Effects of Acute Hypoxic Exposure in Simulated Altitude in Healthy Adults on Cognitive Performance: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Methodological Quality Assessment
2.5. Statistical Analyses
3. Results
3.1. Search Results
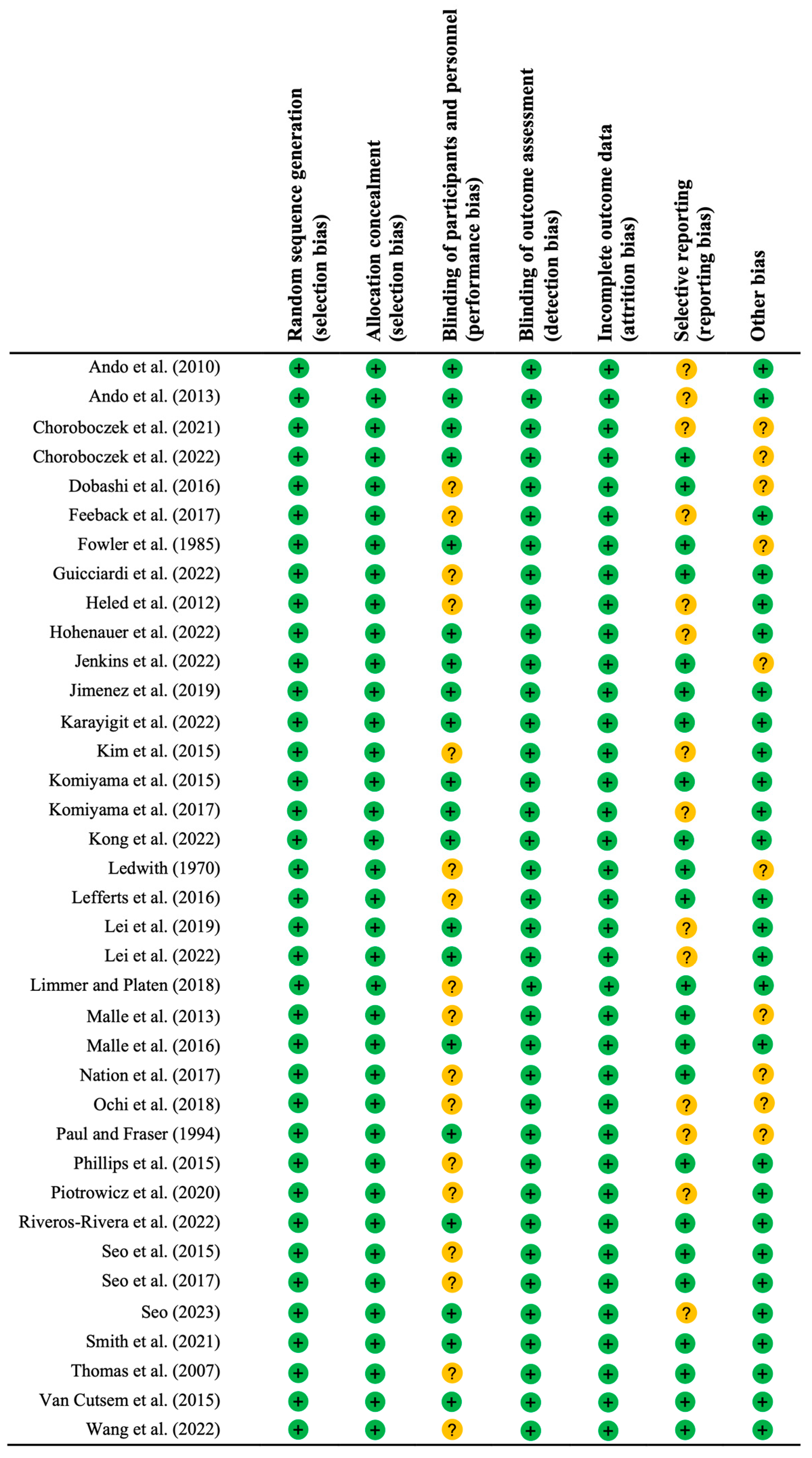
3.2. Assessment of Methodological Quality
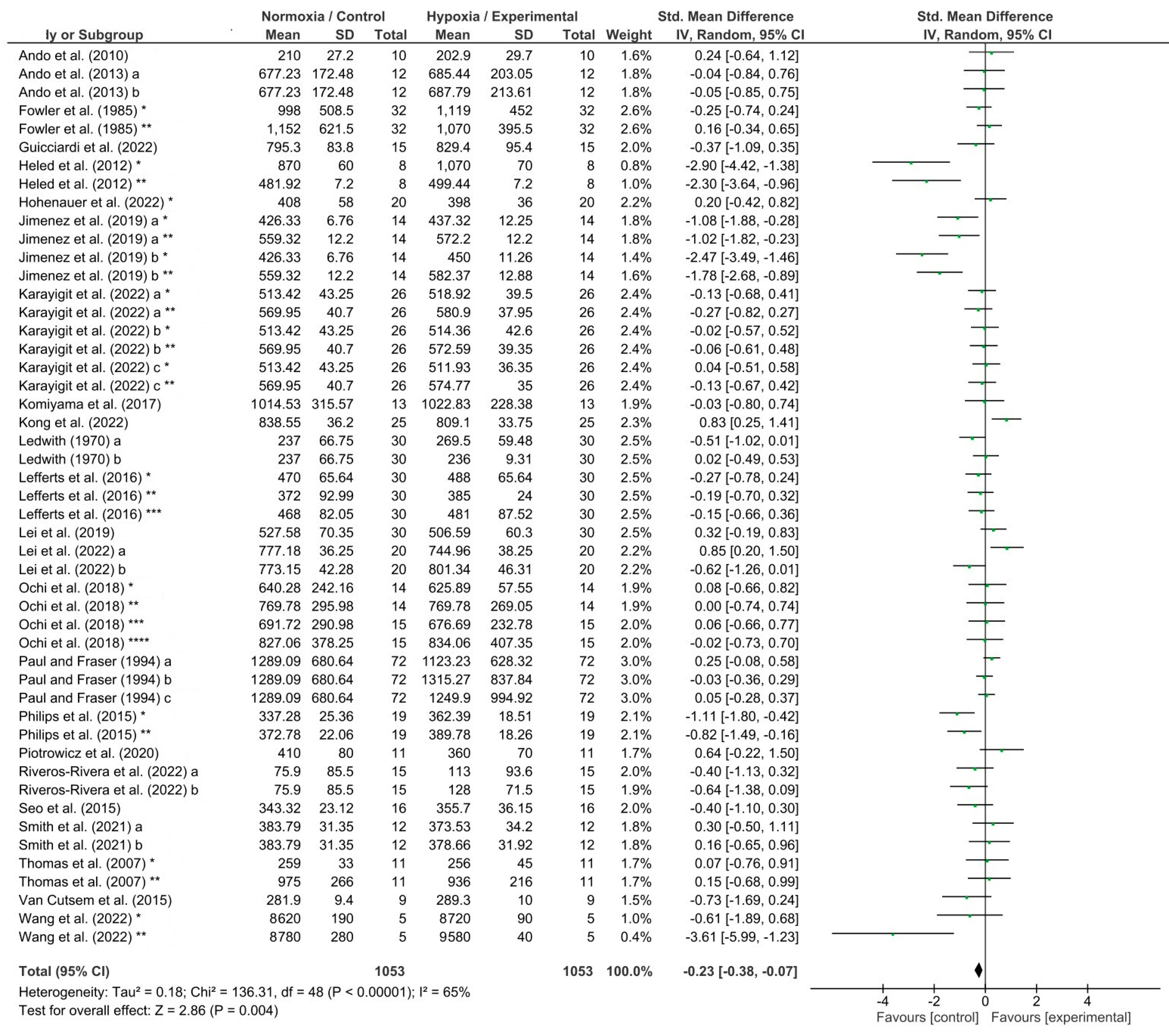
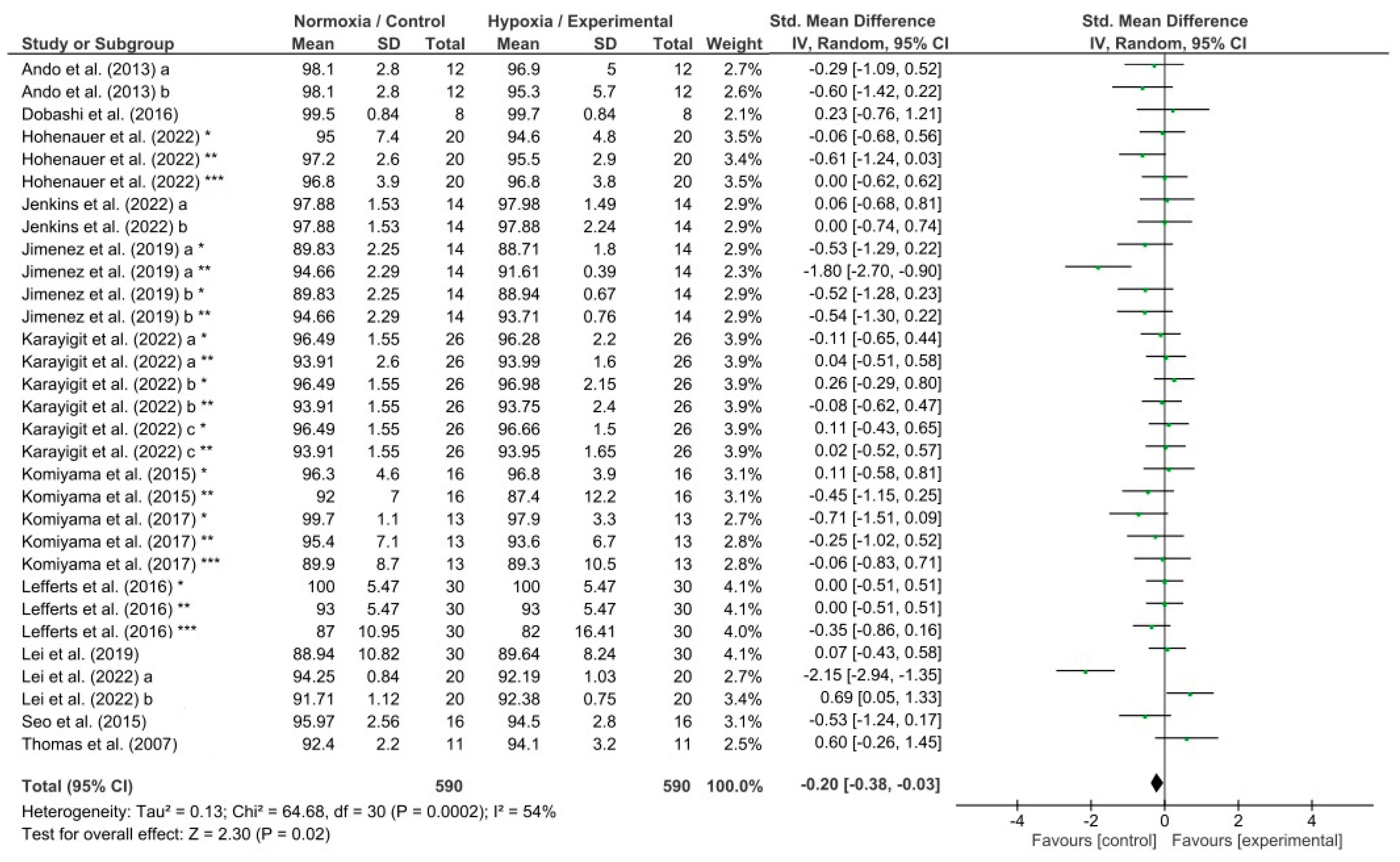
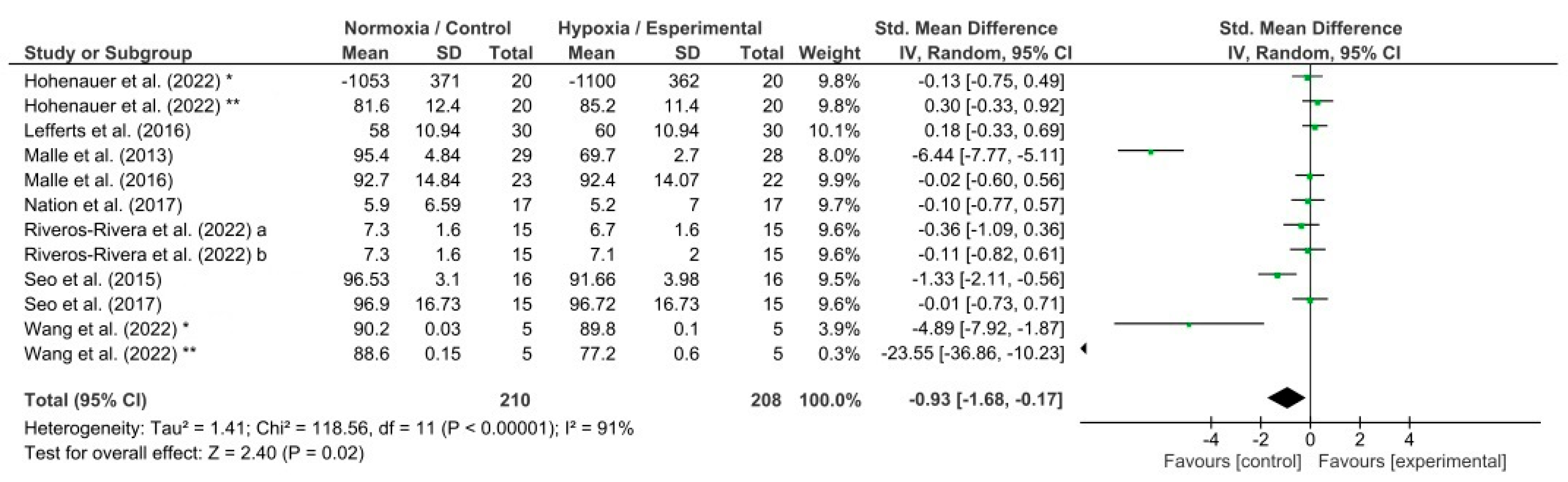
3.3. Meta-Analysis Results
4. Discussion
4.1. Effects of Hypoxia on Reaction Time
4.2. Effects of Hypoxia on Response Accuracy
4.3. Effects of Hypoxia on Memory
4.4. Effects of Hypoxia on Attention
4.5. Harmful Effects of Hypoxia Exposure
4.6. Study Limitations
5. Conclusions
6. Practical Application
7. Key Points
- Altitude causes detrimental effects on cognitive performance due to hypoxia; however, the response induced by simulated altitude was unknown.
- Acute hypoxic exposure in simulated altitude produces an impairment in reaction time, accuracy response, and memory on different cognitive tests in healthy adults.
- Nevertheless, attention shows no significant changes under hypoxic exposure in simulated altitude.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- PUBMED (18 September 2023)
- ((((((((((“training” [Title/Abstract]) OR (“exercise” [Title/Abstract])) OR (“program” [Title/Abstract])) OR (“programme” [Title/Abstract])) OR (“intervention” [Title/Abstract])) OR (“proceeding” [Title/Abstract])) OR (“participation” [Title/Abstract])) AND ((((((((“oxygen deficiency” [Title/Abstract]) OR (“deficiencies oxygen” [Title/Abstract])) OR (“hypoxia” [Title/Abstract])) OR (“hypoxemia” [Title/Abstract])) OR (“anoxia” [Title/Abstract])) OR (“anoxemia” [Title/Abstract])) OR (“intermittent hypoxia” [Title/Abstract])) OR (“altitude” [Title/Abstract]))) AND ((((((((((((((“test” [Title/Abstract]) OR (“testing” [Title/Abstract])) OR (“task” [Title/Abstract])) OR (“exam” [Title/Abstract])) OR (“examination” [Title/Abstract])) OR (“battery” [Title/Abstract])) OR (“essay” [Title/Abstract])) OR (“experiment” [Title/Abstract])) OR (“learning” [Title/Abstract])) OR (“measurement” [Title/Abstract])) OR (“work” [Title/Abstract]))) AND ((((((((((((((“cognitive” [Title/Abstract]) OR (“cognitive performance” [Title/Abstract])) OR (“cognitive function” [Title/Abstract])) OR (“psychology” [Title/Abstract])) OR (“neuropsychological” [Title/Abstract])) OR (“neuropsychologic” [Title/Abstract])) OR (“mental” [Title/Abstract])) OR (“psychometric” [Title/Abstract])) OR (“memory” [Title/Abstract])) OR (“reaction time” [Title/Abstract])) OR (“response time” [Title/Abstract])) OR (“anticipation” [Title/Abstract])) OR (“decision making” [Title/Abstract]))
- Results: 340
- SCOPUS (18 September 2023)
- (TITLE-ABS (“training”) OR TITLE-ABS (“exercise”) OR TITLE-ABS (“program”) OR TITLE-ABS (“programme”) OR TITLE-ABS (“intervention”) OR TITLE-ABS (“proceeding”) OR TITLE-ABS (“participation”)) AND (TITLE-ABS (“oxygen deficiency”) OR TITLE-ABS (“deficiencies oxygen”) OR TITLE-ABS (“hypoxia”) OR TITLE-ABS (“hypoxemia”) OR TITLE-ABS (“anoxia”) OR TITLE-ABS (“anoxemia”) OR TITLE-ABS (“intermittent hypoxia”) OR TITLE-ABS (“altitude”)) AND (TITLE-ABS (“test”) OR TITLE-ABS (“testing”) OR TITLE-ABS (“task”) OR TITLE-ABS (“exam”) OR TITLE-ABS (“examination”) OR TITLE-ABS (“battery”) OR TITLE-ABS (“essay”) OR TITLE-ABS (“experiment”) OR TITLE-ABS (“learning”) OR TITLE-ABS (“measurement”) OR TITLE-ABS (“work”)) AND (TITLE-ABS (“cognitive”) OR TITLE-ABS (“cognitive performance”) OR TITLE-ABS (“cognitive function”) OR TITLE-ABS (“psychology”) OR TITLE-ABS (“neuropsychological”) OR TITLE-ABS (“neuropsychologic”) OR TITLE-ABS (“mental”) OR TITLE-ABS (“psychometric”) OR TITLE-ABS (“memory”) OR TITLE-ABS (“reaction time”) OR TITLE-ABS (“response time”) OR TITLE-ABS (“anticipation”) OR TITLE-ABS (“decision making”))
- Results: 678
- WEB OF SCIENCE (18 September 2023)
- (((AB = (“training” OR “exercise” OR “program” OR “programme” OR “intervention” OR “proceeding” OR “participation”)) AND AB = (“oxygen deficiency” OR “deficiencies oxygen” OR “hypoxia” OR “hypoxemia” OR “anoxia” OR “anoxemia” OR “intermittent hypoxia” OR “altitude”)) AND AB = (“test” OR “testing” OR “task” OR “exam” OR “examination” OR “battery” OR “essay” OR “experiment” OR “learning” OR “measurement” OR “work”)) AND AB = (“cognitive” OR “cognitive performance” OR “cognitive function” OR “psychology” OR “neuropsychological” OR “neuropsychologic” OR “mental” OR “psychometric” OR “memory” OR “reaction time” OR “response time” OR “anticipation” OR “decision making”)
- (((TI = (“training” OR “exercise” OR “program” OR “programme” OR “intervention” OR “proceeding” OR “participation”)) AND TI = (“oxygen deficiency” OR “deficiencies oxygen” OR “hypoxia” OR “hypoxemia” OR “anoxia” OR “anoxemia” OR “intermittent hypoxia” OR “altitude”)) AND TI = (“test” OR “testing” OR “task” OR “exam” OR “examination” OR “battery” OR “essay” OR “experiment” OR “learning” OR “measurement” OR “work”)) AND TI = (“cognitive” OR “cognitive performance” OR “cognitive function” OR “psychology” OR “neuropsychological” OR “neuropsychologic” OR “mental” OR “psychometric” OR “memory” OR “reaction time” OR “response time” OR “anticipation” OR “decision making”)
- Results: 408
- MEDLINE (18 September 2023)
- (AB “training” OR TI “training” OR AB “exercise” OR TI “exercise” OR AB “program” OR TI “program” OR AB “programme” OR TI “programme” OR AB “intervention” OR TI “intervention” OR AB “proceeding” OR TI “proceeding” OR AB “participation” OR TI “participation”) AND (AB “oxygen deficiency” OR TI “oxygen deficiency” OR AB “deficiencies oxygen” OR TI “deficiencies oxygen” OR AB “hypoxia” OR TI “hypoxia” OR AB “hypoxemia” OR TI “hypoxemia” OR AB “anoxia” OR TI “anoxia” OR AB “anoxemia” OR TI “anoxemia” OR AB “intermittent hypoxia” OR TI “intermittent hypoxia” OR AB “altitude” OR TI “altitude”) AND (AB “test” OR TI “test” OR AB “testing” OR TI “testing” OR AB “task” OR TI “task” OR AB “exam” OR TI “exam” OR AB “examination” OR TI “examination” OR AB “battery” OR TI “battery” OR AB “essay” OR TI “essay” OR AB “experiment” OR TI “experiment” OR AB “learning” OR TI “learning” OR AB “measurement” OR TI “measurement” OR AB “work” OR TI “work”) AND (AB “cognitive” OR TI “cognitive” OR AB “cognitive performance” OR TI “cognitive performance” OR AB “cognitive function” OR TI “cognitive function” OR AB “psychology” OR TI “psychology” OR AB “neuropsychological” OR TI “neuropsychological” OR AB “neuropsychologic” OR TI “neuropsychologic” OR AB “mental” OR TI “mental” OR AB “psychometric” OR TI “psychometric” OR AB “memory” OR TI “memory” OR AB “reaction time” OR TI “reaction time” OR AB “response time” OR TI “response time” OR AB “anticipation” OR TI “anticipation” OR AB “decision making” OR TI “decision making”)
- Results: 309
- SPORTDISCUS (18 September 2023)
- (AB “training” OR TI “training” OR AB “exercise” OR TI “exercise” OR AB “program” OR TI “program” OR AB “programme” OR TI “programme” OR AB “intervention” OR TI “intervention” OR AB “proceeding” OR TI “proceeding” OR AB “participation” OR TI “participation”) AND (AB “oxygen deficiency” OR TI “oxygen deficiency” OR AB “deficiencies oxygen” OR TI “deficiencies oxygen” OR AB “hypoxia” OR TI “hypoxia” OR AB “hypoxemia” OR TI “hypoxemia” OR AB “anoxia” OR TI “anoxia” OR AB “anoxemia” OR TI “anoxemia” OR AB “intermittent hypoxia” OR TI “intermittent hypoxia” OR AB “altitude” OR TI “altitude”) AND (AB “test” OR TI “test” OR AB “testing” OR TI “testing” OR AB “task” OR TI “task” OR AB “exam” OR TI “exam” OR AB “examination” OR TI “examination” OR AB “battery” OR TI “battery” OR AB “essay” OR TI “essay” OR AB “experiment” OR TI “experiment” OR AB “learning” OR TI “learning” OR AB “measurement” OR TI “measurement” OR AB “work” OR TI “work”) AND (AB “cognitive” OR TI “cognitive” OR AB “cognitive performance” OR TI “cognitive performance” OR AB “cognitive function” OR TI “cognitive function” OR AB “psychology” OR TI “psychology” OR AB “neuropsychological” OR TI “neuropsychological” OR AB “neuropsychologic” OR TI “neuropsychologic” OR AB “mental” OR TI “mental” OR AB “psychometric” OR TI “psychometric” OR AB “memory” OR TI “memory” OR AB “reaction time” OR TI “reaction time” OR AB “response time” OR TI “response time” OR AB “anticipation” OR TI “anticipation” OR AB “decision making” OR TI “decision making”)
Appendix B
| Study | Criterion 1 | Criterion 2 | Criterion 3 | Criterion 4 | Criterion 5 | Criterion 6 | Criterion 7 | Criterion 8 | Criterion 9 | Criterion 10 | Criterion 11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ando et al. (2010) [34] | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Ando et al. (2013) [33] | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Chroboczek et al. (2021) [63] | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Chroboczek et al. (2022) [64] | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Dobashi et al. (2016) [56] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 4 |
| Feeback et al. (2017) [65] | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 4 |
| Fowler et al. (1985) [35] | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Guicciardi et al. (2022) [36] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Heled et al. (2012) [37] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 4 |
| Hohenauer et al. (2022) [38] | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Jenkins et al. (2022) [57] | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Jimenez et al. (2019) [39] | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Karayigit et al. (2022) [40] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Kim et al. (2015) [66] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 4 |
| Komiyama et al. (2015) [58] | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 8 |
| Komiyama et al. (2017) [10] | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Kong et al. (2022) [41] | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Ledwith (1970) [42] | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Lefferts et al. (2016) [43] | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Lei et al. (2019) [44] | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Lei et al. (2022) [45] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 8 |
| Limmer and Platen (2018) [67] | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Malle et al. (2013) [60] | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Malle et al. (2016) [59] | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 8 |
| Nation et al. (2017) [61] | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Ochi et al. (2018) [46] | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Paul and Fraser (1994) [47] | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Phillips et al. (2015) [48] | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Piotrowicz et al. (2020) [49] | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Riveros-Rivera et al. (2022) [50] | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Seo et al. (2015) [51] | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Seo et al. (2017) [62] | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Seo (2023) [68] | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Smith et al. (2021) [52] | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Thomas et al. (2007) [53] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 4 |
| Van Cutsem et al. (2015) [54] | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Wang et al. (2022) [55] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 4 |
Appendix C
| Study | Risk of Bias |
|---|---|
| Ando et al. (2010) [34] | Some concerns |
| Ando et al. (2013) [33] | Some concerns |
| Chroboczek et al. (2021) [63] | Some concerns |
| Chroboczek et al. (2022) [64] | Some concerns |
| Dobashi et al. (2016) [56] | Some concerns |
| Feeback et al. (2017) [65] | Some concerns |
| Fowler et al. (1985) [35] | Some concerns |
| Guicciardi et al. (2022) [36] | Some concerns |
| Heled et al. (2012) [37] | Some concerns |
| Hohenauer et al. (2022) [38] | Some concerns |
| Jenkins et al. (2022) [57] | Some concerns |
| Jimenez et al. (2019) [39] | Low risk of bias |
| Karayigit et al. (2022) [40] | Low risk of bias |
| Kim et al. (2015) [66] | Some concerns |
| Komiyama et al. (2015) [58] | Low risk of bias |
| Komiyama et al. (2017) [10] | Some concerns |
| Kong et al. (2022) [41] | Low risk of bias |
| Ledwith (1970) [42] | Some concerns |
| Lefferts et al. (2016) [43] | Some concerns |
| Lei et al. (2019) [44] | Some concerns |
| Lei et al. (2022) [45] | Some concerns |
| Limmer and Platen (2018) [67] | Some concerns |
| Malle et al. (2013) [60] | Some concerns |
| Malle et al. (2016) [59] | Low risk of bias |
| Nation et al. (2017) [61] | Some concerns |
| Ochi et al. (2018) [46] | Some concerns |
| Paul and Fraser (1994) [47] | Some concerns |
| Phillips et al. (2015) [48] | Some concerns |
| Piotrowicz et al. (2020) [49] | Some concerns |
| Riveros-Rivera et al. (2022) [50] | Low risk of bias |
| Seo et al. (2015) [51] | Some concerns |
| Seo et al. (2017) [62] | Some concerns |
| Seo (2023) [68] | Some concerns |
| Smith et al. (2021) [52] | Low risk of bias |
| Thomas et al. (2007) [53] | Some concerns |
| Van Cutsem et al. (2015) [54] | Low risk of bias |
| Wang et al. (2022) [55] | Some concerns |
References
- Schodel, J.; Ratcliffe, P.J. Mechanisms of hypoxia signalling: New implications for nephrology. Nat. Rev. Nephrol. 2019, 15, 641–659. [Google Scholar] [CrossRef]
- Chen, P.S.; Chiu, W.T.; Hsu, P.L.; Lin, S.C.; Peng, I.C.; Wang, C.Y.; Tsai, S.J. Pathophysiological implications of hypoxia in human diseases. J. Biomed. Sci. 2020, 27, 63. [Google Scholar] [CrossRef]
- Sharp, F.R.; Bernaudin, M. HIF1 and oxygen sensing in the brain. Nat. Rev. Neurosci. 2004, 5, 437–448. [Google Scholar] [CrossRef]
- Hoiland, R.L.; Bain, A.R.; Rieger, M.G.; Bailey, D.M.; Ainslie, P.N. Hypoxemia, oxygen content, and the regulation of cerebral blood flow. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 310, R398–R413. [Google Scholar] [CrossRef]
- Bailey, D.M.; Bain, A.R.; Hoiland, R.L.; Barak, O.F.; Drvis, I.; Hirtz, C.; Lehmann, S.; Marchi, N.; Janigro, D.; MacLeod, D.B.; et al. Hypoxemia increases blood-brain barrier permeability during extreme apnea in humans. J. Cereb. Blood Flow Metab. 2022, 42, 1120–1135. [Google Scholar] [CrossRef]
- Seymour, R.S.; Bosiocic, V.; Snelling, E.P. Fossil skulls reveal that blood flow rate to the brain increased faster than brain volume during human evolution. R. Soc. Open Sci. 2016, 3, 160305. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y. Effects of Long-Term Exposure to High Altitude Hypoxia on Cognitive Function and Its Mechanism: A Narrative Review. Brain Sci. 2022, 12, 808. [Google Scholar] [CrossRef]
- McMorris, T.; Hale, B.J.; Barwood, M.; Costello, J.; Corbett, J. Effect of acute hypoxia on cognition: A systematic review and meta-regression analysis. Neurosci. Biobehav. Rev. 2017, 74, 225–232. [Google Scholar] [CrossRef]
- Taylor, L.; Watkins, S.L.; Marshall, H.; Dascombe, B.J.; Foster, J. The Impact of Different Environmental Conditions on Cognitive Function: A Focused Review. Front. Physiol. 2015, 6, 372. [Google Scholar] [CrossRef]
- Komiyama, T.; Katayama, K.; Sudo, M.; Ishida, K.; Higaki, Y.; Ando, S. Cognitive function during exercise under severe hypoxia. Sci. Rep. 2017, 7, 10000. [Google Scholar] [CrossRef]
- Arnsten, A.F.; Li, B.M. Neurobiology of executive functions: Catecholamine influences on prefrontal cortical functions. Biol. Psychiatry 2005, 57, 1377–1384. [Google Scholar] [CrossRef]
- Bustamante-Sanchez, A.; Delgado-Teran, M.; Clemente-Suarez, V.J. Psychophysiological response of different aircrew in normobaric hypoxia training. Ergonomics 2019, 62, 277–285. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, S.M.; Yuan, C.; Tian, H.J.; Li, P.; Gao, Y.Q. The effects of short-term and long-term exposure to a high altitude hypoxic environment on neurobehavioral function. High Alt. Med. Biol. 2013, 14, 338–341. [Google Scholar] [CrossRef]
- Ando, S.; Komiyama, T.; Sudo, M.; Higaki, Y.; Ishida, K.; Costello, J.T.; Katayama, K. The interactive effects of acute exercise and hypoxia on cognitive performance: A narrative review. Scand. J. Med. Sci. Sports 2020, 30, 384–398. [Google Scholar] [CrossRef]
- Pun, M.; Guadagni, V.; Bettauer, K.M.; Drogos, L.L.; Aitken, J.; Hartmann, S.E.; Furian, M.; Muralt, L.; Lichtblau, M.; Bader, P.R.; et al. Effects on Cognitive Functioning of Acute, Subacute and Repeated Exposures to High Altitude. Front. Physiol. 2018, 9, 1131. [Google Scholar] [CrossRef]
- Shaw, D.M.; Cabre, G.; Gant, N. Hypoxic Hypoxia and Brain Function in Military Aviation: Basic Physiology and Applied Perspectives. Front. Physiol. 2021, 12, 665821. [Google Scholar] [CrossRef]
- Champigneulle, B.; Davranche, K.; Brugniaux, J.V.; Baillieul, S.; Gajdos, T.; Doutreleau, S.; Robach, P.; Bouzat, P.; Verges, S. Effect of a speed ascent to the top of Europe on cognitive function in elite climbers. Eur. J. Appl. Physiol. 2022, 122, 635–649. [Google Scholar] [CrossRef]
- Moller, K.; Paulson, O.B.; Hornbein, T.F.; Colier, W.N.; Paulson, A.S.; Roach, R.C.; Holm, S.; Knudsen, G.M. Unchanged cerebral blood flow and oxidative metabolism after acclimatization to high altitude. J. Cereb. Blood Flow Metab. 2002, 22, 118–126. [Google Scholar] [CrossRef]
- Nisha, S.N.; Fathinul Fikri, A.S.; Aida, A.R.; Salasiah, M.; Hamed, S.; Rohit, T.; Amei Farina, A.R.; Loh, J.L.; Mazlyfarina, M.; Subapriya, S. The objective assessment of the effects on cognition functioning among military personnel exposed to hypobaric-hypoxia: A pilot fMRI study. Med. J. Malays. 2020, 75, 62–67. [Google Scholar]
- Williams, T.B.; Corbett, J.; McMorris, T.; Young, J.S.; Dicks, M.; Ando, S.; Thelwell, R.C.; Tipton, M.J.; Costello, J.T. Cognitive performance is associated with cerebral oxygenation and peripheral oxygen saturation, but not plasma catecholamines, during graded normobaric hypoxia. Exp. Physiol. 2019, 104, 1384–1397. [Google Scholar] [CrossRef]
- Millet, G.P.; Debevec, T. CrossTalk proposal: Barometric pressure, independent of PO2, is the forgotten parameter in altitude physiology and mountain medicine. J. Physiol. 2020, 598, 893–896. [Google Scholar] [CrossRef]
- Richalet, J.P. CrossTalk opposing view: Barometric pressure, independent of PO2, is not the forgotten parameter in altitude physiology and mountain medicine. J. Physiol. 2020, 598, 897–899. [Google Scholar] [CrossRef]
- Savourey, G.; Launay, J.C.; Besnard, Y.; Guinet, A.; Travers, S. Normo- and hypobaric hypoxia: Are there any physiological differences? Eur. J. Appl. Physiol. 2003, 89, 122–126. [Google Scholar] [CrossRef]
- Systematic Reviews and Meta-Analyses. Available online: https://www.prisma-statement.org/ (accessed on 10 February 2024).
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Stojanovic, E.; Ristic, V.; McMaster, D.T.; Milanovic, Z. Effect of Plyometric Training on Vertical Jump Performance in Female Athletes: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 975–986. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Khan, K.S. Systematic Reviews to Support Evidence -Based Medicine: How to Review and Apply Findings of Healthcare Research, 2nd ed.; Arnold, H., Ed.; Hodder Arnold: London, UK, 2011. [Google Scholar]
- Sole, S.; Ramirez-Campillo, R.; Andrade, D.C.; Sanchez-Sanchez, J. Plyometric jump training effects on the physical fitness of individual-sport athletes: A systematic review with meta-analysis. PeerJ 2021, 9, e11004. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences; Associates, L.E., Ed.; Lawrence Erlbaum Associates: Hillside, NJ, USA, 1988. [Google Scholar]
- Ando, S.; Hatamoto, Y.; Sudo, M.; Kiyonaga, A.; Tanaka, H.; Higaki, Y. The effects of exercise under hypoxia on cognitive function. PLoS ONE 2013, 8, e63630. [Google Scholar] [CrossRef]
- Ando, S.; Yamada, Y.; Kokubu, M. Reaction time to peripheral visual stimuli during exercise under hypoxia. J. Appl. Physiol. (1985) 2010, 108, 1210–1216. [Google Scholar] [CrossRef]
- Fowler, B.; Paul, M.; Porlier, G.; Elcombe, D.D.; Taylor, M. A re-evaluation of the minimum altitude at which hypoxic performance decrements can be detected. Ergonomics 1985, 28, 781–791. [Google Scholar] [CrossRef]
- Guicciardi, M.; Pazzona, R.; Manca, A.; Monni, A.; Scalas, L.F.; Perra, F.; Leban, B.; Roberto, S.; Mulliri, G.; Ghiani, G.; et al. Executive Functions and Mood States in Athletes Performing Exercise Under Hypoxia. Front. Psychol. 2022, 13, 906336. [Google Scholar] [CrossRef]
- Heled, Y.; Peled, A.; Yanovich, R.; Shargal, E.; Pilz-Burstein, R.; Epstein, Y.; Moran, D.S. Heat acclimation and performance in hypoxic conditions. Aviat. Space Environ. Med. 2012, 83, 649–653. [Google Scholar] [CrossRef]
- Hohenauer, E.; Freitag, L.; Costello, J.T.; Williams, T.B.; Kung, T.; Taube, W.; Herten, M.; Clijsen, R. The effects of normobaric and hypobaric hypoxia on cognitive performance and physiological responses: A crossover study. PLoS ONE 2022, 17, e0277364. [Google Scholar] [CrossRef]
- Jimenez, L.Q.; Arwari, B.; Perry, A.; Signorile, J.; Ahn, S.; Kamakawiwo’ole, S.; Jacobs, K.A. Moderate intensity exercise reduces impacts of simulated altitude on cognition. Biol. Exerc. 2019, 15, 5. [Google Scholar]
- Karayigit, R.; Eser, M.C.; Sahin, F.N.; Sari, C.; Sanchez-Gomez, A.; Dominguez, R.; Koz, M. The Acute Effects of Normobaric Hypoxia on Strength, Muscular Endurance and Cognitive Function: Influence of Dose and Sex. Biology 2022, 11, 309. [Google Scholar] [CrossRef]
- Kong, Z.; Yu, Q.; Sun, S.; Lei, O.K.; Tian, Y.; Shi, Q.; Nie, J.; Burtscher, M. The Impact of Sprint Interval Exercise in Acute Severe Hypoxia on Executive Function. High Alt. Med. Biol. 2022, 23, 135–145. [Google Scholar] [CrossRef]
- Ledwith, F. The effects of hypoxia on choice reaction time and movement time. Ergonomics 1970, 13, 465–482. [Google Scholar] [CrossRef]
- Lefferts, W.K.; Babcock, M.C.; Tiss, M.J.; Ives, S.J.; White, C.N.; Brutsaert, T.D.; Heffernan, K.S. Effect of hypoxia on cerebrovascular and cognitive function during moderate intensity exercise. Physiol. Behav. 2016, 165, 108–118. [Google Scholar] [CrossRef]
- Lei, O.K.; Kong, Z.; Loprinzi, P.D.; Shi, Q.; Sun, S.; Zou, L.; Hu, Y.; Nie, J. Severe Hypoxia Does Not Offset the Benefits of Exercise on Cognitive Function in Sedentary Young Women. Int. J. Environ. Res. Public Health 2019, 16, 1003. [Google Scholar] [CrossRef]
- Lei, O.K.; Sun, S.; Nie, J.; Shi, Q.; Kong, Z. Sprint Interval Exercise Improves Cognitive Performance Unrelated to Postprandial Glucose Fluctuations at Different Levels of Normobaric Hypoxia. J. Clin. Med. 2022, 11, 3159. [Google Scholar] [CrossRef]
- Ochi, G.; Yamada, Y.; Hyodo, K.; Suwabe, K.; Fukuie, T.; Byun, K.; Dan, I.; Soya, H. Neural basis for reduced executive performance with hypoxic exercise. Neuroimage 2018, 171, 75–83. [Google Scholar] [CrossRef]
- Paul, M.A.; Fraser, W.D. Performance during mild acute hypoxia. Aviat. Space Environ. Med. 1994, 65, 891–899. [Google Scholar]
- Phillips, J.B.; Horning, D.; Funke, M.E. Cognitive and perceptual deficits of normobaric hypoxia and the time course to performance recovery. Aerosp. Med. Hum. Perform. 2015, 86, 357–365. [Google Scholar] [CrossRef]
- Piotrowicz, Z.; Chalimoniuk, M.; Ploszczyca, K.; Czuba, M.; Langfort, J. Exercise-Induced Elevated BDNF Level Does Not Prevent Cognitive Impairment Due to Acute Exposure to Moderate Hypoxia in Well-Trained Athletes. Int. J. Mol. Sci. 2020, 21, 5569. [Google Scholar] [CrossRef]
- Riveros-Rivera, A.; Penzel, T.; Gunga, H.C.; Opatz, O.; Paul, F.; Klug, L.; Boschmann, M.; Mahler, A. Hypoxia Differentially Affects Healthy Men and Women During a Daytime Nap With a Dose-Response Relationship: A Randomized, Cross-Over Pilot Study. Front. Physiol. 2022, 13, 899636. [Google Scholar] [CrossRef]
- Seo, Y.; Burns, K.; Fennell, C.; Kim, J.H.; Gunstad, J.; Glickman, E.; McDaniel, J. The Influence of Exercise on Cognitive Performance in Normobaric Hypoxia. High Alt. Med. Biol. 2015, 16, 298–305. [Google Scholar] [CrossRef]
- Smith, C.M.; Salmon, O.F.; Jenkins, J.R. Effect of moderate and Severe Hypoxic exposure coupled with fatigue on psychomotor vigilance testing, muscle tissue oxygenation, and muscular performance. Curr. Res. Physiol. 2021, 4, 243–251. [Google Scholar] [CrossRef]
- Thomas, R.J.; Tamisier, R.; Boucher, J.; Kotlar, Y.; Vigneault, K.; Weiss, J.W.; Gilmartin, G. Nocturnal hypoxia exposure with simulated altitude for 14 days does not significantly alter working memory or vigilance in humans. Sleep 2007, 30, 1195–1203. [Google Scholar] [CrossRef]
- Van Cutsem, J.; Pattyn, N.; Vissenaeken, D.; Dhondt, G.; De Pauw, K.; Tonoli, C.; Meeusen, R.; Roelands, B. The influence of a mild thermal challenge and severe hypoxia on exercise performance and serum BDNF. Eur. J. Appl. Physiol. 2015, 115, 2135–2148. [Google Scholar] [CrossRef]
- Wang, L.; Sang, L.; Cui, Y.; Li, P.; Qiao, L.; Wang, Q.; Zhao, W.; Hu, Q.; Zhang, N.; Zhang, Y.; et al. Effects of acute high-altitude exposure on working memory: A functional near-infrared spectroscopy study. Brain Behav. 2022, 12, e2776. [Google Scholar] [CrossRef]
- Dobashi, S.; Horiuchi, M.; Endo, J.; Kiuchi, M.; Koyama, K. Cognitive Function and Cerebral Oxygenation During Prolonged Exercise Under Hypoxia in Healthy Young Males. High Alt. Med. Biol. 2016, 17, 214–221. [Google Scholar] [CrossRef]
- Jenkins, J.R.; Salmon, O.F.; Smith, C.M. Cognitive Function is Unaffected during Acute Hypoxic Exposure but was Improved Following Exercise. Int. J. Exerc. Sci. 2022, 15, 1481–1491. [Google Scholar]
- Komiyama, T.; Sudo, M.; Higaki, Y.; Kiyonaga, A.; Tanaka, H.; Ando, S. Does moderate hypoxia alter working memory and executive function during prolonged exercise? Physiol. Behav. 2015, 139, 290–296. [Google Scholar] [CrossRef]
- Malle, C.; Bourrilhon, C.; Quinette, P.; Laisney, M.; Eustache, F.; Pierard, C. Physiological and Cognitive Effects of Acute Normobaric Hypoxia and Modulations from Oxygen Breathing. Aerosp. Med. Hum. Perform. 2016, 87, 3–12. [Google Scholar] [CrossRef]
- Malle, C.; Quinette, P.; Laisney, M.; Bourrilhon, C.; Boissin, J.; Desgranges, B.; Eustache, F.; Pierard, C. Working memory impairment in pilots exposed to acute hypobaric hypoxia. Aviat. Space Environ. Med. 2013, 84, 773–779. [Google Scholar] [CrossRef]
- Nation, D.A.; Bondi, M.W.; Gayles, E.; Delis, D.C. Mechanisms of Memory Dysfunction during High Altitude Hypoxia Training in Military Aircrew. J. Int. Neuropsychol. Soc. 2017, 23, 1–10. [Google Scholar] [CrossRef]
- Seo, Y.; Gerhart, H.D.; Stavres, J.; Fennell, C.; Draper, S.; Glickman, E.L. Normobaric Hypoxia and Submaximal Exercise Effects on Running Memory and Mood State in Women. Aerosp. Med. Hum. Perform. 2017, 88, 627–632. [Google Scholar] [CrossRef]
- Chroboczek, M.; Kostrzewa, M.; Micielska, K.; Grzywacz, T.; Laskowski, R. Effect of Acute Normobaric Hypoxia Exposure on Executive Functions among Young Physically Active Males. J. Clin. Med. 2021, 10, 1560. [Google Scholar] [CrossRef]
- Chroboczek, M.; Kujach, S.; Luszczyk, M.; Grzywacz, T.; Soya, H.; Laskowski, R. Acute Normobaric Hypoxia Lowers Executive Functions among Young Men despite Increase of BDNF Concentration. Int. J. Environ. Res. Public. Health 2022, 19, 10802. [Google Scholar] [CrossRef]
- Feeback, M.R.; Seo, Y.; Dancy, M.; Glickman, E.L. The Effect of Psychomotor Performance, Cerebral and Arterial Blood Saturation between African-American and Caucasian Males Before, During and After Normobaric Hypoxic Exercise. Int. J. Exerc. Sci. 2017, 10, 655–665. [Google Scholar] [CrossRef]
- Kim, C.H.; Ryan, E.J.; Seo, Y.; Peacock, C.; Gunstad, J.; Muller, M.D.; Ridgel, A.L.; Glickman, E.L. Low intensity exercise does not impact cognitive function during exposure to normobaric hypoxia. Physiol. Behav. 2015, 151, 24–28. [Google Scholar] [CrossRef]
- Limmer, M.; Platen, P. The influence of hypoxia and prolonged exercise on attentional performance at high and extreme altitudes: A pilot study. PLoS ONE 2018, 13, e0205285. [Google Scholar] [CrossRef]
- Seo, Y. Added Inspiratory Resistance Does Not Impair Cognitive Function and Mood State. Int. J. Environ. Res. Public. Health 2023, 20, 2743. [Google Scholar] [CrossRef]
- Ainslie, P.N.; Subudhi, A.W. Cerebral blood flow at high altitude. High Alt. Med. Biol. 2014, 15, 133–140. [Google Scholar] [CrossRef]
- Pringle, R.K. Motor learning and performance: A problem-based learning approach. J. Manip. Physiol. Ther. 2000, 23, 300–301. [Google Scholar] [CrossRef]
- Ando, S.; Kimura, T.; Hamada, T.; Kokubu, M.; Moritani, T.; Oda, S. Increase in reaction time for the peripheral visual field during exercise above the ventilatory threshold. Eur. J. Appl. Physiol. 2005, 94, 461–467. [Google Scholar] [CrossRef]
- Davis, J.E.; Wagner, D.R.; Garvin, N.; Moilanen, D.; Thorington, J.; Schall, C. Cognitive and psychomotor responses to high-altitude exposure in sea level and high-altitude residents of Ecuador. J. Physiol. Anthropol. 2015, 34, 2. [Google Scholar] [CrossRef][Green Version]
- Petrassi, F.A.; Hodkinson, P.D.; Walters, P.L.; Gaydos, S.J. Hypoxic hypoxia at moderate altitudes: Review of the state of the science. Aviat. Space Environ. Med. 2012, 83, 975–984. [Google Scholar] [CrossRef]
- Yan, X. Cognitive impairments at high altitudes and adaptation. High Alt. Med. Biol. 2014, 15, 141–145. [Google Scholar] [CrossRef]
- McFarland, R.A. Psycho-physiological studies at high altitude in the Andes. I. The effect of rapid ascents by aeroplane and train. J. Comp. Psychol. 1937, 23, 191–225. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, R.; Wu, Y.; Li, K.; Wang, D.; Liu, Y.; Li, Y. An EMG study on characteristics of premotor and motor components in an agility reaction time test on athletes. J. Sports Med. Phys. Fit. 2013, 53, 566–572. [Google Scholar]
- Pramsohler, S.; Wimmer, S.; Kopp, M.; Gatterer, H.; Faulhaber, M.; Burtscher, M.; Netzer, N.C. Normobaric hypoxia overnight impairs cognitive reaction time. BMC Neurosci. 2017, 18, 43. [Google Scholar] [CrossRef]
- Zauner, A.; Doppenberg, E.; Woodward, J.J.; Allen, C.; Jebraili, S.; Young, H.F.; Bullock, R. Multiparametric continuous monitoring of brain metabolism and substrate delivery in neurosurgical patients. Neurol. Res. 1997, 19, 265–273. [Google Scholar] [CrossRef]
- Kolb, J.C.; Ainslie, P.N.; Ide, K.; Poulin, M.J. Protocol to measure acute cerebrovascular and ventilatory responses to isocapnic hypoxia in humans. Respir. Physiol. Neurobiol. 2004, 141, 191–199. [Google Scholar] [CrossRef]
- Peltonen, J.E.; Kowalchuk, J.M.; Paterson, D.H.; DeLorey, D.S.; duManoir, G.R.; Petrella, R.J.; Shoemaker, J.K. Cerebral and muscle tissue oxygenation in acute hypoxic ventilatory response test. Respir. Physiol. Neurobiol. 2007, 155, 71–81. [Google Scholar] [CrossRef]
- Brimmell, J.; Edwards, E.J.; Vaughan, R.S. Executive function and visual attention in sport: A systematic review. Int. Rev. Sport Exerc. Psychol. 2022, 1–34. [Google Scholar] [CrossRef]
- Sun, S.; Loprinzi, P.D.; Guan, H.; Zou, L.; Kong, Z.; Hu, Y.; Shi, Q.; Nie, J. The Effects of High-Intensity Interval Exercise and Hypoxia on Cognition in Sedentary Young Adults. Medicina 2019, 55, 43. [Google Scholar] [CrossRef]
- De Waelle, S.; Laureys, F.; Lenoir, M.; Bennett, S.J.; Deconinck, F.J.A. Children Involved in Team Sports Show Superior Executive Function Compared to Their Peers Involved in Self-Paced Sports. Children 2021, 8, 264. [Google Scholar] [CrossRef]
- Steinman, Y.; Groen, E.; Frings-Dresen, M.H.W. Hypoxia impairs reaction time but not response accuracy in a visual choice reaction task. Appl. Ergon. 2023, 113, 104079. [Google Scholar] [CrossRef]
- Kitamura, T.; Ogawa, S.K.; Roy, D.S.; Okuyama, T.; Morrissey, M.D.; Smith, L.M.; Redondo, R.L.; Tonegawa, S. Engrams and circuits crucial for systems consolidation of a memory. Science 2017, 356, 73–78. [Google Scholar] [CrossRef]
- Kumaran, D.; Hassabis, D.; McClelland, J.L. What Learning Systems do Intelligent Agents Need? Complementary Learning Systems Theory Updated. Trends Cogn. Sci. 2016, 20, 512–534. [Google Scholar] [CrossRef]
- Gilbert, P.E.; Brushfield, A.M. The role of the CA3 hippocampal subregion in spatial memory: A process oriented behavioral assessment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 774–781. [Google Scholar] [CrossRef]
- Koolschijn, R.S.; Emir, U.E.; Pantelides, A.C.; Nili, H.; Behrens, T.E.J.; Barron, H.C. The Hippocampus and Neocortical Inhibitory Engrams Protect against Memory Interference. Neuron 2019, 101, 528–541.e6. [Google Scholar] [CrossRef]
- Schmidt-Kastner, R.; Freund, T.F. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience 1991, 40, 599–636. [Google Scholar] [CrossRef]
- Jung, M.; Brizes, I.; Wages, S.; Ponce, P.; Kang, M.; Loprinzi, P.D. Combined effects of acute exercise and hypoxia on memory. Physiol. Int. 2020, 107, 337–348. [Google Scholar] [CrossRef]
- Zhang, Z.A.; Sun, Y.; Yuan, Z.; Wang, L.; Dong, Q.; Zhou, Y.; Zheng, G.; Aschner, M.; Zou, Y.; Luo, W. Insight into the Effects of High-Altitude Hypoxic Exposure on Learning and Memory. Oxidative Med. Cell. Longev. 2022, 2022, 4163188. [Google Scholar] [CrossRef]
- Song, R.; Tao, G.; Guo, F.; Ma, H.; Zhang, J.; Wang, Y. The change of attention network functions and physiological adaptation during high-altitude hypoxia and reoxygenation. Physiol. Behav. 2023, 268, 114240. [Google Scholar] [CrossRef]
- Posner, M.I.; Petersen, S.E. The attention system of the human brain. Annu. Rev. Neurosci. 1990, 13, 25–42. [Google Scholar] [CrossRef]
- Hackett, P.H.; Roach, R.C. High-altitude illness. N. Engl. J. Med. 2001, 345, 107–114. [Google Scholar] [CrossRef]
- DiPasquale, D.M.; Strangman, G.E.; Harris, N.S.; Muza, S.R. Hypoxia, Hypobaria, and Exercise Duration Affect Acute Mountain Sickness. Aerosp. Med. Hum. Perform. 2015, 86, 614–619. [Google Scholar] [CrossRef]
- Imray, C.H.; Barnett, N.J.; Walsh, S.; Clarke, T.; Morgan, J.; Hale, D.; Hoar, H.; Mole, D.; Chesner, I.; Wright, A.D. Near-infrared spectroscopy in the assessment of cerebral oxygenation at high altitude. Wilderness Environ. Med. 1998, 9, 198–203. [Google Scholar] [CrossRef]
- Wagner, D.R.; Tatsugawa, K.; Parker, D.; Young, T.A. Reliability and utility of a visual analog scale for the assessment of acute mountain sickness. High Alt. Med. Biol. 2007, 8, 27–31. [Google Scholar] [CrossRef]
- Roach, R.C.; Hackett, P.H.; Oelz, O.; Bartsch, P.; Luks, A.M.; MacInnis, M.J.; Baillie, J.K.; Lake Louise, A.M.S.S.C.C. The 2018 Lake Louise Acute Mountain Sickness Score. High Alt Med. Biol 2018, 19, 4–6. [Google Scholar] [CrossRef]
- West, J.B. Con: Headache should not be a required symptom for the diagnosis of acute mountain sickness. High Alt. Med. Biol. 2011, 12, 23–25, discussion 27. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Savourey, G.; Guinet, A.; Besnard, Y.; Garcia, N.; Hanniquet, A.M.; Bittel, J. Evaluation of the Lake Louise acute mountain sickness scoring system in a hypobaric chamber. Aviat. Space Environ. Med. 1995, 66, 963–967. [Google Scholar]
- Kanwisher, N. Functional specificity in the human brain: A window into the functional architecture of the mind. Proc. Natl. Acad. Sci. USA 2010, 107, 11163–11170. [Google Scholar] [CrossRef]
- Costello, J.T.; Bhogal, A.S.; Williams, T.B.; Bekoe, R.; Sabir, A.; Tipton, M.J.; Corbett, J.; Mani, A.R. Effects of Normobaric Hypoxia on Oxygen Saturation Variability. High Alt. Med. Biol. 2020, 21, 76–83. [Google Scholar] [CrossRef]
- Richalet, J.P.; Gore, C.J. Live and/or sleep high:train low, using normobaric hypoxia. Scand. J. Med. Sci. Sports 2008, 18 (Suppl. S1), 29–37. [Google Scholar] [CrossRef]



| Study | Population | Characteristics of Hypoxia Exposure | Selected Variables | |||||
|---|---|---|---|---|---|---|---|---|
| Sample Size (N): Male (M)/Female (F) | Age (Years) | Characteristics | Reaction Time | Response Accuracy | Memory | Attention | ||
| Ando et al. (2010) [34] | N = 10 (M) | 25.1 ± 3.4 | Any history of cardiovascular, cerebrovascular, or respiratory disease | FiO2 16% (2200 m) | RT measurement apparatus (Qtec, Osaka) (ms) | |||
| Ando et al. (2013) [33] | N = 12 (M) | 22.9 ± 1.5 | No regular training, physically active, any history of cardiovascular, cerebrovascular, or respiratory disease | FiO2 18% (1300 m) FiO2 15% (2600 m) | Go/No Go Test (ms) | Go/No Go Test (%) | ||
| Chroboczek et al. (2021) [63] | N = 15 | 23.1 ± 2.1 | Healthy, non-obese young adults | 30 min → FiO2 13% (3500 m) 30 min → FiO2 12% (4500 m) 30 min → FiO2 11% (5500 m) Washout: 1 week | Stroop reading interference (s) Stroop naming interference (s) | |||
| Chroboczek et al. (2022) [64] | N = 32 (M) | 20.4 ± 0.6 | Physical Education and Sport students | 30 min →FiO2 13% (3500 m) Washout: 2 weeks | Stroop reading interference (s) Stroop naming interference (s) | |||
| Dobashi et al. (2016) [56] | N = 8 | 23.5 ± 2.2 | People capable of high-intensity cycling. No history of cardiovascular, cerebrovascular, or respiratory disease | FiO2 14.1% (3200 m) | Stroop Test (%) | |||
| Feeback et al. (2017) [65] | N = 12 (M) | 18 to 25 | Healthy and non-smokers. African Americans (N = 6) and Caucasian (N = 6) | FiO2 12% (4300 m) | TMT-A (s) TMT-B (s) | |||
| Fowler et al. (1985) [35] | N = 32 | 19 to 32 | Students | FiO2 11–16% | Mannikin Test (ms) | |||
| Guicciardi et al. (2022) [36] | N = 15 (M) | 30.2 ± 6.6 | Athletes involved in regular endurance training for at least 3 years (8 h/week), without chronic cardiopulmonary, metabolic, or neurological disease | 18 min → FiO2 13% | The Bivalent Shape Task (ms) | |||
| Heled et al. (2012) [37] | N = 8 (M) | 23 ± 3 | Healthy young people | 10 min → FiO2 15.6% (2400 m) | Visual Vigilance Task (ms) 4-Choice RT (ms) | |||
| Hohenauer et al. (2022) [38] | N = 20 (10M/10F) | Males = 30.3 ± 6.3 Females = 24.8 ± 5.1 | Healthy, non-smokers, recreationally trained, and free of any known cardiovascular, respiratory, or neurological disorders | 15 min → FiO2 14.4% (2980 m) Washout: 1 week | 2-Choice RT (ms) | Mannikin Test (%) Switching Test (%) N-back (%) | N-back (ms) N-back (%) | |
| Jenkins et al. (2022) [57] | N = 14 (10M/4F) | Males = 27.6 ± 1.5 Females = 26.7 ± 1.3 | Recreationally active people (8.54 ± 1.44 h/week of physical activity), without musculoskeletal, neurological, or cardiovascular disorders | 60 min → FiO2 16% (2133 m) 60 min → FiO2 14.3% (3048 m) Washout: 48 h | Stroop Test (%) | Stroop Test (points) | ||
| Jimenez et al. (2019) [39] | N = 14 (9M/5F) | Males = 24.7 ± 3.6 Females = 27.6 ± 4.4 | Recreationally active, right-handed individuals. No history of physical or mental health problems, no medication, and no neuroactive drugs | 45 min → FiO2 15.4%, (2400 m) 45 min → FiO2 12.8% (3900 m) Washout: 48 h | Eriksen Flanker Test (ms) Stroop Test (ms) | Eriksen Flanker Test (%) Stroop Test (%) | ||
| Karayigit et al. (2022) [40] | N = 26 (13M/13F) | Males = 23.6 ± 2.8 Females = 22.8 ± 1.4 | Healthy, non-smokers. With at least three years of resistance training experience, and who trains four times per week (squats and bench presses) | 40 min → FiO2 16%, (2000 m) 40 min → FiO2 14% (3000 m) 40 min → FiO2 12% (4000 m) Washout: 72 h | Eriksen Flanker Test (ms) | Eriksen Flanker Test (%) | ||
| Kim et al. (2015) [66] | N = 8 (M) | 41.0 ± 2.0 | Healthy, low-altitude residents who had not been exposed to normobaric hypoxia or altitudes above 2500 m in the previous 2 months | FiO2 12.5% (4300 m) | TMT-A (s) TMT-B (s) | |||
| Komiyama et al. (2015) [58] | N = 16 (M) | 23.0 ± 2.3 | Physically active people with no history of cardiovascular, respiratory, or cerebrovascular diseases | 10 min → FiO2 15% (2600 m) Washout: non-consecutive sessions | Spatial Delayed Response Task (%) Go/No Go Test (%) | |||
| Komiyama et al. (2017) [10] | N = 13 (M) | 21.5 ± 3.5 | Physically active people with no history of cardiovascular, respiratory, or cerebrovascular diseases | FiO2 12–13% (4500 m–3800 m) | Go/No Go Test (ms) | Spatial Delayed Response Task (%) Go/No Go Test—Go Trial (%) Go/No Go Test—No Go Trial (%) | ||
| Kong et al. (2022) [41] | N = 25 (M) | 22.2 ± 2.4 | Physically active men | 30 min → FiO2 11% (5000 m) | Stroop Test (ms) | |||
| Ledwith (1970) [42] | N = 30 (24M/6F) | 18 to 45 | First-year psychology students (N = 19) or members of the St. John Ambulance Society (N = 11) | 2133 m 4267 m | Choice RT (ms) | |||
| Lefferts et al. (2016) [43] | N = 30 (15M/15F) | 21.0 ± 4.0 | Healthy recreationally active people | 120 min → FiO2 12.5% Washout: at least 24 h | Eriksen Flanker Test (ms) N-back (ms) | Eriksen Flanker Test (%) N-back (%) | N-back (%) | Eriksen Flanker Test (ms) |
| Lei et al. (2019) [44] | N = 30 (F) | 22.6 ± 3.2 | Healthy and sedentary young women | FiO2 12% (4000 m) Washout: 72h | Go/No Go Test (ms) | Go/No Go Test (%) | ||
| Lei et al. (2022) [45] | N = 20 (M) | 21.4 ± 2.0 | Recreationally active men | FiO2 15.4% (2500 m) FiO2 11.2% (5000 m) Washout: 3–7 days | Stroop Test (ms) | Stroop Test (%) | ||
| Limmer and Platen (2018) [67] | N = 80 (51M/29F) HYP = 25 NOR = 21 | Males = 25.5 ± 6.0 Females = 24.8 ± 5.9 | Healthy young adults | FiO2 10% (5800 m) | Learning Effect—(attentional performance value) | |||
| Malle et al. (2013) [60] | N = 57 (M) HYP = 28 NOR = 29 | HYP = 23.9 ± 1.7 NOR = 23.9 ± 2.8 | Healthy, non-smoking, right-handed male pilots. | Progressive ascent up to 9500 m and return to ground level (750 m/min) | Paced Auditory Serial Addition Test (%) | |||
| Malle et al. (2016) [59] | N = 86 (M) NOR = 23 HYP = 22 | 29.4 ± 0.9 | Healthy young men | FiO2 6% | Paced Auditory Serial Addition Test (%) | |||
| Nation et al. (2017) [61] | N = 17 (14M/3F) | 30.4 ± 4.7 | U.S. Marine Corps and Navy military pilots and aircrews undergoing altitude exposure training | 15 min → 6096 m | California Verbal Learning (words) | Wechsler Adult Intelligence Scale (# correct) | ||
| Ochi et al. (2018) [46] | N = 29 (20M/9F) EXP 1 = 14 (13M/1F) EXP 2 = 15 (7M/8F) | EXP 1 = 23.4 ± 2.2 EXP 2 = 20.7 ± 2.1 | Healthy, dexterous young adults. Native Japanese speakers and naive about experimental procedures. | FiO2 13.5% Washout: non-consecutive sessions | Stroop Test—Neutral (ms) Stroop Test—Incongruent (ms) | |||
| Paul and Fraser (1994) [47] | N = 144 | 19 to 25 | Canadian Forces youths awaiting vocational training, with no experience of decompression at altitude in a hypobaric chamber. | 1524 m 2438 m 3048 m 3658 m | Mannikin Task (ms) | |||
| Phillips et al. (2015) [48] | N = 19 | Not defined | Active military personnel with a valid flight physical examination | 30 min → FiO2 9.96% (5486 m) | Simple RT (ms) Choice RT (ms) | |||
| Piotrowicz et al. (2020) [49] | N = 11 | 20.0 ± 1.4 | Healthy young cyclists | FiO2 14.7% (3000 m) Washout: 5 days | Choice RT (ms) | |||
| Riveros-Rivera et al. (2022) [50] | N = 15 (7M/8F) | 29.3 ± 6.6 | Healthy people | 90 min → FiO2 14.7% 90 min → FiO2 12.5% Washout: 1 week | Stroop Test Incongruent—Congruent (ms) | Digit Span Test (not defined) | Psychomotor Vigilance Test (not defined) | |
| Seo et al. (2015) [51] | N = 16 (M) | 24.0 ± 4.0 | Young, healthy men | 60 min → FiO2 12.5% (4300 m) | Go/No Go Test (ms) | Go/No Go Test (%) | Running Memory Continuous Performance Task (%) | |
| Seo et al. (2017) [62] | N = 15 (F) | 22.0 ± 2.0 | Young, healthy women | 60 min → FiO2 12.5% (4300 m) | Running Memory Continuous Performance Task (correct response/min) | |||
| Seo (2023) [68] | N = 9 (M) | 25.0 ± 2.0 | Healthy men, without cardiovascular diseases, metabolic disorders, or respiratory diseases. | 30 min → FiO2 17% Washout: 3 days | Stroop Test (interference score) | |||
| Smith et al. (2021) [52] | N = 12 (M) | 20.9 ± 3.4 | Trained persons (3 days/week), non-smokers, without asthma, neuromusculoskeletal disorders, or history of acute mountain sickness. | FiO2 15.4% FiO2 12.9% | Psychomotor vigilance test (ms) | |||
| Thomas et al. (2007) [53] | N = 11 (7M/4F) | 27.0 ± 1.5 | Healthy people and non-smokers | 540 min → FiO2 13% (3962 m) | Psychomotor vigilance test (ms) Verbal 2-back (ms) | Verbal 2-back (%) | ||
| Van Cutsem et al. (2015) [54] | N = 9 (M) | 23.0 ± 3.0 | Trained athletes | 3800 m | Psychomotor vigilance test (ms) | |||
| Wang et al. (2022) [55] | N = 5 (3M/2F) | 21.6 ± 0.3 | Healthy adults at Army Medical University. | 120 min → FiO2 12.8% (4000 m) | Digit Span Task (ms) | Digit Span Test (%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-delaCruz, M.; Bravo-Sánchez, A.; Sánchez-Infante, J.; Abián, P.; Abián-Vicén, J. Effects of Acute Hypoxic Exposure in Simulated Altitude in Healthy Adults on Cognitive Performance: A Systematic Review and Meta-Analysis. Biology 2024, 13, 835. https://doi.org/10.3390/biology13100835
Ramírez-delaCruz M, Bravo-Sánchez A, Sánchez-Infante J, Abián P, Abián-Vicén J. Effects of Acute Hypoxic Exposure in Simulated Altitude in Healthy Adults on Cognitive Performance: A Systematic Review and Meta-Analysis. Biology. 2024; 13(10):835. https://doi.org/10.3390/biology13100835
Chicago/Turabian StyleRamírez-delaCruz, María, Alfredo Bravo-Sánchez, Jorge Sánchez-Infante, Pablo Abián, and Javier Abián-Vicén. 2024. "Effects of Acute Hypoxic Exposure in Simulated Altitude in Healthy Adults on Cognitive Performance: A Systematic Review and Meta-Analysis" Biology 13, no. 10: 835. https://doi.org/10.3390/biology13100835
APA StyleRamírez-delaCruz, M., Bravo-Sánchez, A., Sánchez-Infante, J., Abián, P., & Abián-Vicén, J. (2024). Effects of Acute Hypoxic Exposure in Simulated Altitude in Healthy Adults on Cognitive Performance: A Systematic Review and Meta-Analysis. Biology, 13(10), 835. https://doi.org/10.3390/biology13100835








