, T.; Waterhouse, G.I.N.; Zhao, M.; Su, G. Identification of post-digestion angiotensin-I converting enzyme (ACE) inhibitory peptides from soybean protein isolate: Their production conditions and in silico molecular docking with ACE. Food Chem. 2021, 345, 12885. [Google Scholar] [CrossRef]
- Liu, W.; Fang, L.; Feng, X.; Li, G.; Gu, R. In vitro antioxidant and angiotensin-I converting enzyme inhibitory properties of peptides derived from corn gluten meal. Eur. Food Res. Technol. 2020, 246, 2017–2027. [Google Scholar] [CrossRef]
- de Fátima Garcia, B.; de Barros, M.; de Souza Rocha, T. Bioactive peptides from beans with the potential to decrease the risk of developing non-communicable chronic diaseases. Crit. Rev. Food Sci. Nutr. 2021, 61, 2003–2021. [Google Scholar] [CrossRef] [PubMed]
- Tonin, S.; Borba, H.H.; Wiens, A.; Fernandez-Llimos, F.; Pontarolo, R. Vitamins, antioxidants, and type 2 diabetes. In Diabetes; Academic Press: Cambridge, MA, USA, 2020; pp. 373–383. [Google Scholar]
- Ding, L.; Wang, L.; Zhang, T.; Yu, Z.; Liu, J. Hydrolysis and transepithelial transport of two corn gluten derived bioactive peptides in human Caco-2 cell monolayers. Food Res. Int. 2018, 106, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Cui, Z.; Wang, L.; Xu, H.; Zhang, Y. Production of bioactive peptides from corn gluten meal by solid-state fermentation with Bacillus subtilis MTCC5480 and evaluation of its antioxidant capacity in vivo. LWT—Food Sci. Technol. 2020, 131, 109767. [Google Scholar] [CrossRef]
- Lv, J.; Nie, Z.; Zhang, J.; Liu, F.; Wang, Z.; Ma, Z.; He, H. Corn peptides protect against thioacetamide-induced hepatic fibrosis in rats. J. Med. Food 2013, 16, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Salouti, M.; Mirzaei, F.; Shapuri, R.; Ahangari, A. Synergistic antibacterial activity of plant peptide MBP-1 and silver nanoparticles combination on healing of infected wound due to staphylococcus aureus. Jundishapur J. Microbiol. 2016, 9, e27997. [Google Scholar] [CrossRef]
- Sharma, S.; Pradhan, R.; Manickavasagan, A.; Thimmanagari, M.; Dutta, A. Corn distillers solubles as a novel bioresource of bioactive peptides with ACE and DPP IV inhibition activity: Characterization, in silico evaluation, and molecular docking. Food Funct. 2022, 13, 8179–8203. [Google Scholar] [CrossRef]
- Chanajon, P.; Girgih, A.T.; Oluwagunwa, O.A.; Aluko, R.E.; Yongsawatdigul, J. Long-term intake of corn gluten meal protein hydrolysate attenuated hypertension development and modulated associated plasma metabolite levels in spontaneously hypertensive rats. J. Funct. Foods 2024, 117, 106231. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, X.; Zhang, S.; Wang, W.; Cai, M.; Li, Y.; Yang, F.; Guo, H. Protective effect of corn peptides against alcoholic liver injury in men with chronic alcohol consumption: A randomized double-blind placebo-controlled study. Lipids Health Dis. 2014, 13, 192–201. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Wang, J.; Xing, L. Preparation of active corn peptides from zein through double enzymes immobilized with calcium alginate–chitosan beads. Process Biochem. 2014, 49, 1682–1690. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Lin, S.; Liu, X.; Yang, S.; Jones, G.S. Analysis of DPPH inhibition and structure change of corn peptides treated by pulsed electric field technology. J. Food Sci. Technol. 2015, 52, 4342–4350. [Google Scholar] [CrossRef]
- Jin, D.; Liu, X.; Zheng, X.; Wang, X.; He, J. Preparation of antioxidative corn protein hydrolysates, purification and evaluation of three novel corn antioxidant peptides. Food Chem. 2016, 204, 427–436. [Google Scholar] [CrossRef]
- Wang, C.; He, H.; Zhang, J.; Li, X.; Ma, Z. High performance liquid chromatography (HPLC) fingerprints and primary structure identification of corn peptides by HPLC-diode array detection and HPLC-electrospray ionization tandem mass. J. Food Drug Anal. 2016, 24, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Chen, G.; Li, Y. Production and characterization of antioxidative hydrolysates and peptides from corn gluten meal using papin, ficin, and bromelain. Molecules 2020, 25, 4091. [Google Scholar] [CrossRef]
- Li, C.; Lee, Y.; Lo, H.; Huang, Y.; Hsiang, C.; Ho, T. Antihypertensive effects of corn silk extract and its novel bioactive constituent in spontaneously hypertensive rats: The involvement of angiotensin-converting enzyme inhibition. Molecules 2019, 24, 1886. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, K.; Wu, B.; Wu, P.; Duan, Y.; Ma, H. Production of ACE inhibitory peptides from corn germ meal by an enzymatic membrane reactor with a novel gradient diafiltration feeding working-mode and in vivo evaluation of antihypertensive effect. J. Funct. Foods 2020, 64, 103584. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Zheng, X.; Qu, Y. Antagonistic effect of the glycopeptide from zein on acute alcohol-induced liver injury in mice. J. Funct. Foods 2022, 92, 105062. [Google Scholar] [CrossRef]
- Iwaniak, A.; Darewicz, M.; Mogut, D.; Minkiewicz, P. Elucidation of the role of in silico methodologies in approaches to studying bioactive peptides derived from foods. J. Funct. Foods 2019, 61, 103486. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicv, P.; Darewicz, M. BIOPEP-UWM database—present and future. Curr. Opin. Food Sci. 2024, 55, 101108. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, C.; Hu, Y.; Gao, M.; Luan, G. Zein as a structural protein in gluten-free systems: An overview. Food Sci. Hum. Wellness 2021, 10, 270–277. [Google Scholar] [CrossRef]
- Argos, P.; Pedersen, K.; Marks, M.D.; Larkins, B.A. A structural model for maize zein proteins. J. Biol. Chem. 1982, 257, 9984–9990. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, L.; Li, F.; Shi, N.; Li, C.; Yu, X.; Chen, Y.; Kong, W. Design, fabrication and biomedical applications of zein-based nano/microcarrier system. Int. J. Pharm. 2016, 513, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Jao, C.; Hung, C.; Tung, Y.; Lin, P.; Chen, M.; Hsu, K. The development of bioactive peptides from dietary proteins as a dipeptidyl peptidase IV inhibitor for the management of type 2 diabetes. Biomedicine 2015, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Khaket, T.P.; Redhu, D.; Dhanda, S.; Singh, J. In silico evaluation of potential DPP-III inhibitor precursors from dietary proteins. Int. J. Food Prop. 2014, 18, 499–507. [Google Scholar] [CrossRef]
- Yano, S.; Suzuki, K.; Funatsu, G. Isolation from α-Zein of thermolysin peptides with angiotensin I-converting enzyme inhibitor activity. Biosci. Biotechnol. Biochem. 1996, 60, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Brown, M.R.; Lee, T.D.; Crim, J.W. RF-amide peptides isolated from the midgut of the corn earworm, Helicoverpa zea, resemble pancreatic polypeptide. Insect Biochem. Mol. Biol. 1998, 28, 345–356. [Google Scholar] [CrossRef]
- Maruyama, S.; Miyoshi, S.; Kaneko, T.; Tanaka, H. Angiotensin I-converting enzyme inhibitory activities of synthetic peptides related to the tandem repeated sequence of a maize endosperm protein. Agric. Biol. Chem. 1989, 53, 1077–1081. [Google Scholar]
- García, M.; Puchalska, P.; Esteve, C.; Marina, M. Vegetables foods: A cheap source of proteins and peptides with antihypertensive, antioxidant, and other less occurrence bioactivities. Talanta 2013, 106, 328–349. [Google Scholar] [CrossRef]
- Miyoshi, S.; Ishikawa, H.; Kaneko, T.; Fukui, F.; Tanaka, H.; Maruyama, S. Structures and activity of angiotensin-converting enzyme inhibitors in an α-zein hydrolysate. Agric. Biol. Chem. 1991, 55, 1313–1318. [Google Scholar]
- Mochida, T.; Hira, T.; Hara, H. The corn protein, zein hydrolysate, administered into the ileum attenuates hyperglycemia via its dual action on glucagon-like peptide-1 secretion and dipeptidyl peptidase-IV activity in rats. Endocrinology 2010, 151, 3095–3104. [Google Scholar] [CrossRef]
- Zhuang, H.; Tang, N.; Dong, S.; Sun, B.; Liu, J. Optimisation of antioxidant peptide preparation from corn gluten meal. J. Sci. Food Agric. 2013, 93, 3264–3270. [Google Scholar] [CrossRef]
- Kaur, A.; Kehinde, B.A.; Sharma, P.; Sharma, D.; Kaur, S. Recently isolated food-derived antihypertensive hydrolysates and peptides: A review. Food Chem. 2021, 346, 128719. [Google Scholar] [CrossRef] [PubMed]
- Daskaya-Dickmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-converting enzyme (ACE)—inhibitory peptides from plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, J. Research progress in structure-activity relationship of bioactive peptides. J. Med. Food 2015, 18, 147–156. [Google Scholar] [CrossRef]
- Zhu, B.; He, H.; Hou, T. A comprehensive review of corn protein-derived bioactive peptides: Production, characterization, bioactivities, and transport pathways. CRFSFS 2019, 18, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Rao, X.; Braunstein, Z.; Toomey, A.C.; Zhong, J. Role of incretin axis in inflammatory bowel disease. Front. Immunol. 2017, 8, 1734. [Google Scholar] [CrossRef]
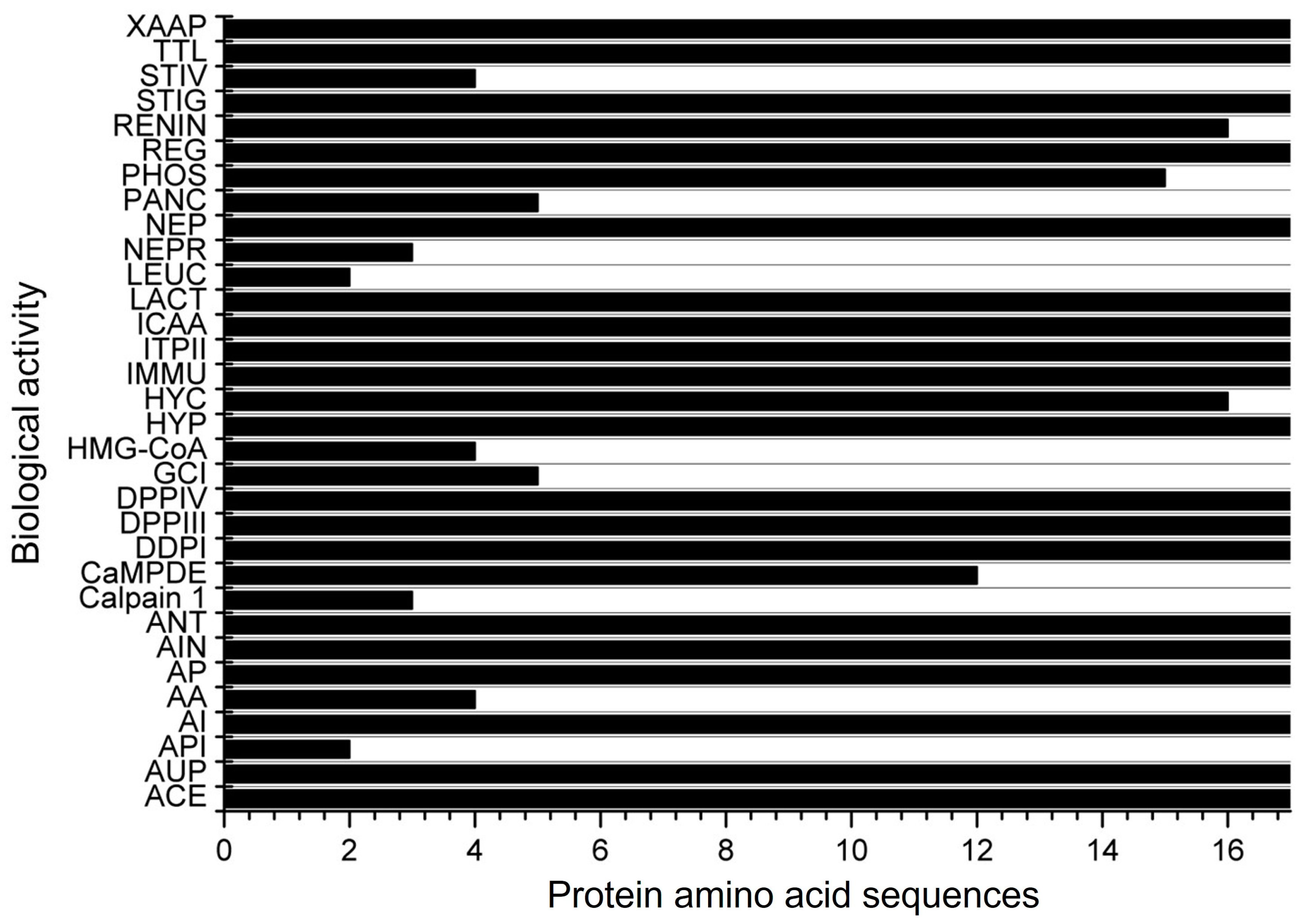
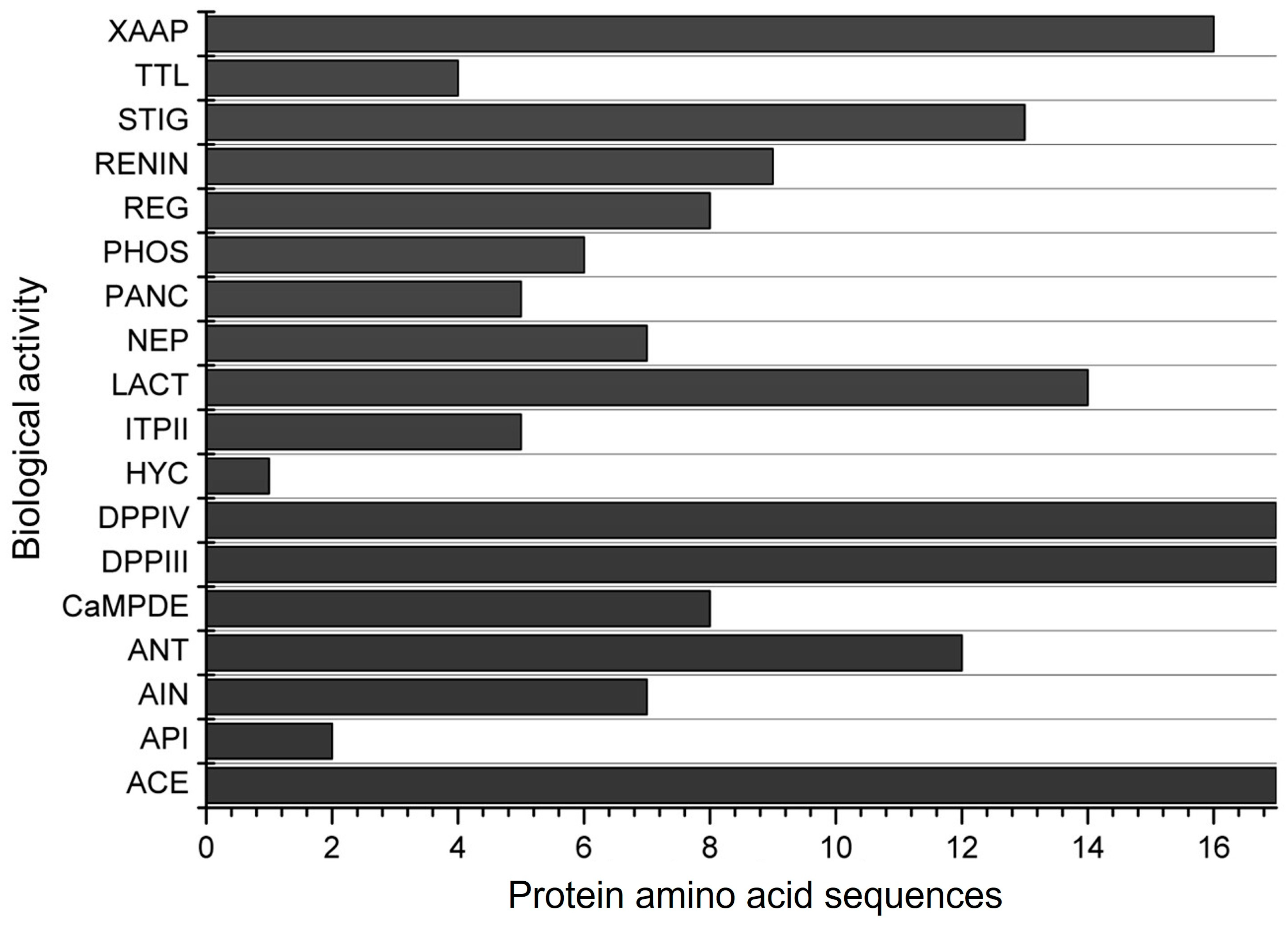
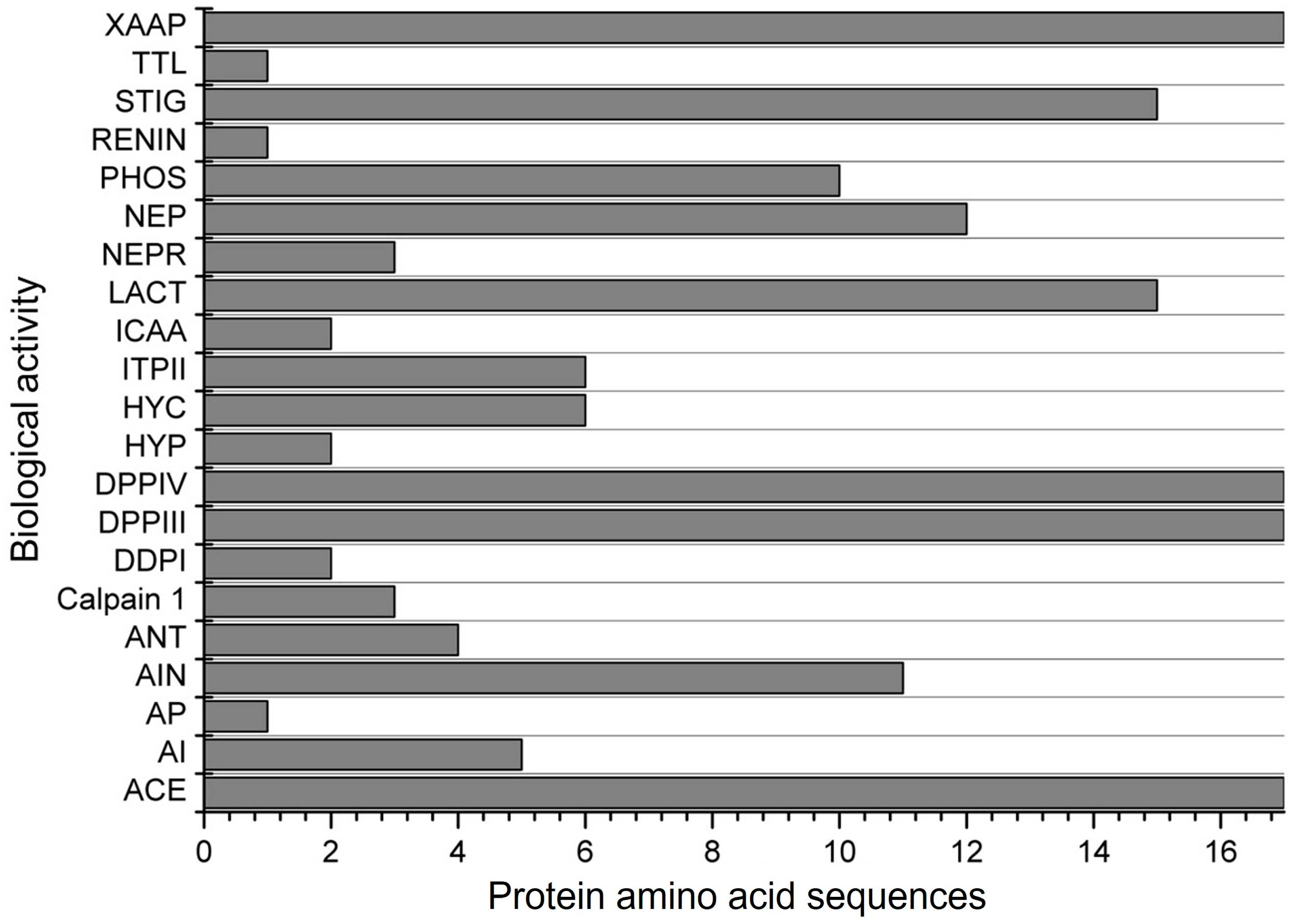
| Zea mays—Alpha Zein | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UniProt Data | Identity (BLAST) | |||||||||||||||||||
| Protein Name | UniProt Code | Length | Q94IM1 | P04698 | P06679 | P04700 | P06678 | P04704 | P06676 | P04703 | P06674 | P06675 | P04702 | P02859 | P04705 | Q548E6 | Q548E7 | B6SIF7 | B6SHV3 | |
| 22 kDa alpha-zein 14 | Q94IM1 | 266 | Q94IM1 | - | 98.9% | 88.9% | 86.2% | 49.1% | 44.2% | 46.7% | 46.7% | 43.6% | 46.0% | 46.7% | 46.4% | 41.9% | 46.4% | 45.5% | 43.8% | 44.0% |
| 22 kDa alpha-zein 14 | P04698 | 267 | P04698 | - | 89.3% | 87.3% | 49.1% | 44.4% | 46.7% | 46.7% | 43.8% | 43.5% | 46.7% | 43.9% | 41.7% | 46.4% | 45.3% | 43.7% | 43.9% | |
| 22 kDa alpha-zein 8b | P06679 | 261 | P06679 | - | 90.1% | 49.1% | 43.5% | 42.4% | 46.0% | 42.9% | 42.8% | 46.8% | 43.1% | 43.2% | 40.6% | 45.4% | 43.1% | 43.1% | ||
| 22 kDa alpha-zein 16 | P04700 | 263 | P04700 | - | 48.9% | 44.0% | 46.7% | 46.7% | 40.7% | 42.8% | 47.1% | 43.5% | 42.3% | 46.7% | 43.1% | 42.5% | 42.8% | |||
| Zein-alpha 19D1 | P06678 | 240 | P06678 | - | 56.0% | 58.5% | 58.5% | 56.3% | 56.0% | 58.1% | 56.5% | 48.7% | 58.1% | 52.2% | 56.5% | 56.5% | ||||
| Zein-alpha ZG99 | P04704 | 235 | P04704 | - | 71.8% | 71.8% | 91.4% | 91.1% | 69.8% | 92.3% | 87.2% | 71.0% | 87.3% | 91.9% | 91.5% | |||||
| Zein-alpha 19C1 | P06676 | 240 | P06676 | - | 99.6% | 70.5% | 72.5% | 96.7% | 72.1% | 61.4% | 99.9% | 65.4% | 72.1% | 72.1% | ||||||
| Zein-alpha A20 | P04703 | 240 | P04703 | - | 70.5% | 72.5% | 96.3% | 72.1% | 61.4% | 98.3% | 62.8% | 72.1% | 72.1% | |||||||
| Zein-alpha 19A2 | P06674 | 230 | P06674 | - | 90.5% | 68.5% | 92.2% | 79.3% | 69.7% | 68.7% | 91.8% | 90.9% | ||||||||
| Zein-alpha 19B1 | P06675 | 234 | P06675 | - | 70.5% | 97.4% | 81.8% | 71.7% | 80.1% | 96.6% | 99.6% | |||||||||
| Zein-alpha M6 | P04702 | 240 | P04702 | - | 70.1% | 57.9% | 97.9% | 63.9% | 70.1% | 70.1% | ||||||||||
| Zein-alpha A30 | P02859 | 234 | P02859 | - | 82.4% | 71.3% | 80.5% | 98.3% | 97.9% | |||||||||||
| Zein-alpha PZ19.1 | P04705 | 186 | P04705 | - | 60.4% | 87.8% | 81.3% | 82.4% | ||||||||||||
| 19kD alpha zein B3 | Q548E6 | 240 | Q548E6 | - | 97.0% | 71.3% | 80.5% | |||||||||||||
| 19kD alpha zein B2 | Q548E7 | 267 | Q548E7 | - | 80.1% | 97.0% | ||||||||||||||
| Zein-alpha PMS1 | B6SIF7 | 234 | B6SIF7 | - | 97.0% | |||||||||||||||
| Zein-alpha 19B1 | B6SHV3 | 234 | B6SHV3 | - | ||||||||||||||||
| α-Zein—19 kDa | ||||||
|---|---|---|---|---|---|---|
| P06674—Protein amino acid sequence: KIFCFLMLLGLSASAATATIFPQCSQAPITSLLPPYLSPAVSSVCENPILQPYRIQQAIAAGILPLSPLFLQQ PSALLQQLPLVHLLAQNIRAQQLQQLVLGNLAAYSQQHQFLPFNQLAALNSAAYLQQQLPFSQLAAAYPQQFLPFNQLAALNSAAYLQQQQLPPFSQLADVSPAAFLTQQQLLPFYLHAAPNAGTVLQLQQLLPFDQLALTNPTAFYQQPIIGGALF | ||||||
| Activity | Profiles of potential biological activity | GI: pepsin, trypsin, chymotrypsin | Subtilisin | |||
| Bioactive fragment | A | Bioactive fragment | A | Bioactive fragment | A | |
| ACE inhibitor | PF, LPP, ILP, IR, AAP, LPP, LVL, FP, IRAQQ, VSP, LAA, LSP, LQP, IRA, YL, LF, FY, FNQ, AY, LSPA, YP, FYQQ, LLP, LQQ, HLL, PL, IA, AF, AP, LA, RA, AA, IF, IG, GI, GA, GL, AG, HL, GT, GG, AI, LG, TNP, FCF, CF, LQ, LN, PT, TQ, PP, PQ, AV, LPF, AFL, IL, QP, LPL, LQL, LP, GIL | 0.5609 | IR, PL, IF, GL, CF, PF | 0.0261 | PL, GL, CF, PF | 0.0217 |
| Antibacterial peptide | AA | 0.0478 | ||||
| Immunomodulating | PFNQL, FLPFNQL | 0.0174 | ||||
| Stimulating (glucose uptake stimulating peptide) | VL, LV, IL, II, LL | 0.0565 | VL | 0.0043 | VL | 0.0087 |
| Neuropeptide | YL, YR, PY, IL | 0.0391 | ||||
| Regulating (phosphoglycerate kinase activity) | SL | 0.0043 | ||||
| Anti-inflammatory | PY, LLPF, LPF | 0.0391 | ||||
| Hypotensive | AA, FY | 0.0565 | ||||
| Antioxidative | LH, HL, AY, LHA, IR, LLPF, FC, LPL, LQL, YSQ, YPQ, FY, YL, LT | 0.1043 | IR | 0.0043 | ||
| Activating ubiquitin-mediated proteolysis | RA, LA | 0.0348 | ||||
| Alpha-glucosidase inhibitor | FY, YP, PP, AD, FY | 0.0261 | AD | 0.0043 | ||
| Dipeptidyl peptidase IV inhibitor | PP, LA, AP, PA, LP, LL, HA, SP, FP, YP, GA, IA, RA, NP, TA, QP, FL, HL, AL, SL, GL, LPL, AA, PL, LQP, AD, AF, AG, AS, AT, AV, AY, DQ, FN, GG, GI, II, IL, IQ, IR, KI, LH, LM, LN, LT, LV, ML, NA, NL, NQ, PF, PI, PN, PQ, PS, PT, PY, QA, QF, QH, QL, QN, QQ, RI, SV, TI, TN, TQ, TS, TV, VH, VL, VS, YL, YQ, YR, YS, APIT, LPF | 0.8130 | AL, GL, PL, IR, PF, QF, QL, TN, VH, VL | 0.0739 | PA, AL, GL, PL, AD, AS, ML, PF, QL, VL, VS | 0.0957 |
| Dipeptidyl peptidase III inhibitor | YL, YR, HL, LA, FL, PF | 0.1043 | PF | 0.0217 | PF | 0.0217 |
| CaMPDE inhibitor | IR | 0.0043 | IR | 0.0043 | ||
| Renin inhibitor | IR, QF, LPL, LQL | 0.0261 | IR, QF | 0.0087 | ||
| Xaa-Pro inhibitor | PL | 0.0130 | PL | 0.0087 | PL | 0.0130 |
| Lactocepin inhibitor | PL, LL, LP | 0.0783 | PL | 0.0087 | PL | 0.0130 |
| Inhibitor of tripeptidyl peptidase II | AAA, AA, AF, AP | 0.0696 | ||||
| Phospholipase A2 inhibitor | PY | 0.0087 | ||||
| Tubulin-tyrosine ligase inhibitor | AY | 0.0174 | ||||
| Inhibitor of cytosol alanyl aminopeptidase | AA, AAA, GGA, LL | 0.0826 | ||||
| Hypouricemic | LT, PT | 0.0130 | ||||
| D-Ala-D-Ala dipeptidase inhibitor | AA | 0.0478 | ||||
| P06676—Protein amino acid sequence: MATKIFSLLMLLALSACVANATIFPQCSQAPIASLLPPYLPSMIASVCENPALQPYRLQQAIAASNIPLSPLLF QQSPALSLVQSLVQTIRAQQLQQLVLPLINQVALANLSPYSQQQQFLPFNQLSTLNPAAYLQQQLLPFSQLATAYSQQQQLLPFNQLAALNPAAYLQQQILLPFSQLAAANRASFLTQQQLLPFYQQFAANPATLLQLQQLLPFVQLALTDPAASYQQHIIGGALF | ||||||
| Activity | Profiles of potential biological activity | GI: pepsin, trypsin, chymotrypsin | Subtilisin | |||
| Bioactive fragment | A | Bioactive fragment | A | Bioactive fragment | A | |
| ACE inhibitor | VLP, RL, IR, LPP, LVL, FP, IRAQQ, LAA, LSP, LQP, LNP, IRA, YL, LF, FY, FNQ, AY, FYQQ, LLP, PF, LPP LQQ, YSQQQQ, LNPA, PL, IA, IP, AP, LA, RA, AA, IF, IG, GA, GG, AI, SY, SF, LLF, LQ, LN, TQ, PP, PQ, ASL, LPF, LVQ, FQ, IL, ST, QP, LPL, LQL, LP | 0.4625 | PL, IF, PF | 0.0083 | RL, PL, PF | 0.0125 |
| Antibacterial peptide | AA | 0.0333 | ||||
| Immunomodulating | PFNQL, FLPFNQL | 0.0125 | ||||
| Stimulating (glucose uptake stimulating peptide) | VL, LV, IL, LI, II, LL | 0.0708 | VL | 0.0042 | VL | 0.0042 |
| Neuropeptide | YL, YR, PY, IL | 0.0333 | PY | 0.0042 | ||
| Regulating (phosphoglycerate kinase activity) | SL | 0.0167 | SL | 0.0083 | ||
| Anti-inflammatory | PY, SACV, LLPF, ANP, LPF | 0.0667 | PY | 0.0042 | ||
| Hypotensive | AA, FY | 0.0375 | ||||
| Antioxidative | AY, IR, LLPF, LPL, LQL, LAN, YSQ, FY, YL, LT | 0.0833 | ||||
| Activating ubiquitin-mediated proteolysis | RA, LA | 0.0333 | ||||
| Alpha-glucosidase inhibitor | FY, PP, FY | 0.0042 | ||||
| Dipeptidyl peptidase IV inhibitor | PP, VA, MA, LA, FA, AP, PA, LP, LL, IP, SP, FP, GA, IA, RA, NP, TA, QP, FL, AL, SL, LPL, AA, PL, LQP, AS, AT, AY, DQ, FN, FQ, GG, HI, II, IL, IN, IR, KI, LI, LM, LN, LT, LV, MI, ML, NA, NL, NQ, NR, PF, PI, PQ, PS, PY, QA, QF, QH, QI, QL, QQ, QS, QT, QV, RL, SF, SV, SY, TD, TI, TK, TL, TQ, VL, VQ, YL, YQ, YR, YS, LPF | 0.8417 | AL, SL, PL, IN, PF, QL, VL | 0.0667 | AL, PL, ML, PF, OS, PY, QL, RL, TL, VL | 0.0792 |
| Dipeptidyl peptidase III inhibitor | YL, YR, LA, FA, FL, PF, SM | 0.0833 | PF | 0.0250 | PF | 0.0250 |
| CaMPDE inhibitor | IR | 0.0042 | ||||
| Renin inhibitor | IR, NR, QF, SF, LPL, LQL | 0.0292 | ||||
| Pancreatic lipase inhibitor | ASF | 0.0042 | ASF | 0.0042 | ||
| Xaa-Pro inhibitor | PL | 0.0125 | PL | 0.0042 | PL | 0.0083 |
| Lactocepin inhibitor | PL, LL, LP | 0.0917 | PL | 0.0042 | PL | 0.0083 |
| Inhibitor of tripeptidyl peptidase II | VA, AAA, AA, AP | 0.0500 | ||||
| Phospholipase A2 inhibitor | PY | 0.0125 | PY | 0.0042 | ||
| Tubulin-tyrosine ligase inhibitor | AY | 0.0125 | ||||
| Inhibitor of cytosol alanyl aminopeptidase | AA, AAA, GGA, LL | 0.0833 | ||||
| Hypouricemic | LT, TL, ATL | 0.0208 | TL | 0.0042 | ||
| D-Ala-D-Ala dipeptidase inhibitor | AA | 0.0333 | ||||
| P06678—Protein amino acid sequence: MAAKIFALLALLALSANVATATIIPQCSQQYLSPVTAARFEYPTIQSYRLQQAIAASILRSLALTVQQPYALL QQPSLVNLYLQRIVAQQLQQQLLPTINQVVAANLDAYLQQQQFLPFNQLAGVNPAAYLQAQQLLPFNQLVRSPAAFLLQQQLLPFHLQVVANIAAFLQQQQLLPFYPQVVGNINAFLQQQQLLPFYPQDVANNVAFLQQQQLLPFSQLALTNPTTLLQQPTIGGAIF | ||||||
| Activity | Profiles of potential biological activity | GI: pepsin, trypsin, chymotrypsin | Subtilisin | |||
| Bioactive fragment | A | Bioactive fragment | A | Bioactive fragment | A | |
| Antiammnestic | LPPV | 0.0038 | ||||
| ACE inhibitor | RL, LPP, VAA, LAA, LNP, VAY, YL, LF, FNQ, AY, LLP, LQQ, PL, IA, LAP, IP, AF, AP, LA, VP, AA, GF, IF, VG, GA, AG, HL, MG, GG, AI, MNP, EV, LQ, LN, PT, TQ, AH, PP, PQ, VNP, LSW, AV, LPF, QP, LP, PF, LPP | 0.4253 | PL, AF, GF, AH, PF | 0.0230 | RL, VAY, PL, PP, VNP, PF | 0.0192 |
| Antibacterial peptide | AA | 0.0192 | ||||
| Immunomodulating | PFNQL, FLPPVT | 0.0077 | ||||
| Stimulating (vasoactive substance release) | SSS | 0.0575 | ||||
| Stimulating (glucose uptake stimulating peptide) | VL, LV, IV, II, LL | 0.0575 | VL | 0.0038 | ||
| Immunostimulating | GFL | 0.0038 | ||||
| Neuropeptide | YL, YR, PY | 0.0268 | PY | 0.0038 | PY | 0.0038 |
| Regulating (phosphoglycerate kinase activity) | GFL, SL | 0.0038 | SL | 0.0038 | ||
| Anti-inflammatory | PY, LLPF, ANP, LPF, LSW | 0.0192 | PY | 0.0038 | PY | 0.0038 |
| Hypotensive | AA | 0.0192 | ||||
| Antioxidative | HL, AY, AH, TY, LLPF, SVL, LFV, LAN, LSW, QAY, YSQ, YL | 0.0958 | AH | 0.0038 | ||
| Activating ubiquitin-mediated proteolysis | LA | 0.0498 | ||||
| HMG-CoA reductase inhibitor | IVG | 0.0038 | ||||
| Alpha-glucosidase inhibitor | PP | 0.0038 | PP | 0.0038 | ||
| Dipeptidyl peptidase IV inhibitor | PP, VA, LA, AP, PA, LP, VP, LL, VV, IP, SP, GA, IA, NP, QP, FL, HL, AL, SL, AA, PL, WQ, AF, AG, AH, AS, AT, AV, AY, EH, EV, FN, GF, GG, II, IN, IQ, LM, LN, LT, LV, MG, MN, MV, NA, NQ, NV, PF, PI, PN, PQ, PS, PT, PV, PY, QA, QF, QI, QL, QQ, QS, QT, RL, SV, SW, TI, TN, TQ, TS, TV, TY, VG, VL, VN, VQ, VS, VT, YL, YQ, YR, YS, LPF | 0.8199 | AL, SL, PL, AF, AH, EH, GF, PF, PY, QL, SW, VN | 0.0690 | PP, AL, PL, AS, PF, OS, PY, QF, QL, RL, VL, VS | 0.0690 |
| Dipeptidyl peptidase III inhibitor | YL, YR, GF, HL, LA, FL, PF, SM, GFL | 0.1149 | GF, PF | 0.0077 | PF | 0.0038 |
| Renin inhibitor | QF | 0.0153 | QF | 0.0038 | ||
| Pancreatic lipase inhibitor | SW | 0.0038 | SW | 0.0038 | ||
| Xaa-Pro inhibitor | PL | 0.0153 | PL | 0.0115 | PL | 0.0038 |
| Lactocepin inhibitor | PL, LL, LP | 0.0651 | PL | 0.0115 | PL | 0.0038 |
| Inhibitor of tripeptidyl peptidase II | VA, GF, AA, AF, AP | 0.0536 | GF, AF | 0.0077 | ||
| Phospholipase A2 inhibitor | PY | 0.0038 | PY | 0.0038 | PY | 0.0038 |
| Acylaminoacyl peptidase inhibitor | GF | 0.0077 | GF | 0.0038 | ||
| Tubulin-tyrosine ligase inhibitor | AY | 0.0230 | ||||
| Glutamate carboxypeptidase inhibitor | FE | 0.0038 | ||||
| Inhibitor of cytosol alanyl aminopeptidase | AA, GGA, LL | 0.0536 | ||||
| Hypouricemic | IAT, LT, PT | 0.0192 | ||||
| D-Ala-D-Ala dipeptidase inhibitor | AA | 0.0192 | ||||
| P04705—Protein amino acid sequence: MAAKIFCLIMLLGLSASAATASIFPQCSQAPIASLLPPYLSPAMSSVCENPILLPYRIQQAIAAGILPLSPLFLQ QSSALLQQLPLVHLLAQNIRAQQLQQLVLANLAAYSQQQQLPLVHLLAQNIRAQQLQQLVLANLAAYSQQQQFLPFNQQLAAAYPRQFLPFNQLAALNSHAYVQQ | ||||||
| Activity | Profiles of potential biological activity | GI: pepsin, trypsin, chymotrypsin | Subtilisin | |||
| Bioactive fragment | A | Bioactive fragment | A | Bioactive fragment | A | |
| ACE inhibitor | PF, LPP, ILP, IR, LPP, LVL, FP, IRAQQ, YPR, PR, LAA, LSP, IRA, YL, LF, FNQ, AY, LSPA, YP, LLP, LQQ, YSQQQQ, HLL, PL, IA, AP, LA, RA, AA, IF, GI, GL, AG, HL, AI, LG, LQ, LN, PP, PQ, ASL, LPF, YV, IL, LPL, LP, GIL | 0.5389 | IR, PR, AY, PL, IF, GL, PF | 0.0500 | PL, IF, GL, PF | 0.0333 |
| Antibacterial peptide | AA | 0.0444 | ||||
| Immunomodulating | PFNQL, FLPFNQL | 0.0111 | ||||
| Stimulating (glucose uptake stimulating peptide) | VL, LV, IL, LI, LL | 0.0833 | VL | 0.0111 | VL | 0.0111 |
| Neuropeptide | YL, YR, PY, IL | 0.0333 | PY | 0.0056 | PY | 0.0056 |
| Regulating (phosphoglycerate kinase activity) | SL | 0.0056 | ||||
| Anti-inflammatory | PY, LPF | 0.0222 | PY | 0.0056 | PY | 0.0056 |
| Hypotensive | AA | 0.0444 | ||||
| Antioxidative | HL, AY, IR, FC, LPL, LAN, YSQ, YL | 0.0944 | AY, IR | 0.0167 | ||
| Activating ubiquitin-mediated proteolysis | RA, LA | 0.0556 | ||||
| Alpha-glucosidase inhibitor | YP, PP | 0.0111 | ||||
| Dipeptidyl peptidase IV inhibitor | PP, MA, LA, AP, PA, LP, LL, HA, SP, FP, YP, IA, RA, NP, TA, FL, HL, AL, SL, GL, LPL, AA, PL, AG, AS, AT, AY, FN, GI, IL, IM, IQ, IR, KI, LI, LN, LV, ML, NL, NQ, PF, PI, PQ, PY, QA, QF, QL, QN, QQ, QS, RI, SH, SI, SV, VH, VL, VQ, YL, YR, YS, YV, LPF | 0.7944 | GL, PL, AY, IM, IR, PF, PY, QF, QL, SH, VH, VL | 0.1000 | AL, GL, PL, AS, PF, PY, VL | 0.0667 |
| Dipeptidyl peptidase III inhibitor | YL, YR, PR, HL, LA, FL, PF | 0.1000 | PR, PF | 0.0167 | PF | 0.0111 |
| CaMPDE inhibitor | IR | 0.0111 | IR | 0.0111 | ||
| Renin inhibitor | IR, QF, LPL | 0.0389 | IR, QF | 0.0167 | ||
| Xaa-Pro inhibitor | PL | 0.0222 | PL | 0.0167 | PL | 0.0222 |
| Lactocepin inhibitor | PL, LL, LP | 0.0944 | PL | 0.0167 | PL | 0.0222 |
| Inhibitor of tripeptidyl peptidase II | AAA, AA, AP | 0.0556 | ||||
| Phospholipase A2 inhibitor | PY | 0.0111 | PY | 0.0056 | PY | 0.0056 |
| Tubulin-tyrosine ligase inhibitor | AY | 0.0222 | AY | 0.0056 | ||
| Inhibitor of cytosol alanyl aminopeptidase | AA, AAA, LL | 0.0833 | ||||
| D-Ala-D-Ala dipeptidase inhibitor | AA | 0.0444 | ||||
| α-Zein—22 kDa | ||||||
|---|---|---|---|---|---|---|
| P06679—Protein amino acid sequence: LALLALLALFVSATNAFIIPQCSLAPSAIIPQFLPPVTSMGFEHLAVQAYRLQQALAASVLQQPINQLQQQSL AHLTIQTIATQQQQQFLPSVSQLDVVNPVAYLQQQLLASNPLALANVAAYQQQQQLQQFLPALSQLAMVNPAAYLQQQQLLSSSPLAVGNAPTYLQQQLLQQIVPALTQLAVANPAAYLQQLLPFNQLTVSNSAAYLQQRQQLLNPLEVPNPLVAGFLQQQQLLPYSQFSLMNPALSWQQPIVGGAIF | ||||||
| Profiles of potential biological activity | GI: pepsin, trypsin, chymotrypsin | Subtilisin | ||||
| Activity | Bioactive fragment | A | Bioactive fragment | A | Bioactive fragment | A |
| Alpha-amylase inhibitor | FY | 0.0083 | ||||
| ACE inhibitor | ARF, PF, RL, LY, RF, VAA, LVR, LSP, YL, FY, FNQ, AY, YP, LLP, LQQ, IA, IP, AF, LA, YA, AA, IF, VG, IG, GA, AG, HL, DA, GV, GG, AI, VR, TNP, SY, AR, EY, FAL, LQ, PT, PQ, VAF, VRSP, AFLL, VNP, LPF, AFL, IL, LR, QP, LP, AYLQAQQLLPFNQLVRSPAA | 0.4917 | AF, IF, VR, EY, VAF, PF | 0.0208 | RL, AG, HL, EY, PQ, VAF, IL, PF | 0.0292 |
| Celiac toxic | QQPY | 0.0042 | ||||
| Antibacterial peptide | AA | 0.0292 | ||||
| Immunomodulating | PFNQL, FLPFNQL, SQLALTNPT, PFNQLAG | 0.0208 | ||||
| Stimulating (glucose uptake stimulating peptide) | LV, IV, IL, II, LL | 0.0667 | IL | 0.0042 | ||
| Neuropeptide | YL, YR, PY, IL | 0.0292 | IL | 0.0042 | ||
| Regulating (phosphoglycerate kinase activity) | SL | 0.0083 | SL | 0.0042 | ||
| Anti-inflammatory | PY, LLPF, LPF | 0.0500 | ||||
| Hypotensive | AA, FY | 0.0375 | ||||
| Antioxidative | HL, AY, LY, LLPF, AYLQAQQLLPFNQLVRSPAA, YPQ, YA, FY, YL, LT | 0.0875 | HL | 0.0042 | ||
| Alpha-glucosidase inhibitor | YP, LR, FY | |||||
| Activating ubiquitin-mediated proteolysis | LA | 0.0208 | ||||
| Dipeptidyl peptidase IV inhibitor | VA, MA, LA, FA, PA, LP, LL, VV, IP, SP, YP, GA, IA, NP, TA, QP, FL, HL, AL, SL, VR, AA, AF, AG, AS, AT, AY, EY, FN, GG, GV, II, IL, IN, IQ, KI, LT, LV, NA, NL, NN, NQ, NV, PF, PQ, PS, PT, PV, PY, QA, QD, QF, QL, QQ, QS, QV, QY, RI, RL, SI, SY, TI, TL, TN, TT, TV, VG, VN, VQ, VT, YA, YL, YR, LPF | 0.8125 | AL, SL, VR, AF, EY, IN, PF, QL, TN, VN | 0.0875 | HL, AL, AG, EY, IL, PF, PQ, QL, RL | 0.0792 |
| Dipeptidyl peptidase III inhibitor | LR, YL, YR, RF, DA, HL, LA, FA, FL, PF | 0.1083 | PF | 0.0250 | HL, PF | 0.0292 |
| Renin inhibitor | LR, QF, YA, LY | 0.0167 | ||||
| Leucyltransferase inhibitor | RF | 0.0042 | ||||
| Lactocepin inhibitor | LL, LP | 0.0750 | ||||
| Inhibitor of tripeptidyl peptidase II | VA, AA, AF | 0.0708 | AF | 0.0042 | ||
| Phospholipase A2 inhibitor | PY | 0.0042 | ||||
| Tubulin-tyrosine ligase inhibitor | EY, AY, YA | 0.0167 | EY | 0.0042 | EY | 0.0042 |
| Alanine carboxypeptidase inhibitor | YA | 0.0042 | ||||
| Glutamate carboxypeptidase inhibitor | FE, DA | 0.0042 | ||||
| Inhibitor of cytosol alanyl aminopeptidase | AA, GGA, LL | 0.0792 | ||||
| Hypouricemic | FH, LR, LPT, LT, PT, TL, TT | 0.0458 | ||||
| D-Ala-D-Ala dipeptidase inhibitor | AA | 0.0292 | ||||
| Q94IM1—Protein amino acid sequence: MATKILSLLALLALFASATNASIIPQCSLAPSSIIPQFLPPVTSMAFEHPAVQAYRLQQAIAASVLQQPIAQL QQQSLAHLTIQTIATQQQQQFLPALSHLAMVNPIAYLQQQLLASNPLGLANVVANQQQQQLQQFLPALSQLAMVNPAAYLQQQQLLSSSPLAVANAPTYLQQELLQQIVPALTQLAVANPVAYLQQLLPFNQLTMSNSVAYLQQRQQLLNPLAVANPLVAAFLQQQQLLPYNRFSLMNPVLSRQQPIVGGAIF | ||||||
| Profiles of potential biological activity | GI: pepsin, trypsin, chymotrypsin | Subtilisin | ||||
| Activity | Bioactive fragment | A | Bioactive fragment | A | Bioactive fragment | A |
| Antiammnestic | LPPV | 0.0038 | ||||
| ACE inhibitor | RL, LPP, RF, VAA, LNP, VAY, YL, LF, FNQ, AY, LLP, LQQ, PLG, PL, IA, LAP, IP, AF, AP, LA, VP, AA, IF, VG, GA, GL, HL, GG, AI, LG, MNP, LQ, LN, PT, TQ, AH, PP, TQ, AH, PP, PQ, HP, VNP, AV, LPF, AFL, IAQ, AQL, IAY, IL, YN, QP, LP | 0.4286 | PL, AF, GL, AH, IL, PF | 0.0263 | RL, VAY, PL, GL, HL, MNP, PP, PF | 0.0301 |
| Antibacterial peptide | AA | 0.0113 | ||||
| Immunomodulating | PFNQL, FLPPVT | 0.0075 | ||||
| Opioid | PLG | 0.0038 | ||||
| Stimulating (vasoactive substance release) | SSS | 0.0639 | ||||
| Stimulating (glucose uptake stimulating peptide) | VL, LV, IV, IL, II, LL | 0.0639 | IL | 0.0038 | VL | 0.0075 |
| Neuropeptide | YL, YR, PY, IL | 0.0301 | PY, IL | 0.0075 | PY | 0.0038 |
| Regulating (phosphoglycerate kinase activity) | SL | 0.0150 | SL | 0.0038 | ||
| Anti-inflammatory | PY, LLPF, ANP, LPF | 0.0188 | PY | 0.0038 | PY | 0.0038 |
| Hypotensive | AA | 0.0113 | ||||
| Antioxidative | HL, AY, AH, EL, TY, LLPF, SVL, LAN, QAY, IAY, YL, LT | 0.0865 | AH | 0.0038 | HL | 0.0038 |
| Activating ubiquitin-mediated proteolysis | LA | 0.0414 | ||||
| HMG-CoA reductase inhibitor | IVG | 0.0038 | ||||
| Alpha-glucosidase inhibitor | PP | 0.0038 | PP | 0.0038 | ||
| Dipeptidyl peptidase IV inhibitor | PP, VA, MA, LA, FA, AP, PA, LP, VP, LL, VV, IP, SP, HP, GA, IA, NP, QP, FL, HL, AL, SL, GL, AA, PL, AF, AH, AS, AT, AV, AY, EH, FN, GG, II, IL, IQ, KI, LM, LN, LT, LV, MN, MV, NA, NQ, NR, NV, PF, PI, PQ, PS, PT, PV, PY, QA, QE, QF, QI, QL, QQ, QS, QT, RL, SH, SI, SV, TI, TK, TM, TN, TQ, TS, TY, VG, VL, VN, VQ, VT, YL, YN, YR, LPF | 0.8083 | AL, SL, GL, PL, AF, AH, EH, IL, PF, PY, QL, SH, TM, VN, | 0.0714 | PP, HL, AL, GL, PL, AS, PF, PY, QL, RL, VL | 0.0526 |
| Dipeptidyl peptidase III inhibitor | YL, YR, RF, HL, HP, LA, FA, FL, PF, SM | 0.1053 | PF | 0.0038 | HL, PF | 0.0075 |
| Leucyltransferase inhibitor | RF | 0.0038 | ||||
| Xaa-Pro inhibitor | PL | 0.0150 | PL | 0.0113 | PL | 0.0038 |
| Lactocepin inhibitor | PL, LL, LP | 0.0639 | PL | 0.0113 | PL | 0.0038 |
| Inhibitor of tripeptidyl peptidase II | VA, AA, AF, AP | 0.0526 | AF | 0.0038 | ||
| Phospholipase A2 inhibitor | PY | 0.0038 | PY | 0.0038 | PY | 0.0038 |
| Tubulin-tyrosine ligase inhibitor | AY | 0.0188 | ||||
| Glutamate carboxypeptidase inhibitor | FE | 0.0038 | ||||
| Inhibitor of cytosol alanyl aminopeptidase | AA, GGA, LL | 0.0451 | ||||
| Hypouricemic | IAT, LT, PT | 0.0188 | ||||
| D-Ala-D-Ala dipeptidase inhibitor | AA | 0.0113 | ||||
| Score a | Bioactive Fragment | Biological Activity | Corn Specie | Peptide Amino Acid Sequence b | Reference |
|---|---|---|---|---|---|
| 0.9978 | FCF | ACE inhibitor | |||
| 0.9964 | CF | ACE inhibitor | |||
| 0.9947 | GF | ACE and Renin inhibitor, and DPP-III and DPP-IV | Corn silk; corn | CGFPPAGYLRR; YGGFM | [52,69] |
| 0.9939 | FP | ACE inhibitor and DPP-IV | |||
| 0.9934 | PF | ACE inhibitor and DPP-IV | Corn | LPF | [61] |
| 0.9869 | LF | ACE inhibitor | α-zein | LF | [79] |
| 0.9865 | RF | ACE inhibitor and DPP-III and DPP-IV | Corn | AARPRFNH2 | [80] |
| 0.9824 | FY | ACE and alpha-amylase inhibitor, hypotensive and antioxidative | |||
| 0.9749 | LPF | ACE inhibitor, anti-inflammatory, and DPP-IV | α-zein | FNQLAALNSAAYLQQQQLLPFSQLA; QLADVSPAAFLTQQQLLPFYLHAM; AYLQAQQLLPFNQLVRSPAA; CFFNQLAALNSAAYLQQQQLLPFSQLA; CFAYLQAQQLLPFNQLVRSPAA; CFQLADVSPAAFLTQQQLLPFYLHAM | [16] |
| 0.9732 | AF | ACE inhibitor, Inhibitor of tripeptidyl peptidase II, and DPP-IV | α-zein | AF, QLADVSPAAFLTQQQLLPFYLHAM, CFQLADVSPAAFLTQQQLLPFYLHAM | [16,79] |
| 0.9491 | IF | ACE inhibitor and DPP-IV | Corn | TIFPQ | [67] |
| 0.9487 | SF | ACE and renin inhibitor and DPP-IV | |||
| 0.9439 | MG | ACE inhibitor and DPP-IV | |||
| 0.9389 | LLF | ACE inhibitor | Corn | LLF | [61] |
| 0.9298 | ARF | ACE inhibitor | |||
| 0.9285 | AFL | ACE inhibitor | α-zein | QLADVSPAAFLTQQQLLPFYLHAM, CFQLADVSPAAFLTQQQLLPFYLHAM | [16,79] |
| 0.9160 | FQ | ACE inhibitor and DPP-IV | Corn | FQ | [61] |
| 0.8902 | FAL | ACE inhibitor | |||
| 0.8892 | AFLL | ACE inhibitor | |||
| 0.8873 | GG | ACE inhibitor and DPP-IV | |||
| 0.8869 | PP | ACE and alpha-glucosidase inhibitor and DPP-IV | γ-zein and α-zein | VHLPPP; OGLPPGPPIPP; SLLPPYLSPA; LPP | [10,81] |
| 0.8282 | LSW | ACE inhibitor, anti-inflammatory, and antioxidative | |||
| 0.8280 | LPP | ACE inhibitor and DPP-IV | α-zein, corn gluten meal | VHLPPP | [79,81,82]. |
| 0.8111 | PL | ACE inhibitor and DPP-IV | α-zein; Zein | PPIPPGPPLOG; VPL | [83,84] |
| 0.8087 | GL | ACE, Xaa-Pro, and lactocepin inhibitor, and DPP-IV | |||
| 0.8013 | PLG | ACE inhibitor and DPP-IV | |||
| 0.9802 | GFL | Regulating (phosphoglycerate kinase activity) and DPP-III | Corn | GGFL | [52] |
| 0.9960 | FC | Antioxidant | |||
| 0.9464 | LLPF | Antioxidant and anti-inflammatory | corn gluten meal; α-zein | LLPF; QQLLPF, QQILLPF, QILLPF; QLLPF | [9,67,85] |
| 0.9934 | PF | DPP-IV and DPP-III | |||
| 0.9896 | FL | DPP-IV and DPP-III | |||
| 0.9558 | FA | DPP-IV and DPP-III | |||
| 0.9512 | FN | DPP-IV | |||
| 0.9461 | QF | DPP-IV and renin inhibitor | |||
| 0.9339 | SW | DPP-IV and pancreatic lipase inhibitor | |||
| 0.9285 | AFL | DPP-IV | |||
| 0.8945 | ML | DPP-IV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cagnin, C.; Garcia, B.d.F.; Rocha, T.d.S.; Prudencio, S.H. Bioactive Peptides from Corn (Zea mays L.) with the Potential to Decrease the Risk of Developing Non-Communicable Chronic Diseases: In Silico Evaluation. Biology 2024, 13, 772. https://doi.org/10.3390/biology13100772
Cagnin C, Garcia BdF, Rocha TdS, Prudencio SH. Bioactive Peptides from Corn (Zea mays L.) with the Potential to Decrease the Risk of Developing Non-Communicable Chronic Diseases: In Silico Evaluation. Biology. 2024; 13(10):772. https://doi.org/10.3390/biology13100772
Chicago/Turabian StyleCagnin, Caroline, Bianca de Fátima Garcia, Thais de Souza Rocha, and Sandra Helena Prudencio. 2024. "Bioactive Peptides from Corn (Zea mays L.) with the Potential to Decrease the Risk of Developing Non-Communicable Chronic Diseases: In Silico Evaluation" Biology 13, no. 10: 772. https://doi.org/10.3390/biology13100772








