The Physiological Mechanisms of Transcranial Direct Current Stimulation to Enhance Motor Performance: A Narrative Review
Abstract
:Simple Summary
Abstract
1. Introduction
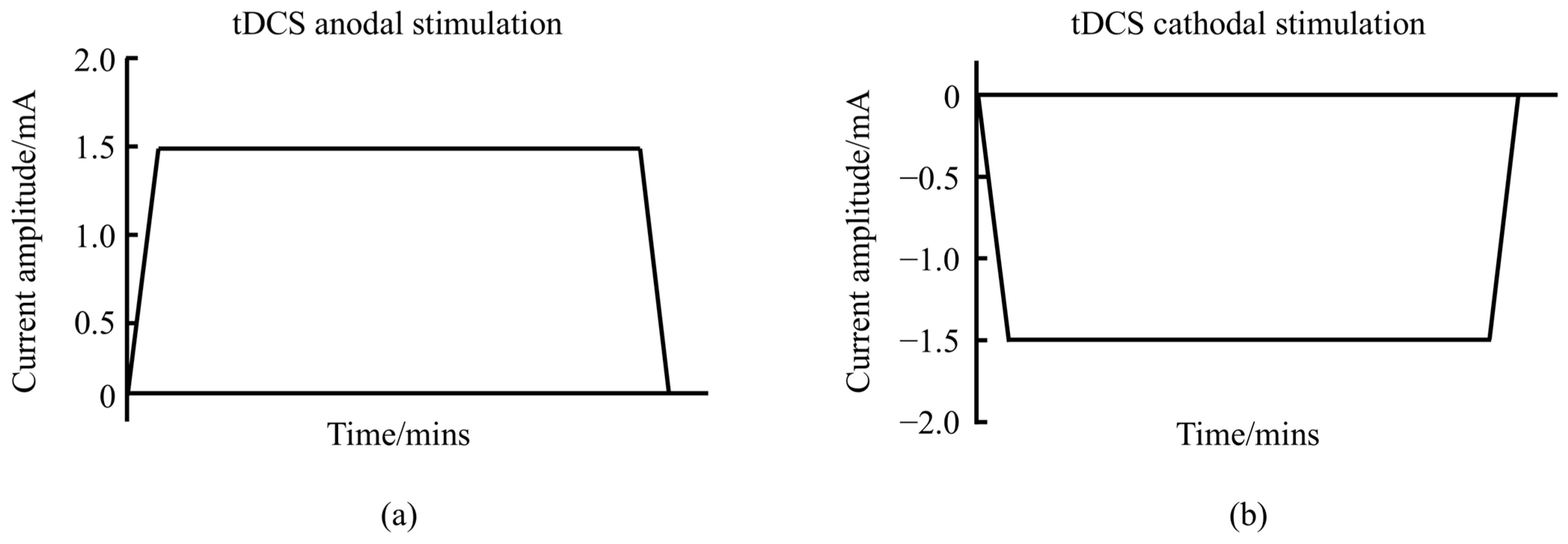
2. Physiological Mechanisms of tDCS Action
2.1. Modulating the Resting Membrane Potential of Neurons to Change the Excitability of the Cerebral Cortex
2.2. Enhancement of Synaptic Plasticity and Modulation of LTP-like Effect
2.3. Modulating Neurovascular Coupling to Improve rCBF in the Brain
2.4. Modulating Brain Network Functional Connectivity to Activate and Strengthen Brain Regions
2.5. Enhancement of Excitatory Neurotransmitter Levels and Release
3. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reardon, S. Neuroscience Performance boost paves way for ‘brain doping’. Nature 2016, 531, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.; Batsikadze, G.; Kuo, H.I.; Labruna, L.; Hasan, A.; Paulus, W.; Nitsche, M.A. Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J. Physiol. 2017, 595, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Mrudula, K.; Sreepada, S.S.; Sathyaprabha, T.N.; Pal, P.K.; Chen, R.; Udupa, K. An Overview of Noninvasive Brain Stimulation: Basic Principles and Clinical Applications. Can. J. Neurol. Sci. 2021, 49, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Unal, G.; Andrade, S.M.; Moreira, A.; Altimari, L.R.; Brunoni, A.R.; Perrey, S.; Mauger, A.R.; Bikson, M.; Okano, A.H. Effect of transcranial direct current stimulation on exercise performance: A systematic review and meta-analysis. Brain Stimul. 2019, 12, 593–605. [Google Scholar] [CrossRef]
- Grosprêtre, S.; Grandperrin, Y.; Nicolier, M.; Gimenez, P.; Vidal, C.; Tio, G.; Haffen, E.; Bennabi, D. Effect of transcranial direct current stimulation on the psychomotor, cognitive, and motor performances of power athletes. Sci. Rep. 2021, 11, 9731. [Google Scholar] [CrossRef]
- Maudrich, T.; Ragert, P.; Perrey, S.; Kenville, R. Single-session anodal transcranial direct current stimulation to enhance sport-specific performance in athletes: A systematic review and meta-analysis. Brain Stimul. 2022, 15, 1517–1529. [Google Scholar] [CrossRef]
- Wang, B.; Xiao, S.; Yu, C.; Zhou, J.; Fu, W. Effects of Transcranial Direct Current Stimulation Combined With Physical Training on the Excitability of the Motor Cortex, Physical Performance, and Motor Learning: A Systematic Review. Front. Neurosci. 2021, 15, 648354. [Google Scholar] [CrossRef]
- Grandperrin, Y.; Grosprêtre, S.; Nicolier, M.; Gimenez, P.; Vidal, C.; Haffen, E.; Bennabi, D. Effect of transcranial direct current stimulation on sports performance for two profiles of athletes (power and endurance) (COMPETE): A protocol for a randomised, crossover, double blind, controlled exploratory trial. Trials 2020, 21, 461. [Google Scholar] [CrossRef]
- Kamali, A.M.; Kazemiha, M.; Keshtkarhesamabadi, B.; Daneshvari, M.; Zarifkar, A.; Chakrabarti, P.; Kateb, B.; Nami, M. Simultaneous transcranial and transcutaneous spinal direct current stimulation to enhance athletic performance outcome in experienced boxers. Sci. Rep. 2021, 11, 19722. [Google Scholar] [CrossRef]
- Hornyak, T. Smarter, not harder. Nature 2017, 549, S1–S3. [Google Scholar] [CrossRef]
- Bestmann, S.; Walsh, V. Transcranial electrical stimulation. Curr. Biol. 2017, 27, R1258–R1262. [Google Scholar] [CrossRef] [PubMed]
- Garner, C.T.; Dykstra, R.M.; Hanson, N.J.; Miller, M.G. Transcranial Direct Current Stimulation with the Halo Sport Does Not Improve Performance on a Three-Minute, High Intensity Cycling Test. Int. J. Exerc. Sci. 2021, 14, 962–970. [Google Scholar] [PubMed]
- Lattari, E.; Rosa Filho, B.J.; Fonseca Junior, S.J.; Murillo-Rodriguez, E.; Rocha, N.; Machado, S.; Maranhao Neto, G.A. Effects on Volume Load and Ratings of Perceived Exertion in Individuals’ Advanced Weight Training After Transcranial Direct Current Stimulation. J. Strength Cond. Res. 2020, 34, 89–96. [Google Scholar] [CrossRef]
- Angius, L.; Pageaux, B.; Hopker, J.; Marcora, S.M.; Mauger, A.R. Transcranial direct current stimulation improves isometric time to exhaustion of the knee extensors. Neuroscience 2016, 339, 363–375. [Google Scholar] [CrossRef]
- Tanaka, S.; Hanakawa, T.; Honda, M.; Watanabe, K. Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. Exp. Brain Res. 2009, 196, 459–465. [Google Scholar] [CrossRef]
- Fridriksson, J.; Rorden, C.; Elm, J.; Sen, S.; George, M.S.; Bonilha, L. Transcranial Direct Current Stimulation vs Sham Stimulation to Treat Aphasia After Stroke: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 1470–1476. [Google Scholar] [CrossRef]
- Flood, A.; Waddington, G.; Keegan, R.J.; Thompson, K.G.; Cathcart, S. The effects of elevated pain inhibition on endurance exercise performance. PeerJ 2017, 5, e3028. [Google Scholar] [CrossRef]
- Hazime, F.A.; da Cunha, R.A.; Soliaman, R.R.; Romancini, A.C.B.; Pochini, A.C.; Ejnisman, B.; Baptista, A.F. Anodal Transcranial Direct Current Stimulation (Tdcs) Increases Isometric Strength of Shoulder Rotators Muscles in Handball Players. Int. J. Sports Phys. Ther. 2017, 12, 402–407. [Google Scholar] [PubMed]
- Vargas, V.Z.; Baptista, A.F.; Pereira, G.O.C.; Pochini, A.C.; Ejnisman, B.; Santos, M.B.; João, S.M.A.; Hazime, F.A. Modulation of Isometric Quadriceps Strength in Soccer Players With Transcranial Direct Current Stimulation: A Crossover Study. J. Strength Cond. Res. 2018, 32, 1336–1341. [Google Scholar] [CrossRef]
- Lattari, E.; Andrade, M.L.; Filho, A.S.; Moura, A.M.; Neto, G.M.; Silva, J.G.; Rocha, N.B.; Yuan, T.F.; Arias-Carrion, O.; Machado, S. Can Transcranial Direct Current Stimulation Improve the Resistance Strength and Decrease the Rating Perceived Scale in Recreational Weight-Training Experience? J. Strength Cond. Res. 2016, 30, 3381–3387. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, B.; Zhang, X.; Zhou, J.; Fu, W. Acute Effects of High-Definition Transcranial Direct Current Stimulation on Foot Muscle Strength, Passive Ankle Kinesthesia, and Static Balance: A Pilot Study. Brain Sci. 2020, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Besson, P.; Muthalib, M.; De Vassoigne, C.; Rothwell, J.; Perrey, S. Effects of Multiple Sessions of Cathodal Priming and Anodal HD-tDCS on Visuo Motor Task Plateau Learning and Retention. Brain Sci. 2020, 10, 875. [Google Scholar] [CrossRef]
- Kaminski, E.; Engelhardt, M.; Hoff, M.; Steele, C.; Villringer, A.; Ragert, P. TDCS effects on pointing task learning in young and old adults. Sci. Rep. 2021, 11, 3421. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.F.; Yeung, A.Y.; Poolton, J.M.; Lee, T.M.; Leung, G.K.; Masters, R.S. Cathodal Transcranial Direct Current Stimulation Over Left Dorsolateral Prefrontal Cortex Area Promotes Implicit Motor Learning in a Golf Putting Task. Brain Stimul. 2015, 8, 784–786. [Google Scholar] [CrossRef]
- Schilberg, L.; Engelen, T.; Ten Oever, S.; Schuhmann, T.; de Gelder, B.; de Graaf, T.A.; Sack, A.T. Phase of beta-frequency tACS over primary motor cortex modulates corticospinal excitability. Cortex 2018, 103, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Antal, A.; Nitsche, M.A. Physiology of Transcranial Direct Current Stimulation. J. ECT 2018, 34, 144–152. [Google Scholar] [CrossRef]
- Fertonani, A.; Miniussi, C. Transcranial Electrical Stimulation: What We Know and Do Not Know About Mechanisms. Neuroscientist 2017, 23, 109–123. [Google Scholar] [CrossRef]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Taylor, J.L.; Amann, M.; Duchateau, J.; Meeusen, R.; Rice, C.L. Neural Contributions to Muscle Fatigue: From the Brain to the Muscle and Back Again. Med. Sci. Sports Exerc. 2016, 48, 2294–2306. [Google Scholar] [CrossRef]
- Taylor, J.L.; Gandevia, S.C. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J. Appl. Physiol. (1985) 2008, 104, 542–550. [Google Scholar] [CrossRef]
- Cogiamanian, F.; Marceglia, S.; Ardolino, G.; Barbieri, S.; Priori, A. Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. Eur. J. Neurosci. 2007, 26, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.S.; Hoffman, R.L.; Clark, B.C. Preliminary evidence that anodal transcranial direct current stimulation enhances time to task failure of a sustained submaximal contraction. PLoS ONE 2013, 8, e81418. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J. Physiol. 2003, 553, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Jaussi, W.; Liebetanz, D.; Lang, N.; Tergau, F.; Paulus, W. Consolidation of human motor cortical neuroplasticity by D-cycloserine. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2004, 29, 1573–1578. [Google Scholar] [CrossRef]
- Ballard, H.K.; Goen, J.R.M.; Maldonado, T.; Bernard, J.A. Effects of cerebellar transcranial direct current stimulation on the cognitive stage of sequence learning. J. Neurophysiol. 2019, 122, 490–499. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Wendling, F. Mechanisms of action of tDCS: A brief and practical overview. Neurophysiol. Clin.=Clin. Neurophysiol. 2019, 49, 269–275. [Google Scholar] [CrossRef]
- Takeuchi, T.; Duszkiewicz, A.J.; Morris, R.G. The synaptic plasticity and memory hypothesis: Encoding, storage and persistence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130288. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Malenka, R.C. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar] [CrossRef]
- Martin, S.J.; Grimwood, P.D.; Morris, R.G. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu. Rev. Neurosci. 2000, 23, 649–711. [Google Scholar] [CrossRef]
- Appelbaum, L.G.; Shenasa, M.A.; Stolz, L.; Daskalakis, Z. Synaptic plasticity and mental health: Methods, challenges and opportunities. Neuropsychopharmacology 2023, 48, 113–120. [Google Scholar] [CrossRef]
- Choi, D.I.; Kaang, B.K. Interrogating structural plasticity among synaptic engrams. Curr. Opin. Neurobiol. 2022, 75, 102552. [Google Scholar] [CrossRef] [PubMed]
- Nimchinsky, E.A.; Sabatini, B.L.; Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 2002, 64, 313–353. [Google Scholar] [CrossRef] [PubMed]
- Cavaleiro, C.; Martins, J.; Gonçalves, J.; Castelo-Branco, M. Memory and Cognition-Related Neuroplasticity Enhancement by Transcranial Direct Current Stimulation in Rodents: A Systematic Review. Neural Plast. 2020, 2020, 4795267. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, I.D.; Lins-Silva, D.H.; Barouh, J.L.; Faria-Guimarães, D.; Dorea-Bandeira, I.; Souza, L.S.; Alves, G.S.; Brunoni, A.R.; Nitsche, M.; Fregni, F.; et al. Neuroplasticity and non-invasive brain stimulation in the developing brain. Prog. Brain Res. 2021, 264, 57–89. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Liebetanz, D.; Lang, N.; Antal, A.; Tergau, F.; Paulus, W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin. Neurophysiol. 2003, 114, 2220–2222; author reply 2222–2223. [Google Scholar] [CrossRef]
- Gellner, A.K.; Reis, J.; Holtick, C.; Schubert, C.; Fritsch, B. Direct current stimulation-induced synaptic plasticity in the sensorimotor cortex: Structure follows function. Brain Stimul. 2020, 13, 80–88. [Google Scholar] [CrossRef]
- Mikuni, T.; Uchigashima, M. Methodological approaches to understand the molecular mechanism of structural plasticity of dendritic spines. Eur. J. Neurosci. 2021, 54, 6902–6911. [Google Scholar] [CrossRef]
- Jiang, T.; Xu, R.X.; Zhang, A.W.; Di, W.; Xiao, Z.J.; Miao, J.Y.; Luo, N.; Fang, Y.N. Effects of transcranial direct current stimulation on hemichannel pannexin-1 and neural plasticity in rat model of cerebral infarction. Neuroscience 2012, 226, 421–426. [Google Scholar] [CrossRef]
- Longo, V.; Barbati, S.A.; Re, A.; Paciello, F.; Bolla, M.; Rinaudo, M.; Miraglia, F.; Alù, F.; Di Donna, M.G.; Vecchio, F.; et al. Transcranial Direct Current Stimulation Enhances Neuroplasticity and Accelerates Motor Recovery in a Stroke Mouse Model. Stroke 2022, 53, 1746–1758. [Google Scholar] [CrossRef]
- Barbati, S.A.; Cocco, S.; Longo, V.; Spinelli, M.; Gironi, K.; Mattera, A.; Paciello, F.; Colussi, C.; Podda, M.V.; Grassi, C. Enhancing Plasticity Mechanisms in the Mouse Motor Cortex by Anodal Transcranial Direct-Current Stimulation: The Contribution of Nitric Oxide Signaling. Cereb. Cortex 2020, 30, 2972–2985. [Google Scholar] [CrossRef] [PubMed]
- Mielke, D.; Wrede, A.; Schulz-Schaeffer, W.; Taghizadeh-Waghefi, A.; Nitsche, M.A.; Rohde, V.; Liebetanz, D. Cathodal transcranial direct current stimulation induces regional, long-lasting reductions of cortical blood flow in rats. Neurol. Res. 2013, 35, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T.; Gozenman, F.; Berryhill, M.E. The strategy and motivational influences on the beneficial effect of neurostimulation: A tDCS and fNIRS study. Neuroimage 2015, 105, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.B.; Lerud, K.D.; Munsch, F.; Alsop, D.C.; Schlaug, G. Effects of tDCS dose and electrode montage on regional cerebral blood flow and motor behavior. Neuroimage 2021, 237, 118144. [Google Scholar] [CrossRef]
- Jamil, A.; Batsikadze, G.; Kuo, H.I.; Meesen, R.L.J.; Dechent, P.; Paulus, W.; Nitsche, M.A. Current intensity- and polarity-specific online and aftereffects of transcranial direct current stimulation: An fMRI study. Hum. Brain Mapp. 2020, 41, 1644–1666. [Google Scholar] [CrossRef]
- Hummel, F.C.; Cohen, L.G. Non-invasive brain stimulation: A new strategy to improve neurorehabilitation after stroke? Lancet. Neurol. 2006, 5, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Jacob, A.; Chowdhury, S.R.; Das, A.; Nitsche, M.A. EEG-NIRS based assessment of neurovascular coupling during anodal transcranial direct current stimulation--a stroke case series. J. Med. Syst. 2015, 39, 205. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Z.; Huang, J.; Wang, T.; Qu, Y. Computer-aided cognitive training combined with tDCS can improve post-stroke cognitive impairment and cerebral vasomotor function: A randomized controlled trial. BMC Neurol. 2024, 24, 132. [Google Scholar] [CrossRef]
- Meinzer, M.; Lindenberg, R.; Antonenko, D.; Flaisch, T.; Flöel, A. Anodal transcranial direct current stimulation temporarily reverses age-associated cognitive decline and functional brain activity changes. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 12470–12478. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Boggio, P.S.; De Raedt, R.; Benseñor, I.M.; Lotufo, P.A.; Namur, V.; Valiengo, L.C.; Vanderhasselt, M.A. Cognitive control therapy and transcranial direct current stimulation for depression: A randomized, double-blinded, controlled trial. J. Affect. Disord. 2014, 162, 43–49. [Google Scholar] [CrossRef]
- Jog, M.S.; Kim, E.; Anderson, C.; Kubicki, A.; Kayathi, R.; Jann, K.; Yan, L.; Leaver, A.; Hellemann, G.; Iacoboni, M.; et al. In-vivo imaging of targeting and modulation of depression-relevant circuitry by transcranial direct current stimulation: A randomized clinical trial. Transl. Psychiatry 2021, 11, 138. [Google Scholar] [CrossRef]
- Boggio, P.S.; Ferrucci, R.; Mameli, F.; Martins, D.; Martins, O.; Vergari, M.; Tadini, L.; Scarpini, E.; Fregni, F.; Priori, A. Prolonged visual memory enhancement after direct current stimulation in Alzheimer’s disease. Brain Stimul. 2012, 5, 223–230. [Google Scholar] [CrossRef]
- Das, N.; Spence, J.S.; Aslan, S.; Vanneste, S.; Mudar, R.; Rackley, A.; Quiceno, M.; Chapman, S.B. Cognitive Training and Transcranial Direct Current Stimulation in Mild Cognitive Impairment: A Randomized Pilot Trial. Front. Neurosci. 2019, 13, 307. [Google Scholar] [CrossRef]
- Hansen, N. Action mechanisms of transcranial direct current stimulation in Alzheimer’s disease and memory loss. Front. Psychiatry 2012, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Bahr-Hosseini, M.; Bikson, M. Neurovascular-modulation: A review of primary vascular responses to transcranial electrical stimulation as a mechanism of action. Brain Stimul. 2021, 14, 837–847. [Google Scholar] [CrossRef] [PubMed]
- MacVicar, B.A.; Newman, E.A. Astrocyte regulation of blood flow in the brain. Cold Spring Harb. Perspect. Biol. 2015, 7, 20388. [Google Scholar] [CrossRef]
- Monai, H.; Ohkura, M.; Tanaka, M.; Oe, Y.; Konno, A.; Hirai, H.; Mikoshiba, K.; Itohara, S.; Nakai, J.; Iwai, Y.; et al. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat. Commun. 2016, 7, 11100. [Google Scholar] [CrossRef]
- Kim, K.; Sherwood, M.S.; McIntire, L.K.; McKinley, R.A.; Ranganath, C. Transcranial Direct Current Stimulation Modulates Connectivity of Left Dorsolateral Prefrontal Cortex with Distributed Cortical Networks. J. Cogn. Neurosci. 2021, 33, 1381–1395. [Google Scholar] [CrossRef]
- Blanken, T.F.; Bathelt, J.; Deserno, M.K.; Voge, L.; Borsboom, D.; Douw, L. Connecting brain and behavior in clinical neuroscience: A network approach. Neurosci. Biobehav. Rev. 2021, 130, 81–90. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, J.; Xiao, Y.; Bi, J.; Biagi, M.C.; Ruffini, G.; Gouskova, N.A.; Manor, B.; Liu, Y.; Lü, J.; et al. Network-Based Transcranial Direct Current Stimulation May Modulate Gait Variability in Young Healthy Adults. Front. Hum. Neurosci. 2022, 16, 877241. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Nichols, T.E. Statistical Challenges in “Big Data” Human Neuroimaging. Neuron 2018, 97, 263–268. [Google Scholar] [CrossRef]
- Friston, K.J. Functional and effective connectivity: A review. Brain Connect. 2011, 1, 13–36. [Google Scholar] [CrossRef]
- Boutet, A.; Madhavan, R.; Elias, G.J.B.; Joel, S.E.; Gramer, R.; Ranjan, M.; Paramanandam, V.; Xu, D.; Germann, J.; Loh, A.; et al. Predicting optimal deep brain stimulation parameters for Parkinson’s disease using functional MRI and machine learning. Nat. Commun. 2021, 12, 3043. [Google Scholar] [CrossRef]
- Pedersen, M.; Zalesky, A. Intracranial brain stimulation modulates fMRI-based network switching. Neurobiol. Dis. 2021, 156, 105401. [Google Scholar] [CrossRef]
- Polanía, R.; Paulus, W.; Antal, A.; Nitsche, M.A. Introducing graph theory to track for neuroplastic alterations in the resting human brain: A transcranial direct current stimulation study. NeuroImage 2011, 54, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Polania, R.; Paulus, W.; Nitsche, M.A. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum. Brain Mapp. 2012, 33, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- Shmuelof, L.; Krakauer, J.W.; Mazzoni, P. How is a motor skill learned? Change and invariance at the levels of task success and trajectory control. J. Neurophysiol. 2012, 108, 578–594. [Google Scholar] [CrossRef]
- Landi, S.M.; Baguear, F.; Della-Maggiore, V. One week of motor adaptation induces structural changes in primary motor cortex that predict long-term memory one year later. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 11808–11813. [Google Scholar] [CrossRef]
- Kuo, M.F.; Paulus, W.; Nitsche, M.A. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage 2014, 85 Pt 3, 948–960. [Google Scholar] [CrossRef]
- Qi, S.; Liang, Z.; Wei, Z.; Liu, Y.; Wang, X. Effects of transcranial direct current stimulation on motor skills learning in healthy adults through the activation of different brain regions: A systematic review. Front. Hum. Neurosci. 2022, 16, 1021375. [Google Scholar] [CrossRef] [PubMed]
- Nissim, N.R.; O’Shea, A.; Indahlastari, A.; Telles, R.; Richards, L.; Porges, E.; Cohen, R.; Woods, A.J. Effects of in-Scanner Bilateral Frontal tDCS on Functional Connectivity of the Working Memory Network in Older Adults. Front. Aging Neurosci. 2019, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Xie, C.; Fan, D.Q.; Lei, X.; Yu, J. High definition-transcranial direct current stimulation changes older adults’ subjective sleep and corresponding resting-state functional connectivity. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2018, 129, 1–8. [Google Scholar] [CrossRef]
- Barbati, S.A.; Podda, M.V.; Grassi, C. Tuning brain networks: The emerging role of transcranial direct current stimulation on structural plasticity. Front. Cell. Neurosci. 2022, 16, 945777. [Google Scholar] [CrossRef]
- Hyman, S.E. Neurotransmitters. Curr. Biol. CB 2005, 15, R154–R158. [Google Scholar] [CrossRef]
- Spitzer, N.C. Neurotransmitter Switching in the Developing and Adult Brain. Annu. Rev. Neurosci. 2017, 40, 1–19. [Google Scholar] [CrossRef]
- Nwaroh, C.; Giuffre, A.; Cole, L.; Bell, T.; Carlson, H.L.; MacMaster, F.P.; Kirton, A.; Harris, A.D. Effects of Transcranial Direct Current Stimulation on GABA and Glx in Children: A pilot study. PLoS ONE 2020, 15, e0222620. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, H.; Thimmashetty, V.H.; Shivakumar, V.; Venkatasubramanian, G.; Narayanswamy, J.C. Effect of transcranial direct current stimulation on in-vivo assessed neuro-metabolites through magnetic resonance spectroscopy: A systematic review. Acta Neuropsychiatr. 2021, 33, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.F.; de Souza, I.C.; Vidor, L.P.; de Souza, A.; Deitos, A.; Volz, M.S.; Fregni, F.; Caumo, W.; Torres, I.L. Neurobiological effects of transcranial direct current stimulation: A review. Front. Psychiatry 2012, 3, 110. [Google Scholar] [CrossRef]
- Agarwal, S.M.; Shivakumar, V.; Bose, A.; Subramaniam, A.; Nawani, H.; Chhabra, H.; Kalmady, S.V.; Narayanaswamy, J.C.; Venkatasubramanian, G. Transcranial direct current stimulation in schizophrenia. Clin. Psychopharmacol. Neurosci. Off. Sci. J. Korean Coll. Neuropsychopharmacol. 2013, 11, 118–125. [Google Scholar] [CrossRef]
- Chang, C.C.; Kao, Y.C.; Chao, C.Y.; Tzeng, N.S.; Chang, H.A. Examining bi-anodal transcranial direct current stimulation (tDCS) over bilateral dorsolateral prefrontal cortex coupled with bilateral extracephalic references as a treatment for negative symptoms in non-acute schizophrenia patients: A randomized, double-blind, sham-controlled trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 96, 109715. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Chen, Z.; Xu, H.; Bu, G.; Zheng, H. Implications of GABAergic Neurotransmission in Alzheimer’s Disease. Front. Aging Neurosci. 2016, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, D.; Walker, P.M.; Bejot, Y.; Aho, S.L.; Tavernier, B.; Rouaud, O.; Ricolfi, F.; Brunotte, F. N-acetylaspartate/creatine and choline/creatine ratios in the thalami, insular cortex and white matter as markers of hypertension and cognitive impairment in the elderly. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2008, 31, 1851–1857. [Google Scholar] [CrossRef]
- Choi, C.H.; Iordanishvili, E.; Shah, N.J.; Binkofski, F. Magnetic resonance spectroscopy with transcranial direct current stimulation to explore the underlying biochemical and physiological mechanism of the human brain: A systematic review. Hum. Brain Mapp. 2021, 42, 2642–2671. [Google Scholar] [CrossRef]
- Marquardt, L.; Kusztrits, I.; Craven, A.R.; Hugdahl, K.; Specht, K.; Hirnstein, M. A multimodal study of the effects of tDCS on dorsolateral prefrontal and temporo-parietal areas during dichotic listening. Eur. J. Neurosci. 2021, 53, 449–459. [Google Scholar] [CrossRef]
- Auvichayapat, N.; Patjanasoontorn, N.; Phuttharak, W.; Suphakunpinyo, C.; Keeratitanont, K.; Tunkamnerdthai, O.; Aneksan, B.; Klomjai, W.; Boonphongsathian, W.; Sinkueakunkit, A.; et al. Brain Metabolite Changes After Anodal Transcranial Direct Current Stimulation in Autism Spectrum Disorder. Front. Mol. Neurosci. 2020, 13, 70. [Google Scholar] [CrossRef]
- Patel, H.J.; Romanzetti, S.; Pellicano, A.; Nitsche, M.A.; Reetz, K.; Binkofski, F. Proton Magnetic Resonance Spectroscopy of the motor cortex reveals long term GABA change following anodal Transcranial Direct Current Stimulation. Sci. Rep. 2019, 9, 2807. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, D.; Thielscher, A.; Saturnino, G.B.; Aydin, S.; Ittermann, B.; Grittner, U.; Flöel, A. Towards precise brain stimulation: Is electric field simulation related to neuromodulation? Brain Stimul. 2019, 12, 1159–1168. [Google Scholar] [CrossRef]
- Puts, N.A.; Edden, R.A.; Evans, C.J.; McGlone, F.; McGonigle, D.J. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J. Neurosci. 2011, 31, 16556–16560. [Google Scholar] [CrossRef]
- Stagg, C.J.; Bachtiar, V.; Johansen-Berg, H. The role of GABA in human motor learning. Curr. Biol. 2011, 21, 480–484. [Google Scholar] [CrossRef]
- Agrawal, B.; Boulos, S.; Khatib, S.; Feuermann, Y.; Panov, J.; Kaphzan, H. Molecular Insights into Transcranial Direct Current Stimulation Effects: Metabolomics and Transcriptomics Analyses. Cells 2024, 13, 205. [Google Scholar] [CrossRef] [PubMed]
- Poh, E.Z.; Hahne, D.; Moretti, J.; Harvey, A.R.; Clarke, M.W.; Rodger, J. Simultaneous quantification of dopamine, serotonin, their metabolites and amino acids by LC-MS/MS in mouse brain following repetitive transcranial magnetic stimulation. Neurochem. Int. 2019, 131, 104546. [Google Scholar] [CrossRef] [PubMed]
- Hosp, J.A.; Pekanovic, A.; Rioult-Pedotti, M.S.; Luft, A.R. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J. Neurosci. 2011, 31, 2481–2487. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, S.; Cao, L.; Wang, Q.; Sheng, Y.; Yu, J.; Liang, Z. The Physiological Mechanisms of Transcranial Direct Current Stimulation to Enhance Motor Performance: A Narrative Review. Biology 2024, 13, 790. https://doi.org/10.3390/biology13100790
Qi S, Cao L, Wang Q, Sheng Y, Yu J, Liang Z. The Physiological Mechanisms of Transcranial Direct Current Stimulation to Enhance Motor Performance: A Narrative Review. Biology. 2024; 13(10):790. https://doi.org/10.3390/biology13100790
Chicago/Turabian StyleQi, Shuo, Lei Cao, Qingchun Wang, Yin Sheng, Jinglun Yu, and Zhiqiang Liang. 2024. "The Physiological Mechanisms of Transcranial Direct Current Stimulation to Enhance Motor Performance: A Narrative Review" Biology 13, no. 10: 790. https://doi.org/10.3390/biology13100790







