Analysis of the Distribution Characteristics of Jellyfish and Environmental Factors in the Seawater Intake Area of the Haiyang Nuclear Power Plant in China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Jellyfish Resource Survey
2.2. Environmental Data
2.3. Data Processing Methods
2.4. Generalized Additive Model (GAM)
+ s(YEAR) + s(LON) + s(LAT) + ∈
3. Results
3.1. Spatial and Temporal Changes in Jellyfish Resource Density
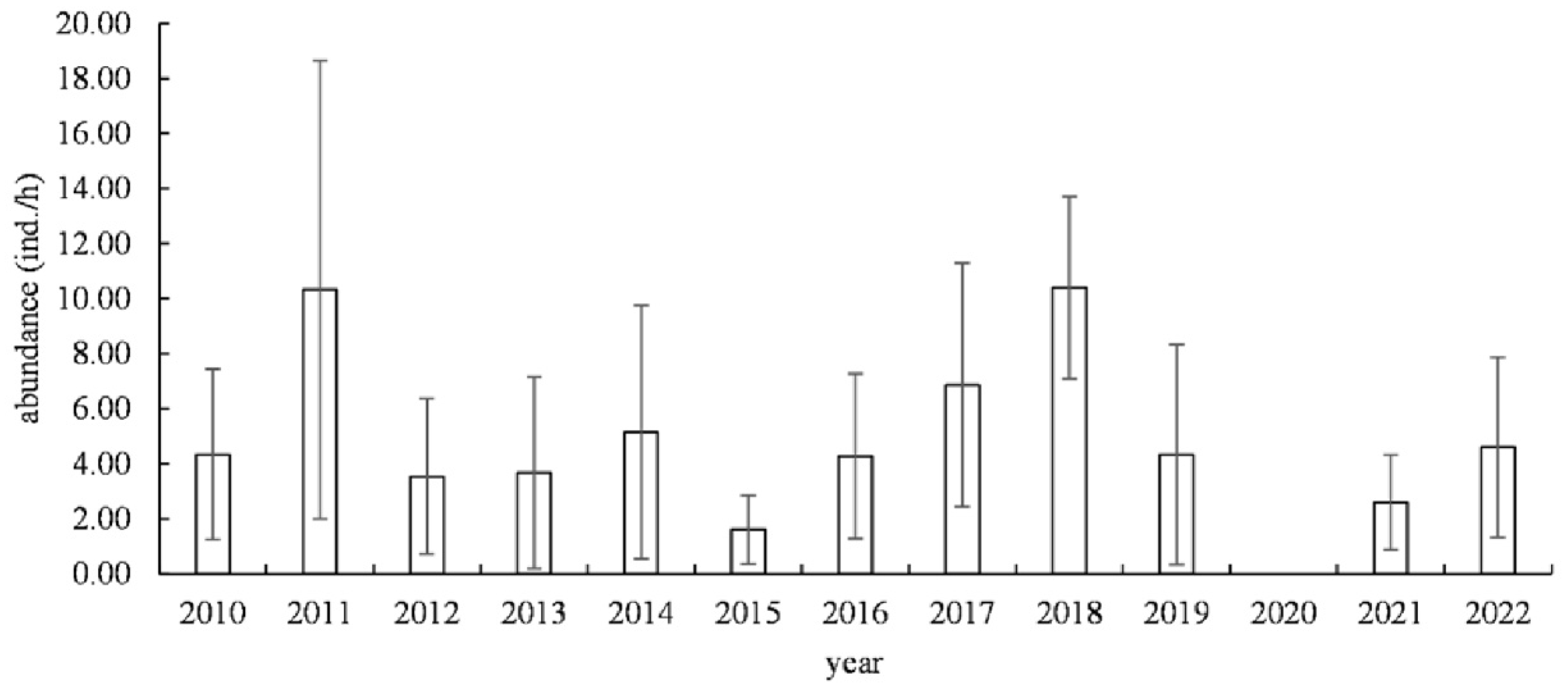
3.1.1. Temporal Changes
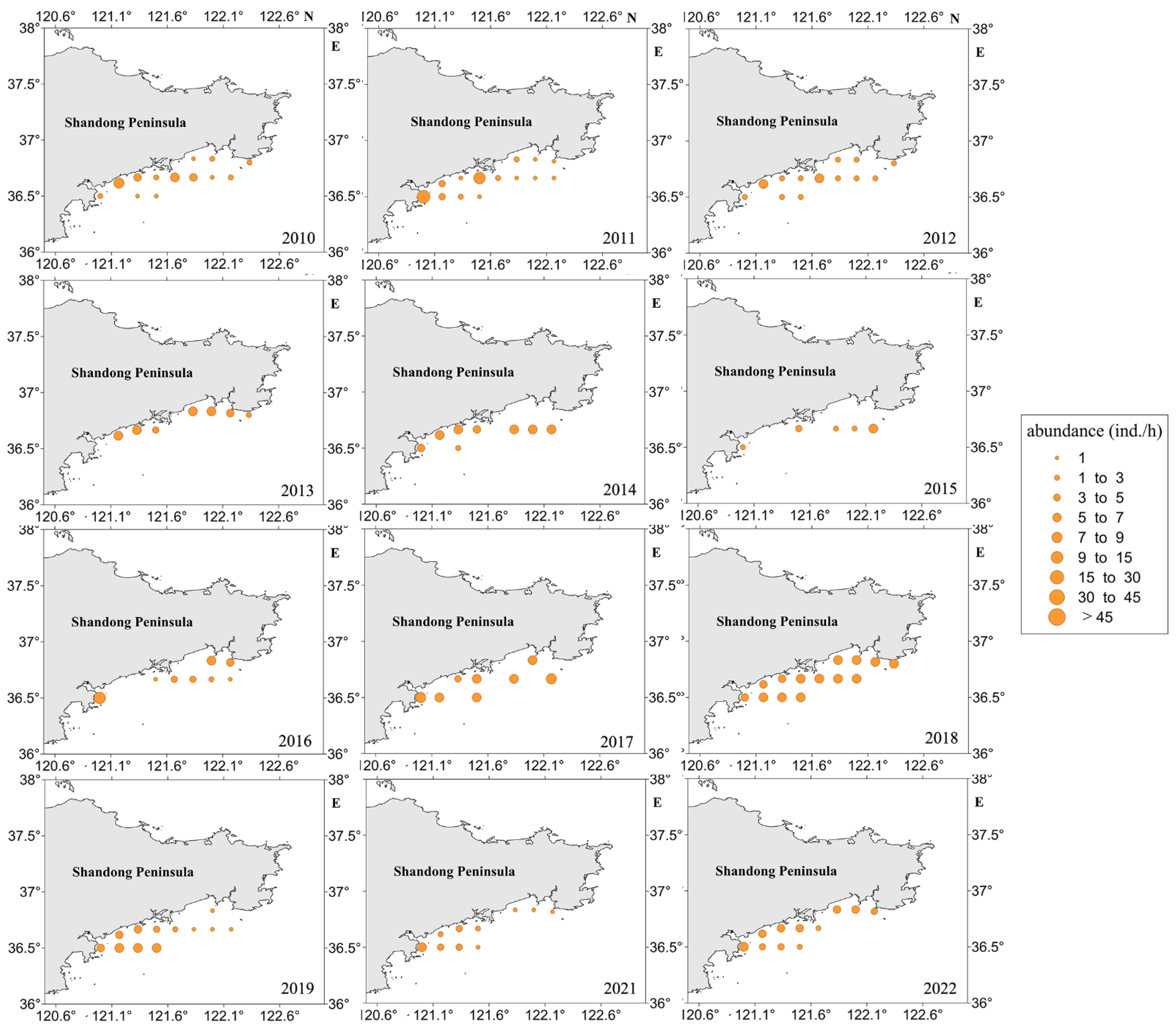
3.1.2. Spatial Changes
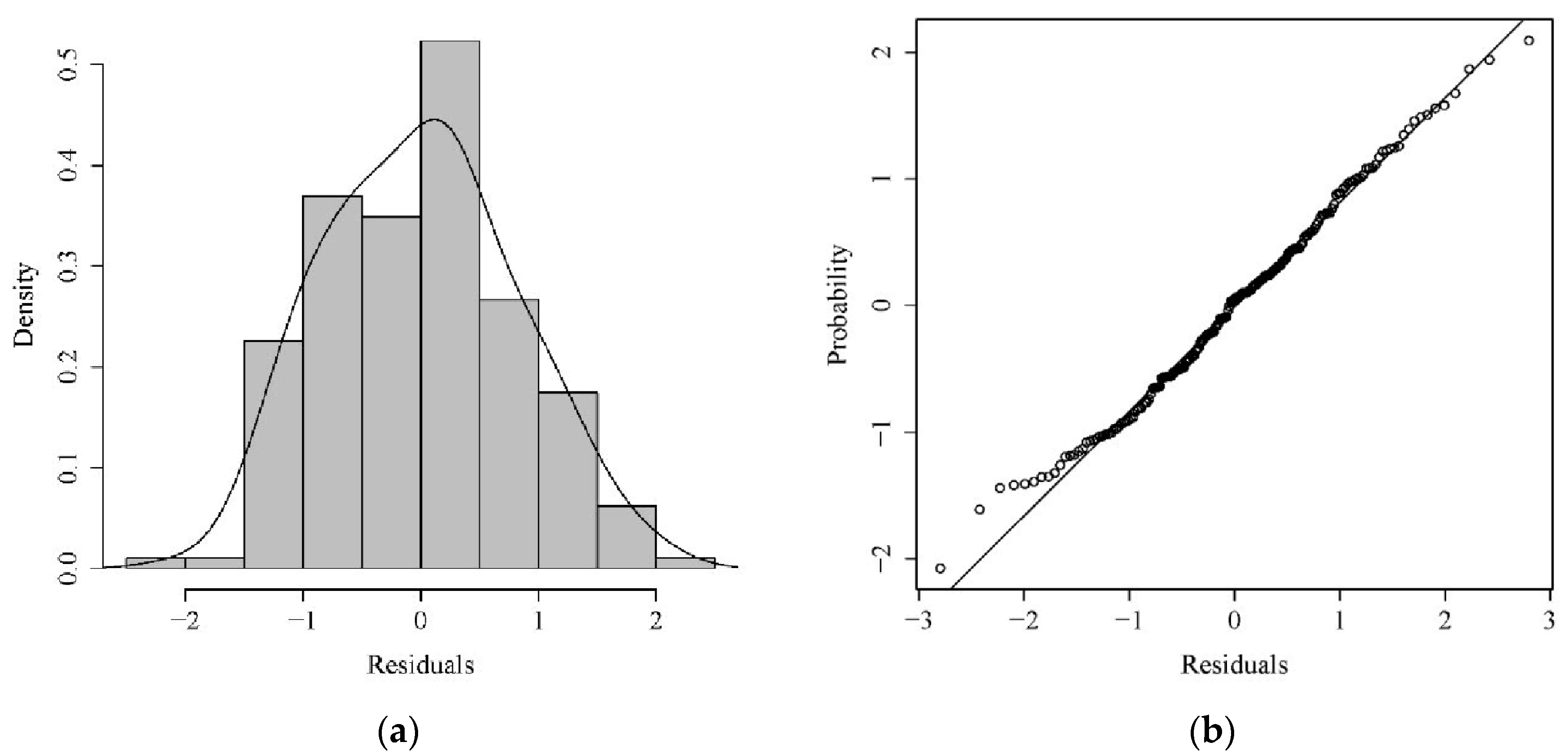
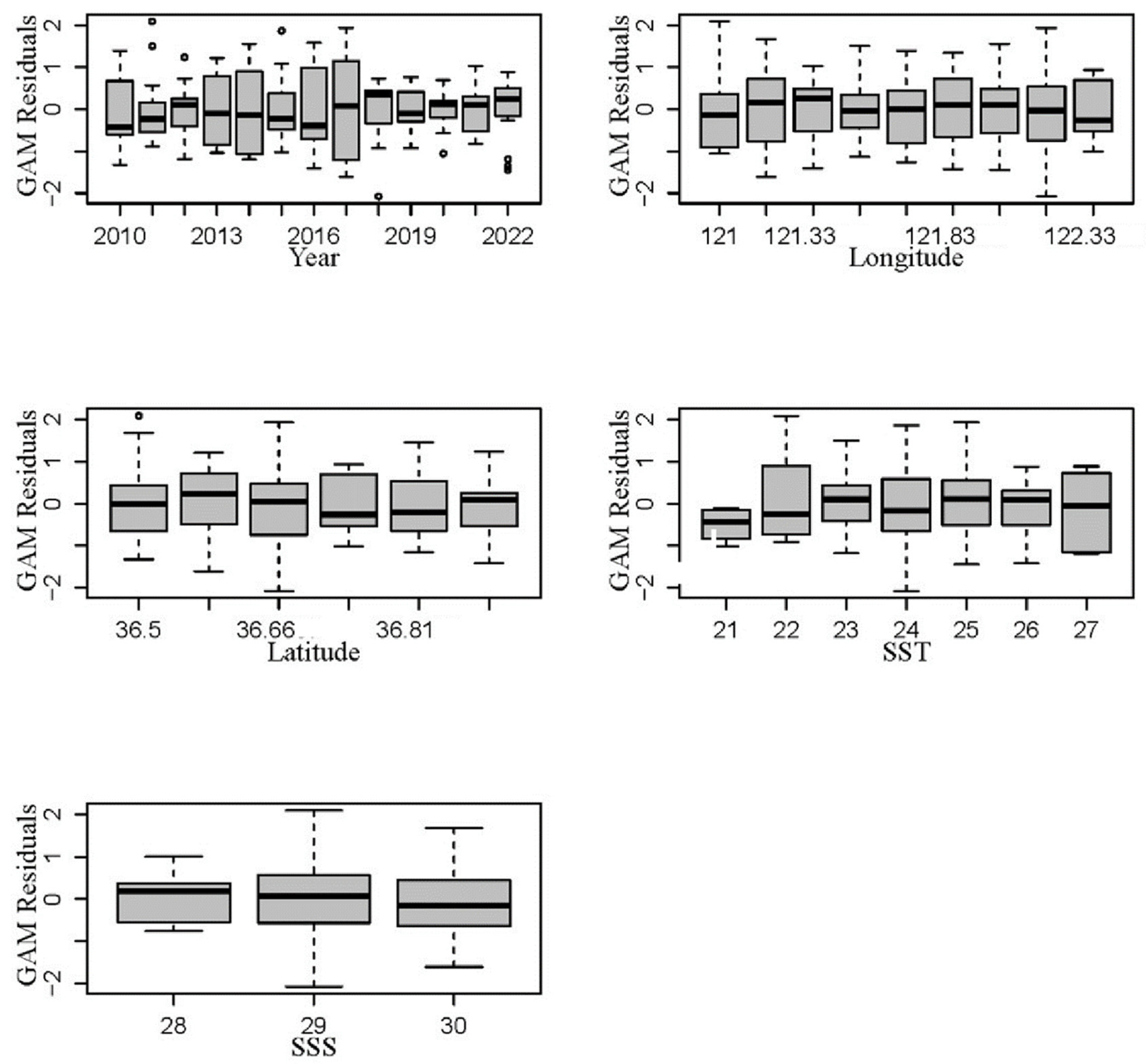
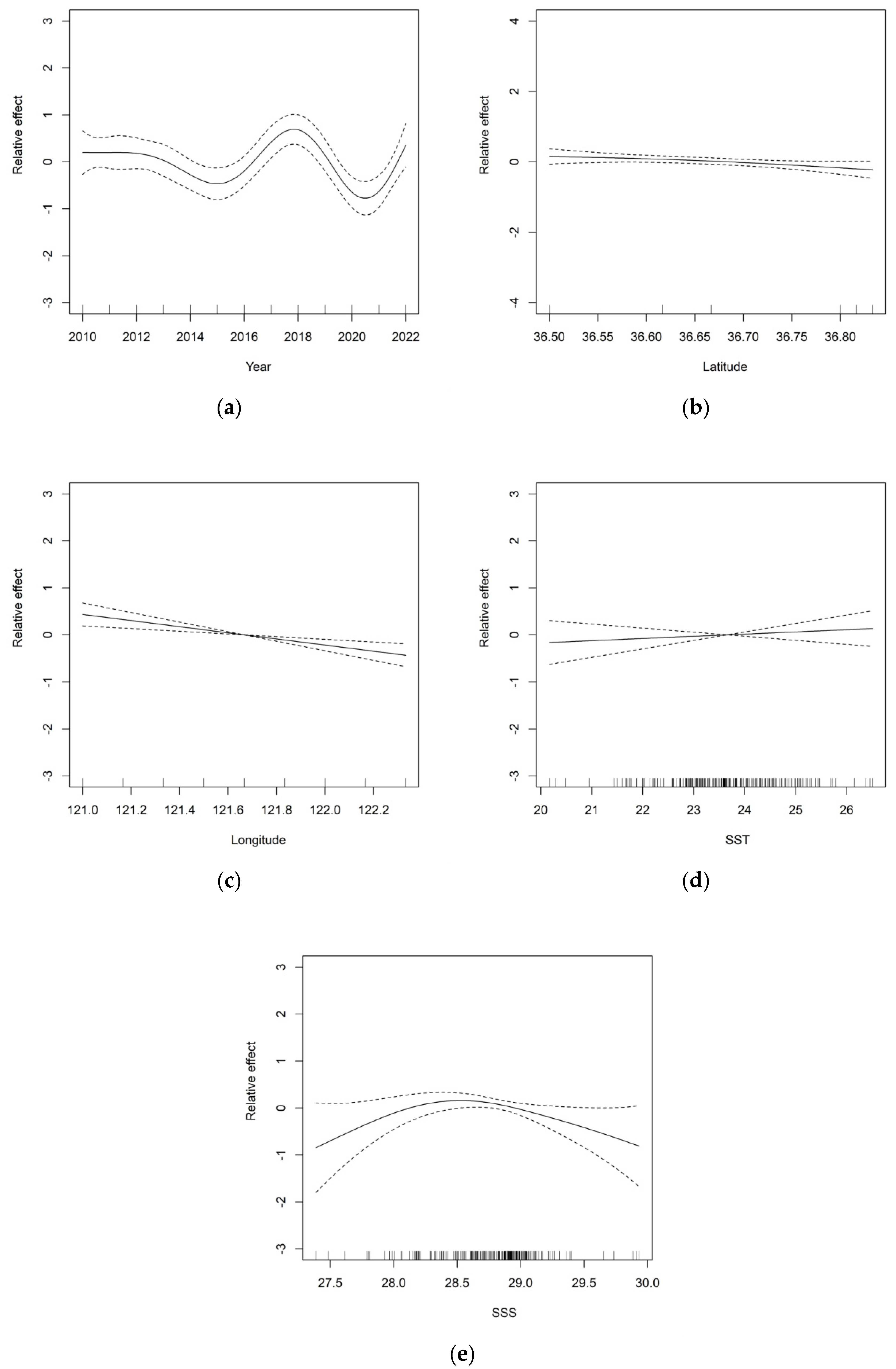
3.2. GAM Analysis of the Distribution of Jellyfish and Environmental Factors
3.3. The Relationship between Jellyfish Resource Density and Environmental Factors
3.3.1. Ocean Current
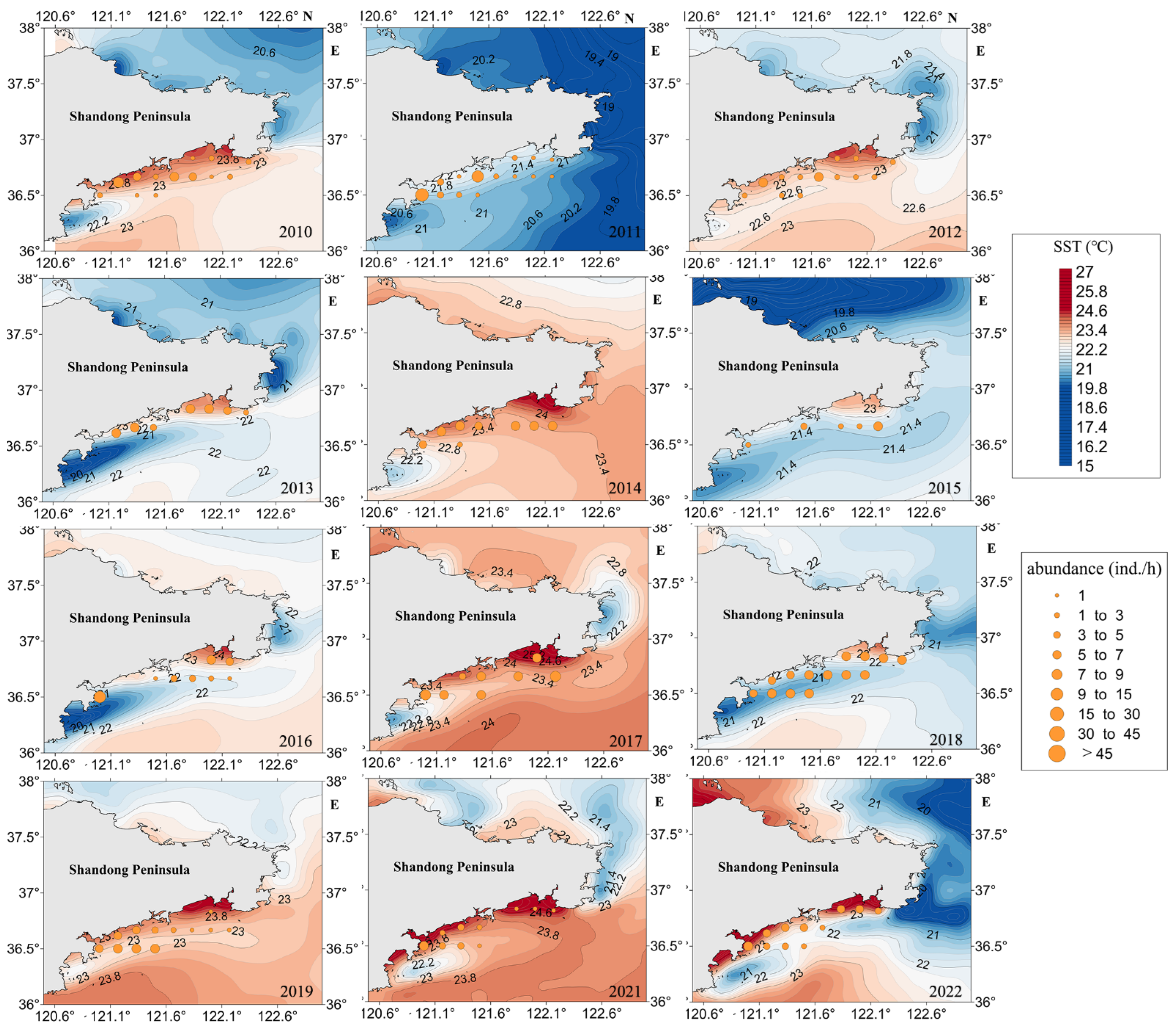
3.3.2. SST
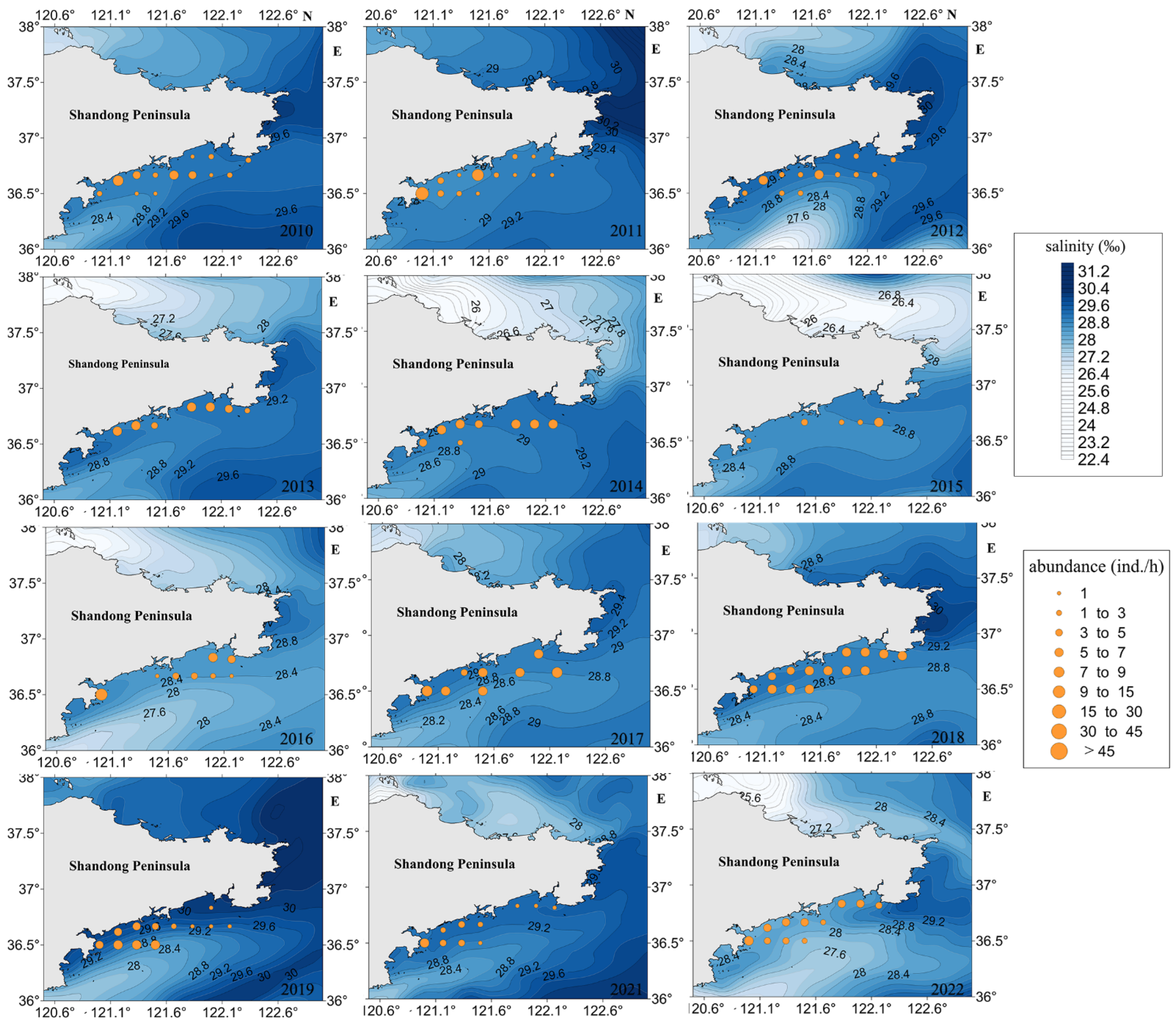
3.3.3. SSS
4. Discussion
4.1. The Spatiotemporal Distribution Characteristics of Jellyfish Resources
4.2. Analysis of GAM
4.3. The Impact of Environmental Factors on Jellyfish Aggregation
4.3.1. Ocean Current and Wind Field
4.3.2. SST and SSS
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q. Genetic Diversity and Population Genetic Structure of Two Jellyfish Species in Chinese Coastal Waters. Master’s Thesis, University of Chinese Academy of Sciences (Yantai Coastal Zone Research Institute, Chinese Academy of Sciences), Yantai, China, 2018. [Google Scholar]
- Zhou, B.; Xu, K. Spatiotemporal variation in community structure of marine benthic ciliates in the Yellow Sea during and after macroalgal and giant jellyfish blooms. Chin. J. Oceanol. Limnol. 2016, 34, 629–641. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Y.; Fu, Y.; Meng, W.; Wang, S.; Liu, X. Monitoring of biomass at Cooling Water System of hongyanhe nuclear power plant by using acoustic methods. E3S Web Conf. 2020, 194, 01007. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, H.; Liang, J.; Zhao, T.; Xu, Y.; Lei, Y. Microalgae removal technology for the cold source of nuclear power plant: A review. Mar. Pollut. Bull. 2022, 183, 114087. [Google Scholar] [CrossRef]
- Ni, J.; Chen, H.; Dai, L.; Ji, J.; Lin, J.; Yu, T. Early warning and monitoring of the safety risk of coastal nuclear power plant cold source under the stress from Phaeocystis globosa blooms. Mar. Freshw. Res. 2024, 75, MF23179. [Google Scholar] [CrossRef]
- Zhang, C.; Guan, C.; Xu, P.; Liu, G.; Xu, Q.; Ye, J.; Wang, X.; Zhao, B. Analysis on risk organisms for the cold source water of nuclear power plant in the eastern waters of Liaodong bay. Mar. Environ. Sci. 2019, 38, 41–45. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Z.; An, C. Research on the detection and early warning technology of harmful marine organisms in the water intake of nuclear power plant by 3D image sonar. E3S Web Conf. 2021, 290, 03013. [Google Scholar] [CrossRef]
- Fischell, E.M.; Schmidt, H. Classification of underwater targets from autonomous underwater vehicle sampled bistatic acoustic scattered fields. J. Acoust. Soc. Am. 2015, 138, 3773–3784. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.H.; Ai, D.W.; Liu, X.L.; Qin, L.; Lu, H.R. Multi-source heterogeneous data model for marine biological monitoring at intake of nuclear power plant. IOP Conf. Ser. Earth Environ. Sci. 2019, 344, 012095. [Google Scholar] [CrossRef]
- Handegard, N.O.; Buisson, L.D.; Brehmer, P.; Chalmers, S.J.; De Robertis, A.; Huse, G.; Kloser, R.; Macaulay, G.; Maury, O.; Ressler, P.H.; et al. Towards an acoustic-based coupled observation and modelling system for monitoring and predicting ecosystem dynamics of the open ocean. Fish Fish. 2013, 14, 605–615. [Google Scholar] [CrossRef]
- Huo, J.; Li, C.; Liu, S.; Sun, L.; Yang, L.; Song, Y.; Li, J. Biomass prediction method of nuclear power cold source disaster based on deep learning. Front. Mar. Sci. 2023, 10. [Google Scholar] [CrossRef]
- Li, S.; Qiu, S.; Wang, Z.; Ren, X.; Yu, W.; Ke, K. Density Distribution and Biological Characteristics of Rhopilema esculentum in Southern Shandong Peninsula. J. Yantai Univ. (Nat. Sci. Eng. Ed.) 2020, 33, 440–445. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, M.; Xu, B.; Feng, Y.; Wang, X.; Zhang, X.; Li, F.; Chen, J. Community structure of ichthyoplankton and its relationship with environmental factors in coastal waters of southern Shandong Peninsula. Ecol. Environ. Sci. 2021, 30, 995–1004. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, S.; Qiao, F.; Li, Z.; Geng, B. Relationship between Distributiono fJellyfish Resources and Zooplankton in Coastal Southern Shandong. Fish. Sci. 2013, 32, 98–101. [Google Scholar] [CrossRef]
- Song, Y.; Xing, X.; Lin, C.; Xiong, M.; Zhang, X.; Zhang, Z.; Shuang, W.; Zhang, S.; Shi, Y.; Wu, Z.; et al. Advances in the investigation and risk assessment of cold source blockages in nuclear power plants in China. Nucl. Eng. Des. 2024, 420, 112998. [Google Scholar] [CrossRef]
- Ren, X.; Jiao, J.; Qiu, S.; Wang, Z.; Li, S.; Liu, S.; Dong, X. Contribution of Releasing and Proliferation on Rhopilema esculentum to Fishing populations in Waters of Southern Shandong Peninsula. J. Yantai Univ. (Nat. Sci. Eng. Ed.) 2020, 33, 360–364+378. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, F.; Wang, P.; Sun, S. Distribution Patterns of Large Jellyfish and Their Effects on the Zooplankton Community in the Northern Chinese Coastal Seas during the Summer of 2021. Diversity 2023, 15, 729. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.; Liu, M.; Liu, J.; Dong, W.; Xu, T.; Wang, Y.; Guo, Y. Analysis of Water Quality and Habitat Suitability for Benthic Macro-Invertebrates in the Majiagou Urban River, China. Water Wastewater Eng. 2023, 15, 2269. [Google Scholar] [CrossRef]
- Li, B.; Cao, J.; Chang, J.-H.; Wilson, C.; Chen, Y. Evaluation of Effectiveness of Fixed-Station Sampling for Monitoring American Lobster Settlement. N. Am. J. Fish. Manag. 2015, 35, 942–957. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, Z.; Yu, Z.; Zhou, K.; Lin, J.; Wu, J.; Mu, J. Spatio-Temporal Variations of Zooplankton and Correlations with Environmental Parameters around Tiaowei Island, Fujian, China. Int. J. Environ. Res. Public Health 2022, 19, 12731. [Google Scholar] [CrossRef]
- Planque, B.; Bellier, E.; Lazure, P. Modelling potential spawning habitat of sardine (Sardina pilchardus) and anchovy (Engraulis encrasicolus) in the Bay of Biscay. Fish. Oceanogr. 2007, 16, 16–30. [Google Scholar] [CrossRef]
- Fang, J.T.; Zhang, J.; Feng, X.; Chen, Z.Z. Relationship between Sthenoteuthis oualaniensis fishing ground and marine environmental factors in Nansha area. J. Shanghai Ocean Univ. 2019, 28, 419–426. [Google Scholar] [CrossRef]
- Wang, Y. Biological and Ecological Characteristics and Resource Distribution of Larimichthys polyactis in Zhoushan Fishery Spawning Ground Reserve and Adjacent Sea Areas. Master′s Thesis, Zhejiang Ocean University, Zhoushan, China, 2023. [Google Scholar]
- Yin, J.; Wang, J.; Zhang, C.L.; Xu, B.; Xue, Y.; Ren, Y.P. Spatial and temporal distribution characteristics of Larimichthys polyactis eggs in Haizhou Bay and adjacent regions based on two-stage GAM. J. Fish. Sci. China 2019, 26, 1164–1174. [Google Scholar] [CrossRef]
- Shi, Y.C.; Zhu, Q.C.; Huang, S.L.; Feng, H.L. Study on CPUE standardization of Chinese Pacific saury (Cololabis saira) fishery in the Northwest Pacific Ocean. Prog. Fish. Sci. 2020, 41, 1–12. [Google Scholar] [CrossRef]
- Yao, L. Environmental Effects of Spatial-Temporal Distribution of Spawning Ground of Major Economic Fishes in the Western Guangdong Waters South China Sea. Master′s Thesis, Shanghai Ocean University, Shanghai, China, 2021. [Google Scholar]
- Kristofersson, D.; Anderson, J.L. Is there a relationship between fisheries and farming? Interdependence of fisheries, animal production and aquaculture. Mar. Policy 2006, 30, 721–725. [Google Scholar] [CrossRef]
- Wootton, J.T.; Power, M.E.; Paine, R.T.; Pfister, C.A. Effects of productivity, consumers, competitors, and El Niño events on food chain patterns in a rocky intertidal community. Proc. Natl. Acad. Sci. USA 1996, 93, 13855–13858. [Google Scholar] [CrossRef] [PubMed]
- Edgar, G.J.; Banks, S.A.; Brandt, M.; Bustamante, R.H.; Chiriboga, A.; Earle, S.A.; Garske, L.E.; Glynn, P.W.; Grove, J.S.; Henderson, S.; et al. El Niño, grazers and fisheries interact to greatly elevate extinction risk for Galapagos marine species. Glob. Chang. Biol. 2010, 16, 2876–2890. [Google Scholar] [CrossRef]
- Fang, Z.; Song, S.; Chao-Lun, L. Estimation on food requirement by large jellyfish Nemopilema nomurai in summer. Oceanol. Limnol. Sin. 2017, 48, 1355–1361. [Google Scholar] [CrossRef]
- Wang, B.; Qi, Y.B.; Dong, J.; Li, Y.L.; Wang, W.B.; Li, Y.P.; Sun, M.; Liu, C.Y. Dynamic distribution of Nemopilema nomurai in inshore waters of the northern Liaodong Bay, Bohai Sea. Acta Ecol. Sin. 2013, 33, 1701–1712. [Google Scholar] [CrossRef]
- Faulk, L.G.; Smart, T.; Stone, J.P. Temporal and spatial distribution of the cannonball jellyfish Stomolophus meleagris in the South Atlantic Bight, USA. Mar. Ecol. Prog. Ser. 2023, 717, 51–65. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, H.; Zhao, M.; Sun, T.; Guo, R. Standardization of catch per unit effort(cpue) in northwest indian ocean Sthenoteuthis oualaniensis based on generalized additive model. Oceanol. Limnol. Sin. 2023, 54, 259–265. [Google Scholar] [CrossRef]
- Lu, H.; Chen, X.; Fang, Z. The spatial and temporal changes of fishing grounds of Illex argentinus and its relationship with sea surface temperature (SST) in the Southwest Atlantic. Mar. Fish. 2013, 35, 382–388. [Google Scholar]
- Xu, J.; Guan, W.; Chen, X. A study of incorporating spatial autocorrelation into CPUE standardization with an application to Ommastrephes bartramii in the northwest Pacific Ocean. J. Fish. China 2015, 39, 754–760. [Google Scholar] [CrossRef]
- Baliarsingh, S.K.; Lotliker, A.A.; Srichandan, S.; Samanta, A.; Kumar, N.; Nair, T.M.B. A review of jellyfish aggregations, focusing on India’s coastal waters. Ecol. Process 2020, 9, 58. [Google Scholar] [CrossRef]
- Chen, X.J.; Tian, S.Q. Effects of sst and temp-spatial factors on abundance of nylon flying squid Ommastrephes bartrami in the northwestern pacific using generalized additive models. Trans. Oceanol. Limnol. 2007, 2, 104–113. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, C.; Lin, N.; Zhong, J.; Xu, Z. Influence of abiotic factors on spatiotemporal patterns of larval fish assemblages in the surf zones of the Yangtze River estuary and Hangzhou Bay. J. Fish. Sci. China 2015, 22, 780–790. [Google Scholar] [CrossRef]
- Ma, J.; Huang, J.; Chen, J.; Li, B.; Zhao, J.; Gao, C.; Wang, X.; Tian, S. Analysis of spatiotemporal fish density distribution and its influential factors based on generalized additive model (GAM) in the Yangtze River estuary. J. Fish. China 2020, 44, 936–946. [Google Scholar] [CrossRef]
- Mu, X.; Li, M.; Yin, J.; Zhang, C.; Ren, Y. Relationship between spatio-temporal distribution of Conger myriaster and the environment factors in the southeast waters of Shandong Peninsula. J. Fish. China 2019, 43, 1759–1767. [Google Scholar] [CrossRef]
- Xu, Y.; Ishizaka, J.; Yamaguchi, H.; Siswanto, E.; Wang, S. Relationships of interannual variability in SST and phytoplankton blooms with giantjellyfish (Nemopilema nomurai) outbreaks in the Yellow Sea and East China Sea. J. Oceanogr. 2013, 69, 511–526. [Google Scholar] [CrossRef]
- Richardson, A.J.; Bakun, A.; Hays, G.C.; Gibbons, M.J. The jellyfish joyride: Causes, consequences and management responses to a more gelatinous future. Trends Ecol. Evol. 2009, 24, 312–322. [Google Scholar] [CrossRef]
- Chang, P.-H.; Isobe, A. A numerical study on the Changjiang diluted water in the Yellow and East China Seas. J. Geophys. Res. Ocean. 2003, 108. [Google Scholar] [CrossRef]
- Moon, J.-H.; Pang, I.-C.; Yang, J.-Y.; Yoon, W.D. Behavior of the giant jellyfish Nemopilema nomurai in the East China Sea and East/Japan Sea during the summer of 2005: A numerical model approach using a particle-tracking experiment. J. Mar. Syst. 2010, 80, 101–114. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Wang, S.; Wang, M.; Zhang, G. Geographical variations in abundance and size of Aequorea coerulescens in the yellow and east China seas in spring 2011. Oceanol. Limnol. Sin. 2012, 43, 1096–1102. [Google Scholar]
- Chi, X.; Mueller-Navarra, D.C.; Hylander, S.; Sommer, U.; Javidpour, J. Food quality matters: Interplay among food quality, food quantity and temperature affecting life history traits of Aurelia aurita (Cnidaria: Scyphozoa) polyps. Sci. Total Environ. 2019, 656, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Alexandra, L.; Lucas, C.H. Transgenerational effects influence acclimation to varying temperatures in Aurelia aurita polyps (Cnidaria: Scyphozoa). Hydrobiologia 2023, 850, 1955–1967. [Google Scholar] [CrossRef]
- Fernández-Alías, A.; Marcos, C.; Quispe, J.I.; Sabah, S.; Pérez-Ruzafa, A. Population dynamics and growth in three scyphozoan jellyfishes, and their relationship with environmental conditions in a coastal lagoon. Estuarine Coast. Shelf Sci. 2020, 243, 106901. [Google Scholar] [CrossRef]
- Leoni, V.; Molinero, J.C.; Meffre, M.; Bonnet, D. Variability of growth rates and thermohaline niches of Rhizostoma pulmo’s pelagic stages (Cnidaria: Scyphozoa). Mar. Biol. 2021, 168, 107. [Google Scholar] [CrossRef]
- Fernández-Alías, A.; Molinero, J.C.; Quispe-Becerra, J.I.; Bonnet, D.; Marcos, C.; Pérez-Ruzafa, A. Phenology of scyphozoan jellyfish species in a eutrophication and climate change context. Mar. Pollut. Bull. 2023, 194, 115286. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, Y.; Yanagi, T.; Guo, X. Tracer experiments for appearance of the giant jellyfish, Nemopilema nomurai, in the southern Japanese coastal area of the Pacific Ocean in summer 2005. Rep. Res. Inst. Appl. Mech. Kyushu Univ. 2008, 134, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Mi, T.Z.; Yao, Q.Z.; Meng, J.; Zhang, X.L.; Liu, S.M. Distributions of nutrients in the southern yellow sea and east China sea in spring and summer 2011. Oceanol. Limnol. Sin. 2012, 43, 678–688. [Google Scholar]
- Zhang, S.; Chen, D.; Liu, J.; Sun, W.; Zhang, J. Effect of pH, salinity and temperature on the oxygen consumption rate and ammonia excretion rate of Rhopilema esculenta. J. Fish. China 2023, 47, 51–61. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, A.; Jiang, Y.; Qi, J. Effects of three levels of salinities on the growth and digestive enzyme activities of Phyllorhiza chinensis at different stages. Mar. Sci. 2023, 47, 97–105. [Google Scholar] [CrossRef]
- Graham, W.M.; Pagès, F.; Hamner, W.M. A physical context for gelatinous zooplankton aggregations: A review. Hydrobiologia 2001, 451, 199–212. [Google Scholar] [CrossRef]
- Meng, W.; Liu, Y.; Li, J.; Liu, X.; Guo, X.; Cheng, Z. Early warning model for marine organism detection in a nuclear power plant based on multi-source information fusion technology. J. Dalian Ocean. Univ. 2019, 34, 840–845. [Google Scholar] [CrossRef]
- Wang, X. The Study of Coping Strategies to the Cold-source Marine Organism in Taipingling Nuclear Power Plant. Guangdong Chem. Ind. 2021, 48, 207–209. [Google Scholar]


| Variable | YEAR | LON | LAT | SST | SSS | SSC_speed | SSC_dir | Wind_speed | Wind_dir |
|---|---|---|---|---|---|---|---|---|---|
| VIF value | 1.52 | 2.85 | 3.4 | 1.94 | 1.22 | 1.71 | 1.64 | 1.19 | 1.21 |
| GAM | R2 (%) | AIC | Explanation Rate (%) |
|---|---|---|---|
| Log(abundance + 1) = s(YEAR) | 14.39 | 550.6193 | 26.85 |
| log(abundance + 1) = s(YEAR) + s(LON) | 20.81 | 542.8741 | 31.77 |
| log(abundance + 1) = s(YEAR) + s(LON) + s(LAT) | 21.59 | 539.7905 | 32.67 |
| log(abundance + 1) = s(YEAR) + s(LON) + s(LAT) + s(SST) | 27.33 | 534.9276 | 37.39 |
| log(abundance + 1) = s(YEAR) + s(LON) + s(LAT) + s(SST) + s(SSS) | 32.47 | 529.0556 | 42.27 |
| log(abundance + 1) = s(YEAR) + s(LON) + s(LAT) + s(SST) + s(SSS) + s(Wind_speed) | 32.11 | 530.8148 | 41.96 |
| log(abundance + 1) = s(YEAR) + s(LON) + s(LAT) + s(SST) + s(SSS) + s(Wind_speed) + s(Wind_dir) | 29.77 | 532.7855 | 40.33 |
| log(abundance + 1) = s(YEAR) + s(LON) + s(LAT) + s(SST) + s(SSS) + s(Wind_speed) + s(Wind_dir) + s(SSC_speed) | 30.69 | 531.2447 | 41.23 |
| log(abundance + 1) = s(YEAR) + s(LON) + s(LAT) + s(SST) + s(SSS) + s(Wind_speed) + s(Wind_dir) + s(SSC_speed) + s(SSC_dir) | 27.51 | 533.1651 | 39.84 |
| Parameter | df | F | p Value |
|---|---|---|---|
| factor(YEAR) | 12 | 5.819337 | 2.28 × 10−8 |
| factor(LON) | 8 | 3.512305 | 8.91 × 10−3 |
| factor(LAT) | 5 | 4.668616 | 5.16 × 10−3 |
| s(SST) | 3 | 0.787265 | 0.416 |
| s(SSS) | 3 | 2.754958 | 0.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Wang, T.; Xiong, M.; Yang, S.; Zhang, H.; Ying, J.; Shi, Y.; Zhao, G.; Zhang, X.; Liu, X.; et al. Analysis of the Distribution Characteristics of Jellyfish and Environmental Factors in the Seawater Intake Area of the Haiyang Nuclear Power Plant in China. Biology 2024, 13, 433. https://doi.org/10.3390/biology13060433
Song Y, Wang T, Xiong M, Yang S, Zhang H, Ying J, Shi Y, Zhao G, Zhang X, Liu X, et al. Analysis of the Distribution Characteristics of Jellyfish and Environmental Factors in the Seawater Intake Area of the Haiyang Nuclear Power Plant in China. Biology. 2024; 13(6):433. https://doi.org/10.3390/biology13060433
Chicago/Turabian StyleSong, Yunpeng, Tiantian Wang, Minsi Xiong, Shenglong Yang, Heng Zhang, Jie Ying, Yongchuang Shi, Guoqing Zhao, Xiumei Zhang, Xiaodan Liu, and et al. 2024. "Analysis of the Distribution Characteristics of Jellyfish and Environmental Factors in the Seawater Intake Area of the Haiyang Nuclear Power Plant in China" Biology 13, no. 6: 433. https://doi.org/10.3390/biology13060433
APA StyleSong, Y., Wang, T., Xiong, M., Yang, S., Zhang, H., Ying, J., Shi, Y., Zhao, G., Zhang, X., Liu, X., Lin, C., Wu, Z., & Wu, Y. (2024). Analysis of the Distribution Characteristics of Jellyfish and Environmental Factors in the Seawater Intake Area of the Haiyang Nuclear Power Plant in China. Biology, 13(6), 433. https://doi.org/10.3390/biology13060433








