Risk Perception: Chemical Stimuli in Predator Detection and Feeding Behaviour of the Invasive Round Goby Neogobius melanostomus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
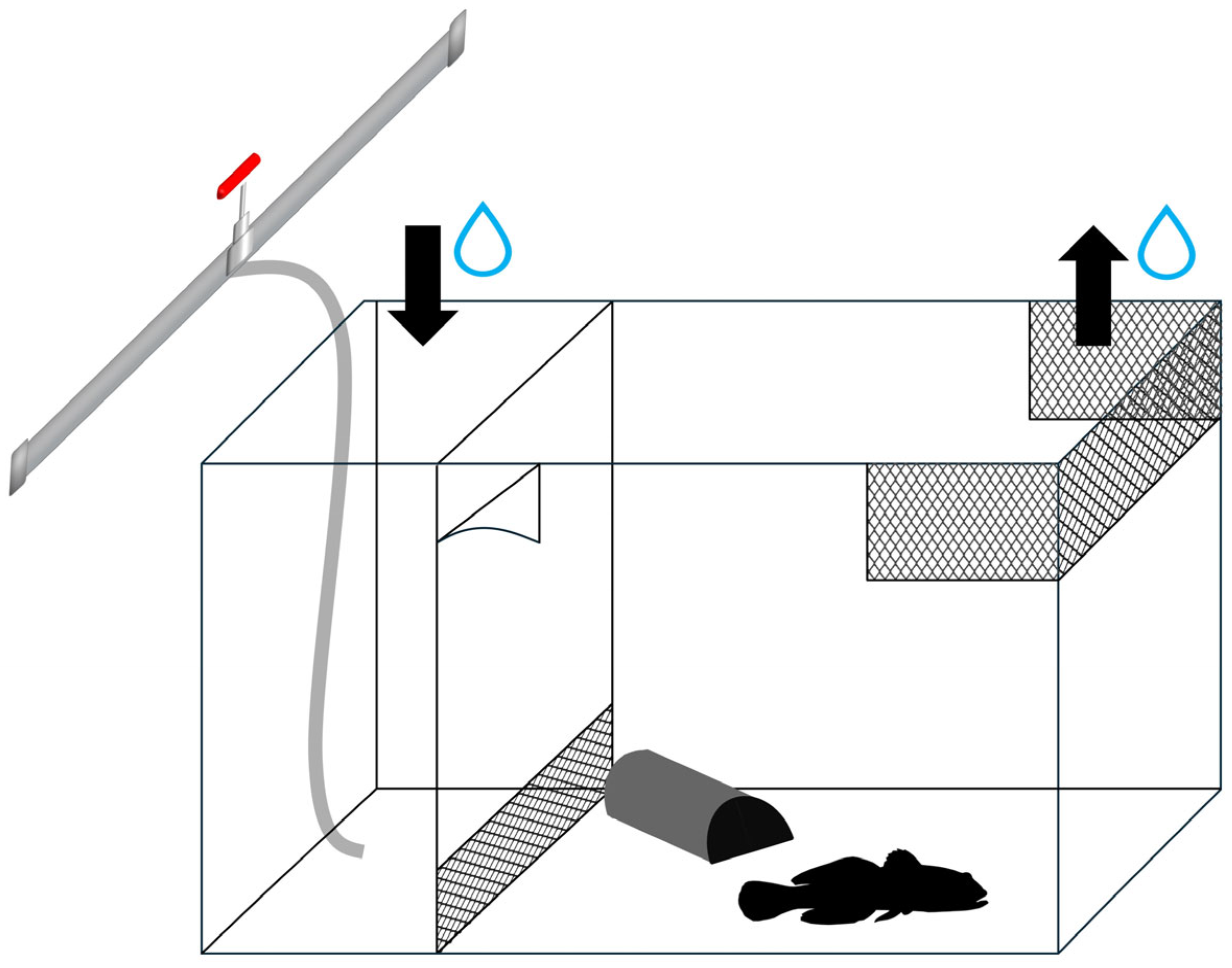
2.1. Collection and Maintenance of Study Organisms
2.2. Preparation of the Alarm Cues
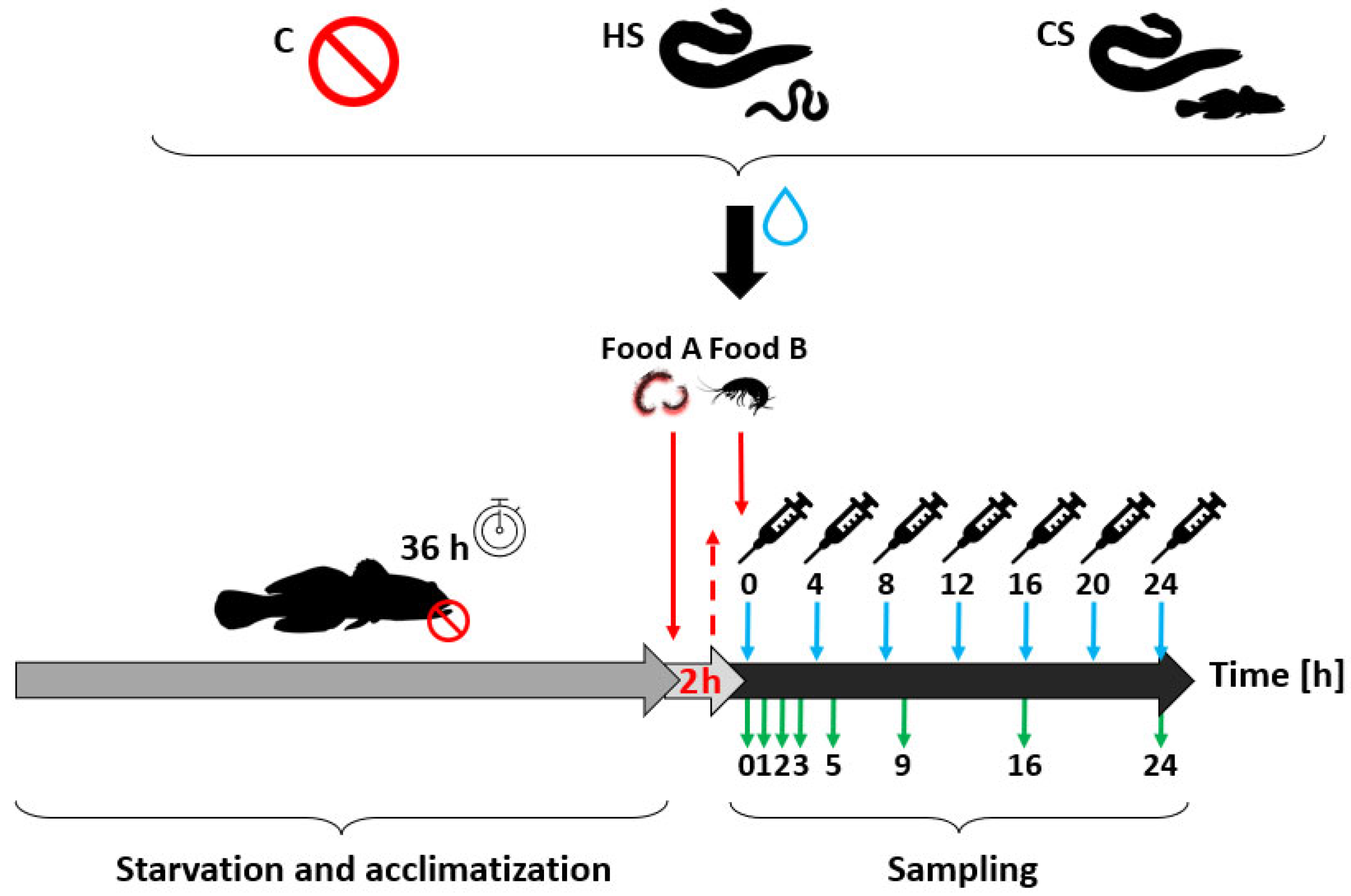
2.3. Experimental Design
2.4. Sample Processing
2.5. Data Analysis
2.5.1. Assumptions of Observation Independence
2.5.2. Gut Evacuation Rate
2.5.3. Consumption Probability
3. Results
3.1. Assumptions of Observation Independence
3.2. Gut Evacuation Rate
3.3. Consumption Probability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Essl, F. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Pyšek, P.; Winter, M.; Arianoitsou, M.; Bacher, S.; et al. Projecting the continental accumulation of alien species through to 2050. Glob. Chang. Biol. 2021, 27, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Penk, M.R.; Jeschke, J.M.; Minchin, D.; Donohue, I. Warming can enhance invasion success through asymmetries in energetic performance. J. Anim. Ecol. 2016, 85, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Jeschke, J.M.; Leroy, B.; Mace, G.M. Insights from modeling studies on how climate change affects invasive alien species geography. Ecol. Evol. 2018, 8, 5688–5700. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Haubrock, P.J.; Cuthbert, R.N.; Ricciardi, A.; Diagne, C.; Courchamp, F. Economic costs of invasive bivalves in freshwater ecosystems. Divers. Distrib. 2022, 28, 1010–1021. [Google Scholar] [CrossRef]
- Vilà, M.; Hulme, P.E. (Eds.) Impact of Biological Invasions on Ecosystem Services; Springer International Publishing: Cham, Switzerland, 2017; Volume 12. [Google Scholar]
- Soto, I.; Cuthbert, R.N.; Ricciardi, A.; Ahmed, D.A.; Altermatt, F.; Schäfer, R.B.; Archambaud-Suard, G.; Bonada, N.; Cañedo-Argüelles, M.; Csabai, Z.; et al. The faunal Ponto-Caspianization of central and western European waterways. Biol. Invasions 2023, 25, 2613–2629. [Google Scholar] [CrossRef]
- Neilson, M.E.; Stepien, C.A. Escape from the Ponto-Caspian: Evolution and biogeography of an endemic goby species flock (Benthophilinae: Gobiidae: Teleostei). Mol. Phylogenetics Evol. 2009, 52, 84–102. [Google Scholar] [CrossRef]
- Copp, G.H.; Bianco, P.G.; Bogutskaya, N.G.; Erős, T.; Falka, I.; Ferreira, M.T.; Fox, M.G.; Freyhof, J.; Gozlan, R.E.; Grabowska, J.; et al. To be, or not to be, a non-native freshwater fish? J. Appl. Ichthyol. 2005, 21, 242–262. [Google Scholar] [CrossRef]
- Corkum, L.D.; Sapota, M.R.; Skora, K.E. The round goby, Neogobius melanostomus, a fish invader on both sides of the Atlantic Ocean. Biol. Invasions 2004, 6, 173–181. [Google Scholar] [CrossRef]
- Cerwenka, A.F.; Brandner, J.; Dashinov, D.; Geist, J. Small but mighty: The round goby (Neogobius melanostomus) as a model species of biological invasions. Diversity 2023, 15, 528. [Google Scholar] [CrossRef]
- Sapota, M.R. The round goby (Neogobius melanostomus) in the Gulf of Gdańsk—A species introduction into the Baltic Sea. In Biology of the Baltic Sea: Proceedings of the 17th BMB Symposium, Stockholm, Sweden, 25–29 November 2001; Developments in Hydrobiology; Kautsky, H., Snoeijs, P., Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 176, pp. 219–224. [Google Scholar] [CrossRef]
- Kornis, M.S.; Mercado-Silva, N.; Vander Zanden, M.J. Twenty years of invasion: A review of round goby Neogobius melanostomus biology, spread and ecological implications. J. Fish Biol. 2012, 80, 235–285. [Google Scholar] [CrossRef] [PubMed]
- Masson, L.; Masson, G.; Beisel, J.N.; Gutowsky, L.F.G.; Fox, M.G. Consistent life history shifts along invasion routes? An examination of round goby populations invading on two continents. Divers. Distrib. 2018, 24, 841–852. [Google Scholar] [CrossRef]
- Christensen, E.A.; Norin, T.; Tabak, I.; van Deurs, M.; Behrens, J.W. Effects of temperature on physiological performance and behavioral thermoregulation in an invasive fish, the round goby. J. Exp. Biol. 2021, 224, jeb237669. [Google Scholar] [CrossRef]
- Diggins, T.P.; Kaur, J.; Chakraborti, R.K.; DePinto, J.V. Diet choice by the exotic round goby (Neogobius melanostomus) as influenced by prey motility and environmental complexity. J. Great Lakes Res. 2002, 28, 411–420. [Google Scholar] [CrossRef]
- Copp, G.H.; Kováč, V.; Zweimüller, I.; Dias, A.; Nascimento, M.; Balážová, M. Preliminary study of dietary interactions between invading Ponto-Caspian gobies and some native fish species in the River Danube near Bratislava (Slovakia). Aquat. Invasions 2008, 3, 193–200. [Google Scholar] [CrossRef]
- Penk, M.; Saul, W.C.; Dick, J.T.A.; Donohue, I.; Alexander, M.E.; Linzmaier, S.; Jeschke, J.M. A trophic interaction framework for identifying the invasive capacity of novel organisms. Methods Ecol. Evol. 2017, 8, 1786–1794. [Google Scholar] [CrossRef]
- Saul, W.C.; Jeschke, J.M.; Heger, T. The role of eco- evolutionary experience in invasion success. NeoBiota 2013, 17, 57–74. [Google Scholar] [CrossRef]
- Morkūnė, R.; Tomczak, M.T.; Bacevičius, E.; Gasiūnaitė, Z.R. Changes in the Baltic Sea coastal food web: A case study on the invasion of Round goby Neogobius melanostomus (Pallas, 1814). Estuar. Coast. Shelf Sci. 2024, 296, 108591. [Google Scholar] [CrossRef]
- Mikl, L.; Adámek, Z.; Všetičková, L.; Janáč, M.; Roche, K.; Šlapanský, L.; Jurajda, P. Response of benthic macroinvertebrate assemblages to round (Neogobius melanostomus, Pallas 1814) and tubenose (Proterorhinus semilunaris, Heckel 1837) goby predation pressure. Hydrobiologia 2017, 785, 219–232. [Google Scholar] [CrossRef]
- Oesterwind, D.; Bock, C.; Förster, A.; Gabel, M.; Henseler, C.; Kotterba, P.; Mengeb, M.; Myts, D.; Winkler, H.M. Predator and prey: The role of the round goby Neogobius melanostomus in the western Baltic. Mar. Biol. Res. 2017, 13, 188–197. [Google Scholar] [CrossRef]
- Vašek, M.; Všetičková, L.; Roche, K.; Jurajda, P. Diet of two invading gobiid species (Proterorhinus semilunaris and Neogobius melanostomus) during the breeding and hatching season: No field evidence of extensive predation on fish eggs and fry. Limnologica 2014, 46, 31–36. [Google Scholar] [CrossRef]
- Lutz, E.; Hirsch, P.E.; Bussmann, K.; Wiegleb, J.; Jermann, H.P.; Muller, R.; Burkhardt-Holm, P.; Adrian-Kalchhauser, I. Predation on native fish eggs by invasive round goby revealed by species-specific gut content DNA analyses. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1566–1577. [Google Scholar] [CrossRef]
- Dashinov, D.; Uzunova, E. Diet and feeding strategies of round goby, Neogobius melanostomus (Pallas, 1814) from the invasion front in the Danube River tributaries (Bulgaria): Ontogenetic shift and seasonal variation. Limnologica 2020, 83, 125796. [Google Scholar] [CrossRef]
- Borcherding, J.; Dolina, M.; Heermann, L.; Knutzen, P.; Krüger, S.; Matern, S.; van Treeck, R.; Gertzen, S. Feeding and niche differentiation in three invasive gobies in the Lower Rhine, Germany. Limnologica 2013, 43, 49–58. [Google Scholar] [CrossRef]
- Bhagat, Y.; Ruetz III, C.R.; Akins, A.L. Differential habitat use by the round goby (Neogobius melanostomus) and Dreissena spp. in coastal habitats of eastern Lake Michigan. J. Great Lakes Res. 2015, 41, 1087–1093. [Google Scholar] [CrossRef]
- Števove, B.; Kováč, V. Ontogenetic variations in the diet of two invasive gobies, Neogobius melanostomus (Pallas, 1814) and Ponticola kessleri (Günther, 1861), from the middle Danube (Slovakia) with notice on their potential impact on benthic invertebrate communities. Sci. Total Environ. 2016, 557, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.R.; Johnson, R.A.; Campbell, L.; Petruniak, J.; Patterson, M. Effects of round gobies (Neogobius melanostomus) on dreissenid mussels and other invertebrates in eastern Lake Erie, 2002–2004. J. Great Lakes Res. 2005, 31, 252–261. [Google Scholar] [CrossRef]
- Lederer, A.M.; Janssen, J.; Reed, T.; Wolf, A. Impacts of the introduced round goby (Apollonia melanostoma) on dreissenids (Dreissena polymorpha and Dreissena bugensis) and on macroinvertebrate community between 2003 and 2006 in the littoral zone of Green Bay, Lake Michigan. J. Great Lakes Res. 2008, 34, 690–697. [Google Scholar] [CrossRef]
- Henseler, C.; Oesterwind, D.; Kotterba, P.; Nordström, M.C.; Snickars, M.; Törnroos, A.; Bonsdorff, E. Impact of round goby on native invertebrate communities-An experimental field study. J. Exp. Mar. Biol. Ecol. 2021, 541, 151571. [Google Scholar] [CrossRef]
- Ustups, D.; Bergström, U.; Florin, A.B.; Kruze, E.; Zilniece, D.; Elferts, D.; Knospina, E.; Uzars, D. Diet overlap between juvenile flatfish and the invasive round goby in the central Baltic Sea. J. Sea Res. 2016, 107, 121–129. [Google Scholar] [CrossRef]
- Jůza, T.; Blabolil, P.; Baran, R.; Bartoň, D.; Čech, M.; Draštík, V.; Frouzová, J.; Holubová, M.; Ketelaars, H.A.M.; Kočvara, L.; et al. Collapse of the native ruffe (Gymnocephalus cernua) population in the Biesbosch lakes (The Netherlands) owing to round goby (Neogobius melanostomus) invasion. Biol. Invasions 2018, 20, 1523–1535. [Google Scholar] [CrossRef]
- Ramler, D.; Keckeis, H. Occurrence of non-native fishes in the Danube east of Vienna (Austria) and potential interactions of invasive gobiids with native fishes. J. Appl. Ichthyol. 2019, 35, 850–862. [Google Scholar] [CrossRef]
- Le Hen, G.; Balzani, P.; Haase, P.; Kouba, A.; Liu, C.; Nagelkerke, L.A.; Theissen, N.; Renault, D.; Soto, I.; Haubrock, P.J. Alien species and climate change drive shifts in a riverine fish community and trait compositions over 35 years. Sci. Total Environ. 2023, 867, 161486. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.; Hoedemakers, K. The round goby Neogobius melanostomus (Pallas, 1814) (Perciformes: Gobiidae), an invasive species in the Albert Canal (Belgium). Belg. J. Zool. 2013, 143, 148–153. [Google Scholar] [CrossRef]
- Taraborelli, A.C.; Fox, M.G.; Johnson, T.B.; Schaner, T. Round goby (Neogobius melanostomus) population structure, biomass, prey consumption and mortality from predation in the Bay of Quinte, Lake Ontario. J. Great Lakes Res. 2010, 36, 625–632. [Google Scholar] [CrossRef]
- Mikl, L.; Adámek, Z.; Roche, K.; Všetičková, L.; Šlapanský, L.; Jurajda, P. Invasive Ponto-Caspian gobies in the diet of piscivorous fish in a European lowland river. Fundam. Appl. Limnol. 2017, 190, 157–171. [Google Scholar] [CrossRef]
- Bruestle, E.L.; Karboski, C.; Hussey, A.; Fisk, A.T.; Mehler, K.; Pennuto, C.; Gorsky, D. Novel trophic interaction between lake sturgeon (Acipenser fulvescens) and non-native species in an altered food web. Can. J. Fish. Aquat. Sci. 2019, 76, 6–14. [Google Scholar] [CrossRef]
- Batabyal, A. Predator–prey systems as models for integrative research in biology: The value of a non-consumptive effects framework. J. Exp. Biol. 2023, 226, jeb245851. [Google Scholar] [CrossRef]
- Lima, S.L. Nonlethal effects in the ecology of predator-prey interactions. Bioscience 1998, 48, 25–34. [Google Scholar] [CrossRef]
- Mitchell, M.D.; Harborne, A.R. Non-consumptive effects in fish predator–prey interactions on coral reefs. Coral Reefs 2020, 39, 867–884. [Google Scholar] [CrossRef]
- Kindinger, T.L.; Albins, M.A. Consumptive and non-consumptive effects of an invasive marine predator on native coral-reef herbivores. Biol. Invasions 2017, 19, 131–146. [Google Scholar] [CrossRef]
- Creel, S.; Christianson, D. Relationships between direct predation and risk effects. Trends Ecol. Evol. 2008, 23, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Preisser, E.L.; Bolnick, D.I. The many faces of fear: Comparing the pathways and impacts of nonconsumptive predator effects on prey populations. PLoS ONE 2008, 3, e2465. [Google Scholar] [CrossRef] [PubMed]
- Madin, E.M.; Gaines, S.D.; Warner, R.R. Field evidence for pervasive indirect effects of fishing on prey foraging behavior. Ecology 2010, 91, 3563–3571. [Google Scholar] [CrossRef] [PubMed]
- Berejikian, B.A.; Tezak, E.P.; LaRae, A.L. Innate and enhanced predator recognition in hatchery-reared chinook salmon. Environ. Biol. Fishes 2003, 67, 241–251. [Google Scholar] [CrossRef]
- Sih, A.; Bolnick, D.I.; Luttbeg, B.; Orrock, J.L.; Peacor, S.D.; Pintor, L.M.; Preisser, E.; Rehage, J.S.; Vonesh, J.R. Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 2010, 119, 610–621. [Google Scholar] [CrossRef]
- Dall, S.R. Managing risk: The perils of uncertainty. In Evolutionary Behavioral Ecology; Westneat, D.F., Fox, C.W., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 194–206. [Google Scholar]
- Smith, R.J.F. The evolution of chemical alarm signals in fishes. In Chemical Signals in Vertebrates 4; Springer: Boston, MA, USA, 1986; pp. 99–115. [Google Scholar]
- Chivers, D.P.; Smith, R.J.F. Chemical alarm signalling in aquatic predator-prey systems: A review and prospectus. Ecoscience 1998, 5, 338–352. [Google Scholar] [CrossRef]
- Kats, L.B.; Dill, L.M. The scent of death: Chemosensory assessment of predation risk by prey animals. Ecoscience 1998, 5, 361–394. [Google Scholar] [CrossRef]
- Ferrari, M.C.O.; Wisenden, B.D.; Chivers, D.P. Chemical ecology of predator-prey interactions in aquatic ecosystems: A review and prospectus. Can. J. Zool. 2010, 88, 698–724. [Google Scholar] [CrossRef]
- Alemadi, S.; Wisenden, B. Antipredator response to injury-released chemical alarm cues by convict cichlid young before and after independence from parental protection. Behaviour 2002, 139, 603–611. [Google Scholar] [CrossRef]
- Hettyey, A.; Tóth, Z.; Thonhauser, K.E.; Frommen, J.G.; Penn, D.J.; Van Buskirk, J. The relative importance of prey-borne and predator-borne chemical cues for inducible antipredator responses in tadpoles. Oecologia 2015, 179, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Burks, R.L.; Lodge, D.M. Cued in: Advances and opportunities in freshwater chemical ecology. J. Chem. Ecol. 2002, 28, 1901–1917. [Google Scholar] [CrossRef] [PubMed]
- Miyai, C.A.; Sanches, F.H.C.; Pinho-Neto, C.F.; Barreto, R.E. Effects of predator odour on antipredator responses of Nile tilapia. Physiol. Behav. 2016, 165, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.E. Learning about danger: Chemical alarm cues and local risk assessment in prey fishes. Fish Fish. 2003, 4, 227–234. [Google Scholar] [CrossRef]
- Wisenden, B.D. Chemically mediated strategies to counter predation. In Sensory Processing in Aquatic Environments; Collin, S.P., Marshall, N.J., Eds.; Springer: New York, NY, USA, 2003; pp. 236–251. [Google Scholar] [CrossRef]
- Barcellos, L.J.G.; Volpato, G.L.; Barreto, R.E.; Coldebella, I.; Ferreira, D. Chemical communication of handling stress in fish. Physiol. Behav. 2011, 103, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Lehtiniemi, M. Swim or hide: Predator cues cause species specific reactions in young fish larvae. J. Fish Biol. 2005, 66, 1285–1299. [Google Scholar] [CrossRef]
- Milano, D.; Lozada, M.; Zagarese, H.E. Predator-induced reaction patterns of landlocked Galaxias maculatus to visual and chemical cues. Aquat. Ecol. 2010, 44, 741–748. [Google Scholar] [CrossRef]
- Sanches, F.H.C.; Miyai, C.A.; Pinho-Neto, C.F.; Barreto, R.E. Stress responses to chemical alarm cues in Nile tilapia. Physiol. Behav. 2015, 149, 8–13. [Google Scholar] [CrossRef]
- Pereira, R.T.; Leutz, J.D.A.C.M.; Valença-Silva, G.; Barcellos, L.J.G.; Barreto, R.E. Ventilation responses to predator odors and conspecific chemical alarm cues in the frillfin goby. Physiol. Behav. 2017, 179, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.E.; Rive, A.C.; Ferrari, M.C.; Chivers, D.P. The dynamic nature of antipredator behavior: Prey fish integrate threat-sensitive antipredator responses within background levels of predation risk. Behav. Ecol. Sociobiol. 2006, 61, 9–16. [Google Scholar] [CrossRef]
- Jónsson, L. Chemical stimuli: Role in the behavior of fishes. In Environmental Physiology of Fishes; NATO Advanced Study Institutes Series; Ali, M.A., Ed.; Springer: Boston, MA, USA, 1980; Volume 35, pp. 353–367. [Google Scholar] [CrossRef]
- Arvigo, A.L.; Miyai, C.A.; Sanches, F.H.; Barreto, R.E.; Costa, T.M. Combined effects of predator odor and alarm substance on behavioral and physiological responses of the pearl cichlid. Physiol. Behav. 2019, 206, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Mirza, R.S.; Chivers, D.P. Are chemical alarm cues conserved within salmonid fishes? J. Chem. Ecol. 2001, 27, 1641–1655. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, P.E.; N’Guyen, A.; Adrian-Kalchhauser, I.; Burkhardt-Holm, P. What do we really know about the impacts of one of the 100 worst invaders in Europe? A reality check. Ambio 2016, 45, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Bromley, P.J. The role of gastric evacuation experiments in quantifying the feeding rates of predatory fish. Rev. Fish Biol. Fish. 1994, 4, 36–66. [Google Scholar] [CrossRef]
- Bajkov, A.D. How to estimate the daily food consumption of fish under natural conditions. Trans. Am. Fish. Soc. 1935, 65, 288–289. [Google Scholar] [CrossRef]
- Elliott, J.M.; Persson, L. The estimation of daily rates of food consumption for fish. J. Anim. Ecol. 1978, 47, 977–991. [Google Scholar] [CrossRef]
- Jobling, M. Mathematical models of gastric emptying and the estimation of daily rates of food consumption for fish. J. Fish Biol. 1981, 19, 245–257. [Google Scholar] [CrossRef]
- Hedden, S.C.; Gido, K.B.; Hedden, C.K.; Pennock, C.A.; Duran, B.R.; Hines, B.A.; Gilbert, E.I.; McKinstry, M.C.; Durst, S.L.; Franssen, N.R. Quantifying consumption of native fishes by nonnative Channel Catfish in a desert river. N. Am. J. Fish. Manag. 2021, 41, 82–94. [Google Scholar] [CrossRef]
- Houston, A.I.; McNamara, J.M.; Hutchinson, J.M. General results concerning the trade-off between gaining energy and avoiding predation. Philos. Trans. R. Soc. Ser. B Biol. Sci. 1993, 341, 375–397. [Google Scholar] [CrossRef]
- Åbjörnsson, K.; Hansson, L.A.; Brönmark, C. Responses of prey from habitats with different predator regimes: Local adaptation and heritability. Ecology 2004, 85, 1859–1866. [Google Scholar] [CrossRef]
- Pettersson, L.B.; Brönmark, C. Trading off safety against food: State dependent habitat choice and foraging in crucian carp. Oecologia 1993, 95, 353–357. [Google Scholar] [CrossRef]
- Kristensen, E.A.; Closs, G.P. Anti-predator response of naïve and experienced common bully to chemical alarm cues. J. Fish Biol. 2004, 64, 643–652. [Google Scholar] [CrossRef]
- McCormick, M.I.; Larson, J.K. Field verification of the use of chemical alarm cues in a coral reef fish. Coral Reefs 2007, 26, 571–576. [Google Scholar] [CrossRef]
- McCormick, M.I.; Manassa, R. Predation risk assessment by olfactory and visual cues in a coral reef fish. Coral Reefs 2008, 27, 105–113. [Google Scholar] [CrossRef]
- del Mar Palacios, M.; Warren, D.T.; McCormick, M.I. Sensory cues of a top-predator indirectly control a reef fish mesopredator. Oikos 2016, 125, 201–209. [Google Scholar] [CrossRef]
- Palacios, M.D.M.; McCormick, M.I. Positive indirect effects of top-predators on the behaviour and survival of juvenile fishes. Oikos 2021, 130, 219–230. [Google Scholar] [CrossRef]
- Foam, P.E.; Harvey, M.C.; Mirza, R.S.; Brown, G.E. Heads up: Juvenile convict cichlids switch to threat-sensitive foraging tactics based on chemosensory information. Anim. Behav. 2005, 70, 601–607. [Google Scholar] [CrossRef]
- Holmes, T.H.; McCormick, M.I. Smell, learn and live: The role of chemical alarm cues in predator learning during early life history in a marine fish. Behav. Process. 2010, 83, 299–305. [Google Scholar] [CrossRef]
- Buřič, M.; Bláha, M.; Kouba, A.; Drozd, B. Upstream expansion of round goby (Neogobius melanostomus)–first record in the upper reaches of the Elbe river. Knowl. Manag. Aquat. Ecosyst. 2015, 416, 32. [Google Scholar] [CrossRef][Green Version]
- Ray, W.J.; Corkum, L.D. Habitat and site affinity of the round goby. J. Great Lakes Res. 2001, 27, 329–334. [Google Scholar] [CrossRef]
- Sadler, K. Effects of temperature on the growth and survival of the European eel, Anguilla anguilla L. J. Fish Biol. 1979, 15, 499–507. [Google Scholar] [CrossRef]
- Seymour, E.A. Devising optimum feeding regimes and temperatures for the warmwater culture of eel, Anguilla anguilla L. Aquac. Res. 1989, 20, 311–324. [Google Scholar] [CrossRef]
- Lee, V.A.; Johnson, T.B. Development of a bioenergetics model for the round goby (Neogobius melanostomus). J. Great Lakes Res. 2005, 31, 125–134. [Google Scholar] [CrossRef]
- Golani, D.; Shefler, D.; Gelman, A. Aspects of growth and feeding habits of the adult European eel (Anguilla anguilla) in Lake Kinneret (Lake Tiberias), Israel. Aquaculture 1988, 74, 349–354. [Google Scholar] [CrossRef]
- Emde, S.; Kochmann, J.; Kuhn, T.; Plath, M.; Klimpel, S. Getting what is served? Feeding ecology influencing parasite-host interactions in invasive round goby Neogobius melanostomus. PLoS ONE 2014, 9, e109971. [Google Scholar] [CrossRef] [PubMed]
- Carman, S.M.; Janssen, J.; Jude, D.J.; Berg, M.B. Diel interactions between prey behaviour and feeding in an invasive fish, the round goby, in a North American river. Freshw. Biol. 2006, 51, 742–755. [Google Scholar] [CrossRef]
- Didenko, A.; Volikov, Y.; Baranov, V.; Kruzhylina, S.; Gurbyk, A.; Bielikova, O. Chironomid diversity in the diets of Ponto-Caspian gobiids in a freshwater habitat: Implications for resource partitioning. Limnologica 2021, 89, 125890. [Google Scholar] [CrossRef]
- Błońska, D.; Grabowska, J.; Kobak, J.; Rachalewski, M.; Bącela-Spychalska, K. Fish predation on sympatric and allopatric prey—A case study of Ponto-Caspian gobies, European bullhead and amphipods. Limnologica 2016, 61, 1–6. [Google Scholar] [CrossRef]
- Verheggen, F.J.; Haubruge, E.; Mescher, M.C. Alarm pheromones—Chemical signaling in response to danger. Vitam. Horm. 2010, 83, 215–239. [Google Scholar] [CrossRef] [PubMed]
- Richter, L.; Schwenkmezger, L.; Becker, J.; Winkelmann, C.; Hellmann, C.; Worischka, S. The very hungry amphipod: The invasive Dikerogammarus villosus shows high consumption rates for two food sources and independent of predator cues. Biol. Invasions 2018, 20, 1321–1335. [Google Scholar] [CrossRef]
- Szydłowska, N.; Let, M.; Franta, P.; Buřič, M.; Worischka, S.; Richter, L.; Drozd, B. Gut evacuation rate as a tool for revealing feeding patterns in the invasive round goby (Neogobius melanostomus) under different feeding modes, food types and temperatures. Aquat. Invasions 2024. submitted. [Google Scholar]
- Hazlett, B. Responses to multiple chemical cues by the crayfish Orconectes virilis. Behaviour 1999, 136, 161–177. [Google Scholar] [CrossRef]
- Manko, P. Stomach content analysis in freshwater fish feeding ecology. University of Prešov 2016, 116, 1–25. [Google Scholar]
- Andersen, N.G. Depletion rates of gastrointestinal content in common goby (Pomatoschistus microps (Kr.)). Effects of temperature and fish size. Dana 1984, 3, 31–42. [Google Scholar]
- Andersen, N.G. The effects of predator size, temperature, and prey characteristics on gastric evacuation in whiting. J. Fish Biol. 1999, 54, 287–301. [Google Scholar] [CrossRef]
- Sveier, H.; Wathne, E.; Lied, E. Growth, feed and nutrient utilisation and gastrointestinal evacuation time in Atlantic salmon (Salmo salar L.): The effect of dietary fish meal particle size and protein concentration. Aquaculture 1999, 180, 265–282. [Google Scholar] [CrossRef]
- Andersen, N.G. A gastric evacuation model for three predatory gadoids and implications of using pooled field data of stomach contents to estimate food rations. J. Fish Biol. 2001, 59, 1198–1217. [Google Scholar] [CrossRef]
- Gillum, Z.D.; Facendola, J.J.; Scharf, F.S. Consumption and gastric evacuation in juvenile red drum Sciaenops ocellatus (Linnaeus): Estimation of prey type effects and validation of field-based daily ration estimates. J. Exp. Mar. Biol. Ecol. 2012, 413, 21–29. [Google Scholar] [CrossRef]
- Seyhan, K.; Bascinar, N.S.; Bascinar, N.; Khan, U. Gastric evacuation in brook trout (Salvelinus fontinalis) fry: Effect of body size. Pak. J. Zool. 2020, 52, 1173. [Google Scholar] [CrossRef]
- Persson, L. Rate of food evacuation in roach (Rutilus rutilus) in relation to temperature, and the application of evacuation rate estimates for studies on the rate of food consumption. Freshw. Biol. 1982, 12, 203–210. [Google Scholar] [CrossRef]
- Temming, A.; Andersen, N.G. Modelling gastric evacuation in cod. ICES CM 1992, 61, 2–13. [Google Scholar]
- Sweka, J.A.; Keith Cox, M.; Hartman, K.J. Gastric evacuation rates of brook trout. Trans. Am. Fish. Soc. 2004, 133, 204–210. [Google Scholar] [CrossRef]
- Chabot, D.; Behrens, J.; Andersen, N.G. Digesting in hypoxia: Impact on gastric evacuation rate and postprandial metabolism (SDA) of Atlantic cod, Gadus morhua. In Proceedings of the ICES Annual Science Conference 2015, Copenhagen, Denmark, 21–25 September 2015. [Google Scholar]
- Barreto, R.E.; Barbosa-Júnior, A.; Urbinati, E.C.; Hoffmann, A. Cortisol influences the antipredator behavior induced by chemical alarm cues in the Frillfin goby. Horm. Behav. 2014, 65, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Archard, G.A.; Earley, R.L.; Hanninen, A.F.; Braithwaite, V.A. Correlated behaviour and stress physiology in fish exposed to different levels of predation pressure. Funct. Ecol. 2012, 26, 637–645. [Google Scholar] [CrossRef]
- Rehnberg, B.G.; Schreck, C.B. Chemosensory detection of predators by coho salmon (Oncorhynchus kisutch): Behavioural reaction and the physiological stress response. Can. J. Zool. 1987, 65, 481–485. [Google Scholar] [CrossRef]
- Barreto, R.E.; Miyai, C.A.; Sanches, F.H.C.; Giaquinto, P.C.; Delicio, H.C.; Volpato, G.L. Blood cues induce antipredator behavior in Nile tilapia conspecifics. PLoS ONE 2013, 8, e54642. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, P.C.; Volpato, G.L. Chemical cues related to conspecific size in pintado catfish, Pseudoplatystoma coruscans. Acta Ethologica 2005, 8, 65–69. [Google Scholar] [CrossRef]
- Giaquinto, P.C.; Hoffmann, A. The scent of stress: Pintado catfish differentially respond to chemical cues from stressed conspecifics. Behaviour 2012, 149, 941–951. [Google Scholar] [CrossRef]
- Fraser, D.F.; Huntingford, F.A. Feeding and avoiding predation hazard: The behavioral response of the prey. Ethology 1986, 73, 56–68. [Google Scholar] [CrossRef]
- Chapple, D.G.; Simmonds, S.M.; Wong, B.B. Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol. Evol. 2012, 27, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, P.; Persson, A.; Behrens, J.W.; Brodin, T.; Hirsch, P.E.; Sundelin, A.; van Deurs, M.; von Friesen, L.W.; Nilsson, P.A. Personality-dependent inter-and intraspecific foraging competition in the invasive round goby, Neogobius melanostomus. J. Fish Biol. 2021, 98, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Galli, A.; Behrens, J.W.; Gesto, M.; Moran, N.P. Boldness and physiological variation in round goby populations along their Baltic Sea invasion front. Physiol. Behav. 2023, 269, 114261. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.J.; Klemet-N’Guessan, S.; Hossie, T.J.; Fox, M.G. Boldness, movement and exploration tendency in round goby (Neogobius melanostomus) in Southern Ontario. J. Fish Biol. 2022, 103, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Mittelbach, G.G.; Ballew, N.G.; Kjelvik, M.K. Fish behavioral types and their ecological consequences. Can. J. Fish. Aquat. Sci. 2014, 71, 927–944. [Google Scholar] [CrossRef]
- Ioannou, C.; Payne, M.; Krause, J. Ecological consequences of the bold-shy continuum: The effect of predator boldness on prey risk. Oecologia 2008, 157, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Thorlacius, M.; Brodin, T. Investigating large-scale invasion patterns using-small scale invasion successions—Phenotypic differentiation of the invasive round goby (Neogobius melanostomus) at invasion fronts. Limnol. Oceanogr. 2018, 63, 702–713. [Google Scholar] [CrossRef]
- Behrens, J.W.; von Friesen, L.W.; Brodin, T.; Ericsson, P.; Hirsch, P.E.; Persson, A.; Sundelin, A.; van Deurs, M.; Nilsson, P.A. Personality-and size-related metabolic performance in invasive round goby (Neogobius melanostomus). Physiol. Behav. 2020, 215, 112777. [Google Scholar] [CrossRef]
- Manassa, R.P.; McCormick, M.I. Social learning and acquired recognition of a predator by a marine fish. Anim. Cogn. 2012, 15, 559–565. [Google Scholar] [CrossRef]
- Pūtys, Ž.; Ložys, L.; Būda, V. Respiratory response to the chemical cues of injured conspecifi cs and histology of skin in round goby, Neogobius melanostomus (Actinopterygii: Perciformes: Gobiidae). Acta Ichthyol. Piscat. 2015, 45, 411–415. [Google Scholar] [CrossRef]
- Chivers, D.P.; Dixson, D.L.; White, J.R.; McCormick, M.I.; Ferrari, M.C. Degradation of chemical alarm cues and assessment of risk throughout the day. Ecol. Evol. 2013, 3, 3925–3934. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ferrar, M.C.; Chivers, D.P. Threat-sensitive learning of predator odours by a prey fish. Behaviour 2006, 143, 1103–1121. [Google Scholar] [CrossRef]
- Zhao, X.; Chivers, D.P. Response of juvenile goldfish (Carassius auratus) to chemical alarm cues: Relationship between response intensity, response duration, and the level of predation risk. In Chemical Signals in Vertebrates 10; Mason, R.T., LeMaster, M.P., Müller-Schwarze, D., Eds.; Springer: Boston, MA, USA, 2005; pp. 334–341. [Google Scholar] [CrossRef]
- Kłosiński, P.; Kobak, J.; Augustyniak, M.; Pawlak, R.; Jermacz, Ł.; Poznańska-Kakareko, M.; Kakareko, T. Behavioural responses to con-and heterospecific alarm cues by an alien and a coexisting native fish. Hydrobiologia 2022, 849, 985–1000. [Google Scholar] [CrossRef]
- Wisenden, B.; Rugg, M.; Korpi, N.; Fuselier, L. Lab and field estimates of active time of chemical alarm cues of a cyprinid fish and an amphipod crustacean. Behaviour 2009, 146, 1423–1442. [Google Scholar]
- Wagner, C.M.; Bals, J.D.; Byford, G.J.; Scott, A.M.; Feder, M.E. Olfactory sensitivity and threat-sensitive responses to alarm cue in an invasive fish. Biol. Invasions 2023, 25, 3083–3101. [Google Scholar] [CrossRef]
- Atema, J. Chemical Senses, Chemical Signals, and Feeding Behavior in Fishes; Bardach, J.E., Magnuson, J.J., May, R.C., Reinhart, J.M., Eds.; FAO: Makati, Philippines, 1980; pp. 57–101. [Google Scholar]
- Kasumyan, A.O. The olfactory system in fish: Structure, function, and role in behavior. J. Ichthyol. 2004, 44, 180. [Google Scholar]
- Belanger, R.M.; Smith, C.M.; Corkum, L.D.; Zielinski, B.S. Morphology and histochemistry of the peripheral olfactory organ in the round goby, Neogobius melanostomus (Teleostei: Gobiidae). J. Morphol. 2003, 257, 62–71. [Google Scholar] [CrossRef]
- Pfeiffer, W. The distribution of fright reaction and alarm substance cells in fishes. Copeia 1977, 653–665. [Google Scholar] [CrossRef]
- Smith, R.J.F. Alarm signals in fishes. Rev. Fish Biol. Fish. 1992, 2, 33–63. [Google Scholar] [CrossRef]
- Marsh-Hunkin, K.E.; Gochfeld, D.J.; Slattery, M. Antipredator responses to invasive lionfish, Pterois volitans: Interspecific differences in cue utilization by two coral reef gobies. Mar. Biol. 2013, 160, 1029–1040. [Google Scholar] [CrossRef]
- McLean, F.; Barbee, N.C.; Swearer, S.E. Avoidance of native versus non-native predator odours by migrating whitebait and juveniles of the common galaxiid, Galaxias maculatus. N. Z. J. Mar. Freshw. Res. 2007, 41, 175–184. [Google Scholar] [CrossRef]
- Utne-Palm, A.C. Response of naïve two-spotted gobies Gobiusculus flavescens to visual and chemical stimuli of their natural predator, cod Gadus morhua. Mar. Ecol. Prog. Ser. 2001, 218, 267–274. [Google Scholar] [CrossRef]
- Ferrari, M.C.; Messier, F.; Chivers, D.P. Degradation of chemical alarm cues under natural conditions: Risk assessment by larval woodfrogs. Chemoecology 2007, 17, 263–266. [Google Scholar] [CrossRef]
- Mathis, A.; Smith, R.J.F. Chemical alarm signals increase the survival time of fathead minnows (Pimephales promelas) during encounters with northern pike (Esox lucius). Behav. Ecol. 1993, 4, 260–265. [Google Scholar] [CrossRef]
- Wisenden, B.D.; Chivers, D.P. The role of public chemical information in antipredator behaviour. Commun. Fishes 2006, 1, 259–278. [Google Scholar]
- Pollock, M.S.; Chivers, D.P.; Mirza, R.S.; Wisenden, B.D. Fathead minnows, Pimephales promelas, learn to recognize chemical alarm cues of introduced brook stickleback, Culaea inconstans. Environ. Biol. Fishes 2003, 66, 313–319. [Google Scholar] [CrossRef]
- Dalesman, S.; Rundle, S.D.; Bilton, D.T.; Cotton, P.A. Phylogenetic relatedness and ecological interactions determine antipredator behavior. Ecology 2007, 88, 2462–2467. [Google Scholar] [CrossRef]
- Vilhunen, S.; Hirvonen, H. Innate antipredator responses of Arctic charr (Salvelinus alpinus) depend on predator species and their diet. Behav. Ecol. Sociobiol. 2003, 55, 1–10. [Google Scholar] [CrossRef]
- Mathis, A.; Smith, R.J.F. Chemical labeling of northern pike (Esox lucius) by the alarm pheromone of fathead minnows (Pimephales promelas). J. Chem. Ecol. 1993, 19, 1967–1979. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.E.; Godin, J.G.J. Who dares, learns: Chemical inspection behaviour and acquired predator recognition in a characin fish. Anim. Behav. 1999, 57, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.E.; Godin, J.G.J. Anti-predator responses to conspecific and heterospecific skin extracts by threespine sticklebacks: Alarm pheromones revisited. Behaviour 1997, 134, 1123–1134. [Google Scholar] [CrossRef]
- Pfeiffer, W. Alarm substances. Experientia 1963, 19, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Wisenden, B. Active space of chemical alarm cue in natural fish populations. Behaviour 2008, 145, 391–407. [Google Scholar] [CrossRef]
- Olson, J.A.; Olson, J.M.; Walsh, R.E.; Wisenden, B.D. A method to train groups of predator-naive fish to recognize and respond to predators when released into the natural environment. N. Am. J. Fish. Manag. 2012, 32, 77–81. [Google Scholar] [CrossRef]
- Rakauskas, V.; Pūtys, Ž.; Dainys, J.; Lesutienė, J.; Ložys, L.; Arbačiauskas, K. Increasing population of the invader round goby, Neogobius melanostomus (Actinopterygii: Perciformes: Gobiidae), and its trophic role in the Curonian Lagoon, SE Baltic Sea. Acta Ichthyol. Piscat. 2013, 43, 95–108. [Google Scholar] [CrossRef]
- Jůza, T.; Blabolil, P.; Bartoň, D.; Čech, M.; Draštík, V.; Frouzova, J.; Holubová, M.; Ketelaars, H.A.M.; Kočvara, L.; Kubečka, J.; et al. Recovery of the ruffe (Gymnocephalus cernua) population after an invasion boom of round goby (Neogobius melanostomus) in De Gijster Lake (The Netherlands). Aquat. Invasions 2021, 16, 499–511. [Google Scholar] [CrossRef]
- Ylönen, H.; Kortet, R.; Myntti, J.; Vainikka, A. Predator odor recognition and antipredatory response in fish: Does the prey know the predator diel rhythm? Acta Oecologica 2007, 31, 1–7. [Google Scholar] [CrossRef]
- Utne, A.C.W.; Bacchi, B. The influence of visual and chemical stimuli from cod Gadus morhua on the distribution of two-spotted goby Gobiusculus flavescens (Fabricius). Sarsia 1997, 82, 129–135. [Google Scholar] [CrossRef]
- Sreedharan, G.; Corkum, L.D.; Johnson, T.B. Response of the round goby, an invasive fish, to food odours. Int. Ver. Theor. Angew. Limnol. Verhandlungen 2009, 30, 1275–1278. [Google Scholar] [CrossRef]
- Holmes, T.H.; McCormick, M.I. Response across a gradient: Behavioural reactions of newly settled fish to predation cues. Anim. Behav. 2011, 81, 543–550. [Google Scholar] [CrossRef]
- Yavno, S.; Corkum, L.D. Round goby Neogobius melanostomus attraction to conspecific and heterospecific egg odours. J. Fish Biol. 2011, 78, 1944–1953. [Google Scholar] [CrossRef]
- Hirvonen, H.; Holopainen, S.; Lempiäinen, N.; Selin, M.; Tulonen, J. Sniffing the trade-off: Effects of eel odours on nocturnal foraging activity of native and introduced crayfish juveniles. Mar. Freshw. Behav. Physiol. 2007, 40, 213–218. [Google Scholar] [CrossRef]


| Model | Predictor | df | Deviance | F Value | p-Value | |
|---|---|---|---|---|---|---|
| Model 1: (GI) ~ Treatment × Time | ||||||
| Treatment | 2 | 0.0015 | 3.13 | 0.047 | * | |
| Time | 1 | 0.0034 | 1.56 | <0.001 | *** | |
| Treatment × Time | 2 | 0.0014 | 0.04 | 0.964 | ||
| Residual df: = 138 | ||||||
| Model 2: (GI) ~ Treatment + Time | ||||||
| Treatment | 2 | 0.0015 | 4.69 | 0.011 | * | |
| Time | 1 | 0.0061 | 453.91 | <0.001 | *** | |
| Residual df: = 140 | ||||||
| Model | Predictor | df | Deviance | F Value | p-Value |
|---|---|---|---|---|---|
| Food B consumption ~ Treatment × Time | |||||
| Treatment | 2 | 347.82 | 1.0178 | 0.3649 | |
| Time | 6 | 352.13 | 0.5607 | 0.7607 | |
| Treatment × Time | 12 | 364.15 | 0.5884 | 0.8473 | |
| Residual df: = 105 | |||||
| Food B consumption ~ Treatment + Time | |||||
| Treatment | 2 | 375.73 | 1.8609 | 0.1601 | |
| Time | 6 | 386.55 | 1.1994 | 0.3115 | |
| Residual df: = 117 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szydłowska, N.Z.; Franta, P.; Let, M.; Mikšovská, V.; Buřič, M.; Drozd, B. Risk Perception: Chemical Stimuli in Predator Detection and Feeding Behaviour of the Invasive Round Goby Neogobius melanostomus. Biology 2024, 13, 406. https://doi.org/10.3390/biology13060406
Szydłowska NZ, Franta P, Let M, Mikšovská V, Buřič M, Drozd B. Risk Perception: Chemical Stimuli in Predator Detection and Feeding Behaviour of the Invasive Round Goby Neogobius melanostomus. Biology. 2024; 13(6):406. https://doi.org/10.3390/biology13060406
Chicago/Turabian StyleSzydłowska, Natalia Z., Pavel Franta, Marek Let, Vendula Mikšovská, Miloš Buřič, and Bořek Drozd. 2024. "Risk Perception: Chemical Stimuli in Predator Detection and Feeding Behaviour of the Invasive Round Goby Neogobius melanostomus" Biology 13, no. 6: 406. https://doi.org/10.3390/biology13060406
APA StyleSzydłowska, N. Z., Franta, P., Let, M., Mikšovská, V., Buřič, M., & Drozd, B. (2024). Risk Perception: Chemical Stimuli in Predator Detection and Feeding Behaviour of the Invasive Round Goby Neogobius melanostomus. Biology, 13(6), 406. https://doi.org/10.3390/biology13060406





