Sexual Dimorphism in Sex Hormone Metabolism in Human Skeletal Muscle Cells in Response to Different Testosterone Exposure
Abstract
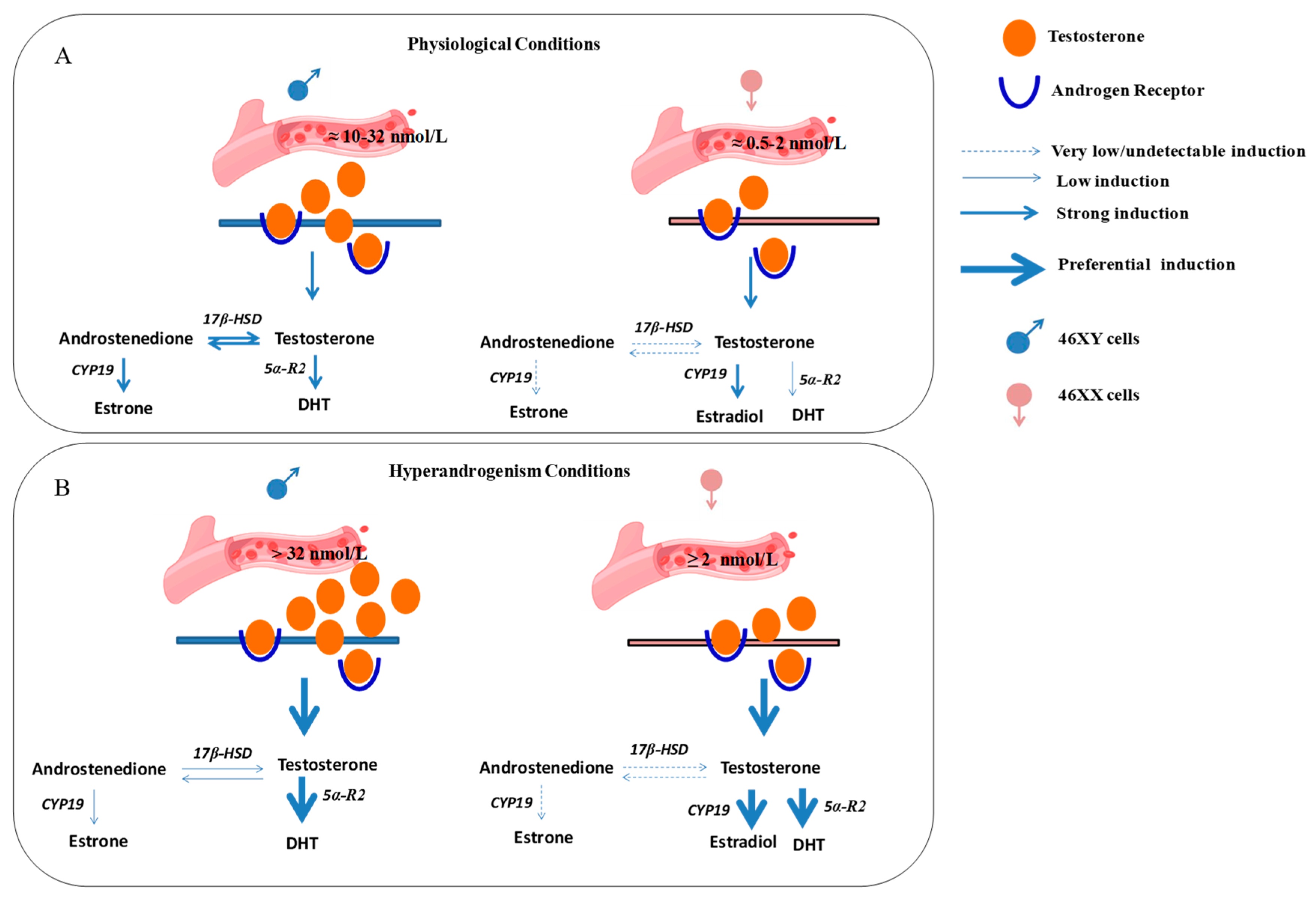
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Treatments
2.2. Measurement of Released Hormones
2.3. Cytokines Assay
2.4. RNA Extraction, Reverse Transcription, and Real-Time Quantitative PCR
2.5. Statistical Analysis
3. Results
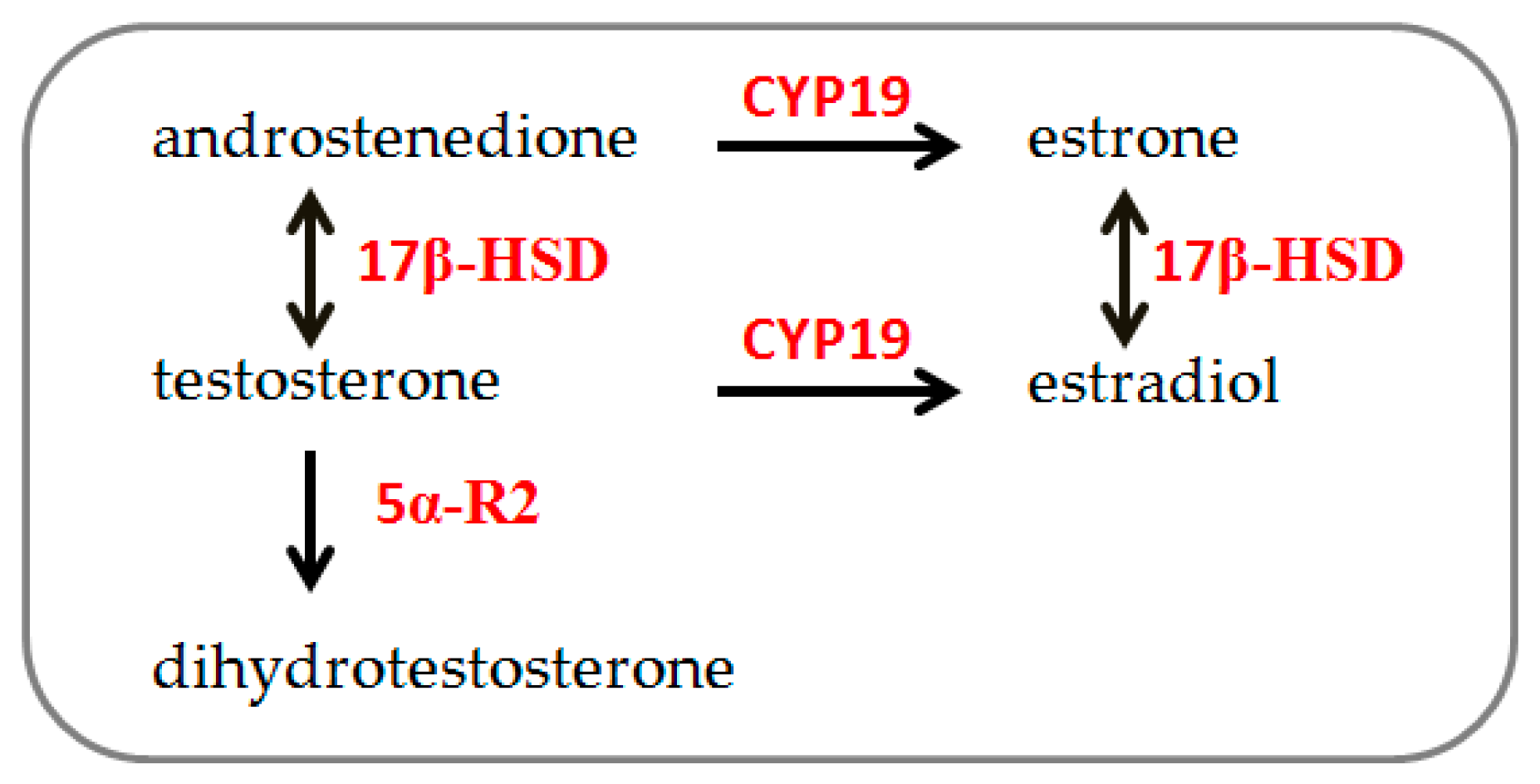
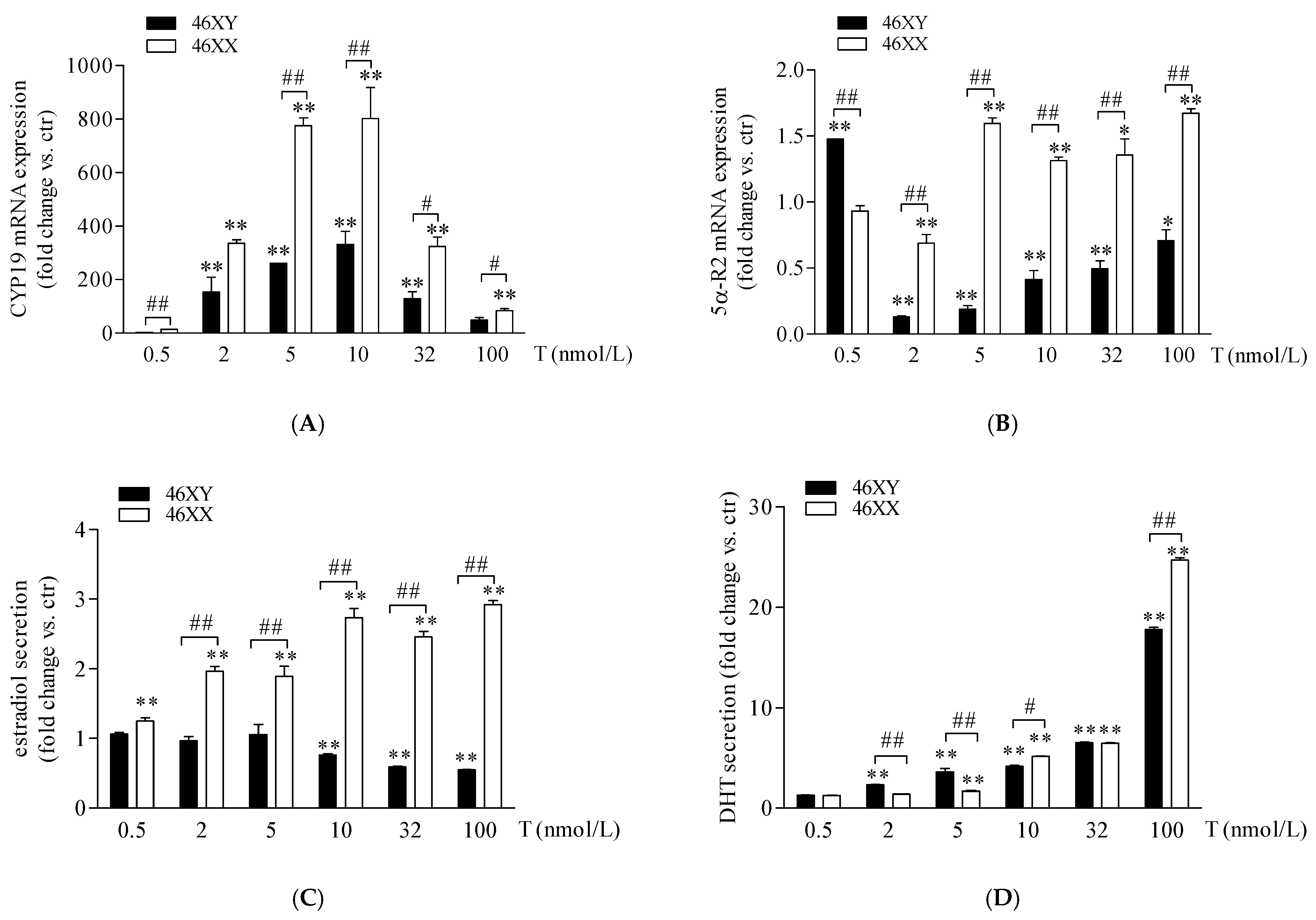
3.1. Sex Dimorphism in Steroidogenic Enzyme Expression and Biosynthesis in Male and Female Human Muscle Cells Exposed to Different Doses of Testosterone
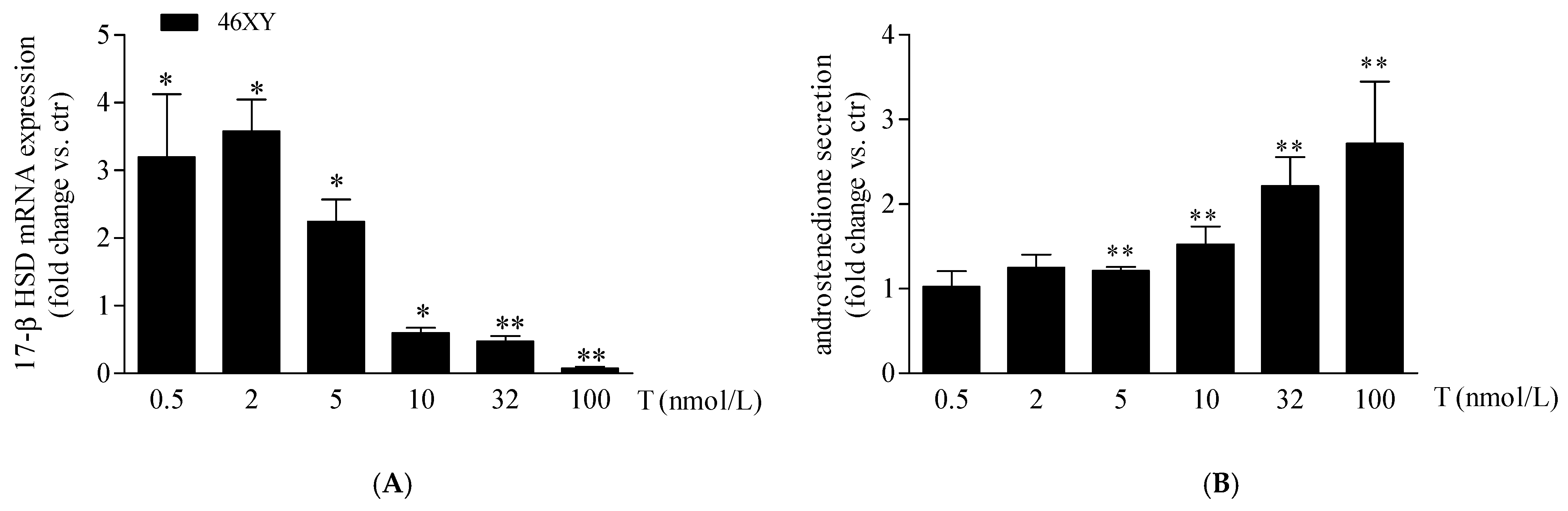
3.2. Increase in 17β-Hydroxysteroid Dehydrogenase Expression and Androstenedione Synthesis in 46XY Cells Following Testosterone Exposure
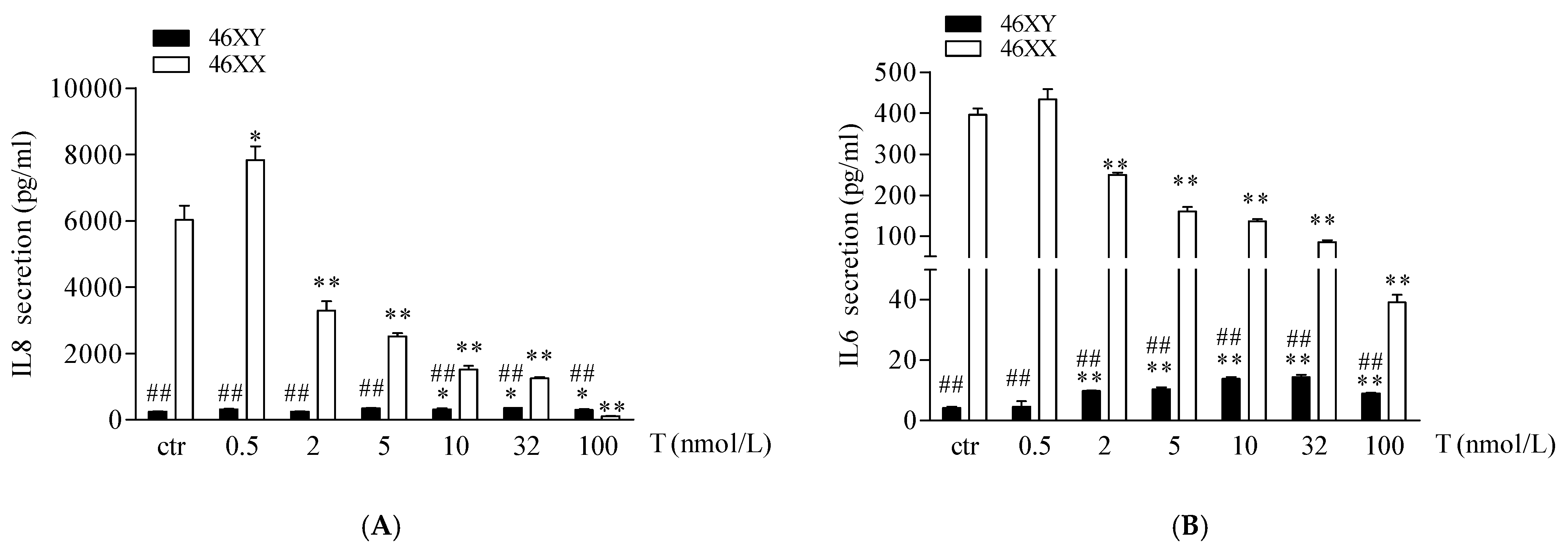
3.3. Sex Dimorphism in IL6 and IL8 Release in Male and Female Human Muscle Cells Exposed to Different Doses of Testosterone
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kasimatis, K.R.; Sánchez-Ramírez, S.; Stevenson, Z.C. Sexual Dimorphism through the Lens of Genome Manipulation, Forward Genetics, and Spatiotemporal Sequencing. Genome Biol. Evol. 2021, 13, evaa243. [Google Scholar] [CrossRef] [PubMed]
- Kroon, J.; Mahmoodzadeh, S.; Helsen, C. Editorial: Sexual Dimorphism in Biomedical Research and Its Therapeutic Implications. Front. Endocrinol. 2022, 13, 1009712. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A. Sex Differences in Body Composition. Adv. Exp. Med. Biol. 2017, 1043, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Ostrer, H. Invited Review: Sex-Based Differences in Gene Expression. J. Appl. Physiol. 2001, 91, 2384–2388. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.L. Sex-Based Differences in Physiology: What Should We Teach in the Medical Curriculum? Adv. Physiol. Educ. 2007, 31, 23–25. [Google Scholar] [CrossRef]
- Raparelli, V.; Proietti, M.; Lenzi, A.; Basili, S. EVA Collaborators Correction to: Sex and Gender Differences in Ischemic Heart Disease: Endocrine Vascular Disease Approach (EVA) Study Design. J. Cardiovasc. Transl. Res. 2020, 13, 26. [Google Scholar] [CrossRef]
- Senefeld, J.W.; Hunter, S.K. Hormonal Basis of Biological Sex Differences in Human Athletic Performance. Endocrinology 2024, bqae036. [Google Scholar] [CrossRef]
- Raparelli, V.; Romiti, G.F.; Spugnardi, V.; Borgi, M.; Cangemi, R.; Basili, S.; Proietti, M. The Eva Collaborative Group Gender-Related Determinants of Adherence to the Mediterranean Diet in Adults with Ischemic Heart Disease. Nutrients 2020, 12, 759. [Google Scholar] [CrossRef]
- Ruggieri, A.; Matarrese, P. Male and Female Cells: Same Stress, Different Response. Ital. J. Gend. Specif. Med. 2020, 6, 1–2. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex Differences in Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Yong, H.J.; Toledo, M.P.; Nowakowski, R.S.; Wang, Y.J. Sex Differences in the Molecular Programs of Pancreatic Cells Contribute to the Differential Risks of Type 2 Diabetes. Endocrinology 2022, 163, bqac156. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, B.C.; Gerard, C.L.; Espinosa da Silva, C. Sex and Gender Differences in Anticancer Treatment Toxicity: A Call for Revisiting Drug Dosing in Oncology. Endocrinology 2022, 163, bqac058. [Google Scholar] [CrossRef]
- O’Reilly, J.; Ono-Moore, K.D.; Chintapalli, S.V.; Rutkowsky, J.M.; Tolentino, T.; Lloyd, K.C.K.; Olfert, I.M.; Adams, S.H. Sex Differences in Skeletal Muscle Revealed through Fiber Type, Capillarity, and Transcriptomics Profiling in Mice. Physiol. Rep. 2021, 9, e15031. [Google Scholar] [CrossRef]
- Balaton, B.P.; Cotton, A.M.; Brown, C.J. Derivation of Consensus Inactivation Status for X-Linked Genes from Genome-Wide Studies. Biol. Sex. Differ. 2015, 6, 35. [Google Scholar] [CrossRef]
- Youness, A.; Miquel, C.-H.; Guéry, J.-C. Escape from X Chromosome Inactivation and the Female Predominance in Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 1114. [Google Scholar] [CrossRef] [PubMed]
- Zito, A.; Roberts, A.L.; Visconti, A.; Rossi, N.; Andres-Ejarque, R.; Nardone, S.; El-Sayed Moustafa, J.S.; Falchi, M.; Small, K.S. Escape from X-Inactivation in Twins Exhibits Intra- and Inter-Individual Variability across Tissues and Is Heritable. PLoS Genet. 2023, 19, e1010556. [Google Scholar] [CrossRef] [PubMed]
- Antinozzi, C.; Duranti, G.; Ceci, R.; Lista, M.; Sabatini, S.; Caporossi, D.; Di Luigi, L.; Sgrò, P.; Dimauro, I. Hydrogen Peroxide Stimulates Dihydrotestosterone Release in C2C12 Myotubes: A New Perspective for Exercise-Related Muscle Steroidogenesis? Int. J. Mol. Sci. 2022, 23, 6566. [Google Scholar] [CrossRef] [PubMed]
- Sgrò, P.; Minganti, C.; Lista, M.; Antinozzi, C.; Cappa, M.; Pitsiladis, Y.; Pigozzi, F.; Di Luigi, L. Dihydrotestosterone (DHT) Rapidly Increase after Maximal Aerobic Exercise in Healthy Males: The Lowering Effect of Phosphodiesterase’s Type 5 Inhibitors on DHT Response to Exercise-Related Stress. J. Endocrinol. Investig. 2021, 44, 1219–1228. [Google Scholar] [CrossRef]
- Di Luigi, L.; Antinozzi, C.; Duranti, G.; Dimauro, I.; Sgrò, P. Sex-Chromosome-Related Dimorphism in Steroidogenic Enzymes and Androgen Receptor in Response to Testosterone Treatment: An In Vitro Study on Human Primary Skeletal Muscle Cells. Int. J. Mol. Sci. 2023, 24, 17382. [Google Scholar] [CrossRef]
- Baquedano, M.S.; Guercio, G.; Costanzo, M.; Marino, R.; Rivarola, M.A.; Belgorosky, A. Mutation of HSD3B2 Gene and Fate of Dehydroepiandrosterone. Vitam. Horm. 2018, 108, 75–123. [Google Scholar] [CrossRef]
- Aizawa, K.; Iemitsu, M.; Otsuki, T.; Maeda, S.; Miyauchi, T.; Mesaki, N. Sex Differences in Steroidogenesis in Skeletal Muscle Following a Single Bout of Exercise in Rats. J. Appl. Physiol. 2008, 104, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Van Der Eerden, B.C.J.; Van De Ven, J.; Lowik, C.W.G.M.; Wit, J.M.; Karperien, M. Sex Steroid Metabolism in the Tibial Growth Plate of the Rat. Endocrinology 2002, 143, 4048–4055. [Google Scholar] [CrossRef] [PubMed]
- Zwain, I.H.; Yen, S.S. Neurosteroidogenesis in Astrocytes, Oligodendrocytes, and Neurons of Cerebral Cortex of Rat Brain. Endocrinology 1999, 140, 3843–3852. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.A.; Manolagas, S.C. Effects of Sex Steroids on Bones and Muscles: Similarities, Parallels, and Putative Interactions in Health and Disease. Bone 2015, 80, 67–78. [Google Scholar] [CrossRef]
- Sato, K.; Iemitsu, M. Exercise and Sex Steroid Hormones in Skeletal Muscle. J. Steroid Biochem. Mol. Biol. 2015, 145, 200–205. [Google Scholar] [CrossRef]
- Seko, D.; Fujita, R.; Kitajima, Y.; Nakamura, K.; Imai, Y.; Ono, Y. Estrogen Receptor β Controls Muscle Growth and Regeneration in Young Female Mice. Stem Cell Rep. 2020, 15, 577–586. [Google Scholar] [CrossRef]
- Clark, R.V.; Wald, J.A.; Swerdloff, R.S.; Wang, C.; Wu, F.C.W.; Bowers, L.D.; Matsumoto, A.M. Large Divergence in Testosterone Concentrations between Men and Women: Frame of Reference for Elite Athletes in Sex-Specific Competition in Sports, a Narrative Review. Clin. Endocrinol. 2019, 90, 15–22. [Google Scholar] [CrossRef]
- Sikaris, K.; McLachlan, R.I.; Kazlauskas, R.; de Kretser, D.; Holden, C.A.; Handelsman, D.J. Reproductive Hormone Reference Intervals for Healthy Fertile Young Men: Evaluation of Automated Platform Assays. J. Clin. Endocrinol. Metab. 2005, 90, 5928–5936. [Google Scholar] [CrossRef]
- Di Luigi, L.; Sgrò, P.; Duranti, G.; Sabatini, S.; Caporossi, D.; Del Galdo, F.; Dimauro, I.; Antinozzi, C. Sildenafil Reduces Expression and Release of IL-6 and IL-8 Induced by Reactive Oxygen Species in Systemic Sclerosis Fibroblasts. Int. J. Mol. Sci. 2020, 21, 3161. [Google Scholar] [CrossRef]
- Dimauro, I.; Grazioli, E.; Lisi, V.; Guidotti, F.; Fantini, C.; Antinozzi, C.; Sgrò, P.; Antonioni, A.; Di Luigi, L.; Capranica, L.; et al. Systemic Response of Antioxidants, Heat Shock Proteins, and Inflammatory Biomarkers to Short-Lasting Exercise Training in Healthy Male Subjects. Oxid. Med. Cell Longev. 2021, 2021, 1938492. [Google Scholar] [CrossRef]
- Paronetto, M.P.; Dimauro, I.; Grazioli, E.; Palombo, R.; Guidotti, F.; Fantini, C.; Sgrò, P.; De Francesco, D.; Di Luigi, L.; Capranica, L.; et al. Exercise-Mediated Downregulation of MALAT1 Expression and Implications in Primary and Secondary Cancer Prevention. Free Radic. Biol. Med. 2020, 160, 28–39. [Google Scholar] [CrossRef]
- Bimonte, V.M.; Marampon, F.; Antonioni, A.; Fittipaldi, S.; Ferretti, E.; Pestell, R.G.; Curreli, M.; Lenzi, A.; Vitale, G.; Brunetti, A.; et al. Phosphodiesterase Type-5 Inhibitor Tadalafil Modulates Steroid Hormones Signaling in a Prostate Cancer Cell Line. Int. J. Mol. Sci. 2021, 22, 754. [Google Scholar] [CrossRef]
- Antinozzi, C.; Marampon, F.; Corinaldesi, C.; Vicini, E.; Sgrò, P.; Vannelli, G.B.; Lenzi, A.; Crescioli, C.; Di Luigi, L. Testosterone Insulin-like Effects: An in Vitro Study on the Short-Term Metabolic Effects of Testosterone in Human Skeletal Muscle Cells. J. Endocrinol. Investig. 2017, 40, 1133–1143. [Google Scholar] [CrossRef]
- Ikeda, K.; Horie-Inoue, K.; Inoue, S. Functions of Estrogen and Estrogen Receptor Signaling on Skeletal Muscle. J. Steroid Biochem. Mol. Biol. 2019, 191, 105375. [Google Scholar] [CrossRef]
- Mohamed, M.K.; Abdel-Rahman, A.A. Effect of Long-Term Ovariectomy and Estrogen Replacement on the Expression of Estrogen Receptor Gene in Female Rats. Eur. J. Endocrinol. 2000, 142, 307–314. [Google Scholar] [CrossRef]
- Romero-Moraleda, B.; Coso, J.D.; Gutiérrez-Hellín, J.; Ruiz-Moreno, C.; Grgic, J.; Lara, B. The Influence of the Menstrual Cycle on Muscle Strength and Power Performance. J. Hum. Kinet. 2019, 68, 123–133. [Google Scholar] [CrossRef]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef]
- Mills, E.G.; Yang, L.; Nielsen, M.F.; Kassem, M.; Dhillo, W.S.; Comninos, A.N. The Relationship Between Bone and Reproductive Hormones Beyond Estrogens and Androgens. Endocr. Rev. 2021, 42, 691–719. [Google Scholar] [CrossRef]
- Niță, A.-R.; Knock, G.A.; Heads, R.J. Signalling Mechanisms in the Cardiovascular Protective Effects of Estrogen: With a Focus on Rapid/Membrane Signalling. Curr. Res. Physiol. 2021, 4, 103–118. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Piquereau, J.; Veksler, V.; Garnier, A. Estrogens, Estrogen Receptors Effects on Cardiac and Skeletal Muscle Mitochondria. Front. Endocrinol. 2019, 10, 557. [Google Scholar] [CrossRef]
- Nara, H.; Watanabe, R. Anti-Inflammatory Effect of Muscle-Derived Interleukin-6 and Its Involvement in Lipid Metabolism. Int. J. Mol. Sci. 2021, 22, 9889. [Google Scholar] [CrossRef]
- Nielsen, A.R.; Pedersen, B.K. The Biological Roles of Exercise-Induced Cytokines: IL-6, IL-8, and IL-15. Appl. Physiol. Nutr. Metab. 2007, 32, 833–839. [Google Scholar] [CrossRef]
- Girasole, G.; Jilka, R.L.; Passeri, G.; Boswell, S.; Boder, G.; Williams, D.C.; Manolagas, S.C. 17 Beta-Estradiol Inhibits Interleukin-6 Production by Bone Marrow-Derived Stromal Cells and Osteoblasts in Vitro: A Potential Mechanism for the Antiosteoporotic Effect of Estrogens. J. Clin. Investig. 1992, 89, 883–891. [Google Scholar] [CrossRef]
- Sunyer, T.; Lewis, J.; Collin-Osdoby, P.; Osdoby, P. Estrogen’s Bone-Protective Effects May Involve Differential IL-1 Receptor Regulation in Human Osteoclast-like Cells. J. Clin. Investig. 1999, 103, 1409–1418. [Google Scholar] [CrossRef]
- Dent, J.R.; Fletcher, D.K.; McGuigan, M.R. Evidence for a Non-Genomic Action of Testosterone in Skeletal Muscle Which May Improve Athletic Performance: Implications for the Female Athlete. J. Sports Sci. Med. 2012, 11, 363–370. [Google Scholar]
- Gao, Z.; Ma, X.; Liu, J.; Ge, Y.; Wang, L.; Fu, P.; Liu, Z.; Yao, R.; Yan, X. Troxerutin Protects against DHT-Induced Polycystic Ovary Syndrome in Rats. J. Ovarian Res. 2020, 13, 106. [Google Scholar] [CrossRef]
- Münzker, J.; Hofer, D.; Trummer, C.; Ulbing, M.; Harger, A.; Pieber, T.; Owen, L.; Keevil, B.; Brabant, G.; Lerchbaum, E.; et al. Testosterone to Dihydrotestosterone Ratio as a New Biomarker for an Adverse Metabolic Phenotype in the Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2015, 100, 653–660. [Google Scholar] [CrossRef]
- Sun, L.-F.; Yang, Y.-L.; Xiao, T.-X.; Li, M.-X.; Zhang, J.V. Removal of DHT Can Relieve Polycystic Ovarian but Not Metabolic Abnormalities in DHT-Induced Hyperandrogenism in Mice. Reprod. Fertil. Dev. 2019, 31, 1597–1606. [Google Scholar] [CrossRef]
- Di Luigi, L.; Greco, E.A.; Fossati, C.; Aversa, A.; Sgrò, P.; Antinozzi, C. Clinical Concerns on Sex Steroids Variability in Cisgender and Transgender Women Athletes. Int. J. Sports Med. 2023, 44, 81–94. [Google Scholar] [CrossRef]
- Hilton, E.N.; Lundberg, T.R. Transgender Women in the Female Category of Sport: Perspectives on Testosterone Suppression and Performance Advantage. Sports Med. 2021, 51, 199–214. [Google Scholar] [CrossRef]
- Cheung, A.S.; Zwickl, S.; Miller, K.; Nolan, B.J.; Wong, A.F.Q.; Jones, P.; Eynon, N. The Impact of Gender-Affirming Hormone Therapy on Physical Performance. J. Clin. Endocrinol. Metab. 2024, 109, e455–e465. [Google Scholar] [CrossRef]
- Hamilton, B.; Brown, A.; Montagner-Moraes, S.; Comeras-Chueca, C.; Bush, P.G.; Guppy, F.M.; Pitsiladis, Y.P. Strength, Power and Aerobic Capacity of Transgender Athletes: A Cross-Sectional Study. Br. J. Sports Med. 2024, 58, 586–597. [Google Scholar] [CrossRef]
- Tebbens, M.; Heijboer, A.C.; T’Sjoen, G.; Bisschop, P.H.; den Heijer, M. The Role of Estrone in Feminizing Hormone Treatment. J. Clin. Endocrinol. Metab. 2022, 107, e458–e466. [Google Scholar] [CrossRef]
- Sasson, S.; Notides, A.C. Estriol and estrone interaction with the estrogen receptor. I. Temperature-induced modulation of the cooperative binding of [3H]estriol and [3H]estrone to the estrogen receptor. J. Biol. Chem. 1983, 10, 8113–8117. [Google Scholar] [CrossRef]
- Ipulan-Colet, L.A. Sexual Dimorphism through Androgen Signaling; from External Genitalia to Muscles. Front. Endocrinol. 2022, 13, 940229. [Google Scholar] [CrossRef]
- Laurent, M.; Antonio, L.; Sinnesael, M.; Dubois, V.; Gielen, E.; Classens, F.; Vanderschueren, D. Androgens and Estrogens in Skeletal Sexual Dimorphism. Asian J. Androl. 2014, 16, 213–222. [Google Scholar] [CrossRef]
- Sharma, S.; Eghbali, M. Influence of Sex Differences on microRNA Gene Regulation in Disease. Biol. Sex. Differ. 2014, 5, 3. [Google Scholar] [CrossRef]
- Fang, H.; Disteche, C.M.; Berletch, J.B. X Inactivation and Escape: Epigenetic and Structural Features. Front. Cell Dev. Biol. 2019, 7, 219. [Google Scholar] [CrossRef]
- Peeters, S.B.; Posynick, B.J.; Brown, C.J. Out of the Silence: Insights into How Genes Escape X-Chromosome Inactivation. Epigenomes 2023, 7, 29. [Google Scholar] [CrossRef]
| Gene Name | Acronym | Forward 5′–3′ | Reverse 5′–3′ |
|---|---|---|---|
| 5α-reductase | 5α-R2 | AGTGGAGGGCATGGTGCTAA | TCTCTCACTTAGCACGGGGA |
| 17β-hydroxysteroid dehydrogenase | 17β-HSD | TTTGCGCTCGAAGGTTTGTG | GCAGTCAAGAAGAGCTCCGT |
| Aromatase | CYP-19 | ATGTTTCTGGAAATGCTGAAC | CTGTTTCAGATATTTTTCGCTG |
| β-ACTIN | ACTB | AAC CTGAACCCCAAGGCC | AGCCTGGATAGCAACGTACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgrò, P.; Antinozzi, C.; Wasson, C.W.; Del Galdo, F.; Dimauro, I.; Di Luigi, L. Sexual Dimorphism in Sex Hormone Metabolism in Human Skeletal Muscle Cells in Response to Different Testosterone Exposure. Biology 2024, 13, 796. https://doi.org/10.3390/biology13100796
Sgrò P, Antinozzi C, Wasson CW, Del Galdo F, Dimauro I, Di Luigi L. Sexual Dimorphism in Sex Hormone Metabolism in Human Skeletal Muscle Cells in Response to Different Testosterone Exposure. Biology. 2024; 13(10):796. https://doi.org/10.3390/biology13100796
Chicago/Turabian StyleSgrò, Paolo, Cristina Antinozzi, Christopher W. Wasson, Francesco Del Galdo, Ivan Dimauro, and Luigi Di Luigi. 2024. "Sexual Dimorphism in Sex Hormone Metabolism in Human Skeletal Muscle Cells in Response to Different Testosterone Exposure" Biology 13, no. 10: 796. https://doi.org/10.3390/biology13100796
APA StyleSgrò, P., Antinozzi, C., Wasson, C. W., Del Galdo, F., Dimauro, I., & Di Luigi, L. (2024). Sexual Dimorphism in Sex Hormone Metabolism in Human Skeletal Muscle Cells in Response to Different Testosterone Exposure. Biology, 13(10), 796. https://doi.org/10.3390/biology13100796








