Mechanobiology of Adipocytes
Abstract
Simple Summary
Abstract
1. Mechanobiology
1.1. Transmission of Mechanical Loads to the Nucleus
1.2. Lamins
1.3. Significance of Mechanobiology in Normal Function and Disease
2. Adipocyte Mechanobiology
2.1. Diverse Roles of Adipocytes
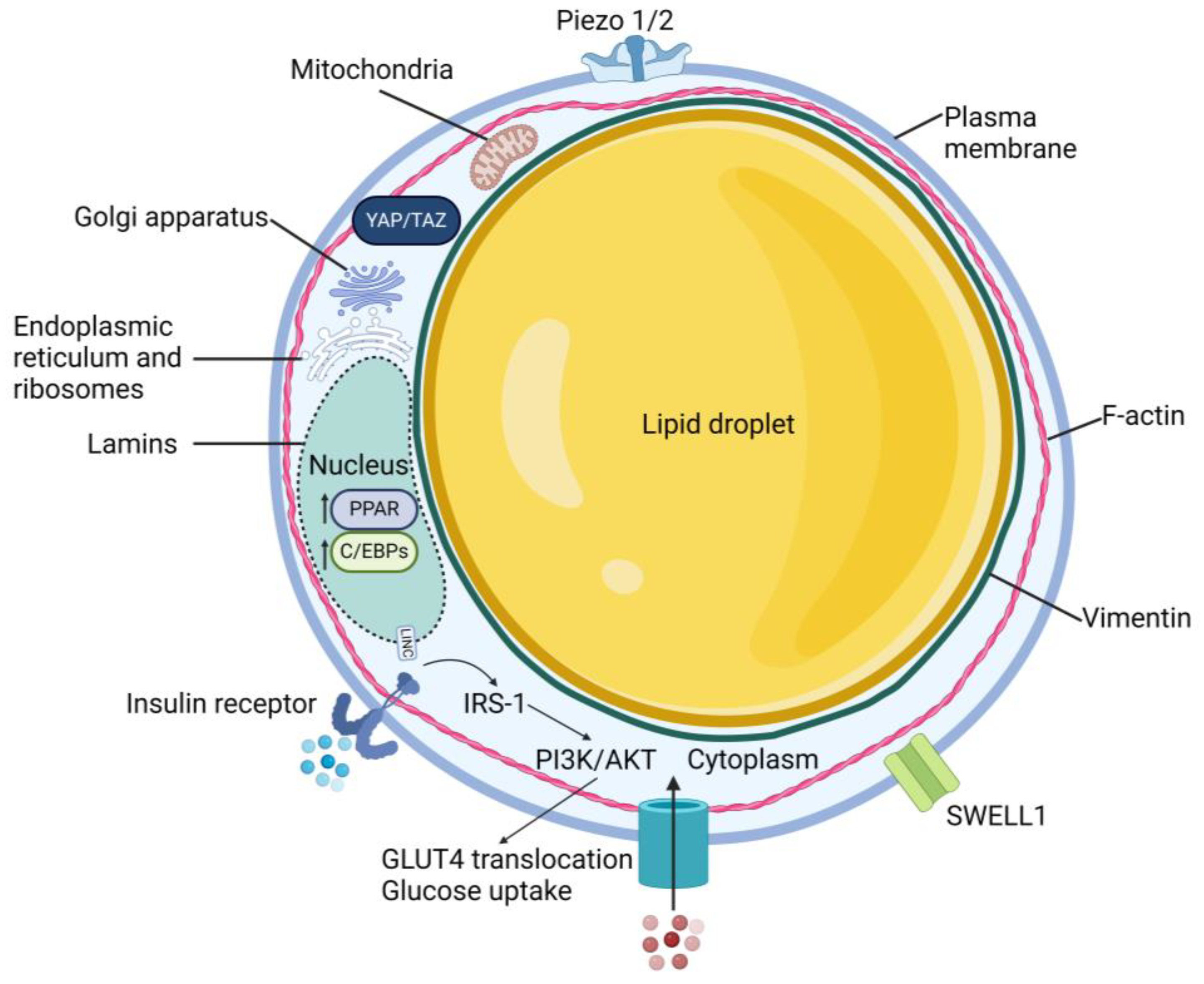
2.2. Cellular Structure
2.3. Yes-Associated Protein
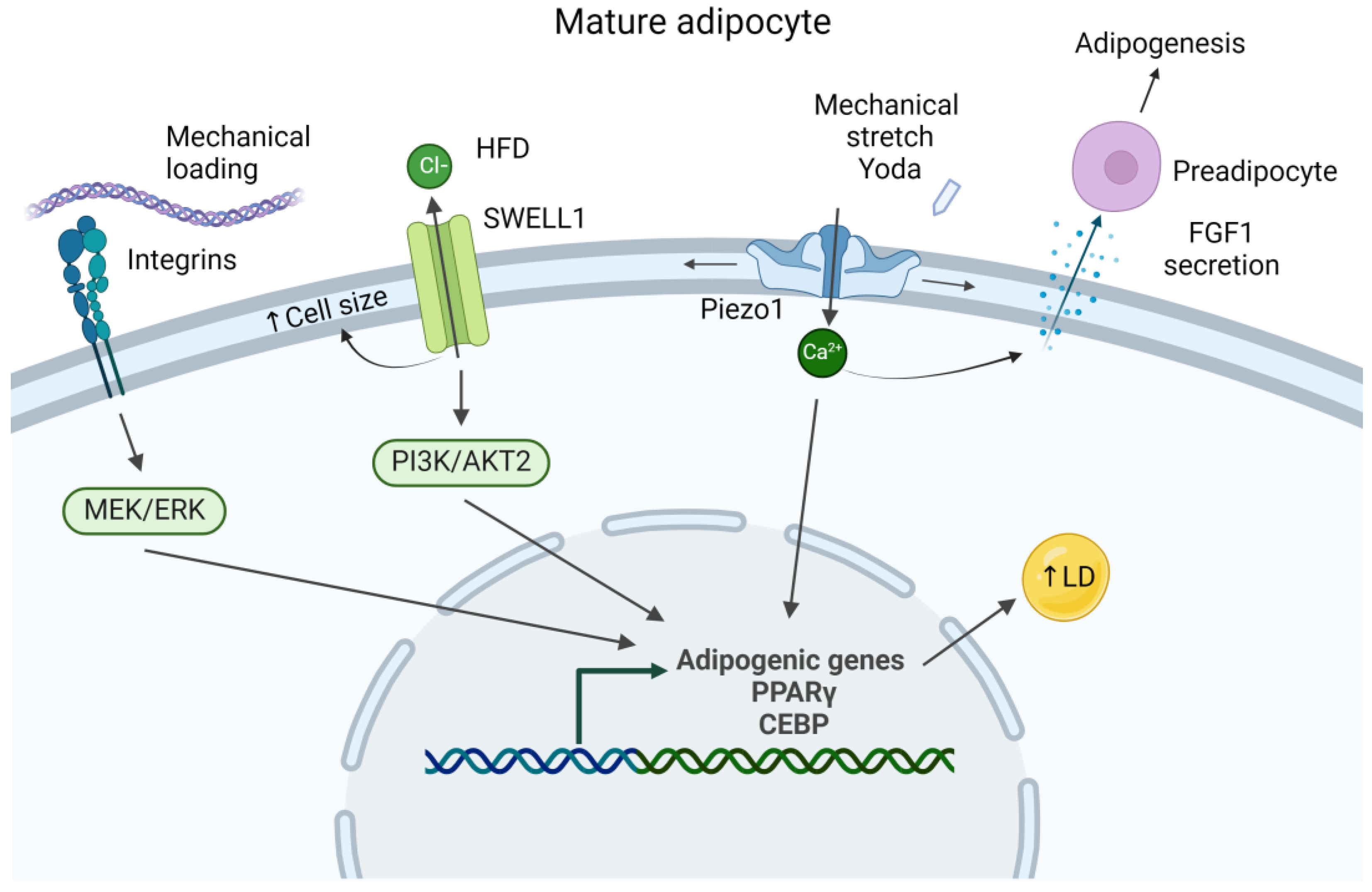
2.4. Piezo Channels
2.5. SWELL1
3. Mechanical Response of Adipocytes
3.1. Response to Tensile Strain
3.2. Response to Shockwaves
3.3. Response to Compression
3.4. Response to Underlying Substrate Stiffness
3.4.1. Effects on Differentiation
3.4.2. Effects on Insulin Sensitivity
4. Cell Stiffness Measurements
5. Tissue Stiffness Measurements
6. Computational Models of Adipose
6.1. Single-Cell Mechanical Models
6.2. Multi-Cellular Mechanical Models
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keller, R. Developmental Biology. Physical Biology Returns to Morphogenesis. Science 2012, 338, 201–203. [Google Scholar] [CrossRef]
- Grolleman, J.; van Engeland, N.C.A.; Raza, M.; Azimi, S.; Conte, V.; Sahlgren, C.M.; Bouten, C.V.C. Environmental Stiffness Restores Mechanical Homeostasis in Vimentin-Depleted Cells. Sci. Rep. 2023, 13, 18374. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Mukherjee, A.; Pisano, S.; Dimri, S.; Knaane, E.; Altshuler, A.; Nasser, W.; Dey, S.; Shi, L.; Mizrahi, I.; et al. The Biophysical Property of the Limbal Niche Maintains Stemness through YAP. Cell Death Differ. 2023, 30, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Divanyan, A.; Jourd’heuil, F.L.; Goldman, R.D.; Ridge, K.M.; Jourd’heuil, D.; Lopez-Soler, R.I. Vimentin Expression Is Required for the Development of EMT-Related Renal Fibrosis Following Unilateral Ureteral Obstruction in Mice. Am. J. Physiol. Renal Physiol. 2018, 315, F769–F780. [Google Scholar] [CrossRef]
- Vashisth, M.; Cho, S.; Irianto, J.; Xia, Y.; Wang, M.; Hayes, B.; Wieland, D.; Wells, R.; Jafarpour, F.; Liu, A.; et al. Scaling Concepts in ’omics: Nuclear Lamin-B Scales with Tumor Growth and Often Predicts Poor Prognosis, Unlike Fibrosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2112940118. [Google Scholar] [CrossRef]
- Pogoda, K.; Pięta, E.; Roman, M.; Piergies, N.; Liberda, D.; Wróbel, T.P.; Janmey, P.A.; Paluszkiewicz, C.; Kwiatek, W.M. In Search of the Correlation between Nanomechanical and Biomolecular Properties of Prostate Cancer Cells with Different Metastatic Potential. Arch. Biochem. Biophys. 2021, 697, 108718. [Google Scholar] [CrossRef]
- Wall, M.; Butler, D.; El Haj, A.; Bodle, J.C.; Loboa, E.G.; Banes, A.J. Key Developments That Impacted the Field of Mechanobiology and Mechanotransduction. J. Orthop. Res. 2018, 36, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Mui, K.L.; Chen, C.S.; Assoian, R.K. The Mechanical Regulation of Integrin-Cadherin Crosstalk Organizes Cells, Signaling and Forces. J. Cell Sci. 2016, 129, 1093–1100. [Google Scholar] [CrossRef]
- Legerstee, K.; Geverts, B.; Slotman, J.A.; Houtsmuller, A.B. Dynamics and Distribution of Paxillin, Vinculin, Zyxin and VASP Depend on Focal Adhesion Location and Orientation. Sci. Rep. 2019, 9, 10460. [Google Scholar] [CrossRef]
- Bouzid, T.; Kim, E.; Riehl, B.D.; Esfahani, A.M.; Rosenbohm, J.; Yang, R.; Duan, B.; Lim, J.Y. The LINC Complex, Mechanotransduction, and Mesenchymal Stem Cell Function and Fate. J. Biol. Eng. 2019, 13, 68. [Google Scholar] [CrossRef]
- Zuela-Sopilniak, N.; Lammerding, J. Can’t Handle the Stress? Mechanobiology and Disease. Trends Mol. Med. 2022, 28, 710–725. [Google Scholar] [CrossRef] [PubMed]
- Gasparski, A.N.; Beningo, K.A. Mechanoreception at the Cell Membrane: More than the Integrins. Arch. Biochem. Biophys. 2015, 586, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, H.; Iwasaki, M.; Wada, K.; Horitani, K.; Tsukamoto, O.; Kamikubo, K.; Nomura, S.; Matsumoto, S.; Harada, T.; Motooka, D.; et al. Wnt5a-YAP Signaling Axis Mediates Mechanotransduction in Cardiac Myocytes and Contributes to Contractile Dysfunction Induced by Pressure Overload. iScience 2023, 26, 107146. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, M.; Mamistvalov, R.; Sprinzak, D.; Vollmar, A.M.; Zahler, S. Matrix Stiffness Regulates Notch Signaling Activity in Endothelial Cells. J. Cell Sci. 2023, 136, jcs260442. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Cui, Y.; Guo, D.; Chen, H.; Li, J.; Zhou, X.; Xie, J. Microenvironmental Stiffness Directs Chondrogenic Lineages of Stem Cells from the Human Apical Papilla via Cooperation between ROCK and Smad3 Signaling. ACS Biomater. Sci. Eng. 2023, 9, 4831–4845. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Bao, M.; Bruekers, S.M.C.; Huck, W.T.S. Collagen Gels with Different Fibrillar Microarchitectures Elicit Different Cellular Responses. ACS Appl. Mater. Interfaces 2017, 9, 19630–19637. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hosaka, T.; Jambaldorj, B.; Nakaya, Y.; Funaki, M. Extracellular Matrix with the Rigidity of Adipose Tissue Helps 3T3-L1 Adipocytes Maintain Insulin Responsiveness. J. Med. Investig. 2009, 56, 142–149. [Google Scholar] [CrossRef]
- Takata, K.; Goto, T.; Kuroda, M.; Kimura, Y.; Harada, I.; Ueda, K.; Kawada, T.; Kioka, N. Stiffness of the Extracellular Matrix Regulates Differentiation into Beige Adipocytes. Biochem. Biophys. Res. Commun. 2020, 532, 205–210. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Bao, M.; Xie, J. Geometric Confinement-Mediated Mechanical Tension Directs Patterned Differentiation of Mouse ESCs into Organized Germ Layers. ACS Appl. Mater. Interfaces 2023, 15, 34397–34406. [Google Scholar] [CrossRef]
- de Leeuw, R.; Gruenbaum, Y.; Medalia, O. Nuclear Lamins: Thin Filaments with Major Functions. Trends Cell Biol. 2018, 28, 34–45. [Google Scholar] [CrossRef]
- Butin-Israeli, V.; Adam, S.A.; Goldman, A.E.; Goldman, R.D. Nuclear Lamin Functions and Disease. Trends Genet. 2012, 28, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Burke, B.; Stewart, C.L. The Nuclear Lamins: Flexibility in Function. Nat. Rev. Mol. Cell Biol. 2013, 14, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Dechat, T.; Adam, S.A.; Taimen, P.; Shimi, T.; Goldman, R.D. Subject Categories Nuclear Lamins Structure and Biochemical Properties of Nuclear Lamins. Cold Spring Harb. Perspect. Biol. 2010, 2, a000547. [Google Scholar]
- Goldman, R.D.; Gruenbaum, Y.; Moir, R.D.; Shumaker, D.K.; Spann, T.P. Nuclear Lamins: Building Blocks of Nuclear Architecture. Genes. Dev. 2002, 16, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, Y.; Keyimu, R.; Hao, J.; Zhao, Z.; Ye, R. The Role of Lamin A/C in Mesenchymal Stem Cell Differentiation. J. Physiol. Biochem. 2019, 75, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Shimi, T.; Pfleghaar, K.; Kojima, S.I.; Pack, C.G.; Solovei, I.; Goldman, A.E.; Adam, S.A.; Shumaker, D.K.; Kinjo, M.; Cremer, T.; et al. The A- and B-Type Nuclear Lamin Networks: Microdomains Involved in Chromatin Organization and Transcription. Genes. Dev. 2008, 22, 3409–3421. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.M.; Burridge, K. Mechanotransduction and Nuclear Function. Curr. Opin. Cell Biol. 2016, 40, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lin, B.Y.; Sun, S.; Dai, C.; Long, F.; Butcher, J.T. Shear and Hydrostatic Stress Regulate Fetal Heart Valve Remodeling through YAP-Mediated Mechanotransduction. Elife 2023, 12, e83209. [Google Scholar] [CrossRef]
- Jarrell, D.K.; Lennon, M.L.; Jacot, J.G. Epigenetics and Mechanobiology in Heart Development and Congenital Heart Disease. Diseases 2019, 7, 52. [Google Scholar] [CrossRef]
- Vasudevan, J.; Jiang, K.; Fernandez, J.G.; Lim, C.T. Extracellular Matrix Mechanobiology in Cancer Cell Migration. Acta Biomater. 2023, 163, 351–364. [Google Scholar] [CrossRef]
- Si, H.; Zhao, N.; Pedroza, A.; Zaske, A.M.; Rosen, J.M.; Creighton, C.J.; Roarty, K. Noncanonical Wnt/Ror2 Signaling Regulates Cell–Matrix Adhesion to Prompt Directional Tumor Cell Invasion in Breast Cancer. Mol. Biol. Cell 2022, 33, ar103. [Google Scholar] [CrossRef] [PubMed]
- Merkher, Y.; Alvarez-Elizondo, M.B.; Weihs, D. Taxol Reduces Synergistic, Mechanobiological Invasiveness of Metastatic Cells. Converg. Sci. Phys. Oncol. 2017, 3, 044002. [Google Scholar] [CrossRef]
- Papavassiliou, K.A.; Basdra, E.K.; Papavassiliou, A.G. The Emerging Promise of Tumour Mechanobiology in Cancer Treatment. Eur. J. Cancer 2023, 190, 112938. [Google Scholar] [CrossRef]
- Chaurasiya, V.; Pham, D.D.; Harju, J.; Juuti, A.; Penttilä, A.; Emmagouni, S.K.G.; Nguyen, V.D.; Zhang, B.; Perttunen, S.; Keskitalo, S.; et al. Human Visceral Adipose Tissue Microvascular Endothelial Cell Isolation and Establishment of Co-Culture with White Adipocytes to Analyze Cell-Cell Communication. Exp. Cell Res. 2023, 433, 113819. [Google Scholar] [CrossRef]
- Crewe, C.; Funcke, J.B.; Li, S.; Joffin, N.; Gliniak, C.M.; Ghaben, A.L.; An, Y.A.; Sadek, H.A.; Gordillo, R.; Akgul, Y.; et al. Extracellular Vesicle-Based Interorgan Transport of Mitochondria from Energetically Stressed Adipocytes. Cell Metab. 2021, 33, 1853–1868. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The Role of Adipokines in Inflammatory Mechanisms of Obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef] [PubMed]
- Attie, A.D.; Scherer, P.E. Adipocyte Metabolism and Obesity. J. Lipid Res. 2009, 50, S395–S399. [Google Scholar] [CrossRef] [PubMed]
- Kenmochi, M.; Kawarasaki, S.; Takizawa, S.; Okamura, K.; Goto, T.; Uchida, K. Involvement of Mechano-Sensitive Piezo1 Channel in the Differentiation of Brown Adipocytes. J. Physiol. Sci. 2022, 72, 13. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Shapiro, S.A.; Bradsell, H.; Frank, R.M. The Essential Roles of Human Adipose Tissue: Metabolic, Thermoregulatory, Cellular, and Paracrine Effects. J. Cartil. Jt. Preserv. 2021, 1, 13. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Małodobra-Mazur, M.; Cierzniak, A.; Pawełka, D.; Kaliszewski, K.; Rudnicki, J.; Dobosz, T. Metabolic Differences between Subcutaneous and Visceral Adipocytes Differentiated with an Excess of Saturated and Monounsaturated Fatty Acids. Genes 2020, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- Wajchenberg, B.L. Subcutaneous and Visceral Adipose Tissue: Their Relation to the Metabolic Syndrome. Endocr. Rev. 2000, 21, 697–738. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.J.; White, U.; Elks, C.M.; Stephens, J.M. Adipose Tissue: Physiology to Metabolic Dysfunction; Endotext MDText Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- Kanzaki, M.; Pessin, J.E. Caveolin-Associated Filamentous Actin (Cav-Actin) Defines a Novel F-Actin Structure in Adipocytes. J. Biol. Chem. 2002, 277, 25867–25869. [Google Scholar] [CrossRef]
- Kim, J.I.; Park, J.; Ji, Y.; Jo, K.; Han, S.M.; Sohn, J.H.; Shin, K.C.; Han, J.S.; Jeon, Y.G.; Nahmgoong, H.; et al. During Adipocyte Remodeling, Lipid Droplet Configurations Regulate Insulin Sensitivity through F-Actin and G-Actin Reorganization. Mol. Cell Biol. 2019, 39, e00210-19. [Google Scholar] [CrossRef]
- Lecoutre, S.; Lambert, M.; Drygalski, K.; Dugail, I.; Maqdasy, S.; Hautefeuille, M.; Clément, K. Importance of the Microenvironment and Mechanosensing in Adipose Tissue Biology. Cells 2022, 11, 2310. [Google Scholar] [CrossRef]
- Franke, W.W.; Hergt, M.; Grund, C. Rearrangement of the Vimentin Cytoskeleton during Adipose Conversion: Formation of an Intermediate Filament Cage around Lipid Globules. Cell 1987, 49, 131–141. [Google Scholar] [CrossRef]
- Ivanovska, I.L.; Tobin, M.P.; Bai, T.; Dooling, L.J.; Discher, D.E. Small Lipid Droplets Are Rigid Enough to Indent a Nucleus, Dilute the Lamina, and Cause Rupture. J. Cell Biol. 2023, 222, e202208123. [Google Scholar] [CrossRef]
- Verstraeten, V.L.R.M.; Renes, J.; Ramaekers, F.C.S.; Kamps, M.; Kuijpers, H.J.; Verheyen, F.; Wabitsch, M.; Steijlen, P.M.; Van Steensel, M.A.M.; Broers, J.L.V. Reorganization of the Nuclear Lamina and Cytoskeleton in Adipogenesis. Histochem. Cell Biol. 2011, 135, 251–261. [Google Scholar] [CrossRef]
- Oliver-De La Cruz, J.; Nardone, G.; Vrbsky, J.; Pompeiano, A.; Perestrelo, A.R.; Capradossi, F.; Melajová, K.; Filipensky, P.; Forte, G. Substrate Mechanics Controls Adipogenesis through YAP Phosphorylation by Dictating Cell Spreading. Biomaterials 2019, 205, 64–80. [Google Scholar] [CrossRef]
- Lorthongpanich, C.; Thumanu, K.; Tangkiettrakul, K.; Jiamvoraphong, N.; Laowtammathron, C.; Damkham, N.; U-Pratya, Y.; Issaragrisil, S. YAP as a Key Regulator of Adipo-Osteogenic Differentiation in Human MSCs. Stem Cell Res. Ther. 2019, 10, 402. [Google Scholar] [CrossRef]
- Heng, B.C.; Zhang, X.; Aubel, D.; Bai, Y.; Li, X.; Wei, Y.; Fussenegger, M.; Deng, X. Role of YAP/TAZ in Cell Lineage Fate Determination and Related Signaling Pathways. Front. Cell Dev. Biol. 2020, 8, 735. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Zhao, J.; Wang, H.; Li, Y. Mechanotransduction Regulates Inflammation Responses of Epicardial Adipocytes in Cardiovascular Diseases. Front. Endocrinol. 2022, 13, 1080383. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.K.; Theise, N.D.; Loneker, A.E.; Janmey, P.A.; Wells, R.G. Lipid Droplets Disrupt Mechanosensing in Human Hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G11–G22. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Yeh, Y.T.; Nguyen, P.; Limqueco, E.; Lopez, J.; Thorossian, S.; Guan, K.L.; Li, Y.S.J.; Chien, S. Flow-Dependent YAP/TAZ Activities Regulate Endothelial Phenotypes and Atherosclerosis. Proc. Natl. Acad. Sci. USA 2016, 113, 11525–11530. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Riehl, B.D.; Bouzid, T.; Yang, R.; Duan, B.; Donahue, H.J.; Lim, J.Y. YAP Mechanotransduction under Cyclic Mechanical Stretch Loading for Mesenchymal Stem Cell Osteogenesis Is Regulated by ROCK. Front. Bioeng. Biotechnol. 2024, 11, 1306002. [Google Scholar] [CrossRef]
- Zhai, M.; Yang, D.; Yi, W.; Sun, W. Involvement of Calcium Channels in the Regulation of Adipogenesis. Adipocyte 2020, 9, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Rendon, C.J.; Flood, E.; Thompson, J.M.; Chirivi, M.; Watts, S.W.; Contreras, G.A. PIEZO1 Mechanoreceptor Activation Reduces Adipogenesis in Perivascular Adipose Tissue Preadipocytes. Front. Endocrinol. 2022, 13, 995499. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.F.; Talbott, H.E.; Guardino, N.J.; Guo, J.L.; Spielman, A.F.; Chen, K.; Parker, J.B.L.; Mascharak, S.; Henn, D.; Liang, N.; et al. Piezo Inhibition Prevents and Rescues Scarring by Targeting the Adipocyte to Fibroblast Transition. bioRxiv 2023. [Google Scholar] [CrossRef]
- Wang, S.P.; Cao, S.; Arhatte, M.; Li, D.; Shi, Y.; Kurz, S.; Hu, J.; Wang, L.; Shao, J.; Atzberger, A.; et al. Adipocyte Piezo1 Mediates Obesogenic Adipogenesis through the FGF1/FGFR1 Signaling Pathway in Mice. Nat. Commun. 2020, 11, 2303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, L.; Gunasekar, S.K.; Tong, D.; Mishra, A.; Gibson, W.J.; Wang, C.; Fidler, T.; Marthaler, B.; Klingelhutz, A.; et al. SWELL1 Is a Regulator of Adipocyte Size, Insulin Signalling and Glucose Homeostasis. Nat. Cell Biol. 2017, 19, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Gunasekar, S.K.; Heebink, J.; Carpenter, D.H.; Kumar, A.; Xie, L.; Zhang, H.; Schilling, J.D.; Sah, R. Adipose-Targeted SWELL1 Deletion Exacerbates Obesity- And Age-Related Nonalcoholic Fatty Liver Disease. JCI Insight 2023, 8, e154940. [Google Scholar] [CrossRef]
- Cho, W.; Kim, S.Y.; Jeong, M.; Park, Y.M. Shockwaves Suppress Adipocyte Differentiation via Decrease in PPARγ. Cells 2020, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Sen, B.; Xie, Z.; Case, N.; Ma, M.; Rubin, C.; Rubin, J. Mechanical Strain Inhibits Adipogenesis in Mesenchymal Stem Cells by Stimulating a Durable β-Catenin Signal. Endocrinology 2008, 149, 6065–6075. [Google Scholar] [CrossRef] [PubMed]
- David, V.; Martin, A.; Lafage-Proust, M.H.; Malaval, L.; Peyroche, S.; Jones, D.B.; Vico, L.; Guignandon, A. Mechanical Loading Down-Regulates Peroxisome Proliferator-Activated Receptor γ in Bone Marrow Stromal Cells and Favors Osteoblastogenesis at the Expense of Adipogenesis. Endocrinology 2007, 148, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Koga, M.; Saito, M.; Matsunaga, Y.; Nakayama, K. Inhibition of Adipocyte Differentiation by Mechanical Stretching through ERK-Mediated Downregulation of PPARγ2. J. Cell Sci. 2004, 117, 3605–3614. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Jones, H.S.; Davies, J.E.; Canfield, A.E. Cyclic Stretch-Induced TGFβ1/Smad Signaling Inhibits Adipogenesis in Umbilical Cord Progenitor Cells. Biochem. Biophys. Res. Commun. 2008, 377, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cai, X.; Wang, J.; Tang, H.; Yuan, Q.; Gong, P.; Lin, Y. Mechanical Stretch Inhibits Adipogenesis and Stimulates Osteogenesis of Adipose Stem Cells. Cell Prolif. 2012, 45, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fu, N.; Yang, X.; Li, M.; Ba, K.; Wei, X.; Fu, Y.; Yao, Y.; Cai, X.; Lin, Y. Mechanical Compressive Force Inhibits Adipogenesis of Adipose Stem Cells. Cell Prolif. 2013, 46, 586–594. [Google Scholar] [CrossRef]
- Hossain, M.G.; Iwata, T.; Mizusawa, N.; Shima, S.W.N.; Okutsu, T.; Ishimoto, K.; Yoshimoto, K. Compressive Force Inhibits Adipogenesis through COX-2-Mediated down-Regulation of PPARγ2 and C/EBPα. J. Biosci. Bioeng. 2010, 109, 297–303. [Google Scholar] [CrossRef]
- Tirkkonen, L.; Halonen, H.; Hyttinen, J.; Kuokkanen, H.; Sievänen, H.; Koivisto, A.M.; Mannerström, B.; Sándor, G.K.B.; Suuronen, R.; Miettinen, S.; et al. The Effects of Vibration Loading on Adipose Stem Cell Number, Viability and Differentiation towards Bone-Forming Cells. J. R. Soc. Interface 2011, 8, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Shoham, N.; Gottlieb, R.; Sharabani-Yosef, O.; Zaretsky, U.; Benayahu, D.; Gefen, A. Static Mechanical Stretching Accelerates Lipid Production in 3T3-L1 Adipocytes by Activating the MEK Signaling Pathway. Am. J. Physiol. Cell Physiol. 2012, 302, C429–C441. [Google Scholar] [CrossRef] [PubMed]
- Mor-Yossef Moldovan, L.; Kislev, N.; Lustig, M.; Pomeraniec, L.; Benayahu, D. Biomechanical Stimulation Effects on the Metabolism of Adipocyte. J. Cell Physiol. 2020, 235, 8702–8713. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Heuvingh, J.; Du Roure, O.; Rouault, C.; Devulder, A.; Klein, C.; Lacasa, M.; Clément, E.; Lacasa, D.; Clément, K. Human Adipocyte Function Is Impacted by Mechanical Cues. J. Pathol. 2014, 233, 183–195. [Google Scholar] [CrossRef]
- Nakajima, I.; Yamaguchi, T.; Ozutsumi, K.; Aso, H. Adipose Tissue Extracellular Matrix: Newly Organized by Adipocytes during Differentiation. Differentiation 1998, 63, 193–200. [Google Scholar] [CrossRef]
- Musale, V.; Wasserman, D.H.; Kang, L. Extracellular Matrix Remodelling in Obesity and Metabolic Disorders. Life Metab. 2023, 2, load021. [Google Scholar] [CrossRef] [PubMed]
- Lustig, M.; Gefen, A.; Benayahu, D. Adipogenesis and Lipid Production in Adipocytes Subjected to Sustained Tensile Deformations and Elevated Glucose Concentration: A Living Cell-Scale Model System of Diabesity. Biomech. Model. Mechanobiol. 2018, 17, 903–913. [Google Scholar] [CrossRef]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the Release of Adipokines by Adipose Tissue, Adipose Tissue Matrix, and Adipocytes from Visceral and Subcutaneous Abdominal Adipose Tissues of Obese Humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef]
- Huang, A.; Lin, Y.; Kao, L.; Chiou, Y.; Lee, G.; Lin, H.; Wu, C.; Chang, C.; Lee, K.; Hsueh, Y.; et al. Inflammation-induced Macrophage Lysyl Oxidase in Adipose Stiffening and Dysfunction in Obesity. Clin. Transl. Med. 2021, 11, e543. [Google Scholar] [CrossRef]
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic Dysregulation and Adipose Tissue Fibrosis: Role of Collagen VI. Mol. Cell Biol. 2009, 29, 1575–1591. [Google Scholar] [CrossRef]
- Young, D.A.; Choi, Y.S.; Engler, A.J.; Christman, K.L. Stimulation of Adipogenesis of Adult Adipose-Derived Stem Cells Using Substrates That Mimic the Stiffness of Adipose Tissue. Biomaterials 2013, 34, 8581–8588. [Google Scholar] [CrossRef] [PubMed]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef]
- St Pierre, R.; Eslami, B.; Bergbreiter, S. Ground Reaction Force Sensing in Milligram-Scale Legged Microrobots. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems and Eurosensors XXXIII, TRANSDUCERS 2019 and EUROSENSORS XXXIII, Berlin, Germany, 23–27 June 2019. [Google Scholar]
- Nikfarjam, M.; López-Guerra, E.A.; Solares, S.D.; Eslami, B. Imaging of Viscoelastic Soft Matter with Small Indentation Using Higher Eigenmodes in Single-Eigenmode Amplitude-Modulation Atomic Force Microscopy. Beilstein J. Nanotechnol. 2018, 9, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Darling, E.M.; Topel, M.; Zauscher, S.; Vail, T.P.; Guilak, F. Viscoelastic Properties of Human Mesenchymally-Derived Stem Cells and Primary Osteoblasts, Chondrocytes, and Adipocytes. J. Biomech. 2008, 41, 454–464. [Google Scholar] [CrossRef]
- Mathur, A.B.; Collinsworth, A.M.; Reichert, W.M.; Kraus, W.E.; Truskey, G.A. Endothelial, Cardiac Muscle and Skeletal Muscle Exhibit Different Viscous and Elastic Properties as Determined by Atomic Force Microscopy. J. Biomech. 2001, 34, 1545–1553. [Google Scholar] [CrossRef]
- Slomka, N.; Oomens, C.W.J.; Gefen, A. Evaluating the Effective Shear Modulus of the Cytoplasm in Cultured Myoblasts Subjected to Compression Using an Inverse Finite Element Method. J. Mech. Behav. Biomed. Mater. 2011, 4, 1559–1566. [Google Scholar] [CrossRef]
- Shoham, N.; Girshovitz, P.; Katzengold, R.; Shaked, N.T.; Benayahu, D.; Gefen, A. Adipocyte Stiffness Increases with Accumulation of Lipid Droplets. Biophys. J. 2014, 106, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Abuhattum, S.; Kotzbeck, P.; Schlüßler, R.; Harger, A.; Ariza de Schellenberger, A.; Kim, K.; Escolano, J.C.; Müller, T.; Braun, J.; Wabitsch, M.; et al. Adipose Cells and Tissues Soften with Lipid Accumulation While in Diabetes Adipose Tissue Stiffens. Sci. Rep. 2022, 12, 10325. [Google Scholar] [CrossRef]
- Bouzid, T.; Esfahani, A.M.; Safa, B.T.; Kim, E.; Saraswathi, V.; Kim, J.K.; Yang, R.; Lim, J.Y. Rho/ROCK Mechanosensor in Adipocyte Stiffness and Traction Force Generation. Biochem. Biophys. Res. Commun. 2022, 606, 42–48. [Google Scholar] [CrossRef]
- Guilak, F.; Tedrow, J.R.; Burgkart, R. Viscoelastic Properties of the Cell Nucleus. Biochem. Biophys. Res. Commun. 2000, 269, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Mihai, L.A.; Chin, L.K.; Janmey, P.A.; Goriely, A. A Comparison of Hyperelastic Constitutive Models Applicable to Brain and Fat Tissues. J. R. Soc. Interface 2015, 12, 0486. [Google Scholar] [CrossRef] [PubMed]
- Wenderott, J.K.; Flesher, C.G.; Baker, N.A.; Neeley, C.K.; Varban, O.A.; Lumeng, C.N.; Muhammad, L.N.; Yeh, C.; Green, P.F.; O’Rourke, R.W. Elucidating Nanoscale Mechanical Properties of Diabetic Human Adipose Tissue Using Atomic Force Microscopy. Sci. Rep. 2020, 10, 20423. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, N.; Mansfield, J.; Green, E.; Bel, J.; Knight, B.; Liversedge, N.; Tham, J.C.; Welbourn, R.; Shore, A.C.; Kos, K.; et al. The Mechanical Properties of Human Adipose Tissues and Their Relationships to the Structure and Composition of the Extracellular Matrix. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1427–E1435. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ashcraft, K.; Jahid, M.J.; April, C.; Ghajar, C.M.; Ruan, J.; Wang, H.; Foster, M.; Hughes, D.C.; Ramirez, A.G.; et al. Regulation of Adipose Oestrogen Output by Mechanical Stress. Nat. Commun. 2013, 4, 1821. [Google Scholar] [CrossRef] [PubMed]
- Perumal, L.; Mon, D.T.T. Finite Elements for Engineering Analysis: A Brief Review. Int. Proc. Comput. Sci. Inf. Technol. 2011, 10, 61–68. [Google Scholar]
- Katzengold, R.; Shoham, N.; Benayahu, D.; Gefen, A. Simulating Single Cell Experiments in Mechanical Testing of Adipocytes. Biomech. Model. Mechanobiol. 2015, 14, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Kasza, K.E.; Rowat, A.C.; Liu, J.; Angelini, T.E.; Brangwynne, C.P.; Koenderink, G.H.; Weitz, D.A. The Cell as a Material. Curr. Opin. Cell Biol. 2007, 19, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Comley, K.; Fleck, N.A. A Micromechanical Model for the Young’s Modulus of Adipose Tissue. Int. J. Solids Struct. 2010, 47, 2982–2990. [Google Scholar] [CrossRef]
- Ben-Or Frank, M.; Shoham, N.; Benayahu, D.; Gefen, A. Effects of Accumulation of Lipid Droplets on Load Transfer between and within Adipocytes. Biomech. Model. Mechanobiol. 2015, 14, 15–28. [Google Scholar] [CrossRef]

| Publication | General Protocol | Samples | Cell Stiffness |
|---|---|---|---|
| Abuhattam, S., Taubenberger, A.V. et al., Scientific Reports 2022 [67] |
| Day 1 of differentiation, single-cell SGBS adipocytes (control) | E = 3.84 ± 0.15 kPa |
| Day 11 of differentiation, single-cell SGBS adipocytes | E = 0.46 ± 0.02 kPa | ||
| Day 1 of differentiation, single-cell SGBS adipocytes (control) | G’ = ~1–1.25 kPa G” = ~0.125–1.25 kPa | |
| Day 1 of differentiation, single-cell SGBS adipocytes | G’ = ~0.125–0.5 kPa G” = ~0.075–0.13 kPa | ||
| Bouzid, T., Lim, J.Y. et al., Biochemical and Biophysical Research Communications 2022 [68] |
| 3T3-L1 preadipocytes (control) | E = ~4 kPa (probing above nucleus or cytoplasm) |
| Differentiated 3T3-L1 adipocytes | E = ~8 kPa (probing above nucleus or lipid droplet) |
| Publication | General Protocol | Samples | Tissue Stiffness |
|---|---|---|---|
| Abuhattam, S., Taubenberger, A.V. et al., Scientific Reports 2022 [67] |
| Gonadal WAT from 46 wk old male C57BL/6-J mice (control) | E = ~2 kPa |
| Gonadal WAT from HFD-fed, 46 wk old male C57BL/6-J mice | E = ~0.9 kPa | ||
| Gonadal WAT from 18 wk old male C57BL/6-J mice (control) | E = ~0.5 kPa | ||
| Gonadal WAT from 18 wk old male db/db mice | E = ~1.2 kPa | ||
| Gonadal WAT from 18 wk old male ob/ob mice | E = ~0.5 kPa | ||
| Fat bodies from WT Drosophila (control) | E = ~0.4 kPa | |
| Fat bodies from HFD-fed Drosophila | E = ~0.25 kPa | ||
| Gonadal WAT from 46 wk old male C57BL/6-J mice (control) | G’ = ~0.4–0.6 kPa G” = ~0.05 kPa | |
| Gonadal WAT from HFD-fed, 46 wk old male C57BL/6-J mice | G’ = ~0.2–0.4 kPa G” = ~0.01–0.12 kPa | ||
| Gonadal WAT from 18 wk old male C57BL/6-J mice (control) | G’ = ~0.5 kPa G” = ~0.05–0.2 kPa | ||
| Gonadal WAT from 18 wk old male db/db mice | G’ = ~0.5–0.85 kPa G” = ~0.05–0.35 kPa | ||
| Gonadal WAT from 46 wk old male C57BL/6-J mice (control) | G’ = ~1–1.5 kPa G” = ~1–2 kPa | |
| Gonadal WAT from HFD-fed, 46 wk old male C57BL/6-J mice | G’ = ~0.9–1.5 kPa G” = ~0.9–2.1 kPa | ||
| Gonadal WAT from 18 wk old male C57BL/6-J mice (control) | G’ = ~1 kPa G” = ~0.5–3 kPa | ||
| Gonadal WAT from 18 wk old male db/db mice | G’ = ~1–2 kPa G” = ~1–2.5 kPa | ||
| Wenderott, J.K, O’Rourke, R.W. et al., Scientific Reports 2020 [71] |
| VAT from non-diabetic human females (control) | E = 4.48 ± 4.81 kPa |
| VAT from diabetic human females | E = 11.50 ± 16.79 kPa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blade, S.P.; Falkowski, D.J.; Bachand, S.N.; Pagano, S.J.; Chin, L. Mechanobiology of Adipocytes. Biology 2024, 13, 434. https://doi.org/10.3390/biology13060434
Blade SP, Falkowski DJ, Bachand SN, Pagano SJ, Chin L. Mechanobiology of Adipocytes. Biology. 2024; 13(6):434. https://doi.org/10.3390/biology13060434
Chicago/Turabian StyleBlade, Sean P., Dylan J. Falkowski, Sarah N. Bachand, Steven J. Pagano, and LiKang Chin. 2024. "Mechanobiology of Adipocytes" Biology 13, no. 6: 434. https://doi.org/10.3390/biology13060434
APA StyleBlade, S. P., Falkowski, D. J., Bachand, S. N., Pagano, S. J., & Chin, L. (2024). Mechanobiology of Adipocytes. Biology, 13(6), 434. https://doi.org/10.3390/biology13060434






