The Effect of Environmental Enrichment on Selected Physiological and Immunological Stress-Related Markers in Dairy Goats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Husbandry
2.2. Ethics Statement
2.3. Environmental Enrichment
2.4. Blood Collection
2.5. Serum Preparation
2.6. Complete Blood Count (CBC) and Blood Biochemistry Analysis
2.7. Blood Chemistry
2.7.1. Determination of Serum Transferrin and AGE by ELISA
2.7.2. Determination of Oxytocin (Serum) and Serotonin (Serum) Levels by ELISA
2.7.3. Peripheral Blood Mononuclear Leukocyte Isolation
2.7.4. RNA Extraction and PCR Analysis
| Sequence | Forward/Reverse | Gene Name | Gene Bank Code |
|---|---|---|---|
| 5′GCAATTATTCCCCATGAACGAGG3′ | F | Capra Hircus 18S | DQ149973.1 |
| 5′GGCAGGGACTTAATCAACGCAA3′ | R | ||
| 5′GGCGAAAGACTAATCGAACCA3′ | F | Capra Hircus 28S | AY894418.1 |
| 5′AGAGCGCCAGCTATCCTGA3′ | R | ||
| 5′CTCCAGCCACAAACACTGACA3′ | F | Capra Hircus IL-6 | NM_001285640.1 |
| 5′ACCTTTGCGTTCTTTACCCAC3′ | R | ||
| 5′GCAACCGTACCTGAACCCA3′ | F | Capra Hircus IL-1β | DQ837160.1 |
| 5′GCCATCAGCCTCAAATAACAGC3′ | R | ||
| 5′TGATGACTGCCCTGATCAAGC3′ | F | Capra Hircus HSP70 | JN604433.1 |
| 5′TACACCTGGATCAGCACACC3′ | R | ||
| 5′CCAACCTGTGTCAACTGTGCAA3′ | F | Capra Hircus Transferrin | GQ149766.1 |
| 5′TCCTTGACAAAAGCCACGTCT3′ | R | ||
| 5′AGTTAATGCCTGTCACATACCCT3′ | F | Capra Hircus Lysozyme | NM_001285711.1 |
| 5′CCATGCTCTAATGCCTTGTGGA3′ | R |
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chrousos, G.P.; Gold, P.W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 1992, 267, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.O.; Kamilaris, T.C.; Chrousos, G.P.; Gold, P.W. Mechanisms of stress: A dynamic overview of hormonal and behavioral homeostasis. Neurosci. Biobehav. Rev. 1992, 16, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J.; Renquist, B.J.; Xiao, Y. A 100-Year Review: Stress physiology including heat stress. J. Dairy. Sci. 2017, 100, 10367–10380. [Google Scholar] [CrossRef]
- Dantzer, R.; Mormede, P. Stress in farm animals: A need for reevaluation. J. Anim. Sci. 1983, 57, 6–18. [Google Scholar] [CrossRef]
- Endris, M.; Feki, E. Review on effect of stress on animal productivity and response of animal to stressors. J. Anim. Vet. Adv. 2021, 20, 1–14. [Google Scholar]
- Kannan, G.; Terrill, T.H.; Kouakou, B.; Gazal, O.S.; Gelaye, S.; Amoah, E.A.; Samake, S. Transportation of goats: Effects on physiological stress responses and live weight loss. J. Anim. Sci. 2000, 78, 1450–1457. [Google Scholar] [CrossRef]
- Barroso, F.G.; Alados, C.L.; Boza, J. Social hierarchy in the domestic goat: Effect on food habits and production. Appl. Anim. Behav. Sci. 2000, 69, 35–53. [Google Scholar] [CrossRef]
- Grandin, T.; Shivley, C. How Farm Animals React and Perceive Stressful Situations Such As Handling, Restraint, and Transport. Animals 2015, 5, 1233–1251. [Google Scholar] [CrossRef]
- Sevi, A.; Casamassima, D.V.; Pulina, G.; Pazzona, A.L. Factors of welfare reduction in dairy sheep and goats. Ital. J. Anim. Sci. 2009, 8, 101–181. [Google Scholar] [CrossRef]
- Kruger, L.P.; Nedambale, T.L.; Scholtz, M.M.; Webb, E.C. The effect of environmental factors and husbandry practices on stress in goats. Small Rumin. Res. 2016, 141, 1–4. [Google Scholar] [CrossRef]
- Miranda-de la Lama, G.C.; Mattiello, S. The importance of social behaviour for goat welfare in livestock farming. Small Rumin. Res. 2010, 90, 1–10. [Google Scholar] [CrossRef]
- Fernández, M.A.; Alvarez, L.; Zarco, L. Regrouping in lactating goats increases aggression and decreases milk production. Small Rumin. Res. 2007, 70, 228–232. [Google Scholar] [CrossRef]
- Papakitsos, G.; Assouad, S.; Papageorgiou, M.; Goliomytis, M.; Charismiadou, M.; Simitzis, P. Regrouping in Dairy Ewes-Effects on Productive Performance and Specific Behavioral Traits. Animals 2023, 13, 1163. [Google Scholar] [CrossRef]
- Wemelsfelder, F. Animal Boredom: Is a Scientific Study of the Subjective Experiences of Animals Possible? In Advances in Animal Welfare Science 1984; Fox, M.W., Mickley, L.D., Eds.; Springer: Dordrecht, The Netherlands, 1985; pp. 115–154. [Google Scholar]
- Miranda-de la Lama, G.C.; Sepúlveda, W.S.; Montaldo, H.H.; María, G.A.; Galindo, F. Social strategies associated with identity profiles in dairy goats. Appl. Anim. Behav. Sci. 2011, 134, 48–55. [Google Scholar] [CrossRef]
- Szabò, S.; Barth, K.; Graml, C.; Futschik, A.; Palme, R.; Waiblinger, S. Introducing young dairy goats into the adult herd after parturition reduces social stress. J. Dairy. Sci. 2013, 96, 5644–5655. [Google Scholar] [CrossRef]
- Moberg, G.P.; Mench, J.A. The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; CABI Pub.: Oxfordshire, UK, 2000. [Google Scholar]
- Dhabhar, F.S. Stress-induced augmentation of immune function—The role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav. Immun. 2002, 16, 785–798. [Google Scholar] [CrossRef]
- Fazio, F.; Ferrantelli, V.; Cicero, A.; Casella, S.; Piccione, G. Utility of Acute Phase Proteins as Biomarkers of Transport Stress in Ewes and Beef Cattle. Ital. J. Food Saf. 2015, 4, 4210. [Google Scholar] [CrossRef][Green Version]
- Haddad, J.J.; Saade, N.E.; Safieh-Garabedian, B. Cytokines and neuro-immune-endocrine interactions: A role for the hypothalamic-pituitary-adrenal revolving axis. J. Neuroimmunol. 2002, 133, 1–19. [Google Scholar] [CrossRef]
- Padgett, D.A.; Glaser, R. How stress influences the immune response. Trends Immunol. 2003, 24, 444–448. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Wein, Y.; Bar Shira, E.; Friedman, A. Avoiding handling-induced stress in poultry: Use of uniform parameters to accurately determine physiological stress. Poult. Sci. 2017, 96, 65–73. [Google Scholar] [CrossRef]
- Apanius, V. Stress and immune defense. In Advances in the Study of Behavior; Elsevier: Amsterdam, The Netherlands, 1998; Volume 27, pp. 133–153. [Google Scholar]
- Ajith, Y.; Dimri, U.; Dixit, S.K.; Singh, S.K.; Gopalakrishnan, A.; Madhesh, E.; Rajesh, J.B.; Sangeetha, S.G. Immunomodulatory basis of antioxidant therapy and its future prospects: An appraisal. Inflammopharmacology 2017, 25, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Alvarez, C.; Bertrand, S.; Devevey, G.; Prost, J.; Faivre, B.; Sorci, G. Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol. Lett. 2004, 7, 363–368. [Google Scholar] [CrossRef]
- Aschbacher, K.; O’Donovan, A.; Wolkowitz, O.M.; Dhabhar, F.S.; Su, Y.; Epel, E. Good stress, bad stress and oxidative stress: Insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 2013, 38, 1698–1708. [Google Scholar] [CrossRef]
- Dubinina, E.E. Anti-oxidant system of blood plasma. Ukr. Biokhim. Zh. 1992, 64, 3–15. [Google Scholar]
- Wein, Y.; Geva, Z.; Bar-Shira, E.; Friedman, A. Transport-related stress and its resolution in turkey pullets: Activation of a pro-inflammatory response in peripheral blood leukocytes. Poult. Sci. 2017, 96, 2601–2613. [Google Scholar] [CrossRef]
- Wein, Y.; Shira, E.B.; Friedman, A. Increased serum levels of advanced glycation end products due to induced molting in hen layers trigger a proinflammatory response by peripheral blood leukocytes. Poult. Sci. 2020, 99, 3452–3462. [Google Scholar] [CrossRef]
- Miranda-de la Lama, G.C.; Pinal, R.; Fuchs, K.; Montaldo, H.H.; Ducoing, A.; Galindo, F. Environmental enrichment and social rank affects the fear and stress response to regular handling of dairy goats. J. Vet. Behav. 2013, 8, 342–348. [Google Scholar] [CrossRef]
- Aschwanden, J.; Gygax, L.; Wechsler, B.; Keil, N.M. Loose housing of small goat groups: Influence of visual cover and elevated levels on feeding, resting and agonistic behaviour. Appl. Anim. Behav. Sci. 2009, 119, 171–179. [Google Scholar] [CrossRef]
- Mandel, R.; Whay, H.R.; Nicol, C.J.; Klement, E. The effect of food location, heat load, and intrusive medical procedures on brushing activity in dairy cows. J. Dairy. Sci. 2013, 96, 6506–6513. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar] [CrossRef]
- Billman, G.E. Homeostasis: The Underappreciated and Far Too Often Ignored Central Organizing Principle of Physiology. Front. Physiol. 2020, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Libretti, S.; Puckett, Y. Physiology, Homeostasis. In StatPearls; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Cabalar, I.; Le, T.H.; Silber, A.; O’Hara, M.; Abdallah, B.; Parikh, M.; Busch, R. The role of blood testing in prevention, diagnosis, and management of chronic diseases: A review. Am. J. Med. Sci. 2024, 368, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Thrall, M.A.; Weiser, G.; Allison, R.W.; Campbell, T.W. Veterinary Hematology and Clinical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Weiss, D.J.; Wardrop, K.J. Schalm’s Veterinary Hematology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Levitt, D.G.; Levitt, M.D. Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int. J. Gen. Med. 2016, 9, 229–255. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.E.; Roussel, A.J. Evaluation of the Ruminant Serum Chemistry Profile. Vet. Clin. N. Am. Food Anim. Pract. 2007, 23, 403–426. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Throop, J.L.; Kerl, M.E.; Cohn, L.A. Albumin in health and disease: Causes and treatment of hypoalbuminemia. Compendium 2004, 26, 940–948. [Google Scholar]
- Prakash, M.; Pathan, M.; Arya, J.S.; Lunagariya, P. Assessment of Glucose, Total Protein, Albumin and Cholesterol Level and Its Correlation with Milk Production during Different Stages of Lactation in Indigenous and Crossbred Cows. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1248–1256. [Google Scholar] [CrossRef]
- Cabrerizo, S.; Cuadras, D.; Gomez-Busto, F.; Artaza-Artabe, I.; Marín-Ciancas, F.; Malafarina, V. Serum albumin and health in older people: Review and meta analysis. Maturitas 2015, 81, 17–27. [Google Scholar] [CrossRef]
- Sheinenzon, A.; Shehadeh, M.; Michelis, R.; Shaoul, E.; Ronen, O. Serum albumin levels and inflammation. Int. J. Biol. Macromol. 2021, 184, 857–862. [Google Scholar] [CrossRef]
- Al-Owaimer, A.N.; Suliman, G.M.; Alobre, M.M.; Swelum, A.A.; Al-Badwi, M.A.; Ba-Awadh, H.; Sazili, A.Q.; Kumar, P.; Kaka, U. Investigating the impact of preslaughter handling intensity on goats: A study on behavior, physiology, blood enzymes, and hormonal responses. Front. Vet. Sci. 2024, 11, 1381806. [Google Scholar] [CrossRef]
- Jia, H.-M.; Li, Q.; Zhou, C.; Yu, M.; Yang, Y.; Zhang, H.-W.; Ding, G.; Shang, H.; Zou, Z.-M. Chronic unpredictive mild stress leads to altered hepatic metabolic profile and gene expression. Sci. Rep. 2016, 6, 23441. [Google Scholar] [CrossRef] [PubMed]
- Joung, J.Y.; Cho, J.H.; Kim, Y.H.; Choi, S.H.; Son, C.G. A literature review for the mechanisms of stress-induced liver injury. Brain Behav. 2019, 9, e01235. [Google Scholar] [CrossRef]
- Li, R.; Wang, L.; Chen, B.; Zhang, Y.; Qi, P. Effects of Transportation on Blood Indices, Oxidative Stress, Rumen Fermentation Parameters and Rumen Microbiota in Goats. Animals 2024, 14, 1616. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, H.R.; Lee, D.W.; Min, J.; Lee, Y.M.; Kang, M.Y. Association between long working hours and liver enzymes: Evidence from the Korea National Health and Nutrition Examination Survey, 2007–2017. Ann. Occup. Environ. Med. 2022, 34, e9. [Google Scholar] [CrossRef]
- Buffington, M.A.; Abreo, K. Hyponatremia: A Review. J. Intensive Care Med. 2016, 31, 223–236. [Google Scholar] [CrossRef]
- Antoni, F. Vasopressin as a stress hormone. In Stress: Neuroendocrinology and Neurobiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 97–108. [Google Scholar]
- Bao, L.-L.; Jiang, W.-Q.; Sun, F.-J.; Wang, D.-X.; Pan, Y.-J.; Song, Z.-X.; Wang, C.-H.; Yang, J. The influence of psychological stress on arginine vasopressin concentration in the human plasma and cerebrospinal fluid. Neuropeptides 2014, 48, 361–369. [Google Scholar] [CrossRef]
- Hydbring, E.; Madej, A.; MacDonald, E.; Drugge-Boholm, G.; Berglund, B.; Olsson, K. Hormonal changes during parturition in heifers and goats are related to the phases and severity of labour. J. Endocrinol. 1999, 160, 75–85. [Google Scholar] [CrossRef][Green Version]
- Celi, P.; Chauhan, S. Oxidative stress management in farm animals: Opportunities and challenges. In Proceedings of the 4th International Conference on Sustainable Animal Agriculture for Developing Countries, Lanzhou, China, 27–31 July 2013. [Google Scholar]
- Gustavo Alberto De La Riva De La, R.; Luis Adrián Saldaña, T.; Juan Carlos, G.-H. Assessment on Oxidative Stress in Animals: From Experimental Models to Animal Production. In Importance of Oxidative Stress and Antioxidant System in Health and Disease; Suna, S., Ahmet, Y., Eds.; IntechOpen: Rijeka, Croatia, 2022; Charpter 2. [Google Scholar]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Puppel, K.; Kapusta, A.; Kuczyńska, B. The etiology of oxidative stress in the various species of animals, a review. J. Sci. Food Agric. 2015, 95, 2179–2184. [Google Scholar] [CrossRef]
- Yusuf, A.O.; Mlambo, V.; Sowande, O.; Solomon, R. Oxidative stress biomarkers in West African Dwarf goats reared under intensive and semi-intensive production systems. South. Afr. J. Anim. Sci. 2017, 47, 281–289. [Google Scholar] [CrossRef][Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Earley, B.; Buckham-Sporer, K.; Gupta, S.; Pang, W.; Ting, S. Biologic response of animals to husbandry stress with implications for biomedical models. Open Access Anim. Physiol. 2010, 2, 25–42. [Google Scholar] [CrossRef]
- Greven, W.L.; Smit, J.M.; Rommes, J.H.; Spronk, P.E. Accumulation of advanced glycation end (AGEs) products in intensive care patients: An observational, prospective study. BMC Clin. Pathol. 2010, 10, 4–5. [Google Scholar] [CrossRef]
- Jaisson, S.; Gillery, P. Evaluation of Nonenzymatic Posttranslational Modification–Derived Products as Biomarkers of Molecular Aging of Proteins. Clin. Chem. 2010, 56, 1401–1412. [Google Scholar] [CrossRef]
- Lane, N. Oxygen: The Molecule That Made the World; Oxford University Press: Oxford, MI, USA, 2002. [Google Scholar]
- Lapolla, A.; Traldi, P.; Fedele, D. Importance of measuring products of non-enzymatic glycation of proteins. Clin. Biochem. 2005, 38, 103–115. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 2008, 153, 6–20. [Google Scholar] [CrossRef]
- de Jong, G.; van Dijk, J.P.; van Eijk, H.G. The biology of transferrin. Clin. Chim. Acta 1990, 190, 1–46. [Google Scholar] [CrossRef]
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Tan, A.X.; Vlassara, H. Antibacterial activity of lysozyme and lactoferrin is inhibited by binding of advanced glycation–modified proteins to a conserved motif. Nat. Med. 1995, 1, 1057–1061. [Google Scholar] [CrossRef]
- López-Rodríguez, G.; Suárez-Dieguez, T. Albumin and transferrin are antioxidants that prevent lipoperoxidation in vitro. Rev. Latinoam. Quím. 2010, 38, 159–167. [Google Scholar]
- Ogun, A.S.; Adeyinka, A. Biochemistry, transferrin. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gallo, D.; Cocchietto, M.; Masat, E.; Agostinis, C.; Harei, E.; Veronesi, P.; Sava, G. Human recombinant lysozyme downregulates advanced glycation endproduct-induced interleukin-6 production and release in an in-vitro model of human proximal tubular epithelial cells. Exp. Biol. Med. 2014, 239, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, F.; Cao, Q.; Ren, B.; Zhu, L.; Striker, G.; Vlassara, H. Amelioration of oxidant stress by the defensin lysozyme. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E824–E832. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Behm, B.; Babilas, P.; Landthaler; Schreml, S. Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 812–820. [Google Scholar] [CrossRef]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef]
- Hübner, G.; Brauchle, M.; Smola, H.; Madlener, M.; Fässler, R.; Werner, S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine 1996, 8, 548–556. [Google Scholar] [CrossRef]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef]
- Chatterjee, S. Oxidative stress, inflammation, and disease. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–58. [Google Scholar]
- Rokutan, K.; Morita, K.; Masuda, K.; Tominaga, K.; Shikishima, M.; Teshima-Kondo, S.; Omori, T.; Sekiyama, A. Gene expression profiling in peripheral blood leukocytes as a new approach for assessment of human stress response. J. Med. Investig. 2005, 52, 137–144. [Google Scholar] [CrossRef]
- Shini, S.; Huff, G.; Shini, A.; Kaiser, P. Understanding stress-induced immunosuppression: Exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult. Sci. 2010, 89, 841–851. [Google Scholar] [CrossRef]
- Shini, S.; Shini, A.; Kaiser, P. Cytokine and chemokine gene expression profiles in heterophils from chickens treated with corticosterone. Stress 2010, 13, 185–194. [Google Scholar] [CrossRef]
- Ranabir, S.; Reetu, K. Stress and hormones. Indian. J. Endocrinol. Metab. 2011, 15, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Tsigos, C.; Kyrou, I.; Kassi, E.; Chrousos, G.P. Stress: Endocrine Physiology and Pathophysiology. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Carter, C.S.; Kenkel, W.M.; MacLean, E.L.; Wilson, S.R.; Perkeybile, A.M.; Yee, J.R.; Ferris, C.F.; Nazarloo, H.P.; Porges, S.W.; Davis, J.M.; et al. Is Oxytocin “Nature’s Medicine”? Pharmacol. Rev. 2020, 72, 829–861. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sato, S. Role of oxytocin in improving the welfare of farm animals—A review. Asian-Australas. J. Anim. Sci. 2017, 30, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, S.F.; Pusapati, S.; Khenhrani, R.R.; Farooqi, M.S.; Sarwar, S.; Alnasarat, A.; Mathur, N.; Metz, C.N.; LeRoith, D.; Tracey, K.J.; et al. Oxytocin and Related Peptide Hormones: Candidate Anti-Inflammatory Therapy in Early Stages of Sepsis. Front. Immunol. 2022, 13, 864007. [Google Scholar] [CrossRef]
- Nagasawa, M.; Okabe, S.; Mogi, K.; Kikusui, T. Oxytocin and mutual communication in mother-infant bonding. Front. Hum. Neurosci. 2012, 6, 31. [Google Scholar] [CrossRef]
- Olff, M.; Frijling, J.L.; Kubzansky, L.D.; Bradley, B.; Ellenbogen, M.A.; Cardoso, C.; Bartz, J.A.; Yee, J.R.; van Zuiden, M. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 2013, 38, 1883–1894. [Google Scholar] [CrossRef]
- Deakin, J.F.W. The role of serotonin in depression and anxiety. Eur. Psychiatry 1998, 13, 57s–63s. [Google Scholar] [CrossRef]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A review. J. Vet. Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef]
- van den Buuse, M.; Hale, M.W. Chapter 10—Serotonin in Stress. In Stress: Physiology, Biochemistry, and Pathology; Fink, G., Ed.; Academic Press: New York, NY, USA, 2019; pp. 115–123. [Google Scholar]
- Mottolese, R.; Redouté, J.; Costes, N.; Le Bars, D.; Sirigu, A. Switching brain serotonin with oxytocin. Proc. Natl. Acad. Sci. USA 2014, 111, 8637–8642. [Google Scholar] [CrossRef]
- Baribeau, D.A.; Anagnostou, E. Oxytocin and vasopressin: Linking pituitary neuropeptides and their receptors to social neurocircuits. Front. Neurosci. 2015, 9, 335. [Google Scholar] [CrossRef]
- Fairhurst, G.D.; Frey, M.D.; Reichert, J.F.; Szelest, I.; Kelly, D.M.; Bortolotti, G.R. Does environmental enrichment reduce stress? An integrated measure of corticosterone from feathers provides a novel perspective. PLoS ONE 2011, 6, e17663. [Google Scholar] [CrossRef]
- Hutchinson, E.; Avery, A.; VandeWoude, S. Environmental Enrichment for Laboratory Rodents. ILAR J. 2005, 46, 148–161. [Google Scholar] [CrossRef]
- Mkwanazi, M.V.; Ncobela, C.N.; Kanengoni, A.T.; Chimonyo, M. Effects of environmental enrichment on behaviour, physiology and performance of pigs—A review. Asian-Australas. J. Anim. Sci. 2019, 32, 138. [Google Scholar] [CrossRef] [PubMed]
- Hopster, H.; Bruckmaier, R.M.; Van der Werf, J.T.N.; Korte, S.M.; Macuhova, J.; Korte-Bouws, G.; van Reenen, C.G. Stress Responses during Milking; Comparing Conventional and Automatic Milking in Primiparous Dairy Cows. J. Dairy. Sci. 2002, 85, 3206–3216. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.A.; Tops, M. Possible involvement of oxytocin in modulating the stress response in lactating dairy cows. Front. Psychol. 2014, 5, 951. [Google Scholar] [CrossRef] [PubMed]

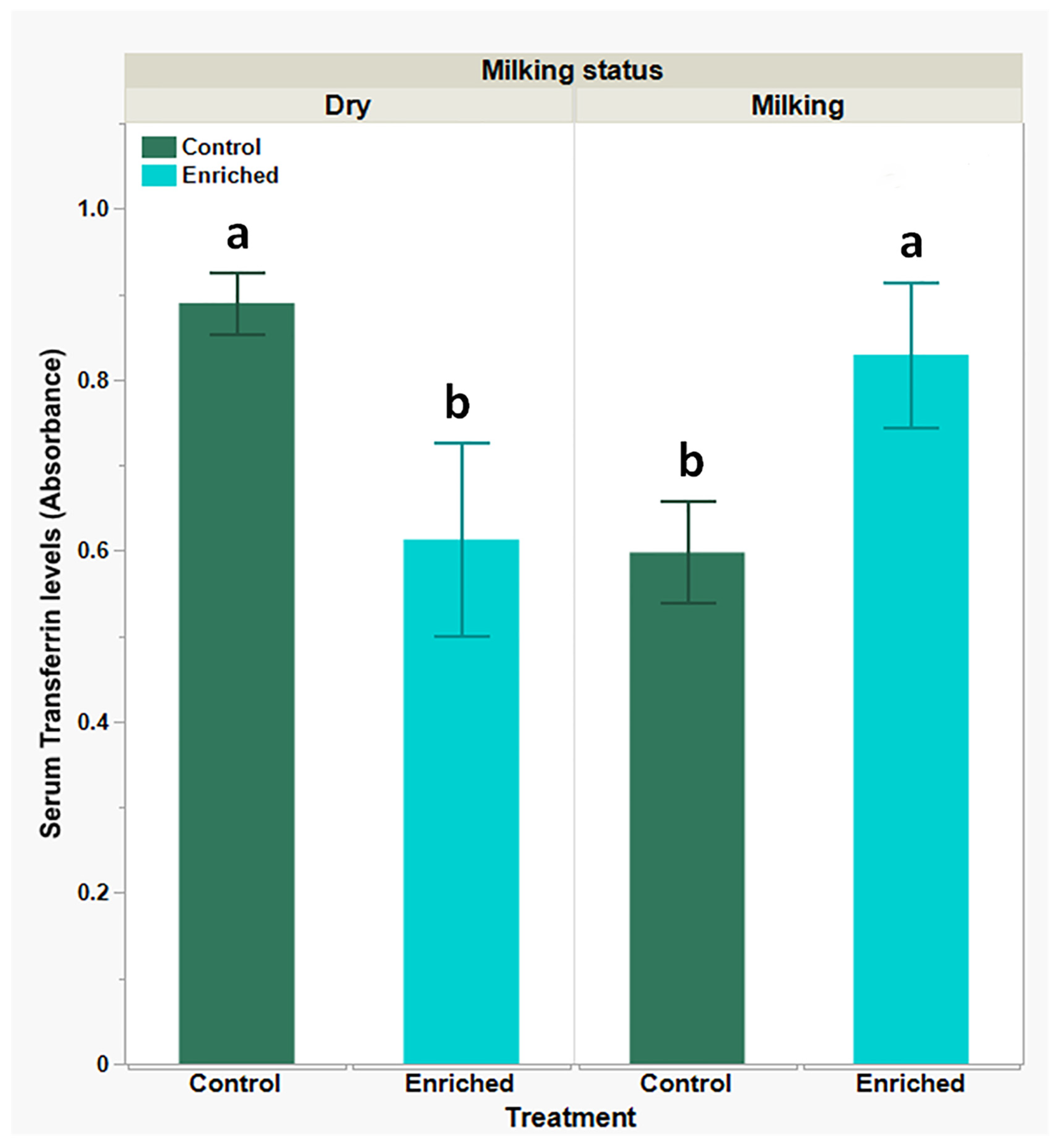
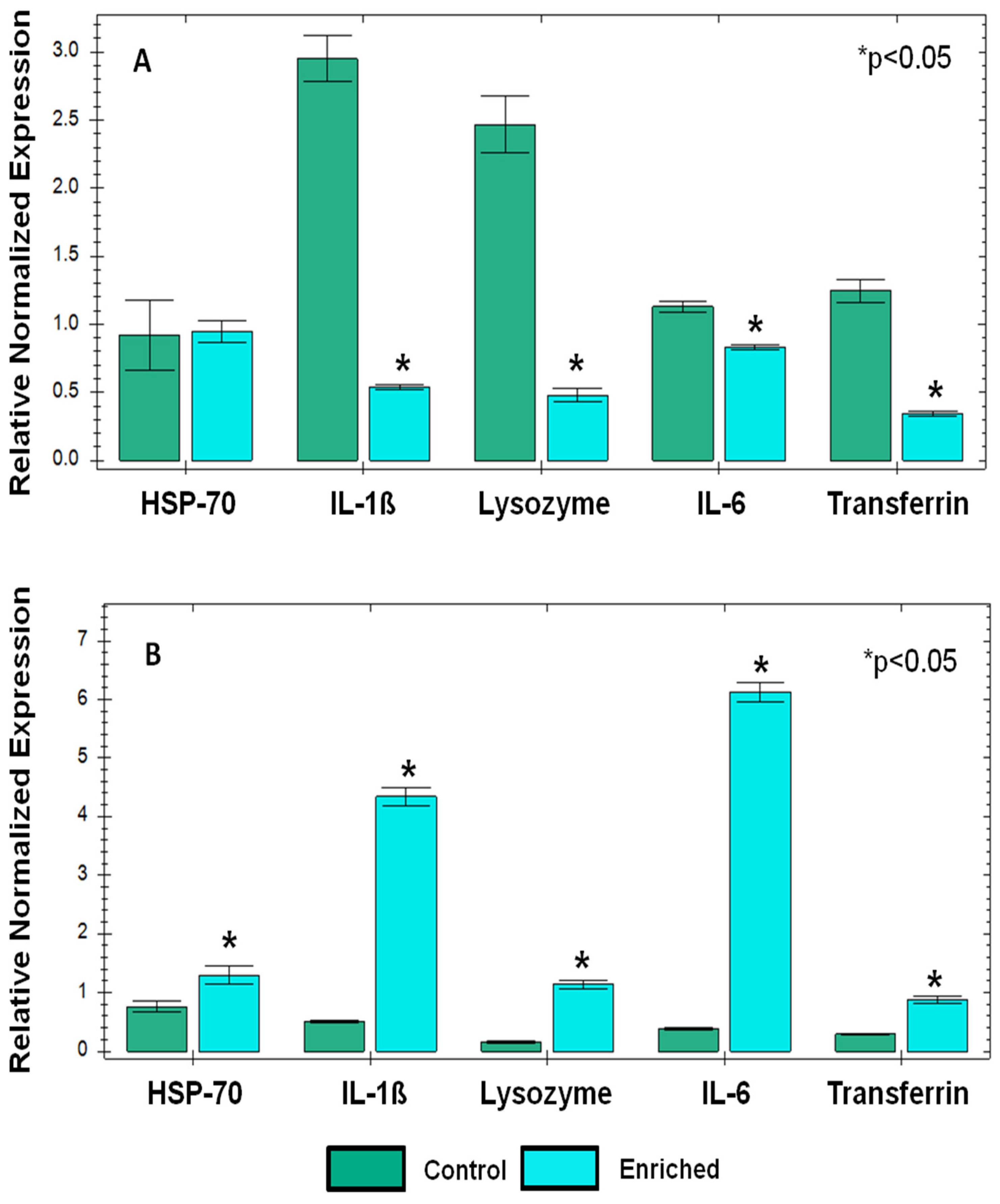
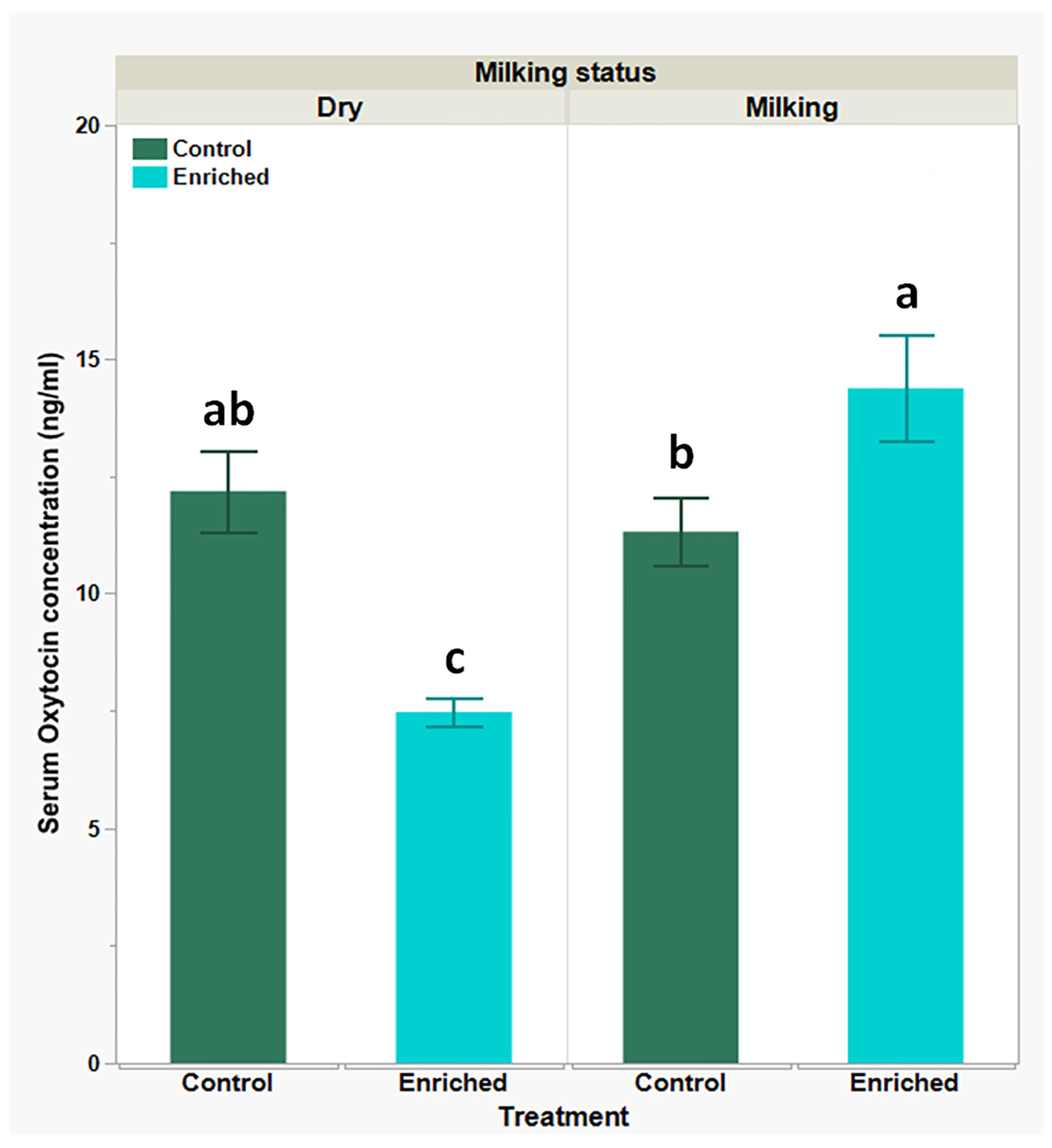
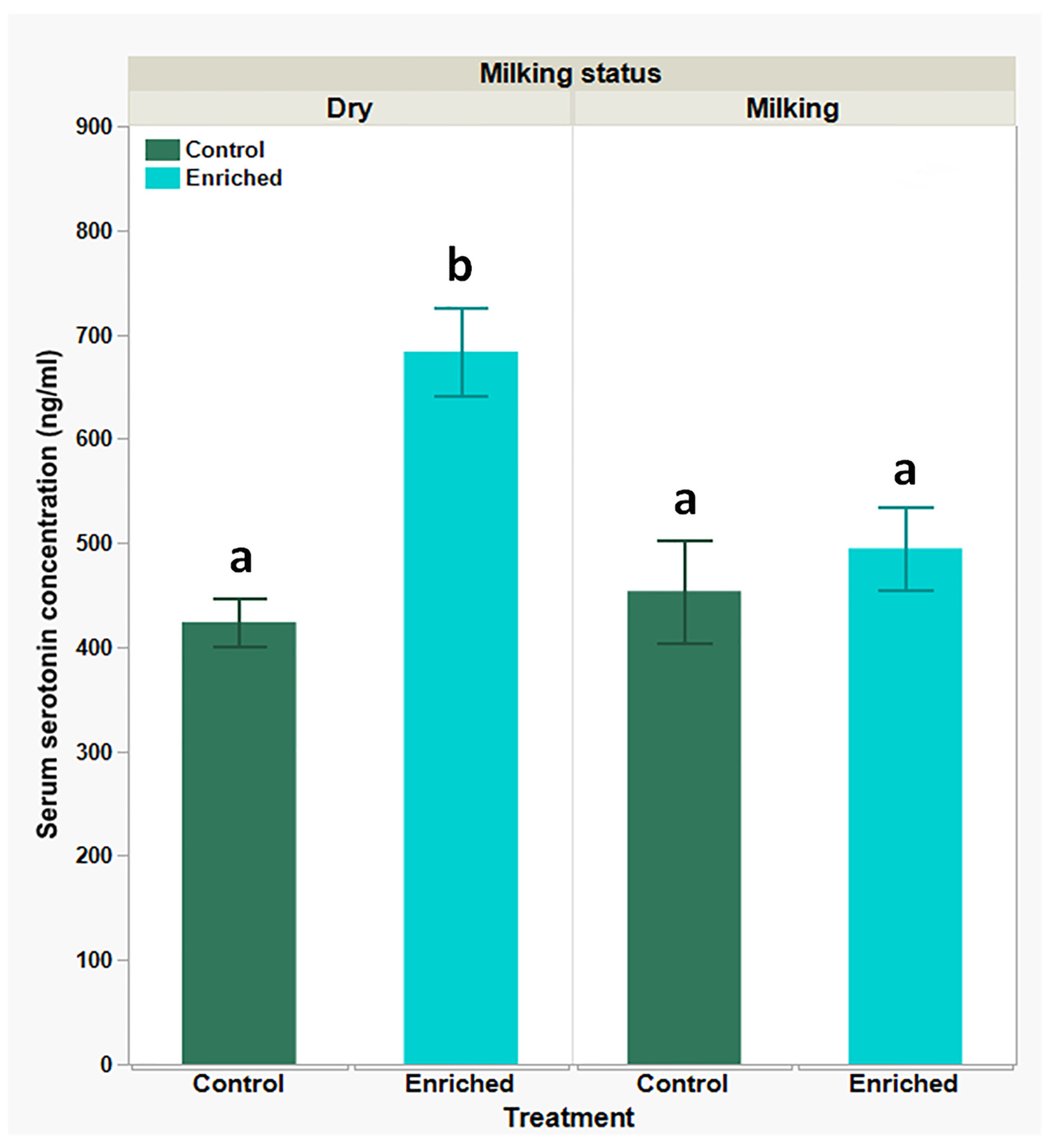
| Parameter | Dry Goats Mean ± SEM | Milking Goats Mean ± SEM | P-Value | ||
|---|---|---|---|---|---|
| Control | Enriched | Control | Enriched | ||
| WBC (103/µL) | 8.63 ± 1.13 | 8.34 ± 0.86 | 9.13 ± 0.96 | 9.67 ± 0.86 | 0.6683 |
| RBC (106/µL) | 14.80 ± 0.56 | 15.24 ± 0.45 | 14.86 ± 0.63 | 14.48 ± 0.55 | 0.4677 |
| HGB (g/dL) | 9.19 ± 0.31 | 9.62 ± 0.24 | 9.40 ± 0.37 | 9.14 ± 0.28 | 0.2589 |
| HCT (%) | 24.96 ± 0.9 | 26.01 ± 0.7 | 25.13 ± 1 | 24.35 ± 0.81 | 0.2999 |
| MCV (fl) | 16.9 ± 0.29 | 17.1 ± 0.3 | 16.99 ± 0.48 | 16.93 ± 0.52 | 0.7623 |
| MCH (pg) | 6.22 ± 0.10 | 6.33 ± 0.10 | 6.44 ± 0.18 | 6.36 ± 0.18 | 0.5588 |
| MCHC (g/dL) | 36.91 ± 0.20 | 37.03 ± 0.15 | 37.44 ± 0.28 | 37.57 ± 0.23 | 0.9944 |
| RDW (%) | 26.37 ± 0.51 | 26.23 ± 0.42 | 26.30 ± 0.5 | 26.33 ± 0.25 | 0.8538 |
| PLT (103/µL) | 260 ± 37.59 | 287.09 ± 43.72 | 309.82 ± 61.53 | 243.5 ± 44.59 | 0.3411 |
| MPV (fl) | 9.93 ± 0.62 | 9.98 ± 0.59 | 9.86 ± 0.77 | 11.5 ± 0.73 | 0.2590 |
| Neut (103/µL) | 3.98 ± 0.52 | 3.22 ± 0.32 | 3.18 ± 0.51 | 3.83 ± 0.35 | 0.1211 |
| Lymph (103/µL) | 4.27 ± 0.51 | 4.59 ± 0.6 | 5.45 ± 0.71 | 5.34 ± 0.70 | 0.7384 |
| Mono (103/µL) | 0.13 ± 0.02 | 0.12 ± 0.02 | 0.12 ± 0.02 | 0.13 ± 0.01 | 0.6146 |
| Eos (103/µL) | 0.44 ± 0.1 | 0.34 ± 0.09 | 0.19 ± 0.08 | 0.27 ± 0.08 | 0.2985 |
| Baso (103/µL) | 0.03 ± 0 | 0.03 ± 0 | 0.05 ± 0.01 | 0.05 ± 0 | 0.7115 |
| LUC (103/µL) | 0.01 ± 0 | 0.01 ± 0 | 0.02 ± 0 | 0.03 ± 0 | 0.1692 |
| Neut (%) | 42 ± 3.1 | 39.84 ± 2.77 | 35.53 ± 4.44 | 40.85 ± 3.12 | 0.2872 |
| Lymph (%) | 46.89 ± 5.03 | 54.21 ± 3 | 54.90 ± 5.94 | 53.86 ± 2.89 | 0.3526 |
| Mono (%) | 1.50 ± 0.29 | 1.64 ± 0.33 | 1.27 ± 0.21 | 1.50 ± 0.23 | 0.8528 |
| Eos (%) | 3.83 ± 0.56 | 3.64 ± 0.51 | 1.98 ± 0.49 | 2.12 ± 0.51 | 0.7528 |
| Baso (%) | 0.40 ± 0.04 | 0.4 ± 0.05 | 0.56 ± 0.08 | 0.57 ± 0.04 | 0.8875 |
| LUC (%) | 0.17 ± 0.02 | 0.25 ± 0.47 | 0.37 ± 0.10 | 0.43 ± 0.17 | 0.9850 |
| Parameter | Dry Goats Mean ± SEM | Milking Goats Mean ± SEM | P-Value | ||
|---|---|---|---|---|---|
| Control | Enriched | Control | Enriched | ||
| CK (U/L) | 151.45 ± 10.92 | 133.63 ± 10.92 | 155.25 ± 10.46 | 145.08 ± 10.46 | 0.7224 |
| Albumin (g/dL) | 3.30 ± 0.13 ab | 3.56 ± 0.16 ab | 3.70 ± 0.19 a | 3.10 ± 0.10 b | * 0.0325 |
| ALKP (U/L) | 344.45 ± 88.14 | 319.45 ± 83.04 | 192.66 ± 56.09 | 203.41 ± 54.4 | 0.8020 |
| ALT (U/L) | 16.50 ± 0.58 a | 14.34 ± 0.72 ab | 13.12 ± 0.33 b | 15.51 ± 0.44 ab | * 0.0030 |
| SuperAMY (U/L) | 17.1 ± 3.82 | 19.27 ± 3.58 | 18.66 ± 2.24 | 19.41 ± 2.40 | 0.8145 |
| AST (U/L) | 62.55 ± 3.51 | 57.66 ± 2.80 | 64.98 ± 2.70 | 66.4 ± 2.85 | 0.2810 |
| Total bile (mg/dL) | 0.03 ± 0.02 | 0.05 ± 0.01 | 0.04 ± 0.02 | 0.06 ± 0.02 | 0.9578 |
| Calcium (mg/dL) | 8.41 ± 0.15 | 8.57 ± 0.15 | 8.49 ± 0.16 | 8.15 ± 0.18 | 0.1380 |
| Cholesterol (mg/dL) | 85.93 ± 3.86 | 85.15 ± 4.34 | 92.73 ± 4.20 | 89.45 ± 4.80 | 0.7761 |
| Creatinine (mg/dL) | 0.74 ± 0.04 | 0.76 ± 0.04 | 0.78 ± 0.03 | 0.74 ± 0.04 | 0.5354 |
| GGT (U/L) | 51.63 ± 4.49 | 54.58 ± 4.3 | 60.08 ± 4.3 | 54.58 ± 4.30 | 0.3347 |
| Glucose (mg/dL) | 43.92 ± 2.85 | 38.07 ± 2.84 | 41 ± 3.16 | 44.25 ± 1.98 | 0.1061 |
| Phosphate (mg/dL) | 6.66 ± 0.45 | 6.36 ± 0.50 | 7.27 ± 0.31 | 7.68 ± 0.44 | 0.4285 |
| Total protein (g/dL) | 7.59 ± 0.12 | 7.70 ± 0.11 | 7.52 ± 0.08 | 7.41 ± 0.11 | 0.3289 |
| Triglycerides (g/dL) | 25.27 ± 3.22 | 24.78 ± 2.27 | 19.37 ± 2.53 | 24.98 ± 2.94 | 0.2795 |
| Urea (mg/dL) | 37.51 ± 3.53 | 37.56 ± 3.38 | 43.35 ± 2.01 | 46.17 ± 1.58 | 0.6087 |
| Sodium (mmol/L) | 146.36 ± 0.60 b | 148.5 ± 0.47 a | 149.75 ± 0.55 a | 146.4 ± 0.46 b | * <0.0001 |
| Potassium (mmol/L) | 4.89 ± 0.11 | 4.79 ± 0.10 | 4.92 ± 0.13 | 4.92 ± 0.11 | 0.3763 |
| Chloride (mmol/L) | 104.47 ± 0.54 b | 107.13 ± 0.93 a | 107.24 ± 0.97 a | 103.35 ± 0.66 b | * <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wein, Y.; Vaidenfeld, O.; Sabastian, C.; Bar Shira, E.; Mabjeesh, S.J.; Tagari, H.; Friedman, A. The Effect of Environmental Enrichment on Selected Physiological and Immunological Stress-Related Markers in Dairy Goats. Biology 2024, 13, 859. https://doi.org/10.3390/biology13110859
Wein Y, Vaidenfeld O, Sabastian C, Bar Shira E, Mabjeesh SJ, Tagari H, Friedman A. The Effect of Environmental Enrichment on Selected Physiological and Immunological Stress-Related Markers in Dairy Goats. Biology. 2024; 13(11):859. https://doi.org/10.3390/biology13110859
Chicago/Turabian StyleWein, Yossi, Ofri Vaidenfeld, Chris Sabastian, Enav Bar Shira, Sameer J. Mabjeesh, Haim Tagari, and Aharon Friedman. 2024. "The Effect of Environmental Enrichment on Selected Physiological and Immunological Stress-Related Markers in Dairy Goats" Biology 13, no. 11: 859. https://doi.org/10.3390/biology13110859
APA StyleWein, Y., Vaidenfeld, O., Sabastian, C., Bar Shira, E., Mabjeesh, S. J., Tagari, H., & Friedman, A. (2024). The Effect of Environmental Enrichment on Selected Physiological and Immunological Stress-Related Markers in Dairy Goats. Biology, 13(11), 859. https://doi.org/10.3390/biology13110859





