Effects of Increasing Oral Deoxynivalenol Gavage on Growth Performance, Blood Biochemistry, Metabolism, Histology, and Microbiome in Rats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Care and Design
2.3. Blood Biochemical Analysis
2.4. Histological Analysis
2.5. Microbial Sequencing and Data Analysis
2.6. Metabolites Preparation and Analysis of Blood, Cecum, Feces, Kidney, and Liver Contaminated with Deoxynivalenol
2.7. Statistical Analysis
3. Results
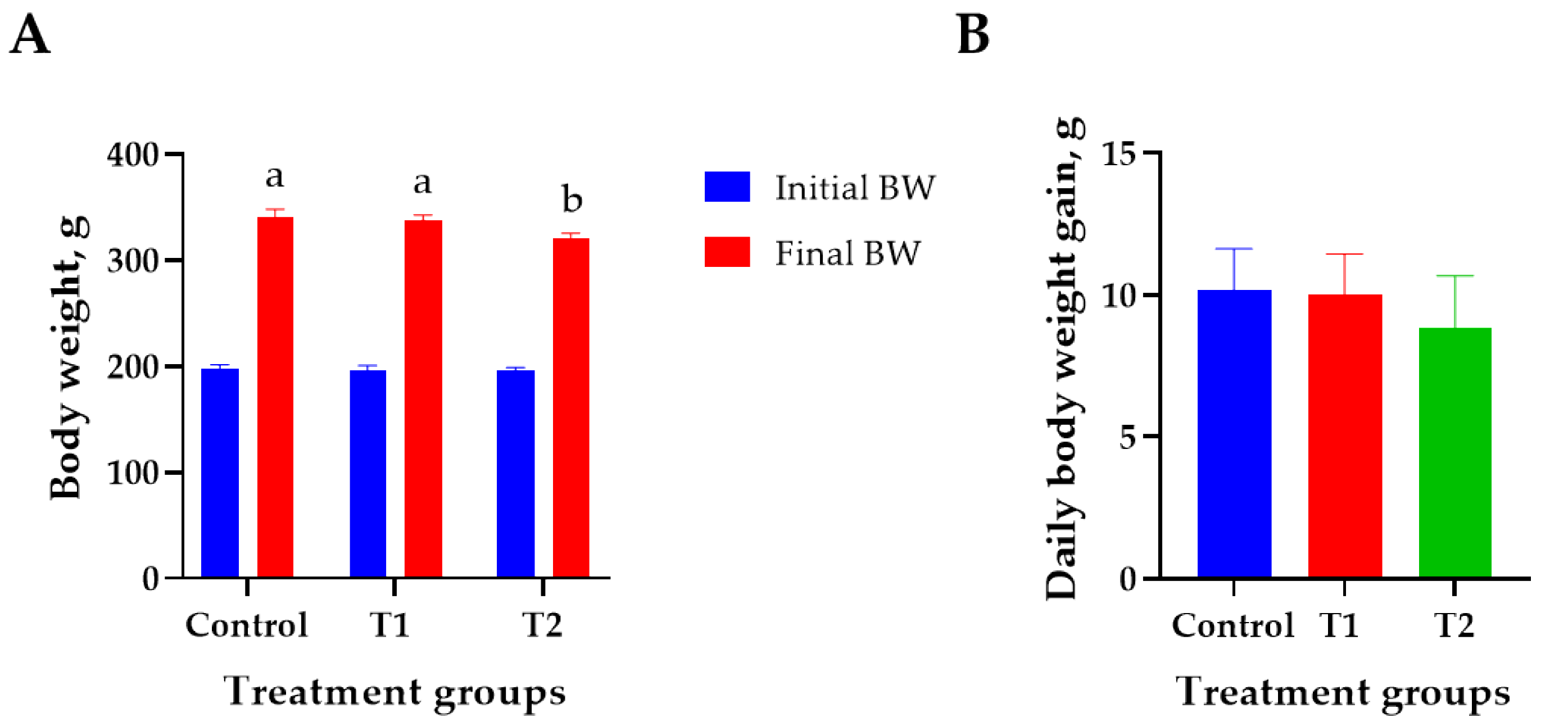
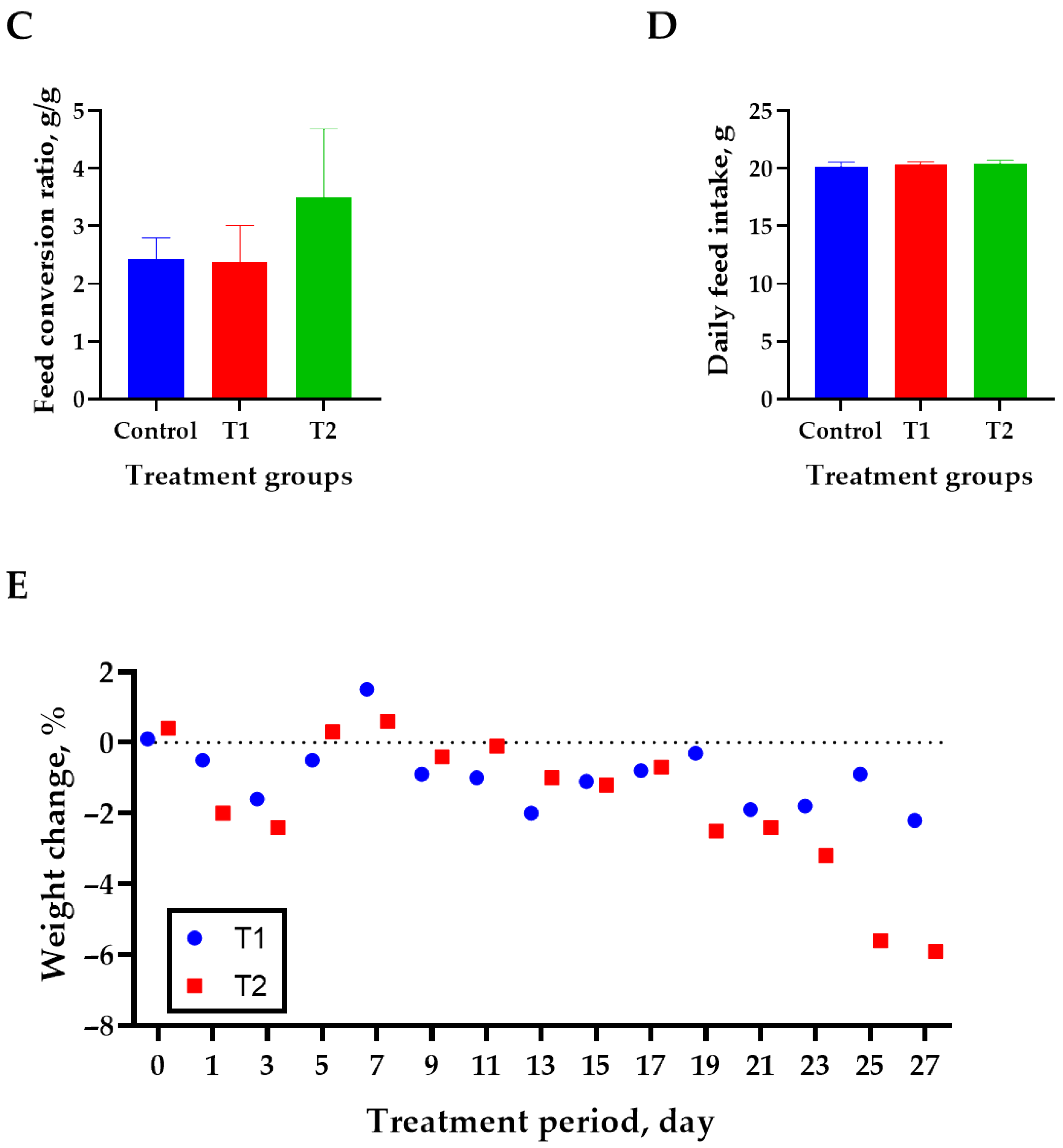
3.1. Growth Performance
3.2. Blood Biochemistry
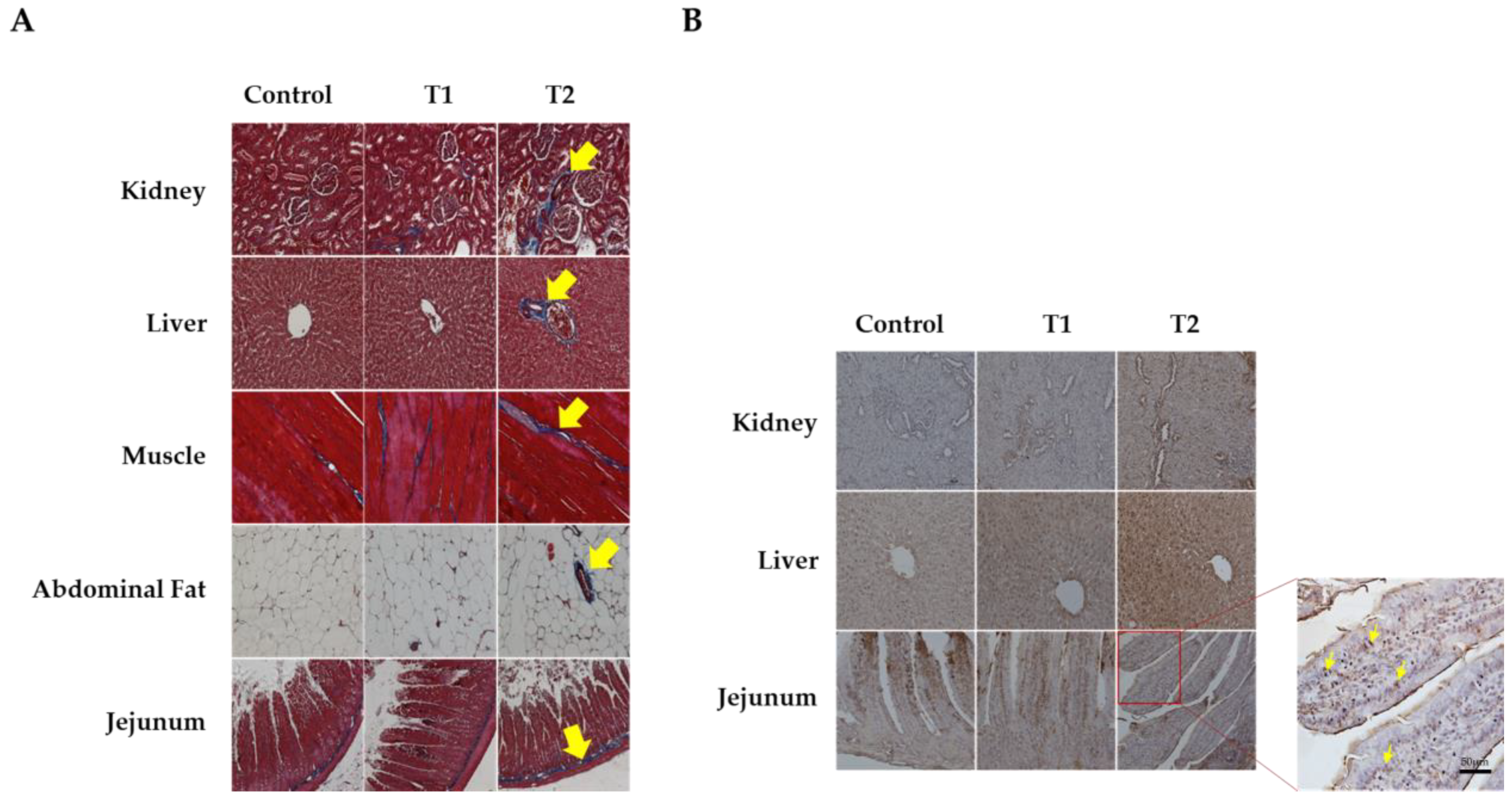
3.3. Histology
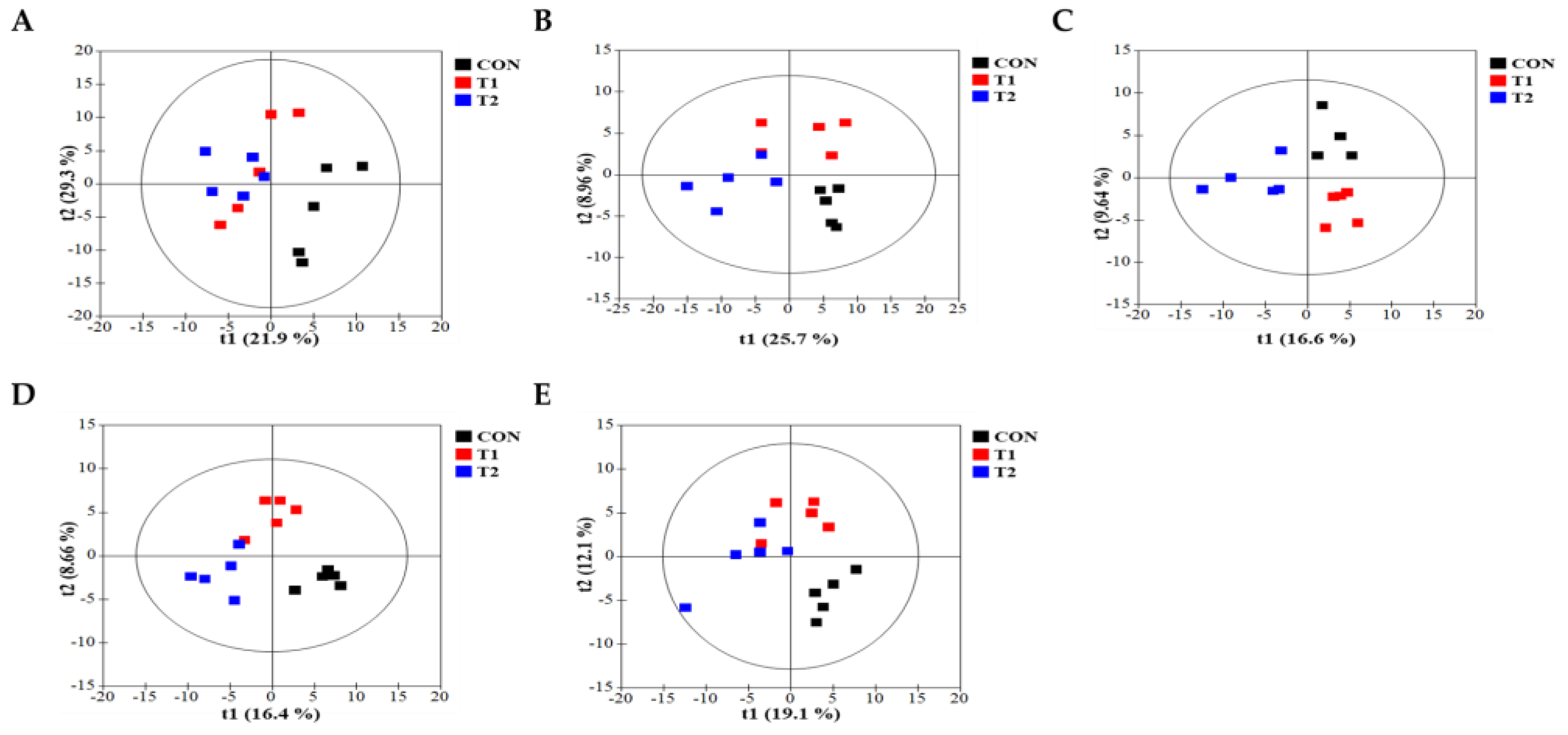
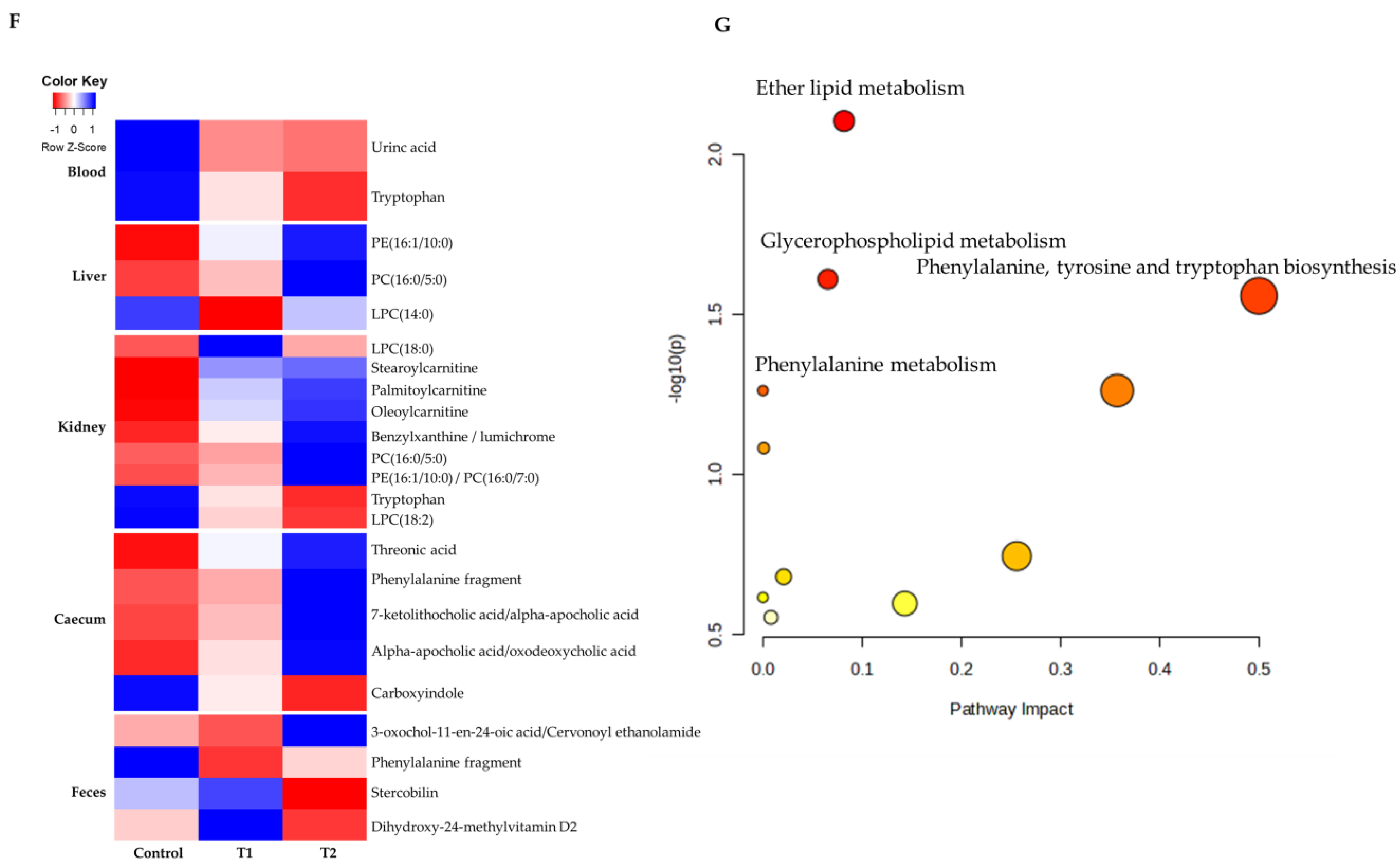
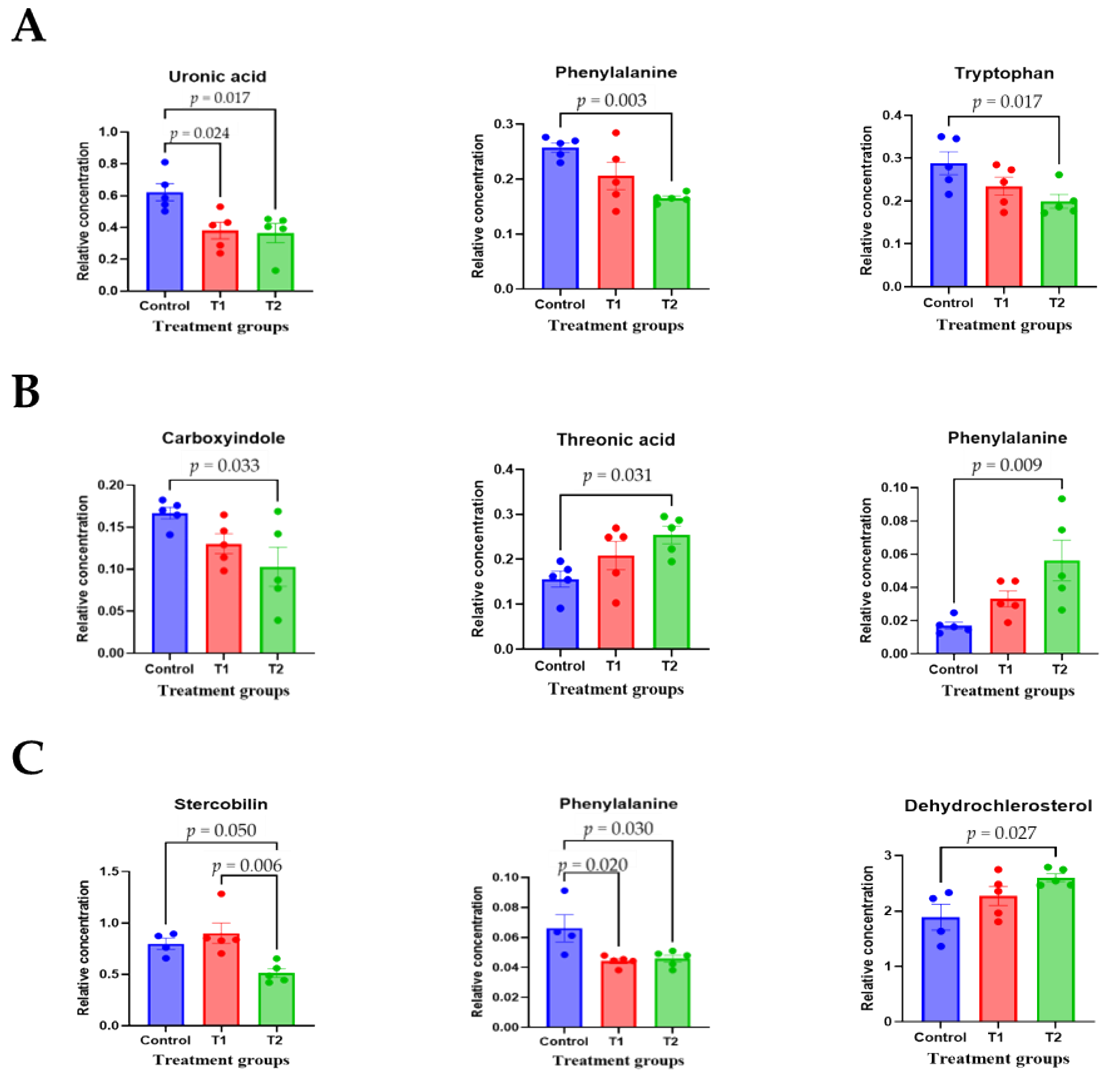
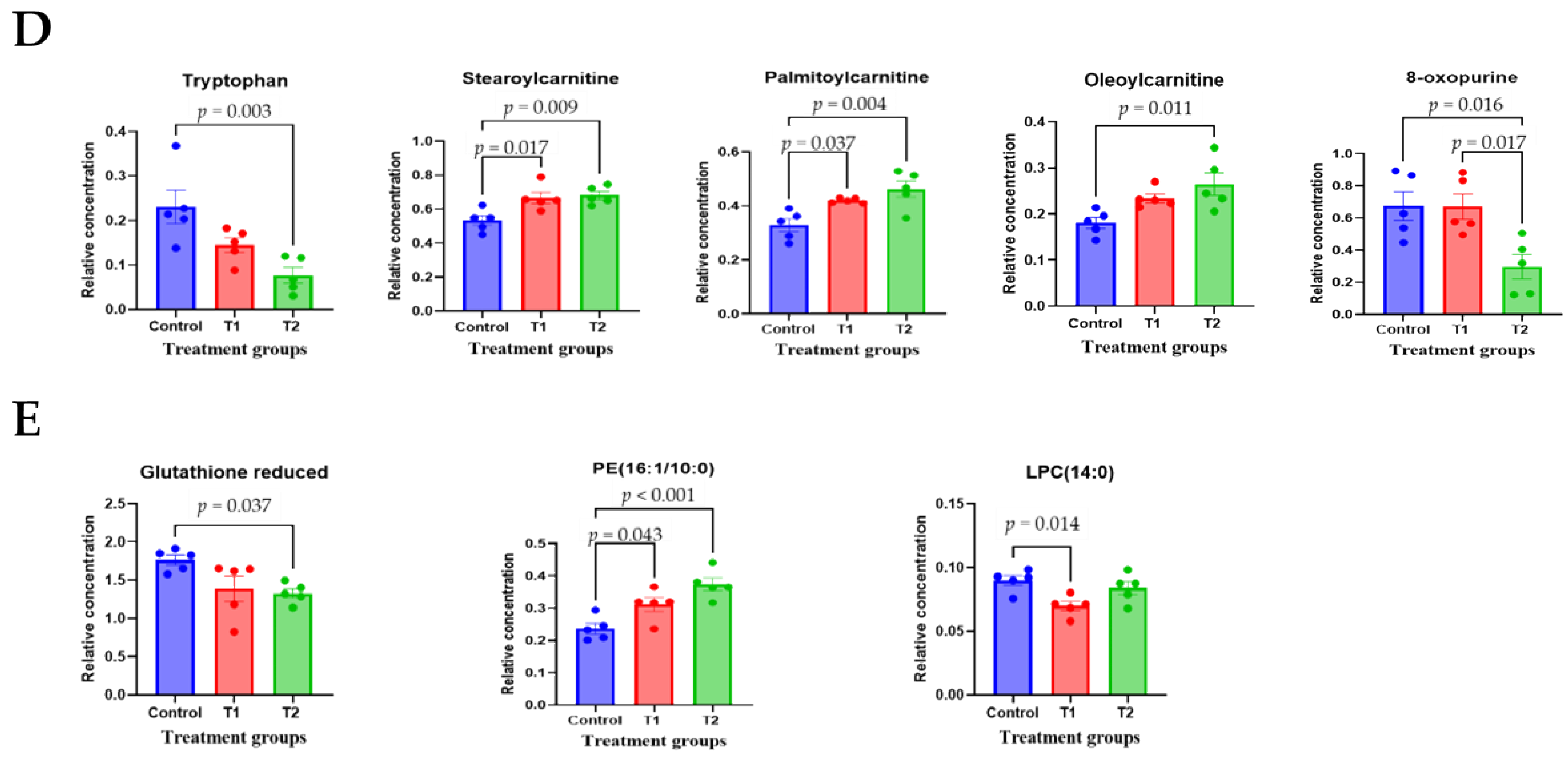
3.4. Metabolomic Profiling in Cecal and Fecal Tissues Contaminated with Deoxynivalenol
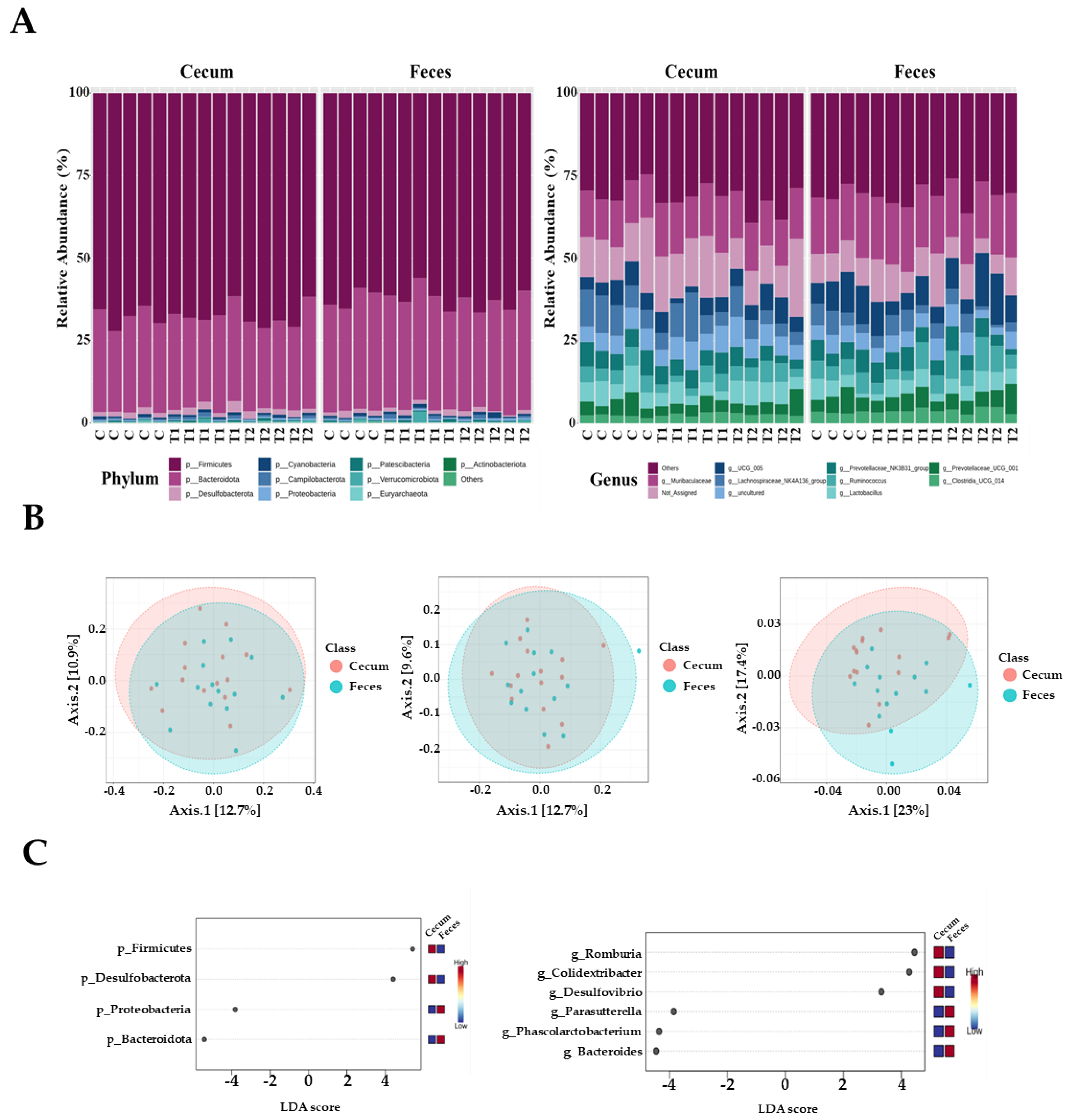
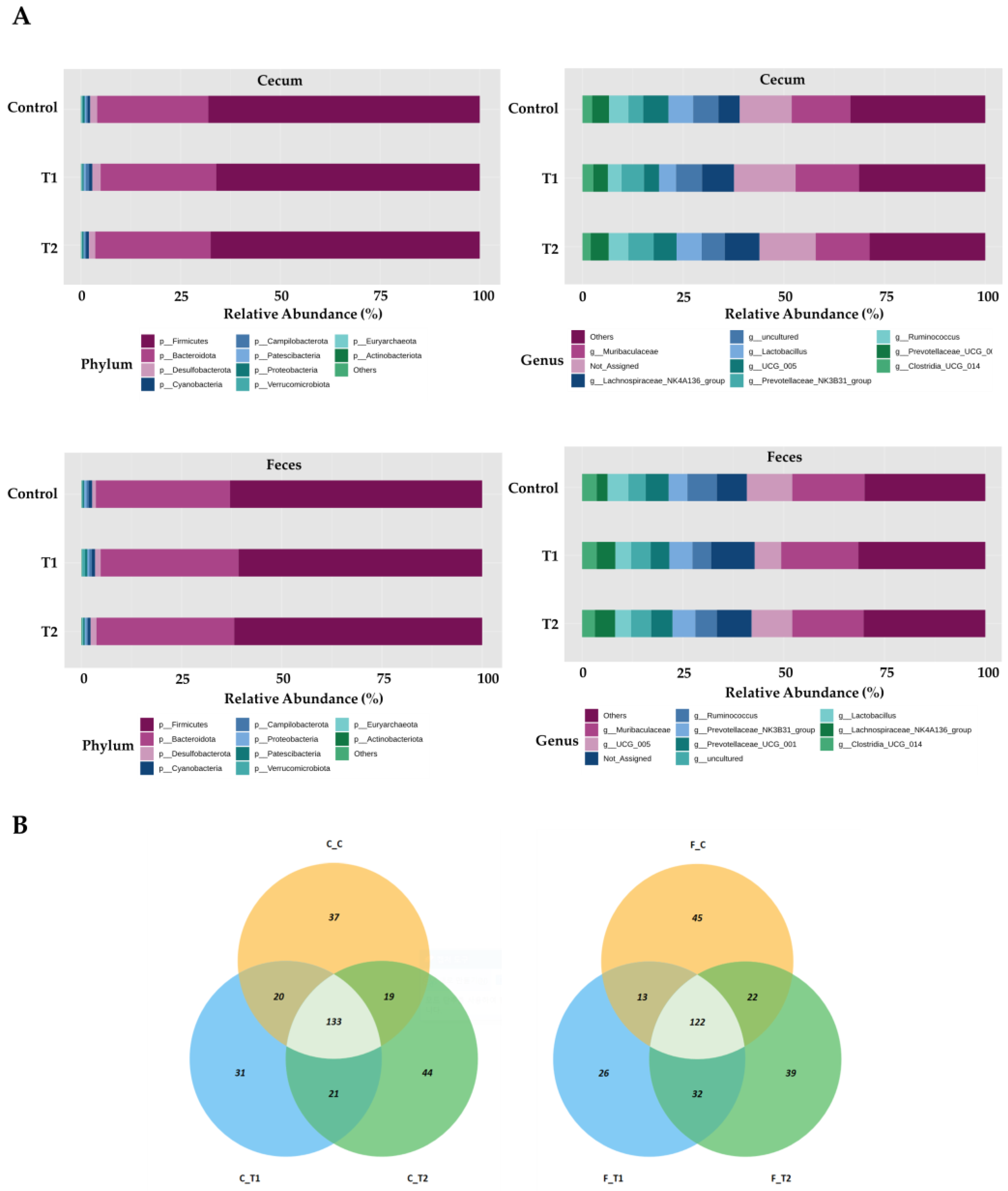
3.5. Microbial Composition of Cecal and Fecal Tissues Contaminated with Deoxynivalenol
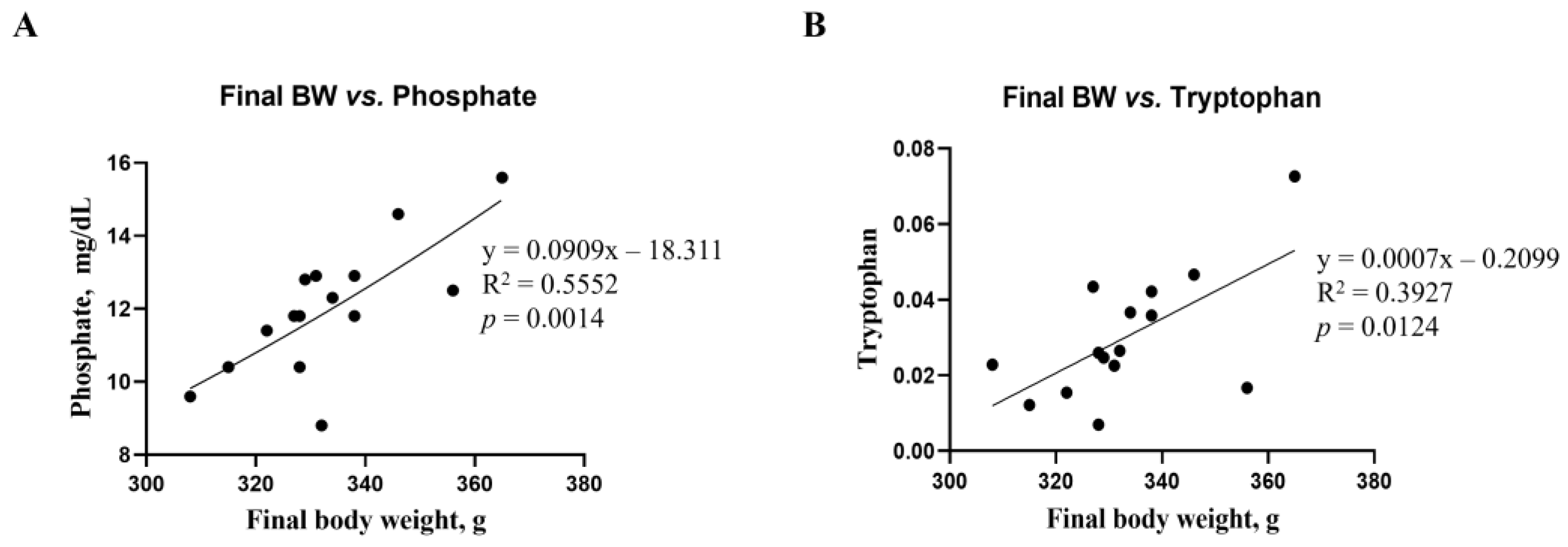
3.6. Correlation Analysis of Biochemical Parameters and Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bracarense, A.P.F.L.; Basso, K.M.; Da Silva, E.O.; Payros, D.; Oswald, I.P. Deoxynivalenol in the liver and lymphoid organs of rats: Effects of dose and duration on immunohistological changes. World Mycotoxin J. 2017, 10, 89–96. [Google Scholar] [CrossRef]
- Schothorst, R.C.; van Egmond, H.P. Report from SCOOP task 3.2.10 “collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states”. Subtask: Trichothecenes. Toxicol. Lett. 2004, 153, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Holanda, D.M.; Kim, S.W. Mycotoxin Occurrence, Toxicity, and Detoxifying Agents in Pig Production with an Emphasis on Deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Saint-Cyr, M.J.; Perrin-Guyomard, A.; Houee, P.; Rolland, J.G.; Laurentie, M. Evaluation of an oral subchronic exposure of deoxynivalenol on the composition of human gut microbiota in a model of human microbiota-associated rats. PLoS ONE 2013, 8, e80578. [Google Scholar] [CrossRef]
- Lattanzio, V.M.; Solfrizzo, M.; De Girolamo, A.; Chulze, S.N.; Torres, A.M.; Visconti, A. LC-MS/MS characterization of the urinary excretion profile of the mycotoxin deoxynivalenol in human and rat. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Deoxynivalenol in food and feed: Occurrence and exposure. EFSA J. 2013, 11, 3379. [CrossRef]
- Park, J.; Chang, H.; Kim, D.; Chung, S.; Lee, C. Long-Term Occurrence of Deoxynivalenol in Feed and Feed Raw Materials with a Special Focus on South Korea. Toxins 2018, 10, 127. [Google Scholar] [CrossRef]
- Pack, E.D.; Weiland, S.; Musser, R.; Schmale, D.G. Survey of zearalenone and type-B trichothecene mycotoxins in swine feed in the USA. Mycotoxin Res. 2021, 37, 297–313. [Google Scholar] [CrossRef]
- European Commission. Commission Recommendation (EU) 2016/1319 of 29 July 2016 amending Recommendation 2006/576/EC as regards deoxynivalenol, zearalenone and ochratoxin A in pet food. Off. J. Eur. Union 2016, 208, 58–60. [Google Scholar]
- Food and Drug Administration. Guidance for industry and FDA: Advisory Levels for Deoxynivalenol (DON) in Finished Wheat Products for Human Consumption and Grains and Grain-by-Products Used for Animal Feed. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-and-fda-advisory-levels-deoxynivalenol-don-finished-wheat-products-human (accessed on 22 May 2019).
- Pestka, J.J.; Clark, E.S.; Schwartz-Zimmermann, H.E.; Berthiller, F. Sex Is a Determinant for Deoxynivalenol Metabolism and Elimination in the Mouse. Toxins 2017, 9, 240. [Google Scholar] [CrossRef]
- Wellington, M.O.; Bosompem, M.A.; Rodrigues, L.A.; Columbus, D.A. Effect of long-term feeding of graded levels of deoxynivalenol on performance, nutrient utilization, and organ health of grower-finisher pigs (35 to 120 kg). J. Anim. Sci. 2021, 99, skab109. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.E.; Jeong, J.Y.; Song, J.; Lee, Y.; Lee, H.J.; Kim, D.W.; Jung, H.J.; Kim, K.H.; Kim, M.; Oh, Y.K.; et al. Colon Microbiome of Pigs Fed Diet Contaminated with Commercial Purified Deoxynivalenol and Zearalenone. Toxins 2018, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Nougayrede, J.P.; Del Rio, J.C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, K.; Awad, W.A.; Bohm, J.; Zebeli, Q. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: Poultry and swine. J. Appl. Toxicol. 2015, 35, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Saenz, J.S.; Kurz, A.; Ruczizka, U.; Bunger, M.; Dippel, M.; Nagl, V.; Grenier, B.; Ladinig, A.; Seifert, J.; Selberherr, E. Metaproteomics Reveals Alteration of the Gut Microbiome in Weaned Piglets Due to the Ingestion of the Mycotoxins Deoxynivalenol and Zearalenone. Toxins 2021, 13, 583. [Google Scholar] [CrossRef]
- Gerez, J.R.; Verri, W.A.; Hohmann, M.S.; Flaiban, K.M.C.; Hasuda, A.L.; Gloria, E.M.; Bracarense, A. Animal performance and biochemical parameters are sex-dependent in peripubertal rats exposed to deoxynivalenol. Toxicon 2022, 220, 106944. [Google Scholar] [CrossRef]
- Skiepko, N.; Przybylska-Gornowicz, B.; Gajecka, M.; Gajecki, M.; Lewczuk, B. Effects of Deoxynivalenol and Zearalenone on the Histology and Ultrastructure of Pig Liver. Toxins 2020, 12, 463. [Google Scholar] [CrossRef]
- Kang, R.; Li, R.; Dai, P.; Li, Z.; Li, Y.; Li, C. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Env. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef]
- Hou, L.; Tong, X.; Lin, S.; Yu, M.; Ye, W.C.; Xie, M. MiR-221/222 Ameliorates Deoxynivalenol-Induced Apoptosis and Proliferation Inhibition in Intestinal Epithelial Cells by Targeting PTEN. Front. Cell Dev. Biol. 2021, 9, 652939. [Google Scholar] [CrossRef]
- Khera, K.S.; Arnold, D.L.; Whalen, C.; Angers, G.; Scott, P.M. Vomitoxin (4-deoxynivalenol): Effects on reproduction of mice and rats. Toxicol. Appl. Pharmacol. 1984, 74, 345–356. [Google Scholar] [CrossRef]
- Rissato, D.F.; de Santi Rampazzo, A.P.; Borges, S.C.; Sousa, F.C.; Busso, C.; Buttow, N.C.; Natali, M.R.M. Chronic ingestion of deoxynivalenol-contaminated diet dose-dependently decreases the area of myenteric neurons and gliocytes of rats. Neurogastroenterol. Motil. 2020, 32, e13770. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.C.; Furr, J.R.; Robinson, V.G.; Betz, L.; Shockley, K.; Cunny, H.; Witt, K.; Waidyanatha, S.; Germolec, D. Oral deoxynivalenol toxicity in Harlan Sprague Dawley (Hsd:Sprague Dawley® SD®) rat dams and their offspring. Food Chem. Toxicol. 2021, 148, 111963. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, X.; Ji, C.; Rong, X.; Liu, S.; Zhang, J.; Ma, Q. Protective effect of Devosia sp. ANSB714 on growth performance, serum chemistry, immunity function and residues in kidneys of mice exposed to deoxynivalenol. Food Chem. Toxicol. 2016, 92, 143–149. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. BioTechniques 2004, 36, 808–812. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Meth 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Azizi, T.; Daneshyar, M.; Allymehr, M.; Jalali, A.S.; Behroozyar, H.K.; Tukmechi, A. The impact of deoxynivalenol contaminated diet on performance, immune response, intestine morphology and jejunal gene expression in broiler chicken. Toxicon 2021, 199, 72–78. [Google Scholar] [CrossRef]
- Li, J.; Bai, Y.; Ma, K.; Ren, Z.; Li, J.; Zhang, J.; Shan, A. Dihydroartemisinin alleviates deoxynivalenol induced liver apoptosis and inflammation in piglets. Ecotoxicol. Env. Saf. 2022, 241, 113811. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health B Crit. Rev. 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Recharla, N.; Park, S.; Kim, M.; Kim, B.; Jeong, J.Y. Protective effects of biological feed additives on gut microbiota and the health of pigs exposed to deoxynivalenol: A review. J. Anim. Sci. Technol. 2022, 64, 640–653. [Google Scholar] [CrossRef]
- Wu, L.; Liao, P.; He, L.; Ren, W.; Yin, J.; Duan, J.; Li, T. Growth performance, serum biochemical profile, jejunal morphology, and the expression of nutrients transporter genes in deoxynivalenol (DON)- challenged growing pigs. BMC Vet. Res. 2015, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Ren, Z.; Gao, S.; Chen, Y.; Yang, Y.; Yang, D.; Deng, J.; Zuo, Z.; Wang, Y.; Shen, L. Individual and combined effects of deoxynivalenol and zearalenone on mouse kidney. Env. Toxicol. Pharmacol. 2015, 40, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Thammitiyagodage, M.G.; de Silva, N.R.; Rathnayake, C.; Karunakaran, R.; Wgss, K.; Gunatillka, M.M.; Ekanayaka, N.; Galhena, B.P.; Thabrew, M.I. Biochemical and histopathological changes in Wistar rats after consumption of boiled and un-boiled water from high and low disease prevalent areas for chronic kidney disease of unknown etiology (CKDu) in north Central Province (NCP) and its comparison with low disease prevalent Colombo, Sri Lanka. BMC Nephrol. 2020, 21, 38. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, M.; Qu, Z.; Zhang, Y.; Yin, S.; Shan, A. Toxic effects of zearalenone on oxidative stress, inflammatory cytokines, biochemical and pathological changes induced by this toxin in the kidney of pregnant rats. Environ. Toxicol. Pharmacol. 2014, 37, 580–591. [Google Scholar] [CrossRef]
- Seki, M.; Nakayama, M.; Sakoh, T.; Yoshitomi, R.; Fukui, A.; Katafuchi, E.; Tsuda, S.; Nakano, T.; Tsuruya, K.; Kitazono, T. Blood urea nitrogen is independently associated with renal outcomes in Japanese patients with stage 3–5 chronic kidney disease: A prospective observational study. BMC Nephrol. 2019, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Fu, S.J.; Guo, Z.Y.; Pang, H.; Ju, W.Q.; Wang, D.P.; Hua, Y.P.; He, X.S. Prognostic value of combined preoperative lactate dehydrogenase and alkaline phosphatase levels in patients with resectable pancreatic ductal adenocarcinoma. Medicine 2016, 95, e4065. [Google Scholar] [CrossRef]
- Ruan, H.; Wang, Y.; Hou, Y.; Zhang, J.; Wu, J.; Zhang, F.; Sui, M.; Luo, J.; Yang, M. Zearalenone-14-Glucoside Is Hydrolyzed to Zearalenone by beta-Glucosidase in Extracellular Matrix to Exert Intracellular Toxicity in KGN Cells. Toxins 2022, 14, 458. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, L.; Nussler, A.K.; Liu, L.; Yang, W. Current sights for mechanisms of deoxynivalenol-induced hepatotoxicity and prospective views for future scientific research: A mini review. J. Appl. Toxicol. 2017, 37, 518–529. [Google Scholar] [CrossRef]
- Lan, T.; Kisseleva, T.; Brenner, D.A. Deficiency of NOX1 or NOX4 Prevents Liver Inflammation and Fibrosis in Mice through Inhibition of Hepatic Stellate Cell Activation. PLoS ONE 2015, 10, e0129743. [Google Scholar] [CrossRef]
- Panizo, S.; Martinez-Arias, L.; Alonso-Montes, C.; Cannata, P.; Martin-Carro, B.; Fernandez-Martin, J.L.; Naves-Diaz, M.; Carrillo-Lopez, N.; Cannata-Andia, J.B. Fibrosis in Chronic Kidney Disease: Pathogenesis and Consequences. Int. J. Mol. Sci. 2021, 22, 408. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhao, X.; Zheng, S.; Wang, S.; Liu, H.; Xu, S. Subacute cadmium exposure promotes M1 macrophage polarization through oxidative stress-evoked inflammatory response and induces porcine adrenal fibrosis. Toxicology 2021, 461, 152899. [Google Scholar] [CrossRef] [PubMed]
- Antar, S.A.; Ashour, N.A.; Marawan, M.E.; Al-Karmalawy, A.A. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int. J. Mol. Sci. 2023, 24, 4004. [Google Scholar] [CrossRef] [PubMed]
- Ranneh, Y.; Ali, F.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A. Crosstalk between reactive oxygen species and pro-inflammatory markers in developing various chronic diseases: A review. Appl. Biol. Chem. 2017, 60, 327–338. [Google Scholar] [CrossRef]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porc. Health Manag. 2016, 2, 21. [Google Scholar] [CrossRef]
- Pasternak, J.A.; Aiyer, V.I.A.; Hamonic, G.; Beaulieu, A.D.; Columbus, D.A.; Wilson, H.L. Molecular and Physiological Effects on the Small Intestine of Weaner Pigs Following Feeding with Deoxynivalenol-Contaminated Feed. Toxins 2018, 10, 40. [Google Scholar] [CrossRef]
- Reddy, K.E.; Kim, M.; Kim, K.H.; Ji, S.Y.; Baek, Y.; Chun, J.L.; Jung, H.J.; Choe, C.; Lee, H.J.; Kim, M.; et al. Effect of commercially purified deoxynivalenol and zearalenone mycotoxins on microbial diversity of pig cecum contents. Anim. Biosci. 2021, 34, 243–255. [Google Scholar] [CrossRef]
- Jia, B.; Lin, H.; Yu, S.; Liu, N.; Yu, D.; Wu, A. Mycotoxin deoxynivalenol-induced intestinal flora disorders, dysfunction and organ damage in broilers and pigs. J. Hazard. Mater. 2023, 451, 131172. [Google Scholar] [CrossRef]
- Miro-Abella, E.; Torrell, H.; Herrero, P.; Canela, N.; Arola, L.; Borrull, F.; Ras, R.; Fontanals, N. Monitoring and evaluation of the interaction between deoxynivalenol and gut microbiota in Wistar rats by mass spectrometry-based metabolomics and next-generation sequencing. Food Chem. Toxicol. 2018, 121, 124–130. [Google Scholar] [CrossRef]
- Lkhagva, E.; Chung, H.J.; Ahn, J.S.; Hong, S.T. Host Factors Affect the Gut Microbiome More Significantly than Diet Shift. Microorganisms 2021, 9, 2520. [Google Scholar] [CrossRef]
- Rao, J.; Xie, R.; Lin, L.; Jiang, J.; Du, L.; Zeng, X.; Li, G.; Wang, C.; Qiao, Y. Fecal microbiota transplantation ameliorates gut microbiota imbalance and intestinal barrier damage in rats with stress-induced depressive-like behavior. Eur. J. Neurosci. 2021, 53, 3598–3611. [Google Scholar] [CrossRef] [PubMed]
- Gryaznova, M.; Smirnova, Y.; Burakova, I.; Morozova, P.; Lagutina, S.; Chizhkov, P.; Korneeva, O.; Syromyatnikov, M. Fecal Microbiota Characteristics in Constipation-Predominant and Mixed-Type Irritable Bowel Syndrome. Microorganisms 2024, 12, 1414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rui, M.; Nie, Y.; Lu, G. Influence of gastrointestinal tract on metabolism of bisphenol A as determined by in vitro simulated system. J. Hazard. Mater. 2018, 355, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Ricaboni, D.; Mailhe, M.; Khelaifia, S.; Raoult, D.; Million, M. Romboutsia timonensis, a new species isolated from human gut. New Microbes New Infect. 2016, 12, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Mangifesta, M.; Mancabelli, L.; Milani, C.; Gaiani, F.; de’Angelis, N.; de’Angelis, G.L.; van Sinderen, D.; Ventura, M.; Turroni, F. Mucosal microbiota of intestinal polyps reveals putative biomarkers of colorectal cancer. Sci. Rep. 2018, 8, 13974. [Google Scholar] [CrossRef]
- Lecomte, V.; Kaakoush, N.O.; Maloney, C.A.; Raipuria, M.; Huinao, K.D.; Mitchell, H.M.; Morris, M.J. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS ONE 2015, 10, e0126931. [Google Scholar] [CrossRef]
- Zhang-Sun, W.; Augusto, L.A.; Zhao, L.; Caroff, M. Desulfovibrio desulfuricans isolates from the gut of a single individual: Structural and biological lipid A characterization. FEBS Lett. 2015, 589, 165–171. [Google Scholar] [CrossRef]
- Ju, T.; Kong, J.Y.; Stothard, P.; Willing, B.P. Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota. ISME J. 2019, 13, 1520–1534. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Duan, Y.; Wang, F.; Guo, F.; Yan, F.; Yang, X.; Yang, X. Intestinal toxicity of deoxynivalenol is limited by supplementation with Lactobacillus plantarum JM113 and consequentially altered gut microbiota in broiler chickens. J. Anim. Sci. Biotechnol. 2018, 9, 74. [Google Scholar] [CrossRef]
| Parameters 2 | Control | T1 | T2 | p Value |
|---|---|---|---|---|
| GLU, mg/dL | 211 ± 24.1 | 166 ± 27.0 | 185 ± 17.8 | 0.4189 |
| CREA, mg/dL | 0.28 ± 0.07 a | 0.10 ± 0.00 b | 0.18 ± 0.02 ab | 0.0413 |
| BUN, mg/dL | 17.6 ± 0.60 | 17.8 ± 1.77 | 19.40 ± 1.69 | 0.6425 |
| BUN/CREA | 83.2 ± 21.43 | 178 ± 17.72 | 122 ± 32.35 | 0.0545 |
| PHOS, mg/dL | 13.06 ± 0.94 | 11.64 ± 0.73 | 11.22 ± 0.57 | 0.2399 |
| CA, mg/dL | 11.46 ± 0.24 | 10.90 ± 0.33 | 11.24 ± 0.64 | 0.6685 |
| TP, g/dL | 6.66 ± 0.05 | 7.22 ± 0.37 | 7.04 ± 0.37 | 0.4387 |
| ALB, g/dL | 3.96 ± 0.12 | 4.42 ± 0.33 | 4.10 ± 0.24 | 0.4205 |
| GLOB, g/dL | 2.70 ± 0.10 | 2.80 ± 0.18 | 2.94 ± 0.14 | 0.5122 |
| ALB/GLOB | 1.48 ± 0.10 | 1.60 ± 0.18 | 1.40 ± 0.04 | 0.5229 |
| ALT, U/L | 88.00 ± 7.40 | 104.20 ± 15.57 | 105.8 ± 24.63 | 0.7309 |
| ALKP, U/L | 253.60 ± 21.94 a | 166.60 ± 13.43 b | 180.80 ± 35.39 b | 0.0172 |
| GGT, U/L | 2.00 ± 2.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.3966 |
| TBIL, mg/dL | 0.44 ± 0.11 | 1.18 ± 0.50 | 1.06 ± 0.79 | 0.5994 |
| CHOL, mg/dL | 72.20 ± 1.62 | 67.00 ± 2.05 | 64.80 ± 8.99 | 0.6217 |
| AMYL, U/L | 1787.8 ± 80.27 | 1686.4 ± 53.20 | 1889.4 ± 122.82 | 0.3163 |
| LIPA, U/L | 172.4 ± 17.79 | 173.0 ± 11.26 | 184.4 ± 12.51 | 0.7990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-Y.; Kim, J.; Kim, M.; Shim, S.-H.; Park, C.; Jung, S.; Jung, H. Effects of Increasing Oral Deoxynivalenol Gavage on Growth Performance, Blood Biochemistry, Metabolism, Histology, and Microbiome in Rats. Biology 2024, 13, 836. https://doi.org/10.3390/biology13100836
Jeong J-Y, Kim J, Kim M, Shim S-H, Park C, Jung S, Jung H. Effects of Increasing Oral Deoxynivalenol Gavage on Growth Performance, Blood Biochemistry, Metabolism, Histology, and Microbiome in Rats. Biology. 2024; 13(10):836. https://doi.org/10.3390/biology13100836
Chicago/Turabian StyleJeong, Jin-Young, Junsik Kim, Minji Kim, Seong-Hoon Shim, Cheolju Park, Sungju Jung, and Hyunjung Jung. 2024. "Effects of Increasing Oral Deoxynivalenol Gavage on Growth Performance, Blood Biochemistry, Metabolism, Histology, and Microbiome in Rats" Biology 13, no. 10: 836. https://doi.org/10.3390/biology13100836
APA StyleJeong, J.-Y., Kim, J., Kim, M., Shim, S.-H., Park, C., Jung, S., & Jung, H. (2024). Effects of Increasing Oral Deoxynivalenol Gavage on Growth Performance, Blood Biochemistry, Metabolism, Histology, and Microbiome in Rats. Biology, 13(10), 836. https://doi.org/10.3390/biology13100836






