Exploring the Potent Roles of an Internally Translated Truncated Connexin-43 Isoform
Simple Summary
Abstract
1. Introduction
2. Cx43 Physiology
3. Cx43 Dysfunction and Pathogenesis
4. Role of Cx43 in the Heart
5. Internal Translation of Cx43
6. Role of GJA1-20k
7. Role of GJA1-20k in Cx43 Trafficking
8. Role of GJA1-20k in Cytoskeletal Remodeling
9. Role of GJA1-20k in Mitochondrial Homeostasis
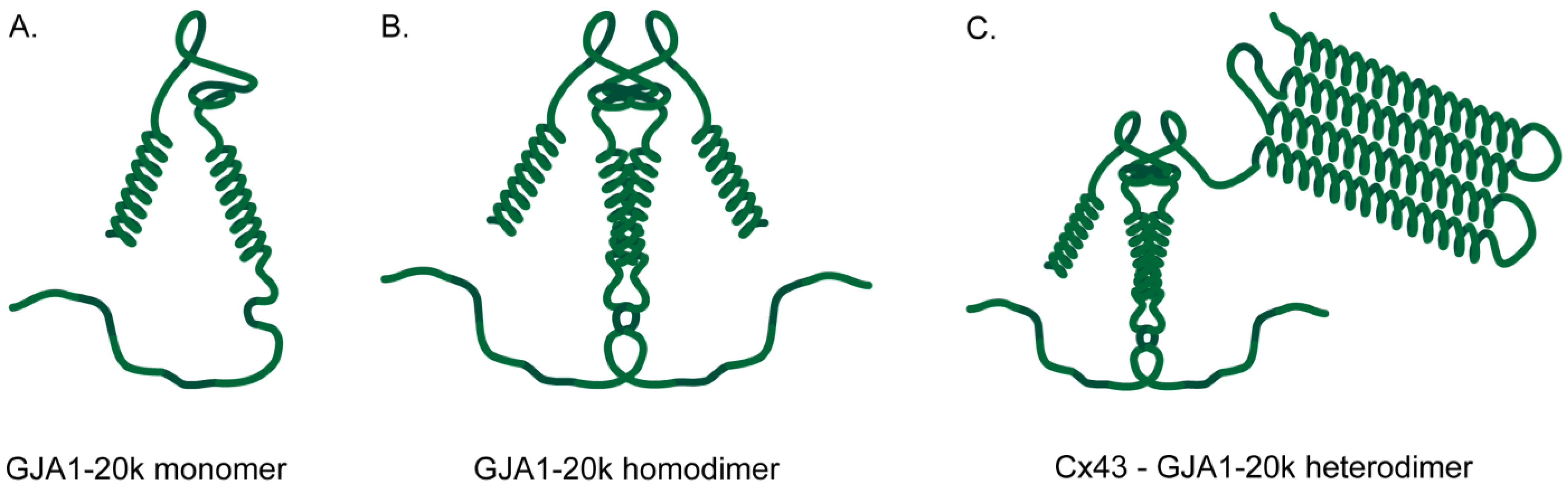
10. The Structure of GJA1-20k
11. Clinical Considerations
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basheer, W.; Shaw, R. The “Tail” of Connexin43: An Unexpected Journey from Alternative Translation to Trafficking. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Kardami, E.; Dang, X.; Iacobas, D.A.; Nickel, B.E.; Jeyaraman, M.; Srisakuldee, W.; Makazan, J.; Tanguy, S.; Spray, D.C. The Role of Connexins in Controlling Cell Growth and Gene Expression. Prog. Biophys. Mol. Biol. 2007, 94, 245–264. [Google Scholar] [CrossRef]
- Schulz, R.; Görge, P.M.; Görbe, A.; Ferdinandy, P.; Lampe, P.D.; Leybaert, L. Connexin 43 Is an Emerging Therapeutic Target in Ischemia/Reperfusion Injury, Cardioprotection and Neuroprotection. Pharmacol. Ther. 2015, 153, 90–106. [Google Scholar] [CrossRef]
- Evans, W.H.; Martin, P.E.M. Gap Junctions: Structure and Function (Review). Mol. Membr. Biol. 2002, 19, 121–136. [Google Scholar] [CrossRef]
- Grek, C.L.; Rhett, J.M.; Bruce, J.S.; Ghatnekar, G.S.; Yeh, E.S. Connexin 43, Breast Cancer Tumor Suppressor: Missed Connections? Cancer Lett. 2016, 374, 117–126. [Google Scholar] [CrossRef]
- Kresh, J.Y. Cell Replacement Therapy: The Functional Importance of Myocardial Architecture and Intercellular Gap-Junction Distribution. J. Thorac. Cardiovasc. Surg. 2006, 131, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- Martins-Marques, T.; Ribeiro-Rodrigues, T.; Batista-Almeida, D.; Aasen, T.; Kwak, B.R.; Girao, H. Biological Functions of Connexin43 Beyond Intercellular Communication. Trends Cell Biol. 2019, 29, 835–847. [Google Scholar] [CrossRef]
- Dbouk, H.A.; Mroue, R.M.; El-Sabban, M.E.; Talhouk, R.S. Connexins: A Myriad of Functions Extending beyond Assembly of Gap Junction Channels. Cell Commun. Signal 2009, 7, 4. [Google Scholar] [CrossRef]
- Xing, L.; Yang, T.; Cui, S.; Chen, G. Connexin Hemichannels in Astrocytes: Role in CNS Disorders. Front. Mol. Neurosci. 2019, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Calder, B.W.; Rhett, J.M.; Bainbridge, H.; Fann, S.A.; Gourdie, R.G.; Yost, M.J. Inhibition of Connexin 43 Hemichannel-Mediated ATP Release Attenuates Early Inflammation During the Foreign Body Response. Tissue Eng. Part A 2015, 21, 1752–1762. [Google Scholar] [CrossRef]
- Siller-Jackson, A.J.; Burra, S.; Gu, S.; Xia, X.; Bonewald, L.F.; Sprague, E.; Jiang, J.X. Adaptation of Connexin 43-Hemichannel Prostaglandin Release to Mechanical Loading. J. Biol. Chem. 2008, 283, 26374–26382. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, S.; Guida, L.; Zocchi, E.; Franco, L.; Flora, A.D. Connexin 43 Hemichannels Mediate Ca2+-Regulated Transmembrane NAD+ Fluxes in Intact Cells. FASEB J. 2001, 15, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Riquelme, M.A.; Gu, S.; Jiang, J.X. Connexin Hemichannels Mediate Glutathione Transport and Protect Lens Fiber Cells from Oxidative Stress. J. Cell Sci. 2018, 131, jcs212506. [Google Scholar] [CrossRef]
- Soares, A.R.; Martins-Marques, T.; Ribeiro-Rodrigues, T.; Ferreira, J.V.; Catarino, S.; Pinho, M.J.; Zuzarte, M.; Isabel Anjo, S.; Manadas, B.; Sluijter, J.P.G.; et al. Gap Junctional Protein Cx43 Is Involved in the Communication between Extracellular Vesicles and Mammalian Cells. Sci. Rep. 2015, 5, 13243. [Google Scholar] [CrossRef]
- Martins-Marques, T.; Pinho, M.J.; Zuzarte, M.; Oliveira, C.; Pereira, P.; Sluijter, J.P.G.; Gomes, C.; Girao, H. Presence of Cx43 in Extracellular Vesicles Reduces the Cardiotoxicity of the Anti-Tumour Therapeutic Approach with Doxorubicin. J. Extracell. Vesicles 2016, 5, 32538. [Google Scholar] [CrossRef] [PubMed]
- Gemel, J.; Kilkus, J.; Dawson, G.; Beyer, E.C. Connecting Exosomes and Connexins. Cancers 2019, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Rodrigues, T.M.; Martins-Marques, T.; Morel, S.; Kwak, B.R.; Girão, H. Role of Connexin 43 in Different Forms of Intercellular Communication—Gap Junctions, Extracellular Vesicles and Tunnelling Nanotubes. J. Cell Sci. 2017, 130, 3619–3630. [Google Scholar] [CrossRef] [PubMed]
- Martins-Marques, T.; Witschas, K.; Ribeiro, I.; Zuzarte, M.; Catarino, S.; Ribeiro-Rodrigues, T.; Caramelo, F.; Aasen, T.; Carreira, I.M.; Goncalves, L.; et al. Cx43 Can Form Functional Channels at the Nuclear Envelope and Modulate Gene Expression in Cardiac Cells. Open Biol. 2023, 13, 230258. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Dodoni, G.; Rodriguez-Sinovas, A.; Cabestrero, A.; Ruiz-Meana, M.; Gres, P.; Konietzka, I.; Lopez-Iglesias, C.; Garcia-Dorado, D.; Di Lisa, F.; et al. Connexin 43 in Cardiomyocyte Mitochondria and Its Increase by Ischemic Preconditioning. Cardiovasc. Res. 2005, 67, 234–244. [Google Scholar] [CrossRef]
- Varela-Eirin, M.; Varela-Vazquez, A.; Rodríguez-Candela Mateos, M.; Vila-Sanjurjo, A.; Fonseca, E.; Mascareñas, J.L.; Eugenio Vázquez, M.; Mayan, M.D. Recruitment of RNA Molecules by Connexin RNA-Binding Motifs: Implication in RNA and DNA Transport through Microvesicles and Exosomes. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Martins-Marques, T.; Costa, M.C.; Catarino, S.; Simoes, I.; Aasen, T.; Enguita, F.J.; Girao, H. Cx43-Mediated Sorting of miRNAs into Extracellular Vesicles. EMBO Rep. 2022, 23, e54312. [Google Scholar] [CrossRef]
- Miro-Casas, E.; Ruiz-Meana, M.; Agullo, E.; Stahlhofen, S.; Rodriguez-Sinovas, A.; Cabestrero, A.; Jorge, I.; Torre, I.; Vazquez, J.; Boengler, K.; et al. Connexin43 in Cardiomyocyte Mitochondria Contributes to Mitochondrial Potassium Uptake. Cardiovasc. Res. 2009, 83, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Basheer, W.A.; Harris, B.S.; Mentrup, H.L.; Abreha, M.; Thames, E.L.; Lea, J.B.; Swing, D.A.; Copeland, N.G.; Jenkins, N.A.; Price, R.L.; et al. Cardiomyocyte-Specific Overexpression of the Ubiquitin Ligase Wwp1 Contributes to Reduction in Connexin 43 and Arrhythmogenesis. J. Mol. Cell. Cardiol. 2015, 88, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Shimura, D.; Baum, R.; Hernandez, D.M.; Agvanian, S.; Nagaoka, Y.; Katsumata, M.; Lampe, P.D.; Kleber, A.G.; Hong, T.; et al. Auxiliary Trafficking Subunit GJA1-20k Protects Connexin-43 from Degradation and Limits Ventricular Arrhythmias. J. Clin. Investig. 2020, 130, 4858–4870. [Google Scholar] [CrossRef]
- Epifantseva, I.; Shaw, R.M. Intracellular Trafficking Pathways of Cx43 Gap Junction Channels. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2018, 1860, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Reaume, A.G.; de Sousa, P.A.; Kulkarni, S.; Langille, B.L.; Zhu, D.; Davies, T.C.; Juneja, S.C.; Kidder, G.M.; Rossant, J. Cardiac Malformation in Neonatal Mice Lacking Connexin43. Science 1995, 267, 1831–1834. [Google Scholar] [CrossRef]
- Liao, Y.; Day, K.H.; Damon, D.N.; Duling, B.R. Endothelial Cell-Specific Knockout of Connexin 43 Causes Hypotension and Bradycardia in Mice. Proc. Natl. Acad. Sci. USA 2001, 98, 9989–9994. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, Y.; Tao, C.; Shao, M.; Zhao, S.; Huang, W.; Yao, T.; Johnson, J.A.; Liu, T.; Cypess, A.M.; et al. Connexin 43 Mediates White Adipose Tissue Beiging by Facilitating the Propagation of Sympathetic Neuronal Signals. Cell Metab. 2016, 24, 420–433. [Google Scholar] [CrossRef]
- Hulsmans, M.; Clauss, S.; Xiao, L.; Aguirre, A.D.; King, K.R.; Hanley, A.; Hucker, W.J.; Wülfers, E.M.; Seemann, G.; Courties, G.; et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell 2017, 169, 510–522.e20. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Bellido, T. Beyond Gap Junctions: Connexin43 and Bone Cell Signaling. Bone 2013, 52, 157–166. [Google Scholar] [CrossRef]
- Watkins, M.; Grimston, S.K.; Norris, J.Y.; Guillotin, B.; Shaw, A.; Beniash, E.; Civitelli, R. Osteoblast Connexin43 Modulates Skeletal Architecture by Regulating Both Arms of Bone Remodeling. Mol. Biol. Cell 2011, 22, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Regan, C.P.; Manabe, I.; Owens, G.K.; Day, K.H.; Damon, D.N.; Duling, B.R. Smooth Muscle–Targeted Knockout of Connexin43 Enhances Neointimal Formation in Response to Vascular Injury. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1037–1042. [Google Scholar] [CrossRef]
- Strauss, R.E.; Gourdie, R.G. Cx43 and the Actin Cytoskeleton: Novel Roles and Implications for Cell-Cell Junction-Based Barrier Function Regulation. Biomolecules 2020, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Retamal, M.A.; Reyes, E.P.; García, I.E.; Pinto, B.; Martínez, A.D.; González, C. Diseases Associated with Leaky Hemichannels. Front. Cell Neurosci. 2015, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.P.; Ramachandran, J.; Himelman, E.; Badr, M.A.; Kang, C.; Nouet, J.; Fefelova, N.; Xie, L.-H.; Shirokova, N.; Contreras, J.E.; et al. Normalization of Connexin 43 Protein Levels Prevents Cellular and Functional Signs of Dystrophic Cardiomyopathy in Mice. Neuromuscul. Disord. 2018, 28, 361–372. [Google Scholar] [CrossRef]
- Michela, P.; Velia, V.; Aldo, P.; Ada, P. Role of Connexin 43 in Cardiovascular Diseases. Eur. J. Pharmacol. 2015, 768, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.M.; Rudy, Y. Ionic Mechanisms of Propagation in Cardiac Tissue. Roles of the Sodium and L-Type Calcium Currents during Reduced Excitability and Decreased Gap Junction Coupling. Circ. Res. 1997, 81, 727–741. [Google Scholar] [CrossRef]
- Akar, F.G.; Nass, R.D.; Hahn, S.; Cingolani, E.; Shah, M.; Hesketh, G.G.; DiSilvestre, D.; Tunin, R.S.; Kass, D.A.; Tomaselli, G.F. Dynamic Changes in Conduction Velocity and Gap Junction Properties during Development of Pacing-Induced Heart Failure. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H1223–H1230. [Google Scholar] [CrossRef]
- Beardslee, M.A.; Lerner, D.L.; Tadros, P.N.; Laing, J.G.; Beyer, E.C.; Yamada, K.A.; Kléber, A.G.; Schuessler, R.B.; Saffitz, J.E. Dephosphorylation and Intracellular Redistribution of Ventricular Connexin43 During Electrical Uncoupling Induced by Ischemia. Circ. Res. 2000, 87, 656–662. [Google Scholar] [CrossRef]
- Smith, J.H.; Green, C.R.; Peters, N.S.; Rothery, S.; Severs, N.J. Altered Patterns of Gap Junction Distribution in Ischemic Heart Disease. An Immunohistochemical Study of Human Myocardium Using Laser Scanning Confocal Microscopy. Am. J. Pathol. 1991, 139, 801–821. [Google Scholar]
- Saffitz, J.E.; Hames, K.Y.; Kanno, S. Remodeling of Gap Junctions in Ischemic and Nonischemic Forms of Heart Disease. J. Membr. Biol. 2007, 218, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Dupont, E.; Matsushita, T.; Kaba, R.A.; Vozzi, C.; Coppen, S.R.; Khan, N.; Kaprielian, R.; Yacoub, M.H.; Severs, N.J. Altered Connexin Expression in Human Congestive Heart Failure. J. Mol. Cell. Cardiol. 2001, 33, 359–371. [Google Scholar] [CrossRef]
- Thibodeau, I.L.; Xu, J.; Li, Q.; Liu, G.; Lam, K.; Veinot, J.P.; Birnie, D.H.; Jones, D.L.; Krahn, A.D.; Lemery, R.; et al. Paradigm of Genetic Mosaicism and Lone Atrial Fibrillation. Circulation 2010, 122, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Van Norstrand, D.W.; Asimaki, A.; Rubinos, C.; Dolmatova, E.; Srinivas, M.; Tester, D.J.; Saffitz, J.E.; Duffy, H.S.; Ackerman, M.J. Connexin43 Mutation Causes Heterogeneous Gap Junction Loss and Sudden Infant Death. Circulation 2012, 125, 474–481. [Google Scholar] [CrossRef]
- Ul-Hussain, M.; Olk, S.; Schoenebeck, B.; Wasielewski, B.; Meier, C.; Prochnow, N.; May, C.; Galozzi, S.; Marcus, K.; Zoidl, G.; et al. Internal Ribosomal Entry Site (IRES) Activity Generates Endogenous Carboxyl-Terminal Domains of Cx43 and Is Responsive to Hypoxic Conditions. J. Biol. Chem. 2014, 289, 20979–20990. [Google Scholar] [CrossRef] [PubMed]
- Salat-Canela, C.; Sesé, M.; Peula, C.; Ramón y Cajal, S.; Aasen, T. Internal Translation of the Connexin 43 Transcript. Cell Commun. Signal 2014, 12, 31. [Google Scholar] [CrossRef]
- Smyth, J.W.; Shaw, R.M. Autoregulation of Connexin43 Gap Junction Formation by Internally Translated Isoforms. Cell Rep. 2013, 5, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Joshi-Mukherjee, R.; Coombs, W.; Burrer, C.; de Mora, I.A.; Delmar, M.; Taffet, S.M. Evidence for the Presence of a Free C-Terminal Fragment of Cx43 in Cultured Cells. Cell Commun. Adhes. 2007, 14, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Basheer, W.A.; Fu, Y.; Shimura, D.; Xiao, S.; Agvanian, S.; Hernandez, D.M.; Hitzeman, T.C.; Hong, T.; Shaw, R.M. Stress Response Protein GJA1-20k Promotes Mitochondrial Biogenesis, Metabolic Quiescence, and Cardioprotection against Ischemia/Reperfusion Injury. JCI Insight 2018, 3, e121900. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Heinzel, F.R.; Boengler, K.; Schulz, R.; Heusch, G. Role of Connexin 43 in Ischemic Preconditioning Does Not Involve Intercellular Communication through Gap Junctions. J. Mol. Cell Cardiol. 2004, 36, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dorado, D.; Ruiz-Meana, M.; Padilla, F.; Rodriguez-Sinovas, A.; Mirabet, M. Gap Junction-Mediated Intercellular Communication in Ischemic Preconditioning. Cardiovasc. Res. 2002, 55, 456–465. [Google Scholar] [CrossRef]
- Padilla, F.; Garcia-Dorado, D.; Rodríguez-Sinovas, A.; Ruiz-Meana, M.; Inserte, J.; Soler-Soler, J. Protection Afforded by Ischemic Preconditioning Is Not Mediated by Effects on Cell-to-Cell Electrical Coupling during Myocardial Ischemia-Reperfusion. Am. J. Physiol.-Heart Circ. Physiol. 2003, 285, H1909–H1916. [Google Scholar] [CrossRef] [PubMed]
- Shimura, D.; Nuebel, E.; Baum, R.; Valdez, S.E.; Xiao, S.; Warren, J.S.; Palatinus, J.A.; Hong, T.; Rutter, J.; Shaw, R.M. Protective Mitochondrial Fission Induced by Stress-Responsive Protein GJA1-20k. eLife 2021, 10, e69207. [Google Scholar] [CrossRef]
- Leithe, E.; Mesnil, M.; Aasen, T. The Connexin 43 C-Terminus: A Tail of Many Tales. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2018, 1860, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Fontana, F.; Sugatani, T.; Castillo, I.P.; Leanza, G.; Coler-Reilly, A.; Civitelli, R. Connexin43 in Mesenchymal Lineage Cells Regulates Body Adiposity and Energy Metabolism in Mice. JCI Insight 2024, 9, e170016. [Google Scholar] [CrossRef] [PubMed]
- Basheer, W.A.; Xiao, S.; Epifantseva, I.; Fu, Y.; Kleber, A.G.; Hong, T.; Shaw, R.M. GJA1-20k Arranges Actin to Guide Cx43 Delivery to Cardiac Intercalated Discs. Circ. Res. 2017, 121, 1069–1080. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, S.-S.; Xiao, S.; Basheer, W.A.; Baum, R.; Epifantseva, I.; Hong, T.; Shaw, R.M. Cx43 Isoform GJA1-20k Promotes Microtubule Dependent Mitochondrial Transport. Front. Physiol. 2017, 8, 905. [Google Scholar] [CrossRef]
- Baum, R.; Nguyen, V.; Maalouf, M.; Shimura, D.; Waghalter, M.; Srapyan, S.; Jin, Q.; Kuzmanovich, L.; Gaffney, A.; Bell, B.; et al. A Truncated Isoform of Connexin43 Caps Actin to Organize Forward Delivery of Full Length Connexin43. J. Cell Biol. 2024; in press. [Google Scholar] [CrossRef]
- Shimura, D.; Shaw, R.M. GJA1-20k and Mitochondrial Dynamics. Front. Physiol. 2022, 13, 867358. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.M.; Fay, A.J.; Puthenveedu, M.A.; von Zastrow, M.; Jan, Y.-N.; Jan, L.Y. Microtubule Plus-End-Tracking Proteins Target Gap Junctions Directly from the Cell Interior to Adherens Junctions. Cell 2007, 128, 547–560. [Google Scholar] [CrossRef]
- Shimura, D.; Hunter, J.; Katsumata, M.; Shaw, R.M. Removal of an Internal Translational Start Site from mRNA While Retaining Expression of the Full-Length Protein. J. Vis. Exp. 2022, 181, e63405. [Google Scholar] [CrossRef]
- Frederick, R.L.; Shaw, J.M. Moving Mitochondria: Establishing Distribution of an Essential Organelle. Traffic 2007, 8, 1668–1675. [Google Scholar] [CrossRef]
- Wu, M.; Kalyanasundaram, A.; Zhu, J. Structural and Biomechanical Basis of Mitochondrial Movement in Eukaryotic Cells. Int. J. Nanomed. 2013, 8, 4033–4042. [Google Scholar] [CrossRef]
- Liu, Y.J.; McIntyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Mitochondrial Fission and Fusion: A Dynamic Role in Aging and Potential Target for Age-Related Disease. Mech. Ageing Dev. 2020, 186, 111212. [Google Scholar] [CrossRef]
- Adebayo, M.; Singh, S.; Singh, A.P.; Dasgupta, S. Mitochondrial Fusion and Fission: The Fine-Tune Balance for Cellular Homeostasis. FASEB J. 2021, 35, e21620. [Google Scholar] [CrossRef] [PubMed]
- Sorgen, P.L.; Duffy, H.S.; Spray, D.C.; Delmar, M. pH-Dependent Dimerization of the Carboxyl Terminal Domain of Cx43. Biophys. J. 2004, 87, 574–581. [Google Scholar] [CrossRef]
- Palatinus, J.A.; Valdez, S.; Taylor, L.; Whisenant, C.; Selzman, C.H.; Drakos, S.G.; Ranjan, R.; Hong, T.; Saffitz, J.E.; Shaw, R.M. GJA1-20k Rescues Cx43 Localization and Arrhythmias in Arrhythmogenic Cardiomyopathy. Circ. Res. 2023, 132, 744–746. [Google Scholar] [CrossRef]
- Ren, D.; Zheng, P.; Zou, S.; Gong, Y.; Wang, Y.; Duan, J.; Deng, J.; Chen, H.; Feng, J.; Zhong, C.; et al. GJA1-20K Enhances Mitochondria Transfer from Astrocytes to Neurons via Cx43-TnTs After Traumatic Brain Injury. Cell Mol. Neurobiol. 2022, 42, 1887–1895. [Google Scholar] [CrossRef]
- Nadeem, L.; Shynlova, O.; Mesiano, S.; Lye, S. Progesterone Via Its Type-A Receptor Promotes Myometrial Gap Junction Coupling. Sci. Rep. 2017, 7, 13357. [Google Scholar] [CrossRef]
- Lamiche, C.; Clarhaut, J.; Strale, P.-O.; Crespin, S.; Pedretti, N.; Bernard, F.-X.; Naus, C.C.; Chen, V.C.; Foster, L.J.; Defamie, N.; et al. The Gap Junction Protein Cx43 Is Involved in the Bone-Targeted Metastatic Behaviour of Human Prostate Cancer Cells. Clin. Exp. Metastasis 2012, 29, 111–122. [Google Scholar] [CrossRef]
- Boucher, J.; Balandre, A.-C.; Debant, M.; Vix, J.; Harnois, T.; Bourmeyster, N.; Péraudeau, E.; Chépied, A.; Clarhaut, J.; Debiais, F.; et al. Cx43 Present at the Leading Edge Membrane Governs Promigratory Effects of Osteoblast-Conditioned Medium on Human Prostate Cancer Cells in the Context of Bone Metastasis. Cancers 2020, 12, 3013. [Google Scholar] [CrossRef]
- Tishchenko, A.; Azorín, D.D.; Vidal-Brime, L.; Muñoz, M.J.; Arenas, P.J.; Pearce, C.; Girao, H.; Ramón y Cajal, S.; Aasen, T. Cx43 and Associated Cell Signaling Pathways Regulate Tunneling Nanotubes in Breast Cancer Cells. Cancers 2020, 12, 2798. [Google Scholar] [CrossRef]
- Whisenant, C.C.; Shaw, R.M. Internal Translation of Gja1 (Connexin43) to Produce GJA1-20k: Implications for Arrhythmia and Ischemic-Preconditioning. Front. Physiol. 2022, 13, 1058954. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maalouf, M.; Gaffney, A.T.; Bell, B.R.; Shaw, R.M. Exploring the Potent Roles of an Internally Translated Truncated Connexin-43 Isoform. Biology 2024, 13, 1046. https://doi.org/10.3390/biology13121046
Maalouf M, Gaffney AT, Bell BR, Shaw RM. Exploring the Potent Roles of an Internally Translated Truncated Connexin-43 Isoform. Biology. 2024; 13(12):1046. https://doi.org/10.3390/biology13121046
Chicago/Turabian StyleMaalouf, Mario, Adelaide T. Gaffney, Bridger R. Bell, and Robin M. Shaw. 2024. "Exploring the Potent Roles of an Internally Translated Truncated Connexin-43 Isoform" Biology 13, no. 12: 1046. https://doi.org/10.3390/biology13121046
APA StyleMaalouf, M., Gaffney, A. T., Bell, B. R., & Shaw, R. M. (2024). Exploring the Potent Roles of an Internally Translated Truncated Connexin-43 Isoform. Biology, 13(12), 1046. https://doi.org/10.3390/biology13121046





