Chemical Constituents and Their Bioactivities of Plants from the Genus Eupatorium (2015–Present)
Abstract
Simple Summary
Abstract
1. Introduction
2. Progress on Chemical Components and Their Biological Activities of the Genus Eupatorium
2.1. Chemical Components of E. adenophorum and Their Biological Activities
2.2. Chemical Components of E. chinense and Their Biological Activities
2.3. Chemical Components of E. fortunei and Their Biological Activities
2.4. Chemical Components of E. heterophyllum and Their Biological Activities
2.5. Chemical Components of E. lindleyanum and Their Biological Activities
2.6. Chemical Components of E. macrocephalum and Their Biological Activities
2.7. Chemical Components of E. obtusissmum and Their Biological Activities
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wright, C.W. Recent developments in research on terrestrial plants used for the treatment of malaria. Nat. Prod. Rep. 2010, 27, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.Y.; Qian, K.D.; Morris-Natschke, S.L.; Hsuc, C.S.; Lee, K.H. Recent discovery of plant-derived anti-diabetic natural products. Nat. Prod. Rep. 2012, 29, 580–606. [Google Scholar] [CrossRef] [PubMed]
- Tasneema, S.; Liu, B.; Li, B.; Choudharya, M.I.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Ma, T.T.; Deng, Z.H.; Gutiérrez-Gamboa, G.; Ge, Q.; Xu, P.K.; Zhang, Q.W.; Zhang, J.X.; Meng, J.F.; Reiter, R.J.; et al. Plant-derived melatonin from food: A gift of nature. Food Funct. 2021, 12, 2829–2849. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, N.; Miraei, H.R.; Amiri, A.; Ebrahimi, M.S.; Nahand, J.S.; Tarrahimofrad, H.; Hamblin, M.R.; Khan, H.; Mirzaei, H. Plant-based vaccines and cancer therapy: Where are we now and where are we going? Pharmacol. Res. 2021, 169, 105655. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.M.; Luo, Z.S.; Ban, Z.J.; Reiter, R.J.; Ma, Q.; Liang, Z.; Yang, M.Y.; Lie, X.H.; Li, L. Bioactive peptides of plant origin: Distribution, functionality, and evidence of benefits in food and health. Food Funct. 2022, 13, 3133–3158. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Marquez, L.; Crandall, W.J.; Risener, C.J.; Quave, C.L. Recent advances in the discovery of plant-derived antimicrobial natural products to combat antimicrobial resistant pathogens: Insights from 2018–2022. Nat. Prod. Rep. 2023, 40, 1271–1290. [Google Scholar] [CrossRef] [PubMed]
- Hui, Z.; Wen, H.; Zhu, J.L.; Deng, H.W.; Jiang, X.Y.; Ye, X.Y.; Wang, L.W.; Xie, T.; Bai, R.R. Discovery of plant-derived anti-tumor natural products: Potential leads for anti-tumor drug discovery. Bioorg. Chem. 2024, 142, 106957. [Google Scholar] [CrossRef] [PubMed]
- Shen, B. A new golden age of natural products drug discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef]
- Yang, Y.H.; Mao, J.W.; Tan, X.L. Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin. J. Nat. Med. 2020, 18, 890–897. [Google Scholar] [CrossRef]
- Gornstein, E.; Schwarz, T.L. The paradox of paclitaxel neurotoxicity: Mechanisms and unanswered questions. Neuropharmacology 2014, 76, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Howat, S.; Park, B.; Oh, S.; Jin, Y.W.; Lee, E.K.; Loake, G.J. Paclitaxel: Biosynthesis, production and future prospects. New Biotechnol. 2014, 31, 242–245. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Z.Y.; Liao, F.L.; Jiang, T.L.; Tu, Y.Y. The birth of artemisinin. Pharmacol. Therapeut. 2020, 216, 107658. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.R. Artemisinin anti-malarial drugs in China. Acta Pharm. Sin. B 2016, 6, 115–124. [Google Scholar] [CrossRef]
- Weathers, P.J. Artemisinin as a therapeutic vs. its more complex Artemisia source material. Nat. Prod. Rep. 2023, 40, 1158–1169. [Google Scholar] [CrossRef]
- Wang, X.Z.; Zhuang, Y.M.; Wang, Y.K.; Jiang, M.K.; Yao, L. The recent developments of camptothecin and its derivatives as potential anti-tumor agents. Eur. J. Med. Chem. 2023, 260, 115710. [Google Scholar] [CrossRef]
- Simran, G.; Ranabir, S.; Paramita, P.; Gouranga, N.; Tarun, K.D. An updated review on Eupatorium adenophorum Spreng. [Ageratina adenophora (Spreng.)]: Traditional uses, phytochemistry, pharmacological activities and toxicity. Pharmacol. Res. Mod. Chin. Med. 2022, 2, 100068. [Google Scholar]
- Semmler, F.W. Zur Kenntnis der Bestandteile ätherischer Öle (Zusammensetzung des Ayapana-öls. Berichte Dtsch. Chem. Ges. 1908, 41, 509–512. [Google Scholar] [CrossRef]
- Zhang, M.L.; Wu, M.; Zhang, J.J.; Irwin, D.; Gu, Y.C.; Shi, Q.W. Chemical Constituents of Plants from the Genus Eupatorium. Chem. Boodivers. 2008, 5, 40–54. [Google Scholar] [CrossRef]
- Liu, P.Y.; Liu, D.; Li, W.H.; Zhao, T.; Sauriol, F.; Gu, Y.C.; Shi, Q.W.; Zhang, M.L. Chemical constituents of plants from the genus Eupatorium (1904–2014). Chem. Boodivers. 2015, 12, 1482–1514. [Google Scholar] [CrossRef]
- Hill, R.A.; Sutherland, A. Hot off the press. Nat. Prod. Rep. 2023, 40, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Sutherland, A. Hot off the press. Nat. Prod. Rep. 2024, 41, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Luo, J.H.; Gu, W.J.; Zhang, J.J.; Yang, Y.X.; Yu, Y. Unusual 5/5 fused bicyclosesquiterpenoids from Eupatorium adenophorum. Fitoterapia 2023, 170, 105643. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Li, X.H.; Li, Z.X.; Zhu, X.X.; Zhang, L.; Zhou, X.L.; Xu, J.B.F.; Chen, Z.; Tang, P.; Gao, F. Adenophorone, an unprecedented sesquiterpene from Eupatorium adenophorum: Structural elucidation, bioinspired total synthesis and neuroprotective activity evaluation. Angew. Chem. Int. Ed. 2023, 62, e202306326. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Gu, W.J.; Luo, J.H.; Yang, Y.X.; Yu, Y. Eupatorione A, an unusual sesquiterpenoid from the aerial parts of Eupatorium adenophorum. Rec. Nat. Prod. 2023, 17, 1064–1068. [Google Scholar] [CrossRef]
- Gu, W.J.; Luo, J.H.; Yang, Y.X.; Geng, H. Identification of Diverse Sesquiterpenoids from Eupatorium adenophorum. Rec. Nat. Prod. 2024, 18, 237–277. [Google Scholar]
- Phan, M.G.; Dong, N.P.; Do, T.V.H.; Vu, M.T.; To, P.L.; Nguyen, N.V.; Tran, T.T.T.; Kawakami, S.; Otsuka, H. A new cadinane sesquiterpenoid from Eupatorium adenophorum and α-glycosidase and AChE inhibitory activities of a gossypetin acylglucoside. Med. Chem. Res. 2023, 32, 2168–2175. [Google Scholar] [CrossRef]
- Liang, X.; Yang, X.Z.; Zhou, T.X.; Ma, Y.R.; Peng, Y.; Bahetejiang, Y.L.; Li, Y.Z.; Yuan, J.Q. Three new cadinane-type sesquiterpenes from Eupatorium adenophorum spreng. Nat. Prod. Res. 2023, 36, 4898–4905. [Google Scholar] [CrossRef]
- Luo, J.H.; Gu, W.J.; Zhang, E.B.; Huang, Y.; Zhang, Y.H.; Yang, Y.X.; Geng, H. Three new cadinene-type sesquiterpenoids from the aerial parts of Ageratina adenophora. Nat. Prod. Res. 2023. [Google Scholar] [CrossRef]
- Liang, X.; Yang, X.Z.; Wu, C.Q.; Li, Y.Z.; Yuan, J.Q. A new cadinane-type sesquiterpenoid from Eupatorium adenophorum Spreng. Acta Pharm. Sin. 2020, 55, 2955–2959. [Google Scholar]
- Luo, B.; Dong, L.M.; Xu, Q.L.; Zhang, X.; Zhang, Q.; Liu, W.B.; Tan, J.W. A new monoterpene and a new sesquiterpene from the roots of Ageratina adenophora. Phytochem. Lett. 2018, 24, 67–70. [Google Scholar] [CrossRef]
- Zhang, M.; Ouyang, J.K.; Xu, Q.L.; Liu, S.B.; Qian, T.; Dong, L.M.; Tan, J.W. Thymol derivatives with antibacterial and cytotoxic activity from the aerial parts of Ageratina adenophora. RSC Adv. 2021, 11, 5755–5761. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.M.; Zhang, M.; Xu, Q.L.; Zhang, Q.; Luo, B.; Luo, Q.W.; Liu, W.B.; Tan, J.W. Two new thymol derivatives from the roots of Ageratina adenophora. Molecules 2017, 22, 592. [Google Scholar] [CrossRef]
- Zheng, G.W.; Luo, S.H.; Li, S.F.; Hua, J.; Li, W.Q.; Li, S.H. Specialized metabolites from Ageratina adenophora and their inhibitory activities against pathogenic fungi. Phytochemistry 2018, 148, 57–62. [Google Scholar] [CrossRef]
- Wang, C.F.; Yang, R.; Song, L.; Ning, B.M.; Ouyang, C.B.; Cao, A.C.; He, L. Two new highly-oxygenated flavonoid glycosides from Eupatorium adenophorum Spreng. Phytochem. Lett. 2016, 16, 245–248. [Google Scholar] [CrossRef]
- Wang, W.J.; Wang, L.; Liu, Z.; Jiang, R.W.; Liu, Z.W.; Li, M.M.; Zhang, Q.W.; Dai, Y.; Li, Y.L.; Zhang, X.Q.; et al. Antiviral benzofurans from Eupatorium chinense. Phytochemistry 2016, 122, 238–245. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Zhou, J.H.; Chen, Y.; Zhang, Z.M.; Liu, Z.X.; Guo, Z.Y.; Liu, C.X.; Zou, K. Seven new chemical constituents from the underground parts of Eupatorium chinense. Fitoterapia 2020, 146, 104674. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, L.S.; Zhou, C.X.; Mo, J.X.; Shen, S.N.; Zhang, T.; Li, J.; Lin, L.G.; Wu, R.H.; Gan, L.S. Alkyl-benzofuran dimers from Eupatorium chinense with insulin-sensitizing and anti-inflammatory activities. Bioorg. Chem. 2021, 113, 105030. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Wang, J.Q.; Yu, X.Q.; Deng, R.; Chen, Z.Y.; Liu, Z.X.; Liu, C.X.; He, H.B.; Zou, K. Chemical constituents of the roots of Eupatorium chinense and their anti-inflammatory activities. Phytochem. Lett. 2022, 48, 11–14. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Sun, Z.Y.; Feng, X.Y.; Chen, R.J.; Deng, W.; Tang, Y.L.; Guo, Z.Y.; Liu, C.X.; Chen, J.F.; Zou, K. Thymol derivatives from the roots of Eupatorium chinense and their cytotoxic activities. Phytochem. Lett. 2019, 29, 165–168. [Google Scholar] [CrossRef]
- Chen, Z.A.; Ke, C.Q.; Zhou, S.Z.; Feng, L.; Tang, C.P.; Ye, Y. Ten undescribed cadinane-type sesquiterpenoids from Eupatorium chinense. Fitoterapia 2022, 156, 105091. [Google Scholar] [CrossRef]
- Yu, X.Q.; Zhang, Q.Q.; Yan, W.H.; Wang, L.; Guo, Z.Y.; Yang, Q.Q.; Liu, C.X.; He, H.B.; Zou, K. Three new terpenoids from the Eupatorium chinense. Phytochem. Lett. 2017, 20, 224–227. [Google Scholar] [CrossRef]
- Yu, X.Q.; Zhang, J.J.; Tian, L.; Guo, Z.Y.; Liu, C.X.; Chen, J.F.; Ebrahim, W.; Liu, Z.; Proksch, P.; Zou, K. Germacrane-type sesquiterpenoids with antiproliferative activities from Eupatorium chinense. J. Nat. Prod. 2018, 81, 85–91. [Google Scholar] [CrossRef]
- Phan, M.G.; Vu, M.T.; Do, T.V.H.; Kawakami, S.; Otsuka, H. Thymol Derivatives from Eupatorium fortune. Rec. Nat. Prod. 2019, 13, 434–439. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.G.; Shi, R.R.; Zhang, D.M.; Li, C.J.; Shi, J. New thymol and isothymol derivatives from Eupatorium fortunei and their cytotoxic effects. Bioorg. Chem. 2020, 98, 103644. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Dai, Y.P.; Yuan, M.; Sun, X.M.; Song, C.J.; Liu, Y.G. Two new thymol derivatives from Eupatorium fortunei. Nat. Prod. Res. 2024, 38, 386–392. [Google Scholar] [CrossRef]
- Nguyen-Ngoc, H.; Trang, B.T.T.; Thu, D.T.H.; Nguyen, H.T.; Hoang, V.D.; Tran, Q.D.; Nguyen, T.N.; Quang, D.N.; Pham, G.N.; Dang, Q.L. Characterization of thymol derivatives from Eupatorium fortunei Turcz. aerial parts. Nat. Prod. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.P.; Pham, H.D.; Dinh, K.D.; Thi, T.D.; Thi, P.Q.L.; Quang, D.N.; Dat, N.T. Anticyanobacterial phenolic constituents from the aerial parts of Eupatorium fortunei Turcz. Nat. Prod. Res. 2019, 33, 1345–1348. [Google Scholar]
- Miao, L.; Wang, S.T.; Wei, Q.H.; Ma, R.F.; Zhang, H. Bioactive monoterpenoids and acetophenones from the aerial parts of Eupatorium fortunei. Phytochemistry 2024, 219, 113984. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Wu, S.M.; Hsu, K.C.; Huang, W.J.; Chen, J.J. Dibenzofuran, 4-chromanone, acetophenone, and dithiecine derivatives: Cytotoxic constituents from Eupatorium fortunei. Int. J. Mol. Sci. 2021, 22, 7448. [Google Scholar] [CrossRef]
- Shi, J.; Yuan, M.; Yu, Y.; Shi, S.B.; Liu, Y.G. Chiral resolution, absolute configuration of two pairs of unusual monoterpene enantiomers from Eupatorium fortunei. Tetraheron. Lett. 2020, 61, 151655. [Google Scholar] [CrossRef]
- Miao, L.; Wei, Q.H.; Wang, S.T.; Sun, P.; Zhang, H. Chemical constituents from Eupatorium fortunei and their anti-inflammatory evaluation by in silico and experimental approaches. Fitoterapia 2023, 171, 105700. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.M.; Saito, Y.; Matsuo, Y.; Gong, X.; Tanaka, T. New benzofuran oligomers from the roots of Eupatorium heterophyllum collected in China. Molecules 2022, 27, 8856. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.M.; Saito, Y.; Gong, X.; Matsuo, Y.; Tanaka, T. Dihydrobenzofurans and propynylthiophenes from the roots of Eupatorium heterophyllum. Nat. Prod. Commun. 2022, 17, 1–9. [Google Scholar] [CrossRef]
- Hu, Y.M.; Saito, Y.; Matsuo, Y.; Gong, X.; Tanaka, T. Two new dimeric benzofuran diastereomers from the roots of Eupatorium heterophyllum. Tetrahedron Lett. 2022, 102, 153924. [Google Scholar] [CrossRef]
- Hu, Y.M.; Saito, Y.; Okamoto, Y.; Matsuo, Y.; Gong, X.; Tanaka, T. Chemical compositions of Eupatorium heterophyllum leaf samples from Yunnan and Sichuan provinces of China-Isolation of 13 new sesquiterpene lactones. Molecules 2023, 28, 5107. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhong, H.H.; Fang, S.Q.; Zheng, Y.F.; Li, C.Y.; Peng, G.P.; Shen, X.C. Potential anti-inflammatory sesquiterpene lactones from Eupatorium lindleyanum. Planta Med. 2015, 81, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guo, W.X.; Huo, X.Y.; Hu, Y.J.; Zhou, L.Y.; Xie, X.F.; Pei, J.; Deng, Y.; Xiao, B.; Liu, D.; et al. Eupalinolide N, a previously undescribed sesquiterpene lactone with anti-inflammatory activity from Eupatorium lindleyanum. Rec. Nat. Prod. 2023, 17, 529–535. [Google Scholar]
- Yang, B.; Shen, J.W.; Zhou, D.H.; Zhao, Y.P.; Wang, W.Q.; Zhu, Y.; Zhao, H.J. Precise discovery of a STAT3 inhibitor from Eupatorium lindleyanum and evaluation of its activity of anti-triple negative breast cancer. Nat. Prod. Res. 2019, 33, 477–485. [Google Scholar] [CrossRef]
- Pereira Cabral, M.R.; Cecchetto, M.; Batista, J.M., Jr.; Batista, A.N.L.; Foglio, M.A.; Tasca Gois Ruiz, A.L.; Barrotto do Carmo, M.R.; Ferreira da Costa, W.; Baldoqui, D.C.; Sarragiotto, M.H. Cytotoxic sesquiterpene lactones from Campuloclinium macrocephalum (=Eupatorium macrocephalum). Phytochemistry 2020, 179, 112469. [Google Scholar] [CrossRef]
- Castillo, Q.A.; Triana, J.; Eiroa, J.L.; Calcul, L.; Rivera, E.; Wojtas, L.; Padron, J.M.; Boberieth, L.; Keramane, M.; Abel-Santos, E.; et al. ent-Labdane diterpenoids from the aerial parts of Eupatorium obtusissmum. J. Nat. Prod. 2016, 79, 907–913. [Google Scholar] [CrossRef] [PubMed]
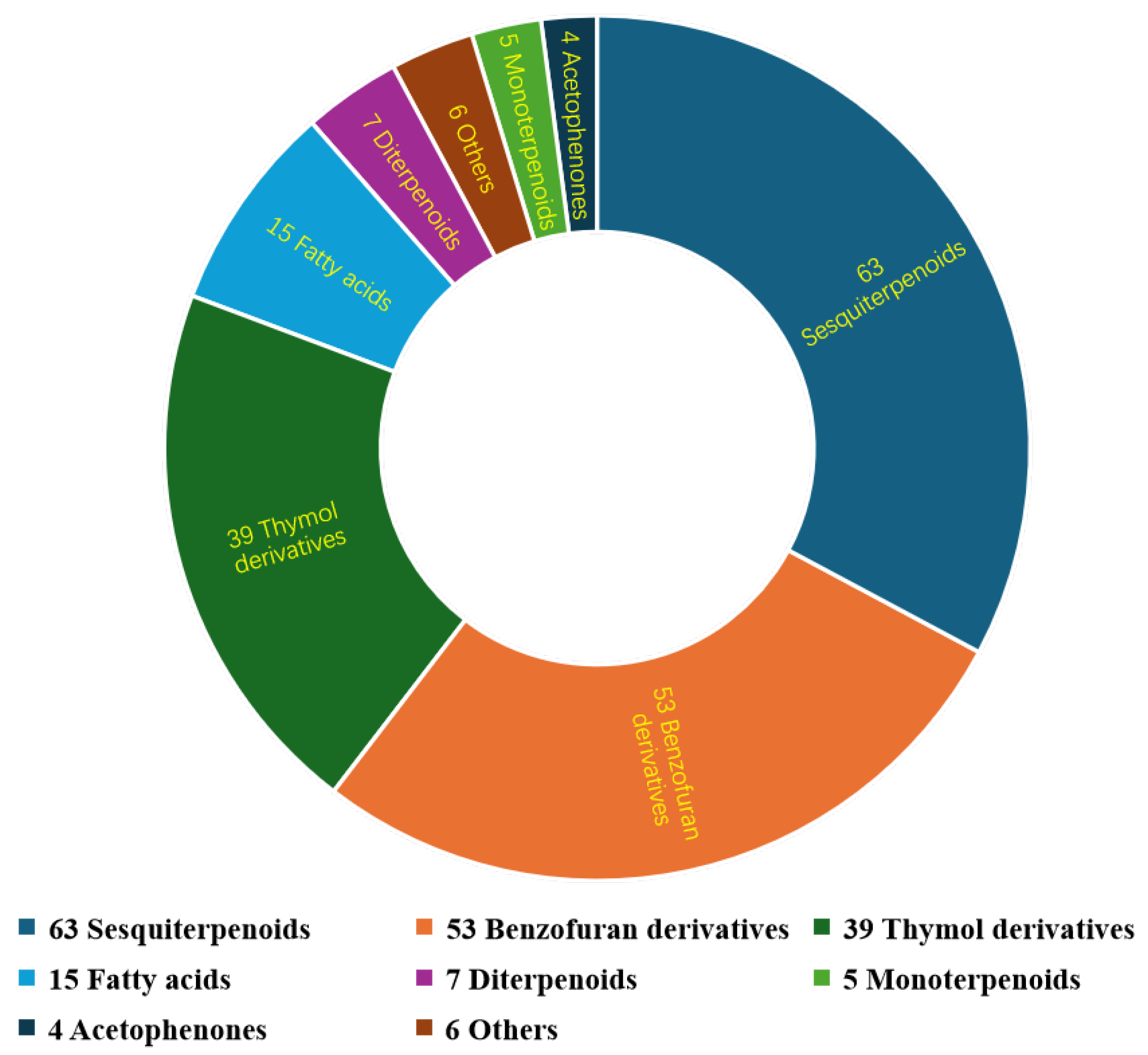



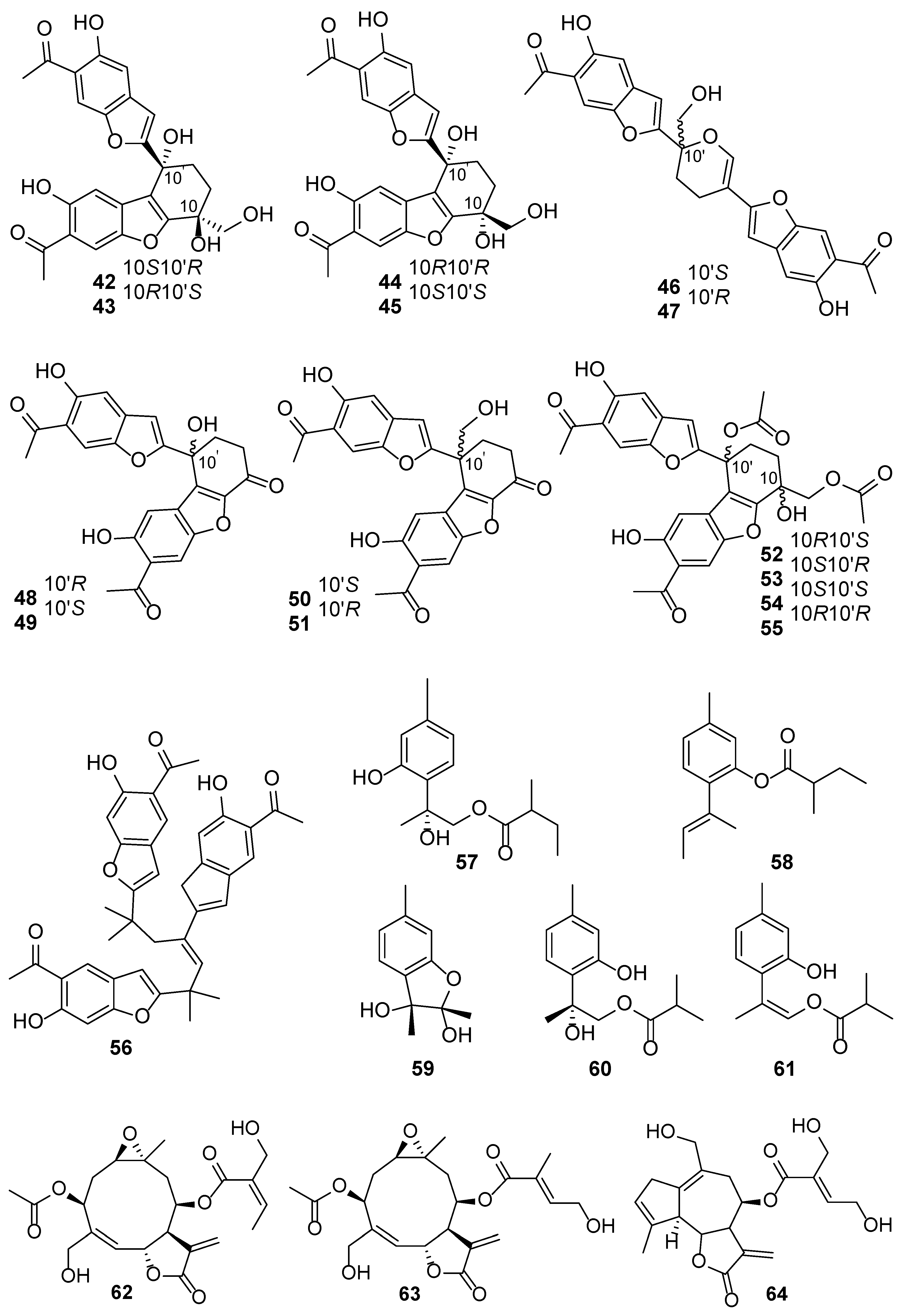
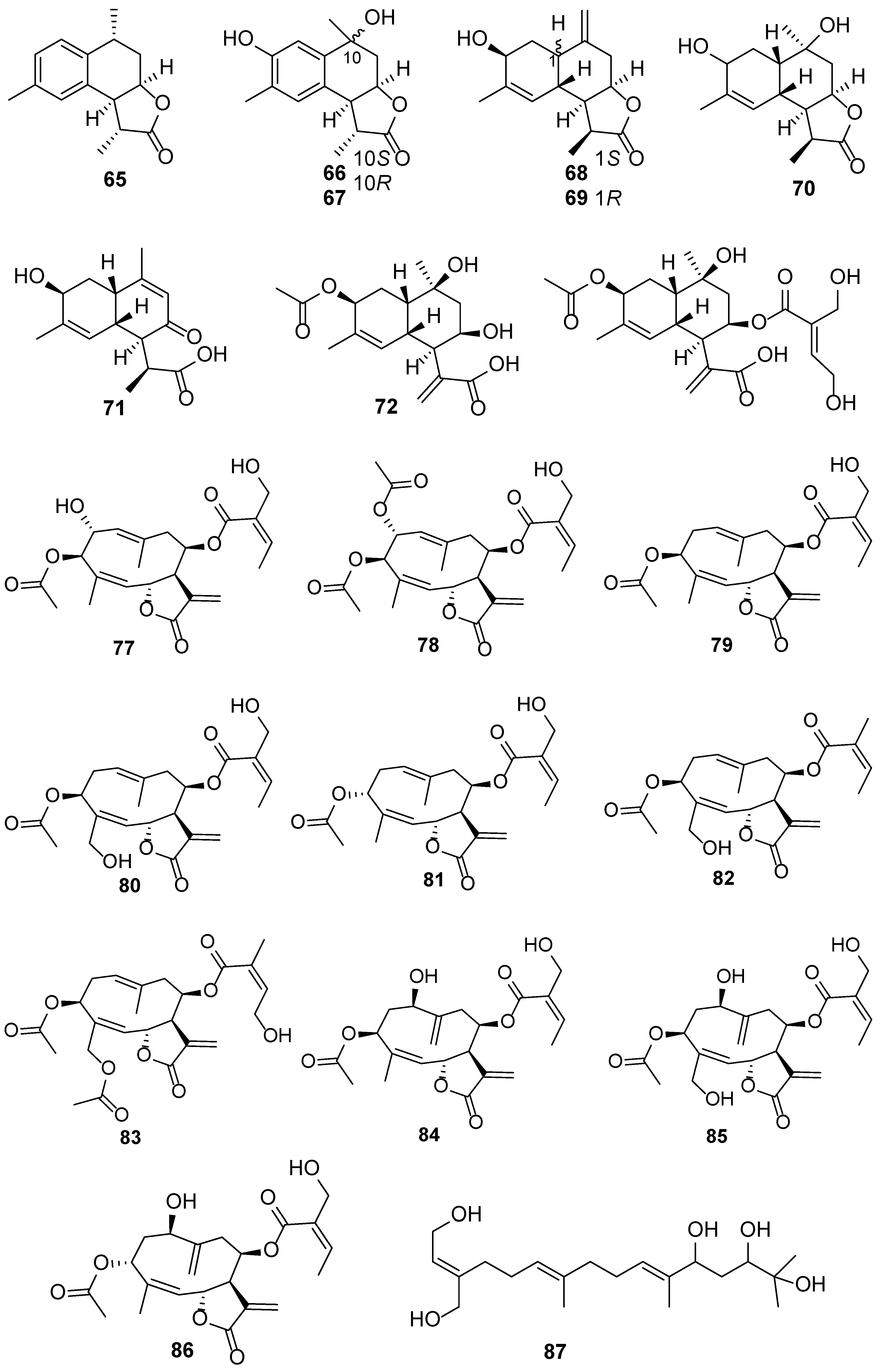




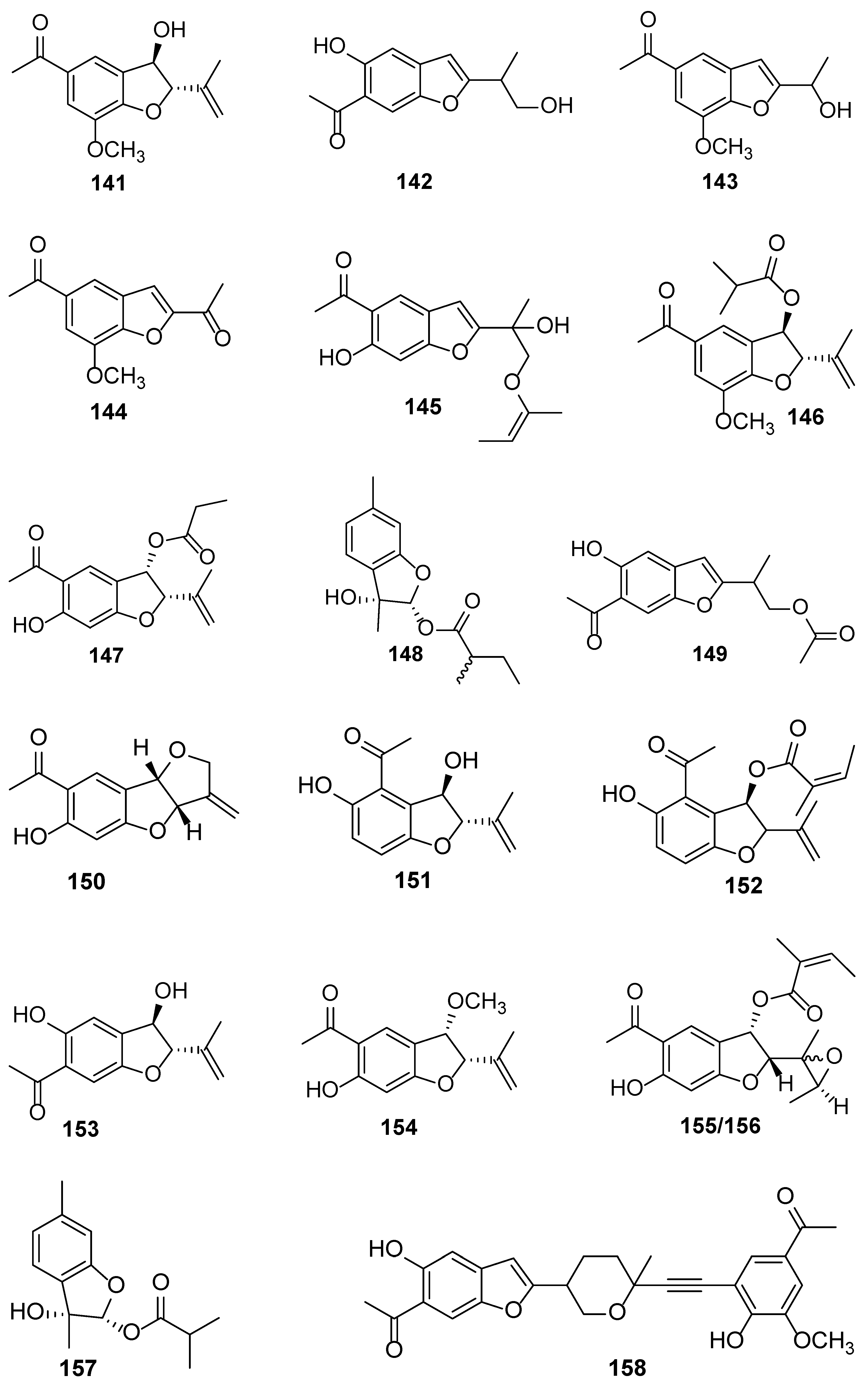
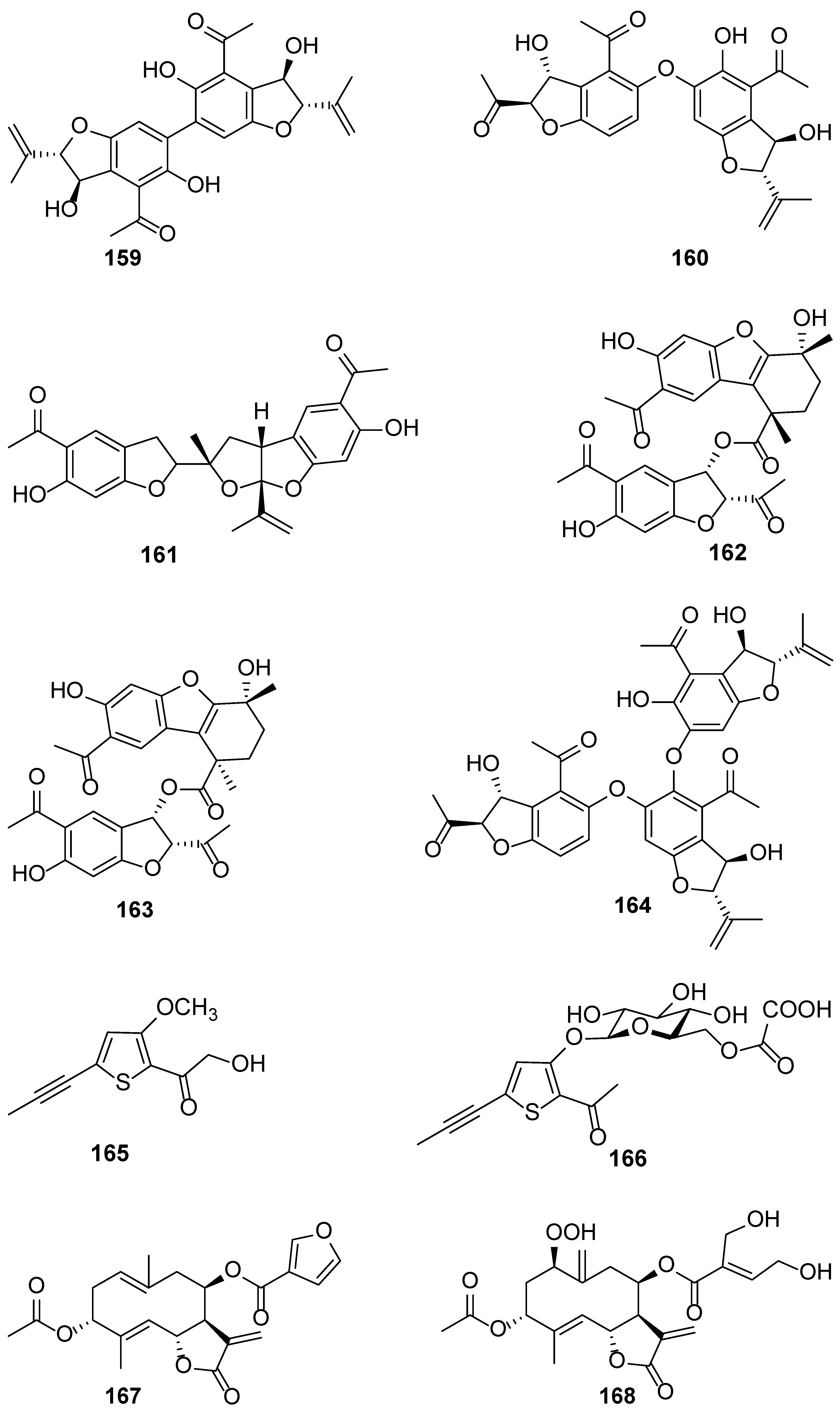
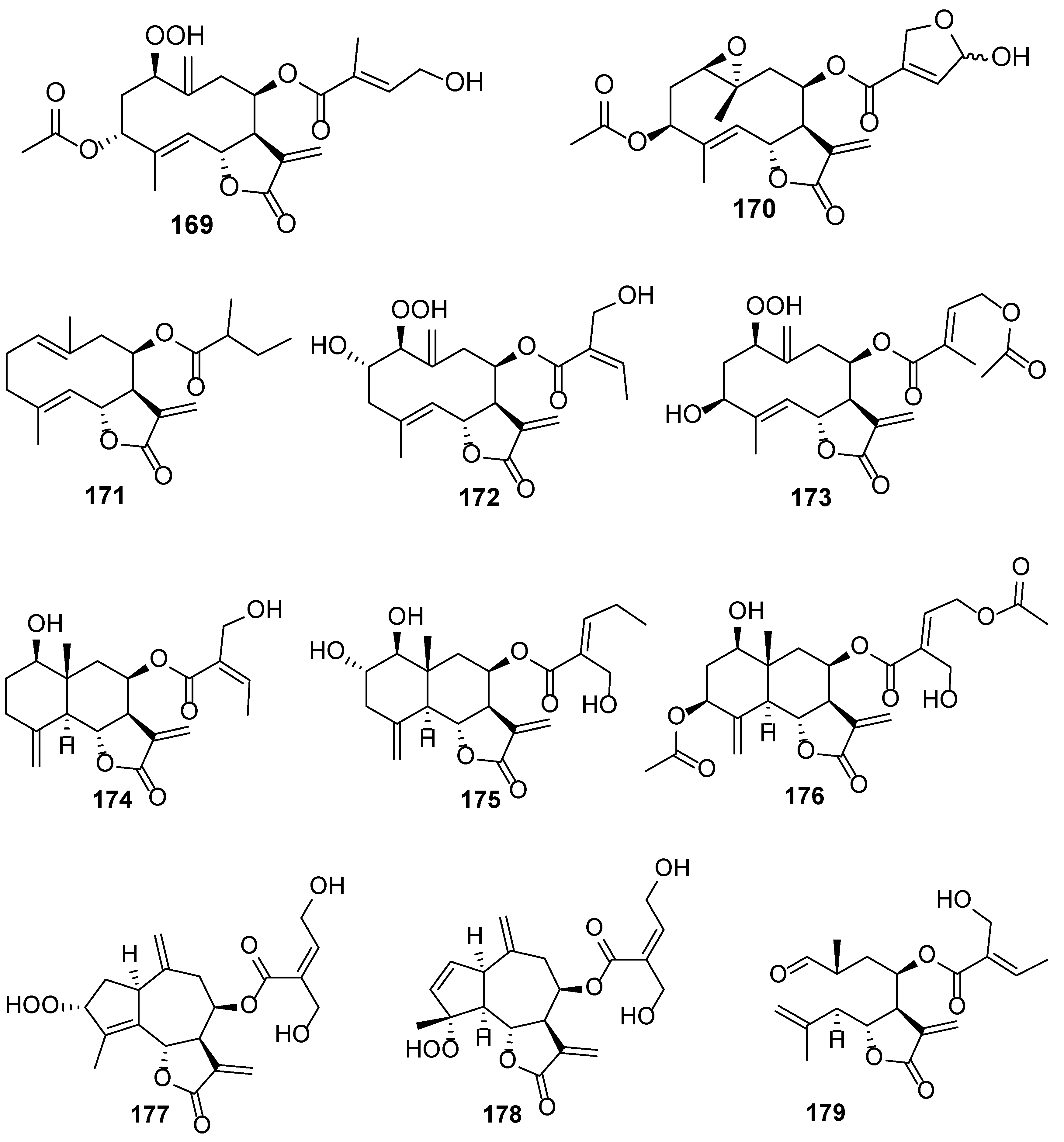

| No. | Plant Source | Compound Name | Structure Classification | Extraction Method | Type of Bioactivity Evaluation | Ref. |
|---|---|---|---|---|---|---|
| 1 | E. adenophorum | Eupatorid A | Sesquiterpenoid | Petroleum ether at room temperature | Anti-inflammatory, antibacterial, and cytotoxic | [23] |
| 2 | E. adenophorum | Eupatorester A | Sesquiterpenoid | Petroleum ether at room temperature | Anti-inflammatory, antibacterial, and cytotoxic | [23] |
| 3 | E. adenophorum | Eupatorester B | Sesquiterpenoid | Petroleum ether at room temperature | Anti-inflammatory, antibacterial, and cytotoxic | [23] |
| 4 | E. adenophorum | Eupatorester C | Sesquiterpenoid | Petroleum ether at room temperature | Anti-inflammatory, antibacterial, and cytotoxic | [23] |
| 5 | E. adenophorum | Adenophorone | Sesquiterpenoid | Reflux with ethyl acetate | Neuroprotective | [24] |
| 6 | E. adenophorum | Eupatorione A | Sesquiterpenoid | Petroleum ether at room temperature | Anti-inflammatory | [25] |
| 7 | E. adenophorum | Dihyroeupatorid A | Sesquiterpenoid | Petroleum ether at room temperature | Anti-inflammatory and cytotoxic | [26] |
| 8 | E. adenophorum | (5S, 6S, 7R, 9R, 10S)-7-Hydroxyageraphorone | Sesquiterpenoid | Petroleum ether at room temperature | Anti-inflammatory and cytotoxic | [26] |
| 9 | E. adenophorum | Adenophorone | Sesquiterpenoid | Methanol at room temperature | α-glycosidase and AChE inhibitory | [27] |
| 10 | E. adenophorum | Eupatorinone A | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic and antidiabetic | [28] |
| 11 | E. adenophorum | Eupatorinone B | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic and antidiabetic | [28] |
| 12 | E. adenophorum | Eupatorinone C | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic and antidiabetic | [28] |
| 13 | E. adenophorum | Ageratinone A | Sesquiterpenoid | Petroleum ether at room temperature | Cytotoxic | [29] |
| 14 | E. adenophorum | Ageratinone B | Sesquiterpenoid | Petroleum ether at room temperature | Cytotoxic | [29] |
| 15 | E. adenophorum | Ageratinone C | Sesquiterpenoid | Petroleum ether at room temperature | Cytotoxic | [29] |
| 16 | E. adenophorum | Eupatorinol | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [30] |
| 17 | E. adenophorum | 1,6-Dihydroxy-1-isopropyl-4,7-dimethyl-3,4- dihydronaphthalen-2(1H)-one | Sesquiterpenoid | 95% ethanol at room temperature | Antibacterial | [31] |
| 18 | E. adenophorum | 2α-Methoxyl-3β-methyl-6-(acetyl-O-methyl)-2,3-dihydrobenzofuran | Thymol | 95% ethanol at room temperature | Antibacterial | [31] |
| 19 | E. adenophorum | 7-Formyl-9-isobutyryloxy-8-hydroxythymol | Thymol | 95% ethanol at room temperature | Antibacterial and cytotoxic | [32] |
| 20 | E. adenophorum | 7,9-Di-isobutyryloxy-8,10-dehydrothymol | Thymol | 95% ethanol at room temperature | Antibacterial and cytotoxic | [32] |
| 21 | E. adenophorum | 2a-Methoxyl-3b-methyl-6-methylol-2,3-dihydrobenzofuran | Thymol | 95% ethanol at room temperature | Antibacterial and cytotoxic | [32] |
| 22 | E. adenophorum | 7,9-Diisobutyryloxy-8-ethoxythymol | Thymol | 95% ethanol at room temperature | Antibacterial and cytotoxic | [33] |
| 23 | E. adenophorum | 7-Acetoxy-8-methoxy-9-isobutyryloxythymol | Thymol | 95% ethanol at room temperature | Antibacterial and cytotoxic | [33] |
| 24 | E. adenophorum | 7-Hydroxy-dehydrotremetone | Benzofuran | Methanol at room temperature | Antipathogenic fungi | [34] |
| 25 | E. adenophorum | 7,10,11-Trihydroxy-dehydrotremetone | Benzofuran | Methanol at room temperature | Antipathogenic fungi | [34] |
| 26 | E. adenophorum | 10-oxo-7-Hydroxy-nordehydrotremetone | Benzofuran | Methanol at room temperature | Antipathogenic fungi | [34] |
| 27 | E. adenophorum | 5-β-Glucosyl-7-demethoxy-encecalin | Chromene | Methanol at room temperature | Antipathogenic fungi | [34] |
| 28 | E. adenophorum | 8-Hydroxy-8-β-glucosyl-2-carene | Monoterpenoid | Methanol at room temperature | Antipathogenic fungi | [34] |
| 29 | E. adenophorum | Gossypetin-5-O-(6″-(E)-caffeoyl)-β-D-glucoside | Flavonoid | Reflux with 70% ethanol | Cytotoxic and antiradical | [35] |
| 30 | E. adenophorum | Herbacetin-5-O-(6″-(E)-caffeoyl)-β-D-glucoside | Flavonoid | Reflux with 70% ethanol | Cytotoxic and antiradical | [35] |
| No. | Plant Source | Compound Name | Structure Classification | Extraction Method | Type of Bioactivity Evaluation | Ref. |
|---|---|---|---|---|---|---|
| 31 | E. chinense | (+)-Dieupachinin A | Benzofuran | Reflux with 70% ethanol | Antiviral | [36] |
| 32 | E. chinense | (−)-Dieupachinin A | Benzofuran | Reflux with 70% ethanol | Antiviral | [36] |
| 33 | E. chinense | (+)-Dieupachinin B | Benzofuran | Reflux with 70% ethanol | Antiviral | [36] |
| 34 | E. chinense | (−)-Dieupachinin B | Benzofuran | Reflux with 70% ethanol | Antiviral | [36] |
| 35 | E. chinense | (+)-Dieupachinin C | Benzofuran | Reflux with 70% ethanol | Antiviral | [36] |
| 36 | E. chinense | (−)-Dieupachinin C | Benzofuran | Reflux with 70% ethanol | Antiviral | [36] |
| 37 | E. chinense | (+)-Dieupachinin D | Benzofuran | Reflux with 70% ethanol | Antiviral | [36] |
| 38 | E. chinense | (−)-Dieupachinin D | Benzofuran | Reflux with 70% ethanol | Antiviral | [36] |
| 39 | E. chinense | (+)-Dieupachinin E | Benzofuran | Reflux with 70% ethanol | Antiviral | [36] |
| 40 | E. chinense | (−)-Dieupachinin E | Benzofuran | Reflux with 70% ethanol | Antiviral | [36] |
| 41 | E. chinense | Dieupachinin F | Benzofuran | Reflux with 70% ethanol | Antiviral | [36] |
| 42 | E. chinense | (+)-Dieupachinin G | Benzofuran | 95% ethanol at room temperature | Cytotoxic | [37] |
| 43 | E. chinense | (−)-Dieupachinin G | Benzofuran | 95% ethanol at room temperature | Cytotoxic | [37] |
| 44 | E. chinense | (+)-Dieupachinin H | Benzofuran | 95% ethanol at room temperature | Cytotoxic | [37] |
| 45 | E. chinense | (−)-Dieupachinin H | Benzofuran | 95% ethanol at room temperature | Cytotoxic | [37] |
| 46 | E. chinense | (+)-Dieupachinin I | Benzofuran | 95% ethanol at room temperature | Anti-inflammatory | [38] |
| 47 | E. chinense | (−)-Dieupachinin I | Benzofuran | 95% ethanol at room temperature | Anti-inflammatory | [38] |
| 48 | E. chinense | (+)-Dieupachinin J | Benzofuran | 95% ethanol at room temperature | Anti-inflammatory | [38] |
| 49 | E. chinense | (−)-Dieupachinin J | Benzofuran | 95% ethanol at room temperature | Anti-inflammatory | [38] |
| 50 | E. chinense | (+)-Dieupachinin K | Benzofuran | 95% ethanol at room temperature | Anti-inflammatory | [38] |
| 51 | E. chinense | (−)-Dieupachinin K | Benzofuran | 95% ethanol at room temperature | Anti-inflammatory | [38] |
| 52 | E. chinense | (+)-Dieupachinin L | Benzofuran | 95% ethanol at room temperature | Anti-inflammatory | [38] |
| 53 | E. chinense | (−)-Dieupachinin L | Benzofuran | 95% ethanol at room temperature | Anti-inflammatory | [38] |
| 54 | E. chinense | (+)-Dieupachinin M | Benzofuran | 95% ethanol at room temperature | Anti-inflammatory | [38] |
| 55 | E. chinense | (−)-Dieupachinin M | Benzofuran | 95% ethanol at room temperature | Anti-inflammatory | [38] |
| 56 | E. chinense | Trieupachinin A | Benzofuran | Reflux with 70% ethanol | Antiviral | [36] |
| 57 | E. chinense | 8R-hydroxy-9-methyl-butyryloxythymol | Thymol | 95% ethanol at room temperature | Cytotoxic and anti-inflammatory | [39] |
| 58 | E. chinense | 10-isobutyryloxy-8, 9-didehydrothymyl-isobutyrate | Thymol | 95% ethanol at room temperature | Cytotoxic and anti-inflammatory | [39] |
| 59 | E. chinense | (8R, 9S)-1, 8-dimethyl-8, 9-dihydro benzofuran-8, 9-diol | Thymol | 95% ethanol at room temperature | Cytotoxic and anti-inflammatory | [39] |
| 60 | E. chinense | 8R-hydroxy-9-isobutyryloxythymol | Thymol | 95% ethanol at room temperature | Cytotoxic | [40] |
| 61 | E. chinense | (Z)-8(9)-ene-9-isobutyryloxythymol | Thymol | 95% ethanol at room temperature | Cytotoxic | [40] |
| 62 | E. chinense | Eupachinsin E | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [37] |
| 63 | E. chinense | Eupachinsin F | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [37] |
| 64 | E. chinense | 14-Deacetylguaiaglehnin A | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [37] |
| 65 | E. chinense | Eupatorinolide A | Sesquiterpenoid | 95% ethanol at room temperature | None | [41] |
| 66 | E. chinense | Eupatorinolide B | Sesquiterpenoid | 95% ethanol at room temperature | None | [41] |
| 67 | E. chinense | Eupatorinolide C | Sesquiterpenoid | 95% ethanol at room temperature | None | [41] |
| 68 | E. chinense | Eupatorinolide D | Sesquiterpenoid | 95% ethanol at room temperature | None | [41] |
| 69 | E. chinense | Eupatorinolide E | Sesquiterpenoid | 95% ethanol at room temperature | None | [41] |
| 70 | E. chinense | Eupatorinolide F | Sesquiterpenoid | 95% ethanol at room temperature | None | [41] |
| 71 | E. chinense | Eupatorinic acid A | Sesquiterpenoid | 95% ethanol at room temperature | None | [41] |
| 72 | E. chinense | Eupatorinic acid B | Sesquiterpenoid | 95% ethanol at room temperature | None | [41] |
| 73 | E. chinense | Eupatorinic acid C | Sesquiterpenoid | 95% ethanol at room temperature | None | [41] |
| 74 | E. chinense | Eupatorinic acid D | Sesquiterpenoid | 95% ethanol at room temperature | None | [41] |
| 75 | E. chinense | Eupaguaiane A | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [42] |
| 76 | E. chinense | Eupaguaiane B | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [42] |
| 77 | E. chinense | Eupachinsin A | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [43] |
| 78 | E. chinense | Eupachinisin A 2-acetate | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [43] |
| 79 | E. chinense | Eupachinsin B | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [43] |
| 80 | E. chinense | 3-Epi-eupachinisin B | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [43] |
| 81 | E. chinense | 15-Hydroxyeupachinisin B | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [43] |
| 82 | E. chinense | Eupachinsin C | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [43] |
| 83 | E. chinense | 4′-Hydroxyeupachinisin C 15-acetate | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [43] |
| 84 | E. chinense | Eupachinsin D | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [43] |
| 85 | E. chinense | 15-Hydroxyeupachinisin D | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [43] |
| 86 | E. chinense | 3-Epi-eupachinisin D | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [43] |
| 87 | E. chinense | Eupaditerpenoid A | Diterpenoid | 95% ethanol at room temperature | Cytotoxic | [42] |
| No. | Plant Source | Compound Name | Structure Classification | Extraction Method | Type of Bioactivity Evaluation | Ref. |
|---|---|---|---|---|---|---|
| 88 | E. fortunei | 9-O-Angeloxy-10-hydroxy-8-methoxythymol | Thymol | Methanol at room temperature | None | [44] |
| 89 | E. fortunei | 9-Angeloyloxy-8,9-dehydrothymol | Thymol | Refluxed with 95% ethanol | Cytotoxic | [45] |
| 90 | E. fortunei | 9-(3-Methyl-2-butenoyloxy)-8,10-dehydrothymol | Thymol | Refluxed with 95% ethanol | Cytotoxic | [45] |
| 91 | E. fortunei | 7-Isobutyryloxythymol | Thymol | Refluxed with 95% ethanol | Cytotoxic | [45] |
| 92 | E. fortunei | 7-Isobutyryloxy-8,9-dehydrothymol | Thymol | Refluxed with 95% ethanol | Cytotoxic | [45] |
| 93 | E. fortunei | 2-Acetyl-7-tigloyloxy-isothymol | Isothymol | Refluxed with 95% ethanol | Cytotoxic | [45] |
| 94 | E. fortunei | 8, 9-dehydrothymol-3-O-β-glucoside | Thymol | 95% ethanol at room temperature | Cytotoxic | [46] |
| 95 | E. fortunei | 3-methylbut-2-enoate | Thymol | 95% ethanol at room temperature | Cytotoxic | [46] |
| 96 | E. fortunei | 2-(2-hydroxy-4-methylphenyl)-2-methyl-3-(5-methylbenzofuran-3-yl)propanoic acid | Thymol | Methanol at room temperature | None | [47] |
| 97 | E. fortunei | 9-acetoxyl-3-isobutyroylthymol | Thymol | Methanol at room temperature | a-Glucosidase and acetylcholinesterase inhibitory | [47] |
| 98 | E. fortunei | 7,8,9-trihydroxythymol | Thymol | 95% ethanol at room temperature | Antibacterial | [48] |
| 99 | E. fortunei | 8,10-didehydro-7,9-dihydroxythymol | Thymol | 95% ethanol at room temperature | Antibacterial | [48] |
| 100 | E. fortunei | (−)-Eupafortunin A | Thymol | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 101 | E. fortunei | (+)-Eupafortunin A | Thymol | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 102 | E. fortunei | (+)-Eupafortunin B | Thymol | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 103 | E. fortunei | (−)-eupafortunin B | Thymol | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 104 | E. fortunei | Eupafortunin C | Thymol | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 105 | E. fortunei | Eupafortunin D | Thymol | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 106 | E. fortunei | Eupafortunin E | Thymol | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 107 | E. fortunei | (+)-Eupafortunin F | Thymol | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 108 | E. fortunei | (−)-Eupafortunin F | Thymol | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 109 | E. fortunei | Eupafortunin G | Thymol | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 110 | E. fortunei | Eupafortunin H | Thymol | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 111 | E. fortunei | Eupafortunin I | Thymol | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 112 | E. fortunei | Eupafortunin J | Thymol | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 113 | E. fortunei | (+)-Eupafortunin K | Thymol | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 114 | E. fortunei | (−)-Eupafortunin K | Thymol | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 115 | E. fortunei | Eupafortunin L | Acetophenone | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 116 | E. fortunei | Eupafortunin M | Acetophenone | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 117 | E. fortunei | Eupafortunin N | Acetophenone | 95% ethanol at room temperature | Antiradical and anti-inflammatory | [49] |
| 118 | E. fortunei | Eupatofortunone | Acetophenone | Methanol at room temperature | Cytotoxic | [50] |
| 119 | E. fortunei | Eupatodibenzofuran A | Benzofuran | Methanol at room temperature | Cytotoxic | [50] |
| 120 | E. fortunei | Eupatodibenzofuran B | Benzofuran | Methanol at room temperature | Cytotoxic | [50] |
| 121 | E. fortunei | 6-acetyl-8-methoxy-2,2-dimethylchroman-4-one | Chromanone | Methanol at room temperature | Cytotoxic | [50] |
| 122 | E. fortunei | Eupatodithiecine | Dithiecine | Methanol at room temperature | Cytotoxic | [50] |
| 123 | E. fortunei | (+)-Eupafortin A | Monoterpenoid | 95% ethanol at room temperature | Anti-inflammatory | [51] |
| 124 | E. fortunei | (−)-Eupafortin A | Monoterpenoid | 95% ethanol at room temperature | Anti-inflammatory | [51] |
| 125 | E. fortunei | (+)-Eupafortin B | Monoterpenoid | 95% ethanol at room temperature | Anti-inflammatory | [51] |
| 126 | E. fortunei | (−)-Eupafortin B | Monoterpenoid | 95% ethanol at room temperature | Anti-inflammatory | [51] |
| 127 | E. fortunei | Eupatorid A | Fatty acid | 95% ethanol at room temperature | Anti-inflammatory | [52] |
| 128 | E. fortunei | Eupatorid A | Fatty acid | 95% ethanol at room temperature | Anti-inflammatory | [52] |
| 129 | E. fortunei | Eupatorid B | Fatty acid | 95% ethanol at room temperature | Anti-inflammatory | [52] |
| 130 | E. fortunei | Eupatorid B | Fatty acid | 95% ethanol at room temperature | Anti-inflammatory | [52] |
| 131 | E. fortunei | Eupatorid C | Fatty acid | 95% ethanol at room temperature | Anti-inflammatory | [52] |
| 132 | E. fortunei | Eupatorid C | Fatty acid | 95% ethanol at room temperature | Anti-inflammatory | [52] |
| 133 | E. fortunei | Eupatorid D | Fatty acid | 95% ethanol at room temperature | Anti-inflammatory | [52] |
| 134 | E. fortunei | Eupatorid D | Fatty acid | 95% ethanol at room temperature | Anti-inflammatory | [52] |
| 135 | E. fortunei | Eupatorid E | Fatty acid | 95% ethanol at room temperature | Anti-inflammatory | [52] |
| 136 | E. fortunei | Eupatorid E | Fatty acid | 95% ethanol at room temperature | Anti-inflammatory | [52] |
| 137 | E. fortunei | Eupatorid F | Fatty acid | 95% ethanol at room temperature | Anti-inflammatory | [52] |
| 138 | E. fortunei | Eupatorid F | Fatty acid | 95% ethanol at room temperature | Anti-inflammatory | [52] |
| 139 | E. fortunei | Eupatorid G | Fatty acid | 95% ethanol at room temperature | Anti-inflammatory | [52] |
| 140 | E. fortunei | Eupatorid G | Fatty acid | 95% ethanol at room temperature | Anti-inflammatory | [52] |
| No. | Plant Source | Compound Name | Structure Classification | Extraction Method | Type of Bioactivity Evaluation | Ref. |
|---|---|---|---|---|---|---|
| 141 | E. heterophyllum | Eupaheterin A | Benzofuran | Methanol at room temperature | None | [53] |
| 142 | E. heterophyllum | Eupaheterin B | Benzofuran | Methanol at room temperature | None | [53] |
| 143 | E. heterophyllum | Eupaheterin C | Benzofuran | Methanol at room temperature | None | [53] |
| 144 | E. heterophyllum | Eupaheterin D | Benzofuran | Methanol at room temperature | None | [53] |
| 145 | E. heterophyllum | Eupaheterin E | Benzofuran | Methanol at room temperature | None | [53] |
| 146 | E. heterophyllum | Eupaheterin F | Benzofuran | Methanol at room temperature | None | [53] |
| 147 | E. heterophyllum | Eupaheterin G | Benzofuran | Methanol at room temperature | None | [53] |
| 148 | E. heterophyllum | Eupaheterin H | Benzofuran | Methanol at room temperature | None | [53] |
| 149 | E. heterophyllum | Eupaheterin I | Benzofuran | Methanol at room temperature | None | [53] |
| 150 | E. heterophyllum | Eupaheterin J | Benzofuran | Methanol at room temperature | None | [53] |
| 151 | E. heterophyllum | 4-Acetyl-3β,5-dihydroxy-2α-(propen-2-yl)-2,3-dihydrobenzofuran | Benzofuran | Methanol at room temperature | None | [54] |
| 152 | E. heterophyllum | 4-Acetyl-3β-angeloyloxy-5-hydroxy-2α-(propen-2-yl)- 2,3-dihydrobenzofuran | Benzofuran | Methanol at room temperature | None | [54] |
| 153 | E. heterophyllum | 6-Acetyl-3β,5-dihydroxy-2α-(propen-2-yl)-2,3-dihydrobenzofuran | Benzofuran | Methanol at room temperature | None | [54] |
| 154 | E. heterophyllum | 5-Acetyl-6-hydroxy-3α-methoxyl-2α-(propen-2-yl)-2,3- dihydrobenzofuran | Benzofuran | Methanol at room temperature | None | [54] |
| 155 | E. heterophyllum | 5-Acetyl-3α-angeloyloxy-6-hydroxy-2α-(2-methyloxiran-2-yl)-2,3-dihydrobenzofuran | Benzofuran | Methanol at room temperature | None | [54] |
| 156 | E. heterophyllum | 5-Acetyl-3α-angeloyloxy-6-hydroxy-2α-(2-methyloxiran-2-yl)-2,3-dihydrobenzofuran | Benzofuran | Methanol at room temperature | None | [54] |
| 157 | E. heterophyllum | 3,9β-Epoxy-9α-isobutanoyloxymentha-13,5-trien-8α-ol | Benzofuran | Methanol at room temperature | None | [54] |
| 158 | E. heterophyllum | Dieupaheterin A | Benzofuran | Methanol at room temperature | None | [53] |
| 159 | E. heterophyllum | Dieupaheterin B | Benzofuran | Methanol at room temperature | None | [53] |
| 160 | E. heterophyllum | Dieupaheterin C | Benzofuran | Methanol at room temperature | None | [53] |
| 161 | E. heterophyllum | Dieupaheterin D | Benzofuran | Methanol at room temperature | None | [53] |
| 162 | E. heterophyllum | Dieupaheterin E | Benzofuran | Methanol at room temperature | None | [55] |
| 163 | E. heterophyllum | Dieupaheterin F | Benzofuran | Methanol at room temperature | None | [55] |
| 164 | E. heterophyllum | Trieupaheterin A | Benzofuran | Methanol at room temperature | None | [53] |
| 165 | E. heterophyllum | 2-(Hydroxyacetyl)-3-methoxy-5-(propyn-1-yl)thiophene | Thiophene | Methanol at room temperature | None | [54] |
| 166 | E. heterophyllum | 2-Acetyl-3-hydroxy-5-(propyn-1-yl)thiophene-3-O-(6-O-malonyl)-β-glucoside | Thiophene | Methanol at room temperature | None | [54] |
| 167 | E. heterophyllum | (3R,6R,7R,8R)-(4Z)-3α-acetoxy-8β-(3-furoyloxy)germacra-1(10),4,11(13)-trien-(12,6α)-olide | Sesquiterpenoid | Methanol at room temperature | None | [56] |
| 168 | E. heterophyllum | (4Z)-3α-acetoxy-8β-(4′,5′ -dihydroxytigloyloxy)-1β-hydroperoxygermacra-4,10(14),11(13)-trien-(12,6α)-olide | Sesquiterpenoid | Methanol at room temperature | None | [56] |
| 169 | E. heterophyllum | 5′-deoxy-(4Z)-3α-acetoxy-8β-(4′,5′ -dihydroxytigloyloxy)-1β-hydroperoxygermacra-4,10(14),11(13)-trien-(12,6α)-olide | Sesquiterpenoid | Methanol at room temperature | None | [56] |
| 170 | E. heterophyllum | (4Z)-3β-acetoxy-1β,10α-epoxy-8β-(4′,5-epoxy-4′-hydroxytigloyloxy)germacra-4,11(13)-dien-(12,6α)-olide | Sesquiterpenoid | Methanol at room temperature | None | [56] |
| 171 | E. heterophyllum | 8β-(2′-methylbutanoyloxy)germacra-1(10),4,11(13)-trien-(12,6α)-olide | Sesquiterpenoid | Methanol at room temperature | None | [56] |
| 172 | E. heterophyllum | 1β-hydroperoxy-2α-hydroxy-8β-(5′-hydroxyangeloyloxy)germacra-4,10(14),11(13)-trien-(12,6α)-olide | Sesquiterpenoid | Methanol at room temperature | None | [56] |
| 173 | E. heterophyllum | 8β-(4′-acetoxytigloyloxy)-1β-hydroperoxy-3β-hydroxygermacra-4,10(14),11(13)-trien-(12,6α)-olide | Sesquiterpenoid | Methanol at room temperature | None | [56] |
| 174 | E. heterophyllum | 1β-hydroxy-8β-(5′-hydroxyangeloyloxy)eudesma-4(15),11(13)-dien-(12,6α)-olide | Sesquiterpenoid | Methanol at room temperature | None | [56] |
| 175 | E. heterophyllum | 1β,2α-dihydroxy-8β-(5′-hydroxyangeloyloxy)eudesma-4(15),11(13)-dien- (12,6α)-olide | Sesquiterpenoid | Methanol at room temperature | None | [56] |
| 176 | E. heterophyllum | Sesquiterpenoid | Methanol at room temperature | None | [56] | |
| 177 | E. heterophyllum | 8β-(4′,5′-dihydroxytigloyloxy)-3α-hydroperoxyguaia-4,10(14),11(13)-trien-(12,6α)-olide | Sesquiterpenoid | Methanol at room temperature | None | [56] |
| 178 | E. heterophyllum | Sesquiterpenoid | Methanol at room temperature | None | [56] | |
| 179 | E. heterophyllum | 8β-(5′-hydroxyangeloyloxy)-1-oxo-2-norelema-3,11(13)-dien-(12,6α)-olid | Sesquiterpenoid | Methanol at room temperature | None | [56] |
| No. | Plant Source | Compound Name | Structure Classification | Extraction Method | Type of Bioactivity Evaluation | Ref. |
|---|---|---|---|---|---|---|
| 180 | E. lindleyanum | Eupalinolide L | Sesquiterpenoid | Boiling water | Anti-inflammatory | [57] |
| 181 | E. lindleyanum | Eupalinolide M | Sesquiterpenoid | Boiling water | Anti-inflammatory | [57] |
| 182 | E. lindleyanum | Eupalinolide N | Sesquiterpenoid | Refluxed with 90% ethanol | Anti-inflammatory | [58] |
| 183 | E. lindleyanum | Eupalinolide O | Sesquiterpenoid | 95% ethanol at room temperature | Cytotoxic | [59] |
| 184 | E. macrocephalum | Macrocephalide A | Sesquiterpenoid | Methanol at room temperature | Cytotoxic | [60] |
| 185 | E. macrocephalum | Macrocephalide B | Sesquiterpenoid | Methanol at room temperature | Cytotoxic | [60] |
| 186 | E. macrocephalum | Macrocephalide C | Sesquiterpenoid | Methanol at room temperature | Cytotoxic | [60] |
| 187 | E. obtusissmum | Uasdlabdane A | Diterpenoid | 95% ethanol at room temperature | Cytotoxic | [61] |
| 188 | E. obtusissmum | Uasdlabdane B | Diterpenoid | 95% ethanol at room temperature | Cytotoxic | [61] |
| 189 | E. obtusissmum | Uasdlabdane C | Diterpenoid | 95% ethanol at room temperature | Cytotoxic | [61] |
| 190 | E. obtusissmum | Uasdlabdane D | Diterpenoid | 95% ethanol at room temperature | Cytotoxic | [61] |
| 191 | E. obtusissmum | Uasdlabdane E | Diterpenoid | 95% ethanol at room temperature | Cytotoxic | [61] |
| 192 | E. obtusissmum | Uasdlabdane F | Diterpenoid | 95% ethanol at room temperature | Cytotoxic | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, H. Chemical Constituents and Their Bioactivities of Plants from the Genus Eupatorium (2015–Present). Biology 2024, 13, 288. https://doi.org/10.3390/biology13050288
Geng H. Chemical Constituents and Their Bioactivities of Plants from the Genus Eupatorium (2015–Present). Biology. 2024; 13(5):288. https://doi.org/10.3390/biology13050288
Chicago/Turabian StyleGeng, Hao. 2024. "Chemical Constituents and Their Bioactivities of Plants from the Genus Eupatorium (2015–Present)" Biology 13, no. 5: 288. https://doi.org/10.3390/biology13050288
APA StyleGeng, H. (2024). Chemical Constituents and Their Bioactivities of Plants from the Genus Eupatorium (2015–Present). Biology, 13(5), 288. https://doi.org/10.3390/biology13050288






