Phylogeography of the Sinica Group of Macaques in the Himalayas: Taxonomic and Evolutionary Implications
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.1.1. Shannan, Xizang, Eastern Himalayas
2.1.2. Jilong Valley, Xizang, Central Himalayas
2.1.3. Eastern Nepal, Central Himalayas
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. DNA Sequence Assembly and Analysis
2.3.1. Sequence Assembly and Alignment
2.3.2. Phylogenetic Analysis and Divergence Time Estimation
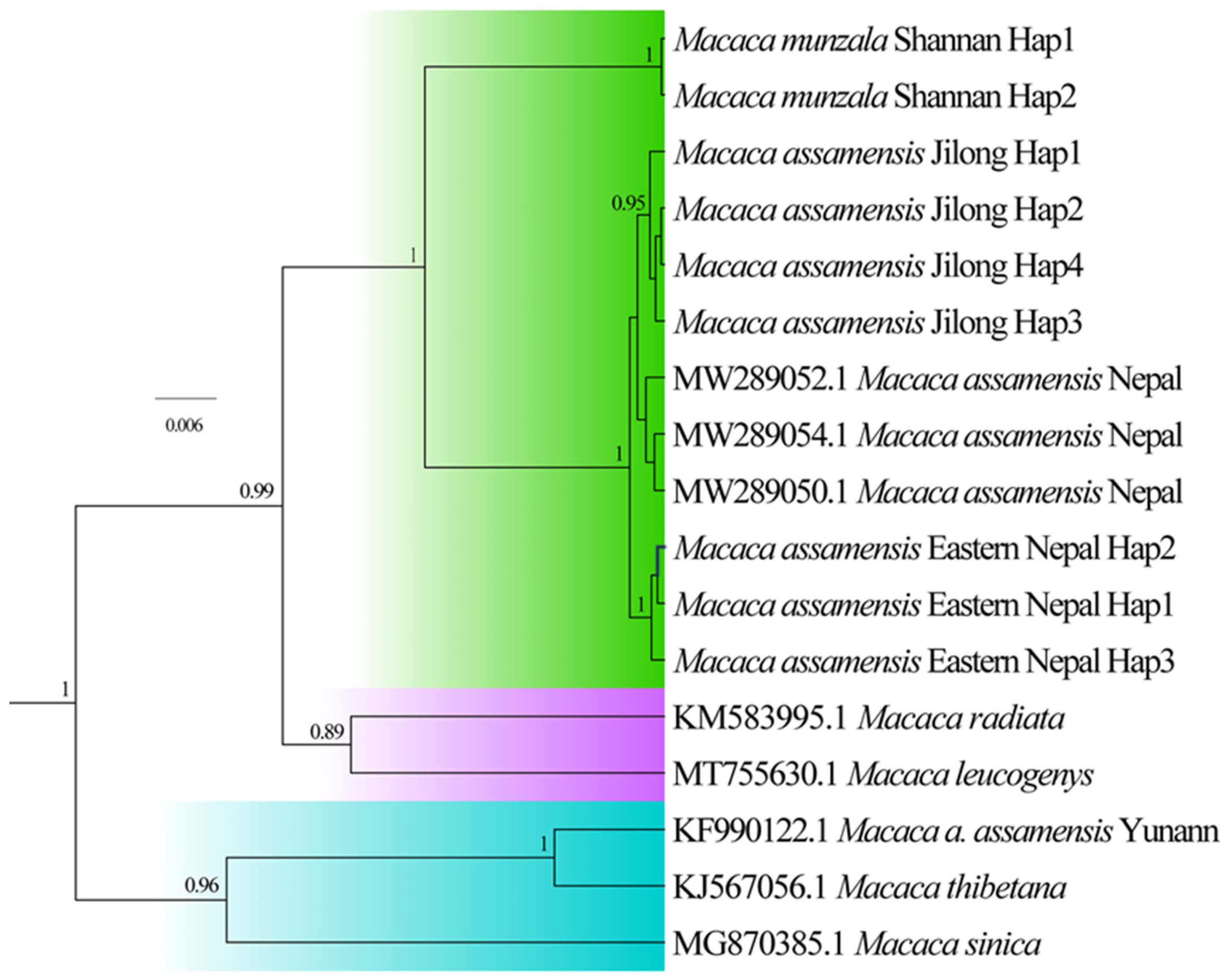
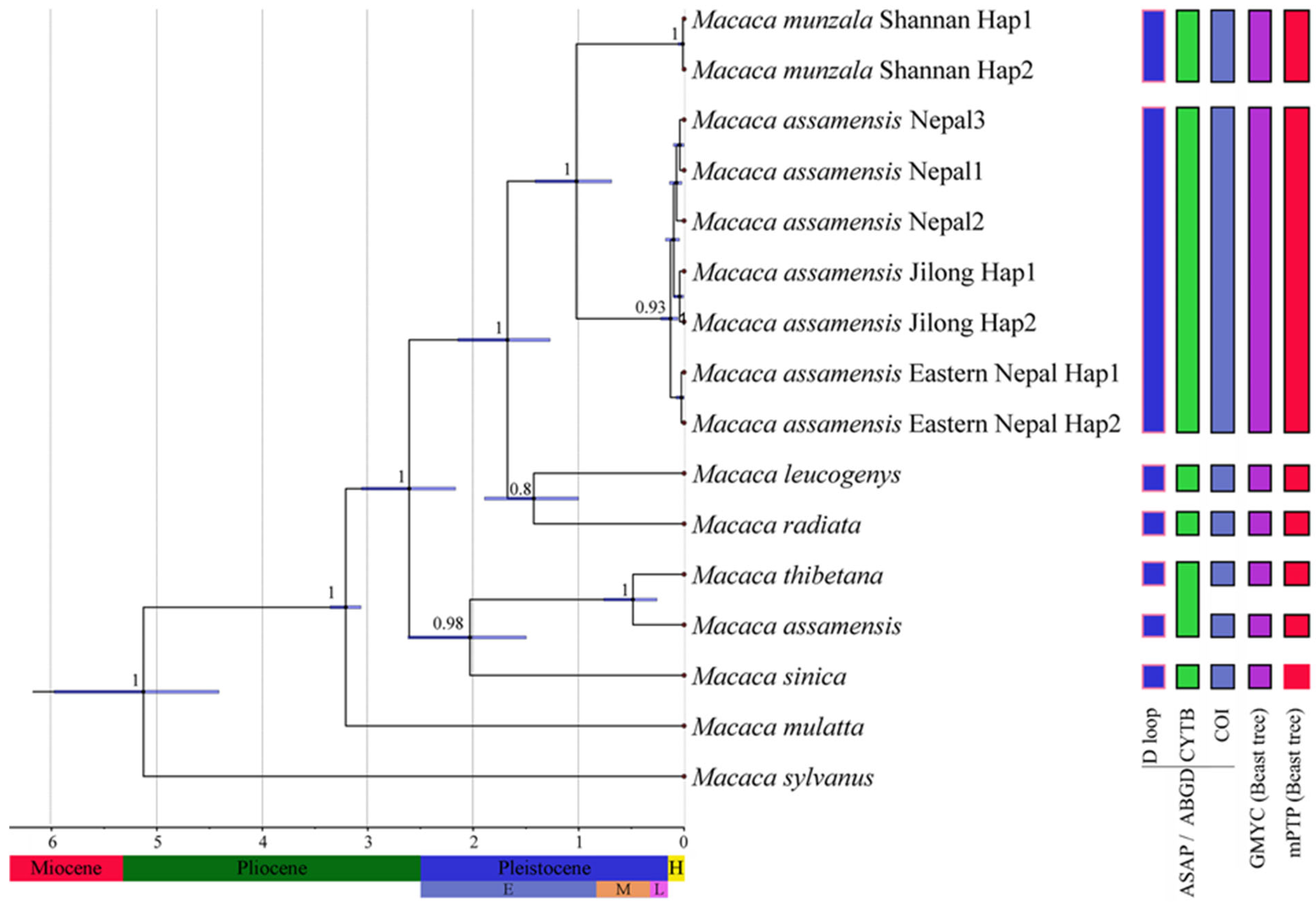
2.3.3. Molecular Species Delimitation
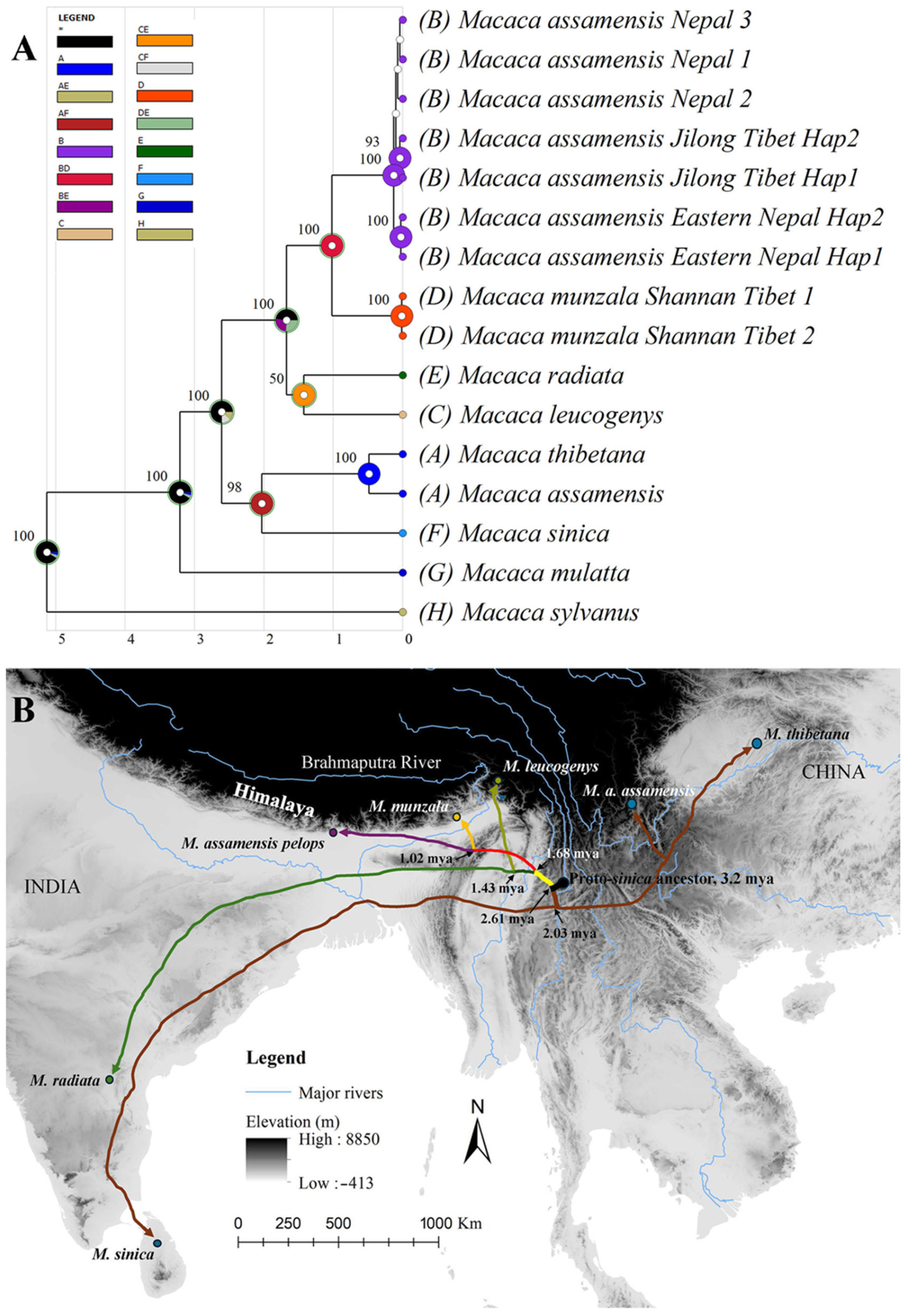
2.3.4. Reconstruction of Ancestral Areas
3. Results
3.1. Genetic Polymorphisms among the Sampled Populations
3.2. Assignment of Study Populations to Known Species
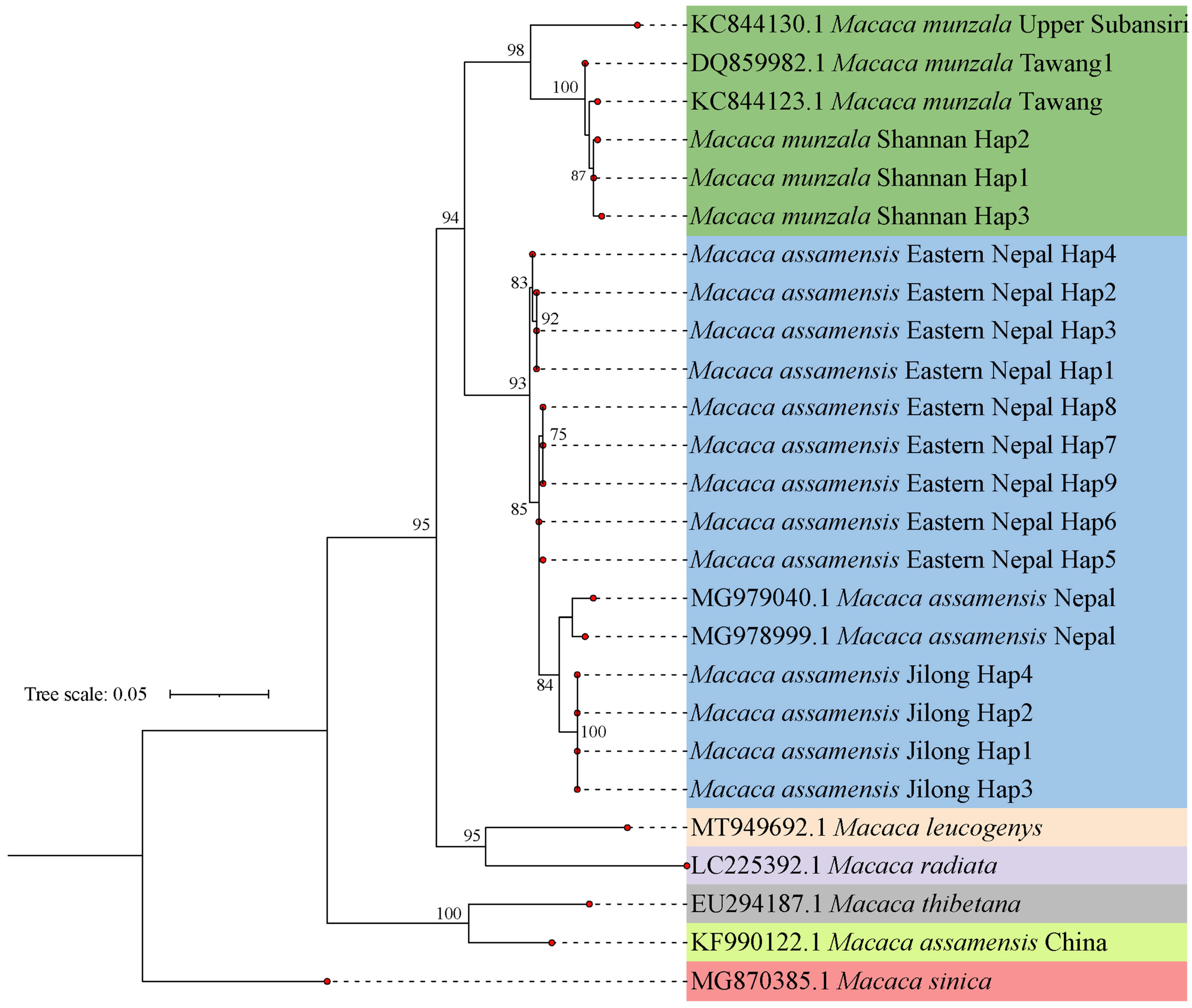
3.3. Phylogenetic Relationships and Evolutionary Distances among Macaques in sinica Group
3.4. Divergence Time Estimates and Species Delimitation
3.5. Ancestral Area Inference for Sinica Group of Macaques
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roos, C.; Kothe, M.; Alba, D.M.; Delson, E.; Zinner, D. The radiation of macaques out of Africa: Evidence from mitogenome divergence times and the fossil record. J. Hum. Evol. 2019, 133, 114–132. [Google Scholar] [CrossRef]
- Liedigk, R.; Roos, C.; Brameier, M.; Zinner, D. Mitogenomics of the Old World monkey tribe Papionini. BMC Evol. Biol. 2014, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Fooden, J. Taxonomy and evolution of the Sinica group of macaques: I. Species and subspecies accounts of Macaca sinica. Primates 1979, 20, 109–140. [Google Scholar] [CrossRef]
- Li, C.; Zhao, C.; Fan, P.F. White-cheeked macaque (Macaca leucogenys): A new macaque species from Modog, southeastern Tibet. Am. J. Primatol. 2015, 77, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Datta, A.; Madhusudan, M.D.; Mishra, C. Macaca munzala: A new species from western Arunachal Pradesh, Northeastern India. Int. J. Primatol. 2005, 26, 977–989. [Google Scholar] [CrossRef]
- Ghosh, A.; Thakur, M.; Singh, S.K.; Dutta, R.; Sharma, L.K.; Chandra, K.; Banerjee, D. The Sela macaque (Macaca selai) is a distinct phylogenetic species that evolved from the Arunachal macaque following allopatric speciation. Mol. Phylogen. Evol. 2022, 174, 107513. [Google Scholar] [CrossRef]
- Choudhury, A. A review of the Assamese macaque Macaca assamensis complex and its geographical variation. Primate Conserv. 2022, 36, 203–231. [Google Scholar]
- Hoelzer, G.A.; Hoelzer, M.A.; Melnick, D.J. The evolutionary history of the sinica-group of macaque monkeys as revealed by mtDNA restriction site analysis. Mol. Phylogen. Evol. 1992, 1, 215–222. [Google Scholar] [CrossRef]
- Zhang, B.-L.; Chen, W.; Wang, Z.; Pang, W.; Luo, M.-T.; Wang, S.; Shao, Y.; He, W.-Q.; Deng, Y.; Zhou, L.; et al. Comparative genomics reveals the hybrid origin of a macaque group. Sci. Adv. 2023, 9, eadd3580. [Google Scholar] [CrossRef]
- Fan, P.; Liu, Y.; Zhang, Z.; Zhao, C.; Li, C.; Liu, W.; Liu, Z.; Li, M. Phylogenetic position of the white-cheeked macaque (Macaca leucogenys), a newly described primate from southeastern Tibet. Mol. Phylogen. Evol. 2017, 107, 80–89. [Google Scholar] [CrossRef]
- Khanal, L.; Chalise, M.K.; Fan, P.F.; Kyes, R.C.; Jiang, X.L. Multilocus phylogeny suggests a distinct species status for the Nepal population of Assam macaques (Macaca assamensis): Implications for evolution and conservation. Zool. Res. 2021, 42, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Biswas, J.; Borah, D.K.; Das, A.; Das, J.; Bhattacharjee, P.C.; Mohnot, S.M.; Horwich, R.H. The enigmatic Arunachal macaque: Its biogeography, biology and taxonomy in Northeastern India. Am. J. Primatol. 2011, 73, 458–473. [Google Scholar] [CrossRef] [PubMed]
- Fooden, J. Taxonomy and evolution of the sinica group of macaques: 3. Species and subspecies accounts of Macaca assamensis. Fieldina Zool. 1982, 10, 1–52. [Google Scholar] [CrossRef]
- Abegg, C.; Thierry, B. Macaque evolution and dispersal in insular south-east Asia. Biol. J. Linn. Soc. 2002, 75, 555–576. [Google Scholar] [CrossRef]
- Kumara, H.N.; Singh, M.; Kumar, S.; Sinha, A. Distribution, abundance, group size and demography of dark-bellied bonnet macaque Macaca radiata radiata in Karnataka, South India. Curr. Sci. 2010, 99, 663–667. [Google Scholar]
- Chalise, M.K. Characteristics of the Assamese monkey (Macaca assamensis) of Nepal. Am. J. Primatol. 2005, 66, 195. [Google Scholar]
- Sinha, A.; Kumar, R.S.; Gama, N.; Madhusudan, M.D.; Mishra, C. Distribution and conservation status of the Arunachal macaque, Macaca munzala, in Western Arunachal Pradesh, Northeastern India. Primate Conserv. 2006, 21, 145–148. [Google Scholar] [CrossRef]
- Sarania, B.; Devi, A.; Kumar, A.; Sarma, K.; Gupta, A.K. Predictive distribution modeling and population status of the endangered Macaca munzala in Arunachal Pradesh, India. Am. J. Primatol. 2017, 79, e22592. [Google Scholar] [CrossRef]
- Yangdon, N.; Dorji, K.; Tobgay, S. Sighting of Arunachal Macaque Macaca munzala Sinha et al., 2005 (Mammalia: Primates: Cercopithecidae) in Sakteng Wildlife Sanctuary, Bhutan. J. Threat. Taxa 2019, 11, 13805–13807. [Google Scholar] [CrossRef]
- Chetry, D.; Borthakur, U.; Das, R. A Short Note on A First Distribution Record of White-Cheeked Macaque Macaca leucogenys from India. Asian Primates J. 2015, 5, 45–47. [Google Scholar]
- Ghosh, A.; Dalui, S.; Mukherjee, T.; Joshi, B.D.; Singh, S.K.; Maheswaran, G.; Sharma, L.K.; Chandra, K.; Thakur, M. Serendipitous discovery of white-cheeked macaque (Macaca leucogenys) from Arunachal Pradesh, India. Anim. Gene 2022, 23, 200124. [Google Scholar] [CrossRef]
- Khanal, L.; Chalise, M.K.; Jiang, X.L. Distribution of the threatened Assamese macaque Macaca assamensis (Mammalia: Primates: Cercopithecidae) population in Nepal. J. Threat. Taxa 2019, 11, 13047–13057. [Google Scholar] [CrossRef]
- Molur, S.; Brandon-Jones, D.; Dittus, W.; Eudey, A.; Kumar, A.; Singh, M.; Feeroz, M.M.; Chalise, M.K.; Priya, P.; Walker, S. Status of South Asian Primates:Conservation Assessment and Management Plan (C.A.M.P.); Zoo Outreach Organisation/CBSG-South Asia: Coimbatore, India, 2003; p. 432. [Google Scholar]
- Boonratana, R.; Chalise, M.K.; Das, J.; Htun, S.; Timmins, R.J. Macaca assamensis. The IUCN Red List of Threatened Species 2020: e.T12549A17950189. Available online: https://www.iucnredlist.org/species/12549/17950189 (accessed on 15 May 2024).
- Chalise, M.K. Assamese monkeys (Macaca assamensis) in Nepal. Primate Conserv. 2003, 19, 99–107. [Google Scholar]
- Chakraborty, D.; Ramakrishnan, U.; Panor, J.; Mishra, C.; Sinha, A. Phylogenetic relationships and morphometric affinities of the Arunachal macaque Macaca munzala, a newly described primate from Arunachal Pradesh, northeastern India. Mol. Phylogen. Evol. 2007, 44, 838–849. [Google Scholar] [CrossRef]
- Fooden, J. Ecogeographic segregation of macaque species. Primates 1982, 23, 574–579. [Google Scholar] [CrossRef]
- Kawamoto, Y.; Aimi, M.; Wangchuk, T.; Sherub. Distribution of Assamese macaques (Macaca assamensis) in the inner Himalayan region of Bhutan and their mtDNA diversity. Primates 2006, 47, 388–392. [Google Scholar] [CrossRef]
- Kumar, A.; Sinha, A.; Kumar, S. Macaca munzala. The IUCN Red List of Threatened Species 2020: e.T136569A17948833. 2020. Available online: https://www.iucnredlist.org/species/136569/17948833 (accessed on 29 February 2024).
- Fan, P.F.; Ma, C. Macaca leucogenys. The IUCN Red List of Threatened Species 2022: e.T205889816A205890248. 2022. Available online: https://www.iucnredlist.org/species/205889816/205890248 (accessed on 29 February 2024).
- Dittus, W.; Watson, A.C. Macaca sinica. The IUCN Red List of Threatened Species 2020: e.T12560A17951229. 2020. Available online: https://www.iucnredlist.org/species/12560/17951229 (accessed on 29 February 2024).
- Singh, M.; Kumara, H.N.; Kumar, A. Macaca radiata. The IUCN Red List of Threatened Species 2020: e.T12558A17951596. 2020. Available online: https://www.iucnredlist.org/species/12558/17951596 (accessed on 29 February 2024).
- Yongcheng, L.; Richardson, M. Macaca thibetana. The IUCN Red List of Threatened Species 2020: e.T12562A17948236. 2020. Available online: https://www.iucnredlist.org/species/12562/17948236 (accessed on 29 February 2024).
- Kumar, R.S.; Gama, N.; Raghunath, R.; Sinha, A.; Mishra, C. In search of the munzala: Distribution and conservation status of the newly-discovered Arunachal macaque Macaca munzala. Oryx 2008, 42, 360–366. [Google Scholar] [CrossRef]
- Khanal, L.; Upadhyaya, L.P.; Pandey, N.; Chand, D.B.; Karki, M.; Chalise, M.K.; Kyes, R.C. Projected distribution of the westernmost subpopulation of Assamese macaques (Macaca assamensis pelops) under climate change: Conservation implications of a threatened population. Front. Conserv. Sci. 2023, 4, 1235595. [Google Scholar] [CrossRef]
- Adhikari, K.; Khanal, L.; Chalise, M.K. Status and effects of food provisoning on ecology of Assamese monkey (Macaca assamensis) in Ramdi area of Palpa, Nepal. J. Inst. Sci. Technol. 2018, 22, 183–190. [Google Scholar] [CrossRef]
- Ellegren, H.; Smith, N.G.C.; Webster, M.T. Mutation rate variation in the mammalian genome. Curr. Opin. Genet. Dev. 2003, 13, 562–568. [Google Scholar] [CrossRef]
- Baer, C.F.; Miyamoto, M.M.; Denver, D.R. Mutation rate variation in multicellular eukaryotes: Causes and consequences. Nat. Rev. Genet. 2007, 8, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Matsudaira, K.; Hamada, Y.; Bunlungsup, S.; Ishida, T.; San, A.M.; Malaivijitnond, S. Whole mitochondrial genomic and Y-chromosomal phylogenies of Burmese long-tailed macaque (Macaca fascicularis aurea) suggest ancient hybridization between fascicularis and sinica species groups. J. Hered. 2018, 108, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.M.; George, M., Jr.; Wilson, A.C. Rapid evolution of animal mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1979, 76, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C. Applications of mitochondrial DNA analysis in conservation: A critical review. Mol. Ecol. 1994, 3, 401–411. [Google Scholar] [CrossRef]
- Hodgson, B.H. Three new species of monkey; with remarks on the genera Semnopithecus et Macacus. J. Asiat. Soc. Bengal Calcutta 1841, 9, 1211–1213. [Google Scholar]
- White, P.S.; Densmore, L.D., III. Mitochondrial DNA Isolation. In Molecular Genetic Analysis of Populations: A practical Approach; Oxford University Press: Oxford, UK, 1992; pp. 29–58. [Google Scholar]
- Li, Q.Q.; Zhang, Y.P. Phylogenetic relationships of the macaques (Cercopithecidae: Macaca), inferred from mitochondrial DNA sequences. Biochem. Genet. 2005, 43, 375–386. [Google Scholar] [CrossRef]
- Hayasaka, K.; Ishida, T.; Horai, S. Heteroplasmy and polymorphism in the major noncoding region of Mitochondrial DNA in Japanese monkeys: Association with tandemly repeated sequences. Mol. Biol. Evol. 1991, 8, 399–415. [Google Scholar] [CrossRef]
- Burland, T.G. DNASTAR’s Lasergene Sequence Analysis Software. In Bioinformatics Methods and Protocols: Methods in Molecular Biology, Volume 132; Misener, S., Krawetz, S.A., Eds.; Humana Press: Totowa, NJ, USA, 2000; pp. 71–91. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S.; Battistuzzi, F.U. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010. [Google Scholar]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.; Jakovlic, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.Y.; Gao, F.; Jakovlić, I.; Lei, H.P.; Hu, Y.; Zhang, H.; Zou, H.; Wang, G.T.; Zhang, D. Using PhyloSuite for molecular phylogeny and tree-based analyses. iMeta 2023, 2, e87. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Suchard, M.; Xie, D.; Drummond, A. Tracer v1.6.—Computer Program and Documentation. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 25 April 2020).
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Tosi, A.J.; Morales, J.C.; Melnick, D.J. Paternal, maternal and biparental molecular markers provide unique windows onto the evolutionary history of macaque monkeys. Evolution 2003, 57, 1419–1435. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Fujisawa, T.; Barraclough, T.G. Delimiting species using single-locus data and the Generalized Mixed Yule Coalescent approach: A revised method and evaluation on simulated data sets. Syst. Biol. 2013, 62, 707–724. [Google Scholar] [CrossRef]
- Kapli, P.; Lutteropp, S.; Zhang, J.; Kobert, K.; Pavlidis, P.; Stamatakis, A.; Flouri, T. Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics 2017, 33, 1630–1638. [Google Scholar] [CrossRef]
- Ezard, T.; Fujisawa, T.; Barraclough, T.G. Splits: SPecies’ Limits by Threshold Statistics. R Package Version 1.0.19. 2009. Available online: http://R-Forge.R-project.org/projects/splits/ (accessed on 6 March 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: http://www.R-project.org/ (accessed on 6 March 2024).
- Yu, Y.; Blair, C.; He, X. RASP 4: Ancestral state reconstruction tool for multiple genes and characters. Mol. Biol. Evol. 2020, 37, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Harris, A.J.; He, X. S-DIVA (Statistical Dispersal-Vicariance Analysis): A tool for inferring biogeographic histories. Mol. Phylogen. Evol. 2010, 56, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Delson, E. Fossil macaques, phyletic relationships and a scenario of development. In The Macaques: Studies in Ecology, Behavior and Evolution; Lindburg, D.G., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1980; pp. 10–30. [Google Scholar]
- Evans, B.J.; Gansauge, M.T.; Tocheri, M.W.; Schillaci, M.A.; Sutikna, T.; Jatmiko; Saptomo, E.W.; Klegarth, A.; Tosi, A.J.; Melnick, D.J.; et al. Mitogenomics of macaques (Macaca) across Wallace’s Line in the context of modern human dispersals. J. Hum. Evol. 2020, 146, 102852. [Google Scholar] [CrossRef] [PubMed]
- Padial, J.M.; Miralles, A.; De la Riva, I.; Vences, M. The integrative future of taxonomy. Front. Zool. 2010, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Groves, C.P. Primate Taxonomy; Smithsonian Series in Comparative Evolutionary Biology; Smithsonian Institution Press: Washington, DC, USA, 2011; p. 350. [Google Scholar]
- Mittermeier, R.A.; Rylands, A.B.; Wilson, D.E. (Eds.) Handbook of the Mammals of the World; Primates; Lynx Edicions: Barcelona, Spain, 2013; Volume 3. [Google Scholar]
- Khanal, L.; Chalise, M.K.; He, K.; Acharya, B.K.; Kawamoto, Y.; Jiang, X.L. Mitochondrial DNA analyses and ecological niche modeling reveal post-LGM expansion of the Assam macaque (Macaca assamensis) in the foothills of Nepal Himalaya. Am. J. Primatol. 2018, 80, e22748. [Google Scholar] [CrossRef]
- McClelland, J. List of Mammalia and birds collected in Assam. Proceeding Zool. Soc. Lond. 1839, 7, 146–167. [Google Scholar]
- McClelland, J. XLIV.—A List of Mammalia and Birds collected in Assam. Ann. Mag. Nat. Hist. 1841, 6, 366–374. [Google Scholar] [CrossRef]
- Roos, C.; Boonratana, R.; Supriatna, J.; Fellowes, J.R.; Groves, C.; Nash, S.D.; Rylands, A.B.; Mittermeier, R.A. An upadated taxonomy and conservation status review of Asian primates. Asian Primates J. 2014, 4, 2–20. [Google Scholar]
- Tosi, A.J.; Disotell, T.R.; Carlos Morales, J.; Melnick, D.J. Cercopithecine Y-chromosome data provide a test of competing morphological evolutionary hypotheses. Mol. Phylogen. Evol. 2003, 27, 510–521. [Google Scholar] [CrossRef]
- Owen, L.A.; Caffee, M.W.; Finkel, R.C.; Seong, Y.B. Quaternary glaciation of the Himalayan-Tibetan orogen. J. Quat. Sci. 2008, 23, 513–531. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, M.C.; Deswal, S.; Manna, I.; Chakraborty, E.; Prakash, S. Last Glacial Maximum and subsequent glacial chronology in the monsoon-dominated Sikkim Himalaya, India. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2023, 617, 111480. [Google Scholar] [CrossRef]

| Locus | Primer | Primer Sequence (5′–3′) | Annealing Temp. | Reference |
|---|---|---|---|---|
| D-loop | LqqF | TCCTAGGGCAATCAGAAAGAAAG | 58 °C | [44] |
| Saru5 | GGCCAGGACCAAGCCTATTT | [45] | ||
| CYTB | CYTF | AACCATCGTTGTACTTCAAC | 56 °C | [11] |
| CYTR | TCTGGTTTACAAGGCCAGTG | [11] | ||
| COI | MCOIF | TCAACAAACCATAAAGACATTGG | 55 °C | This study |
| MCOIR | AGACTTCGGGGTGACCAAAGAATC | This study |
| Species | GenBank Accession Numbers | |||
|---|---|---|---|---|
| D-Loop | CYTB | COI | ||
| Macaca assamensis China * | KF990122.1 | KF990122.1 | KF990122.1 | |
| Macaca assamensis Nepal ** | MG978999.1, MG979040.1, PP874946–PP874958 # | MW289050.1, MW289052.1, PP884086–PP884092 # | PP868421.1–PP868425.1 # | |
| Macaca leucogenys | MT755630.1 | MT755630.1 | MT755630.1 | |
| Macaca munzala | DQ859981.1, DQ859982.1, KC844122.1, KC844123.1, PP874959–PP874961 # | PP884093–PP884094 # | PP868426.1–PP868427.1 # | |
| Macaca radiata | KM583995.1 | KM583995.1 | KM583995.1 | |
| Macaca sinica | MG870385.1 | MG870385.1 | MG870385.1 | |
| Macaca thibetana | KJ567056.1 | KJ567056.1 | KJ567056.1 | |
| Macaca mulatta # | AY612638.1 | AY612638.1 | AY612638.1 | |
| Macaca sylvanus | KJ567054.1 | KJ567054.1 | KJ567054.1 | |
| Species (Location)→ Locus↓ | M. munzala (Shannan, Xizang) | M. a. pelops (Jilong, Xizang) | M. a. pelops (Eastern Nepal) | |
|---|---|---|---|---|
| Samples (Sequences) | N | 9 (6) | 12 (12) | 39 (36) |
| D-loop (1090 bp) | H | 3 | 4 | 9 |
| Hd ± SD | 0.600 ± 0.215 | 0.758 ± 0.081 | 0.903 ± 0.017 | |
| π ± SD | 0.0017 ± 0.0009 | 0.0014 ± 0.0002 | 0.0046 ± 0.0003 | |
| CYTB (1140 bp) | H | 2 | 4 | 3 |
| Hd ± SD | 0.533 ± 0.172 | 0.561 ± 0.154 | 0.686 ± 0.019 | |
| π ± SD | 0.001 ± 0.0003 | 0.0007 ± 0.0003 | 0.0008 ± 0.0001 | |
| COI (668 bp) | H | 2 | 3 | 2 |
| Hd ± SD | 0.333 ± 0.215 | 0.545 ± 0.144 | 0.413 ± 0.068 | |
| π ± SD | 0.0005 ± 0.0003 | 0.0012 ± 0.0003 | 0.0006 ± 0.0001 | |
| D-loop + CYTB + COI (2898 bp) | H | 4 | 8 | 15 |
| Hd ± SD | 0.867 ± 0.129 | 0.924 ± 0.057 | 0.937 ± 0.018 | |
| π ± SD | 0.0011 ± 0.0003 | 0.0011 ± 0.0002 | 0.0023 ± 0.0001 | |
| Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|
| 1 | M. munzala Xizang | 0.0068 | 0.0087 | 0.0082 | 0.0103 | 0.0100 | 0.0116 | |
| 2 | M. a. pelops Nepal and Jilong | 0.0457 | 0.0082 | 0.0079 | 0.0096 | 0.0094 | 0.0112 | |
| 3 | M. radiata | 0.0734 | 0.0658 | 0.0075 | 0.0091 | 0.0088 | 0.0094 | |
| 4 | M. leucogenys | 0.0740 | 0.0675 | 0.0649 | 0.0092 | 0.0091 | 0.0102 | |
| 5 | M. a. assamensis China | 0.0983 | 0.0879 | 0.0855 | 0.0877 | 0.0041 | 0.0092 | |
| 6 | M. thibetana | 0.0950 | 0.0847 | 0.0813 | 0.0837 | 0.0208 | 0.0086 | |
| 7 | M. sinica | 0.1037 | 0.1003 | 0.0866 | 0.0989 | 0.0805 | 0.0722 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanal, L.; Li, X.; Subba, A.; Ulak, S.; Kyes, R.C.; Jiang, X.-L. Phylogeography of the Sinica Group of Macaques in the Himalayas: Taxonomic and Evolutionary Implications. Biology 2024, 13, 795. https://doi.org/10.3390/biology13100795
Khanal L, Li X, Subba A, Ulak S, Kyes RC, Jiang X-L. Phylogeography of the Sinica Group of Macaques in the Himalayas: Taxonomic and Evolutionary Implications. Biology. 2024; 13(10):795. https://doi.org/10.3390/biology13100795
Chicago/Turabian StyleKhanal, Laxman, Xueyou Li, Asmit Subba, Sapana Ulak, Randall C. Kyes, and Xue-Long Jiang. 2024. "Phylogeography of the Sinica Group of Macaques in the Himalayas: Taxonomic and Evolutionary Implications" Biology 13, no. 10: 795. https://doi.org/10.3390/biology13100795
APA StyleKhanal, L., Li, X., Subba, A., Ulak, S., Kyes, R. C., & Jiang, X.-L. (2024). Phylogeography of the Sinica Group of Macaques in the Himalayas: Taxonomic and Evolutionary Implications. Biology, 13(10), 795. https://doi.org/10.3390/biology13100795








