Spheroid Model of Mammary Tumor Cells: Epithelial–Mesenchymal Transition and Doxorubicin Response
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Spheroid Formation Using Cell Line and Primary Culture of Mouse Tumor Cells
2.3. Spheroid Growing and Migration Assays
2.4. Necrotic Core Analysis
2.5. Cell Death and Viability Analysis
2.6. Protein Distribution Analysis Using Immunofluorescence and Confocal Microscopy
2.7. Protein Expression Analysis Using Western Blotting
2.8. Statistical Analysis
3. Results
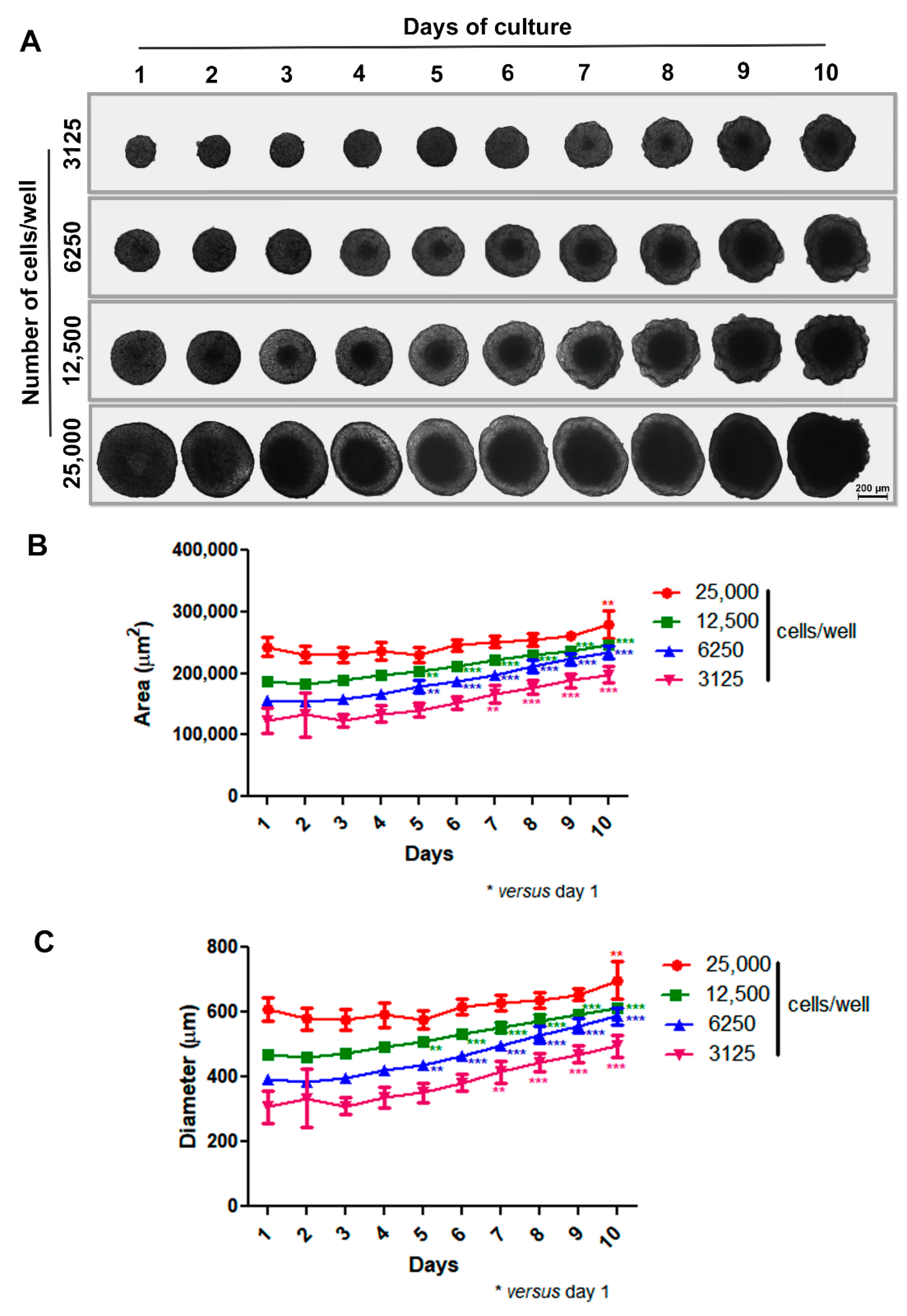
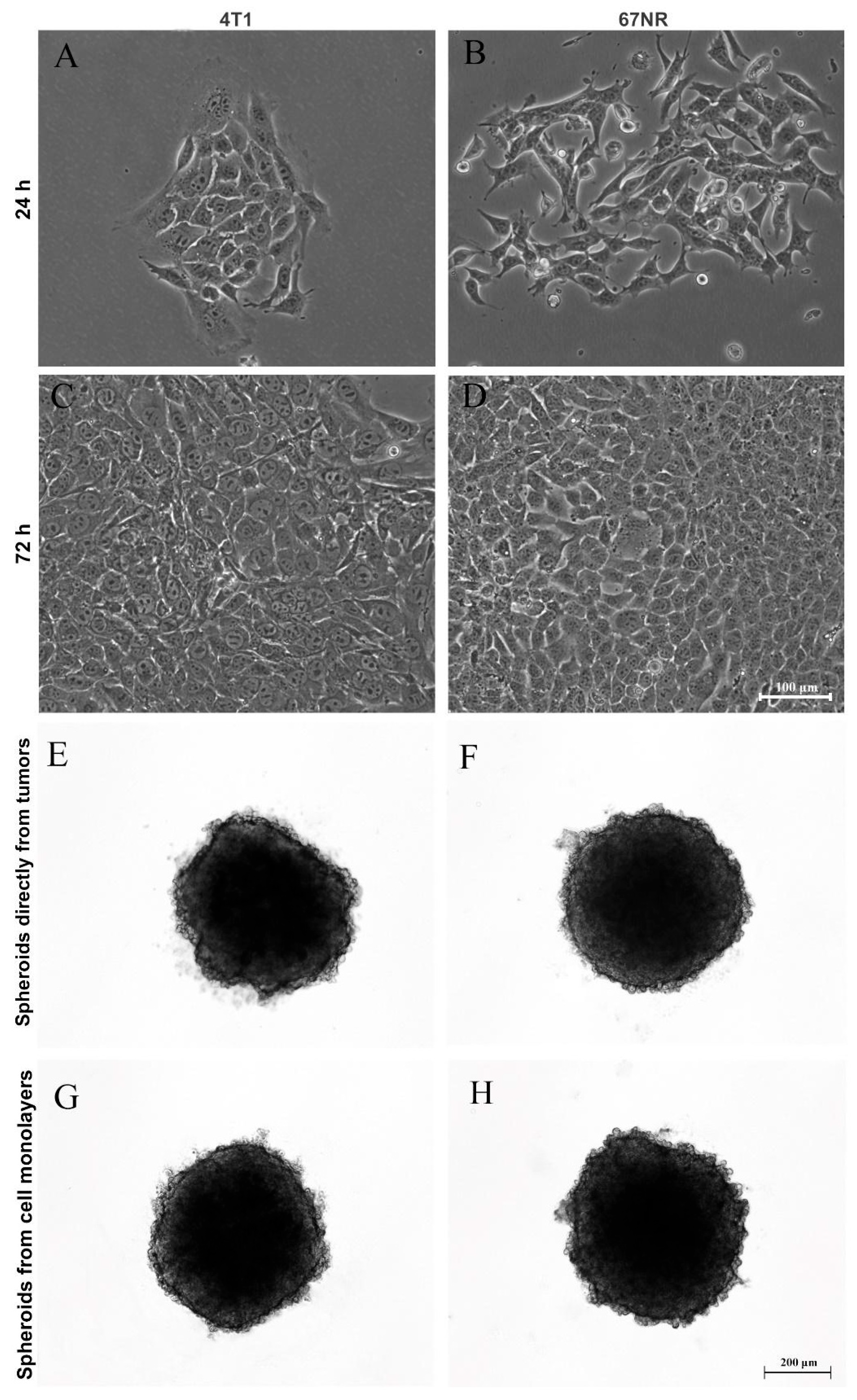
3.1. Production and Characterization of the 3D Mammary Spheroid Model Using Cell Line
3.2. Production of Spheroids from Mice Mammary Tumors
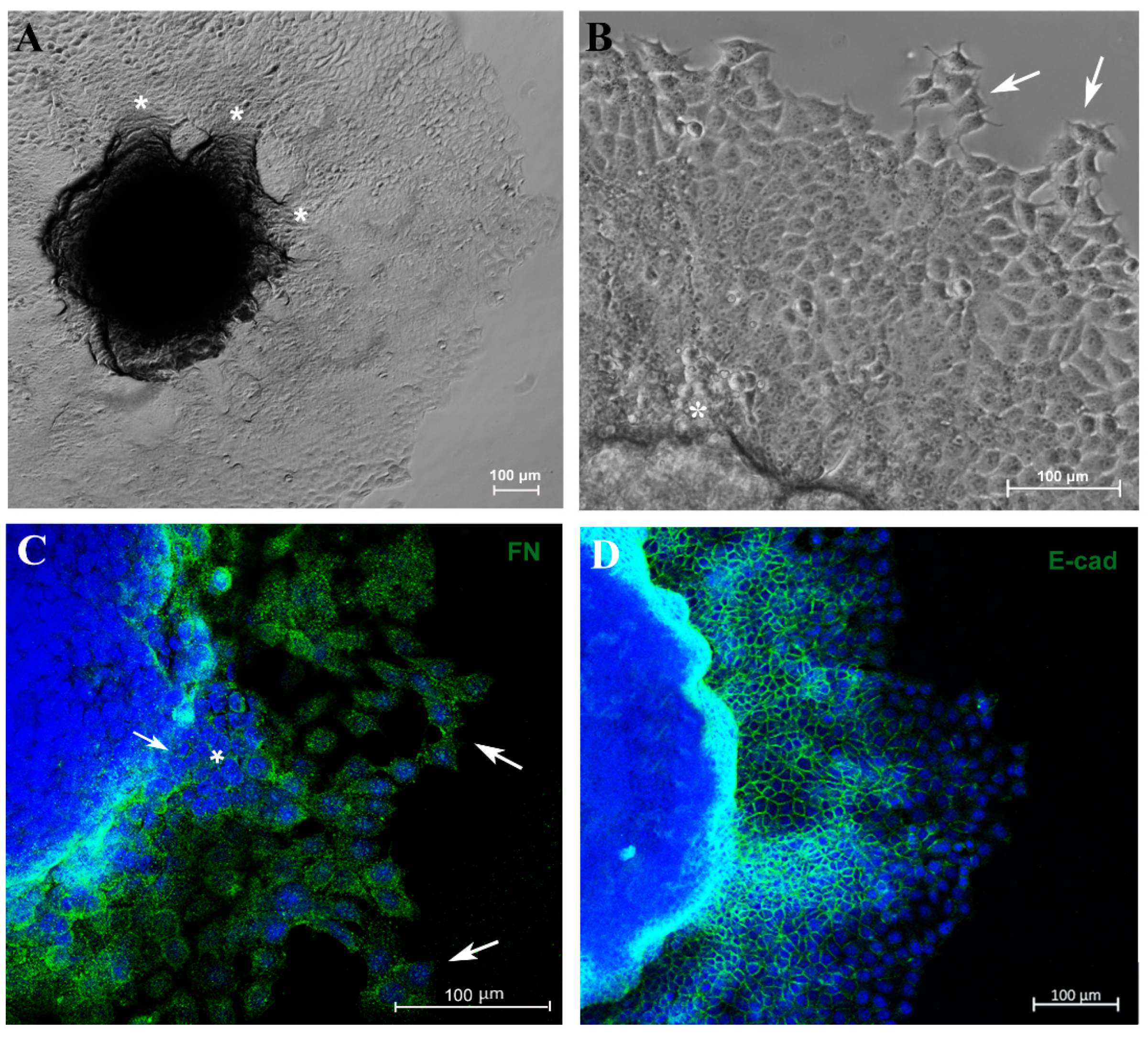
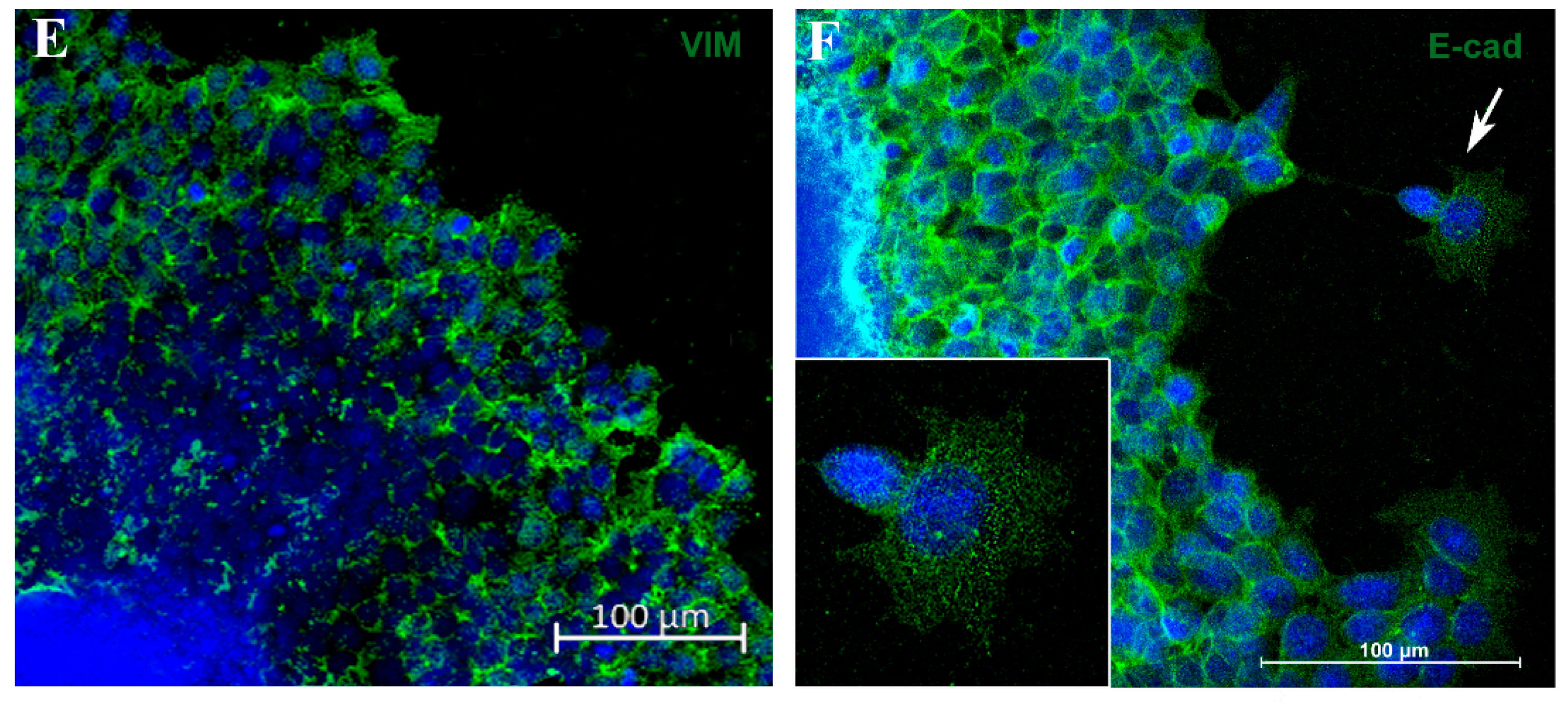
3.3. Migration Potential of Spheroid Cells In Vitro and Expression of EMT Markers E-Cadherin and Vimentin
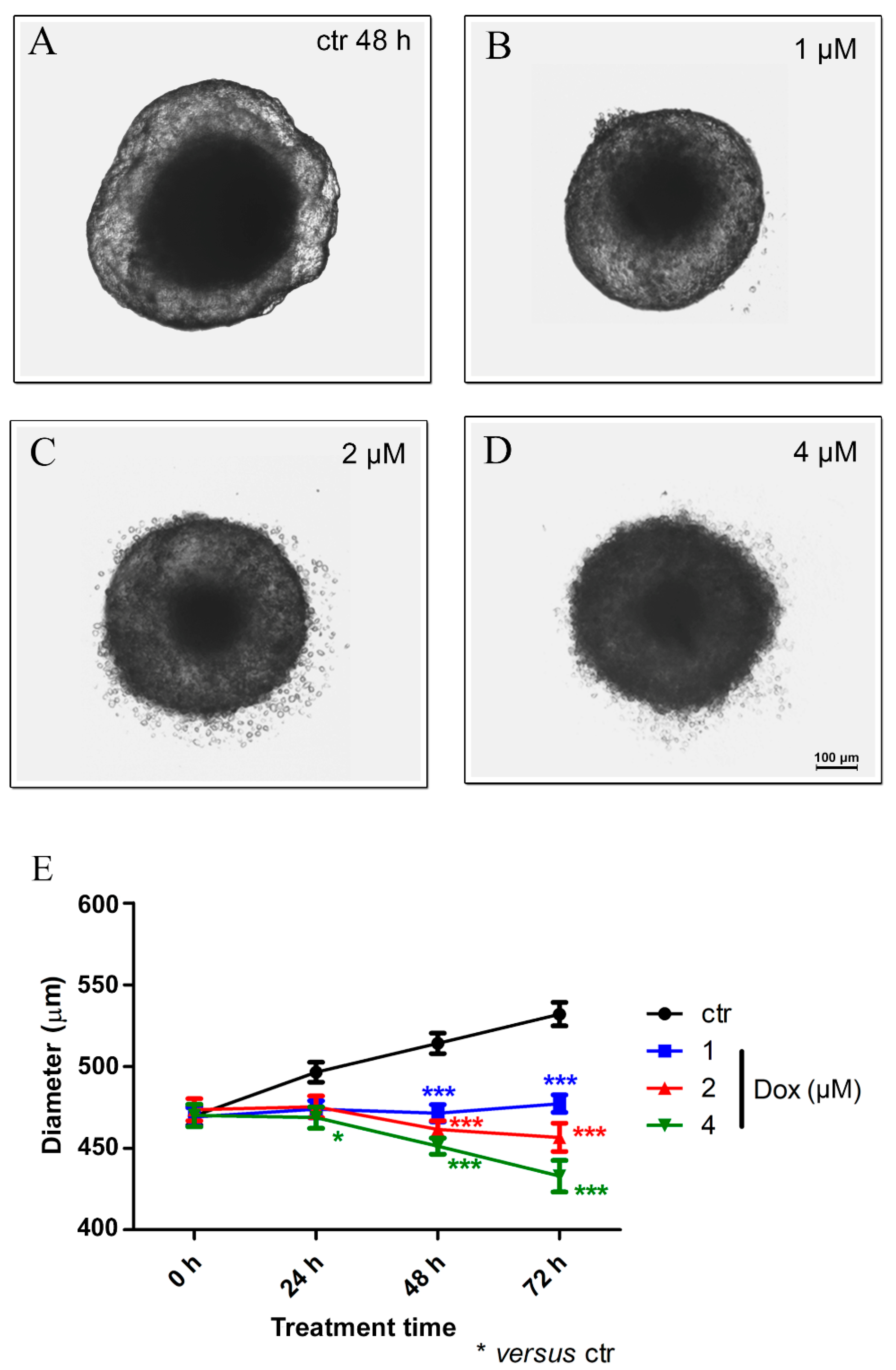
3.4. Doxorubicin Induces Cytotoxicity in MTSs
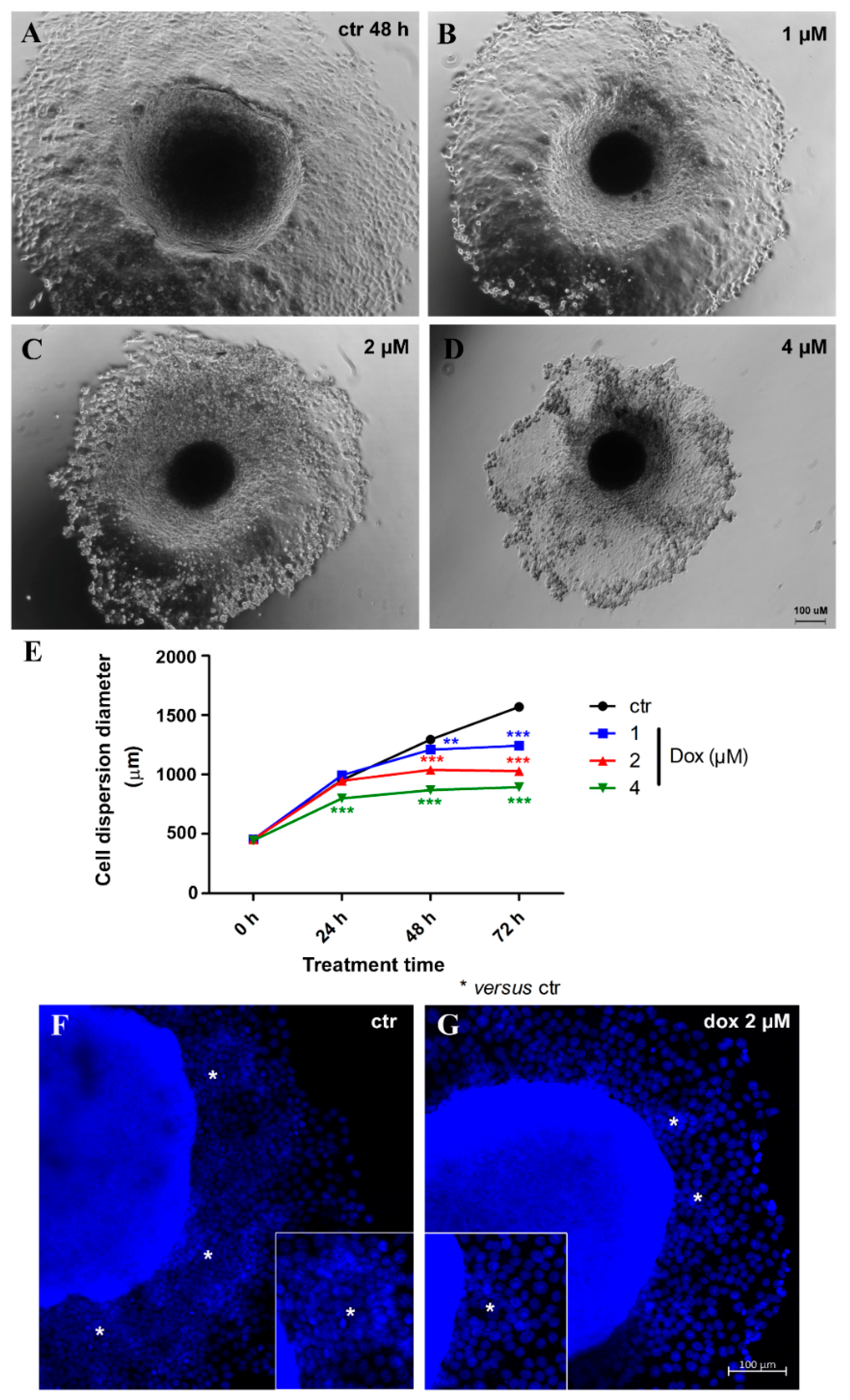
3.5. Doxorubicin Inhibits Cell Migration in MTSs
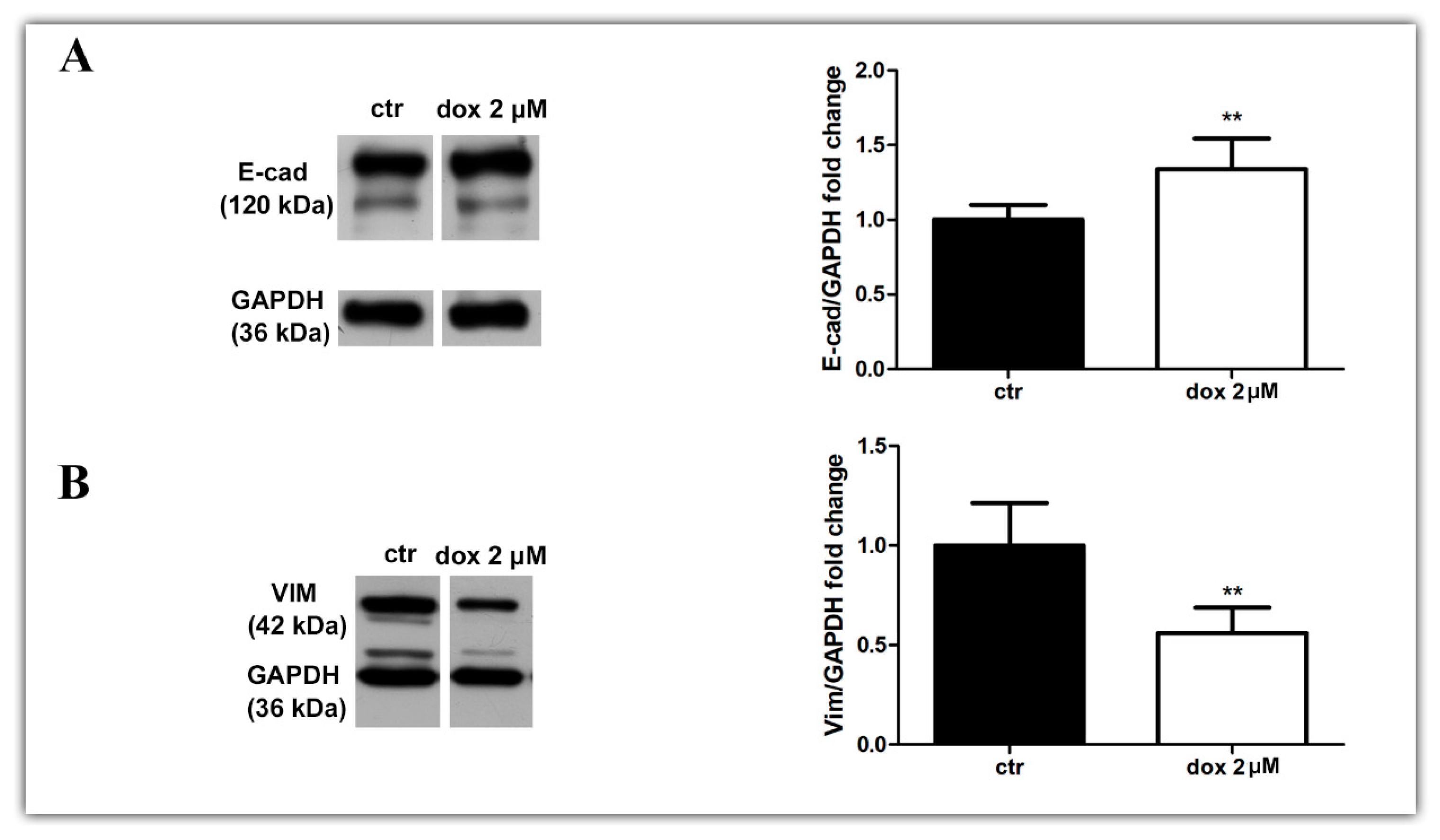
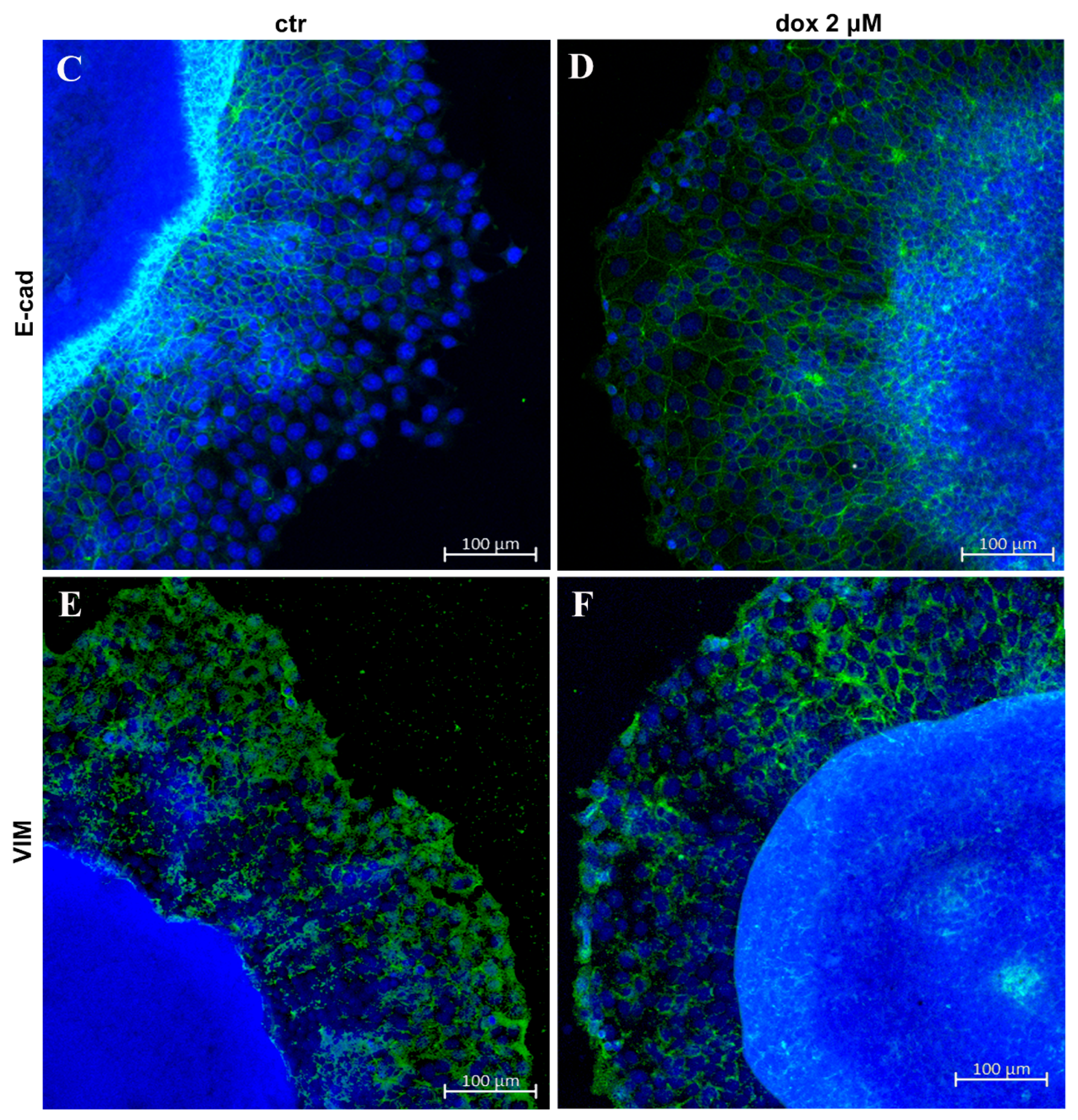
3.6. Doxorubicin Inhibits Aspects of the EMT Process in MTSs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Breast Cancer. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 1 February 2024).
- Cecilio, A.P.; Takakura, E.T.; Jumes, J.J.; Dos Santos, J.W.; Herrera, A.C.; Victorino, V.J.; Panis, C. Breast cancer in Brazil: Epidemiology and treatment challenges. Breast Cancer 2015, 7, 43–49. [Google Scholar] [PubMed]
- BCNA. 2023. Available online: https://www.bcna.org.au/resource-hub/articles/types-of-breast-cancer/ (accessed on 13 October 2023).
- Lee, B.L.; Liedke, P.E.R.; Barrios, C.H.; Simon, S.D.; Finkelstein, D.M.; Goss, P.E. Breast cancer in Brazil: Present status and future goals. Lancet Oncol. 2012, 13, e95–e102. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Drasin, D.J.; Robin, T.P.; Ford, H.L. Breast cancer epithelial-to-mesenchymal transition: Examining the functional consequences of plasticity. Breast Cancer Res. 2011, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Heerboth, S.; Housman, G.; Leary, M.; Longacre, M.; Byler, S.; Lapinska, K.; Willbanks, A.; Sarkar, S. EMT and tumor metastasis. Clin. Transl. Med. 2015, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Seo, J.; Yoon, H.; Cho, S. The Post-Translational Regulation of Epithelial-Mesenchymal Transition-Inducing Transcription Factors in Cancer Metastasis. Int. J. Mol. Sci. 2021, 22, 3591. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Bueno, G.; Portillo, F.; Cano, A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene 2008, 27, 6958–6969. [Google Scholar] [CrossRef] [PubMed]
- Zhitnyak, I.Y.; Rubtsova, S.N.; Litovka, N.I.; Gloushankova, N.A. Early Events in Actin Cytoskeleton Dynamics and E-Cadherin-Mediated Cell-Cell Adhesion during Epithelial-Mesenchymal Transition. Cells 2020, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Z.; Hao, Q.; Li, W.; Xu, Y.; Zhang, J.; Zhang, W.; Wang, S.; Liu, S.; Li, M.; et al. Loss of ERα induces amoeboid-like migration of breast cancer cells by downregulating vinculin. Nat. Commun. 2017, 8, 14483. [Google Scholar] [CrossRef] [PubMed]
- Ilina, O.; Campanello, L.; Gritsenko, P.G.; Vullings, M.; Wang, C.; Bult, P.; Losert, W.; Friedl, P. Intravital microscopy of collective invasion plasticity in breast cancer. Dis. Model. Mech. 2018, 11, dmm034330. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; Ilina, O.; Gritsenko, P.G.; Bult, P.; Span, P.N.; Friedl, P. Collective invasion in ductal and lobular breast cancer associates with distant metastasis. Clin. Exp. Metastasis 2017, 34, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.D.; Sahai, E. Cancer Dissemination-Lessons from Leukocytes. Dev. Cell 2010, 19, 13–26. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, F.B.; Schwartz, G.N.; Wells, W.A.; Tsongalis, G.J. Personalized therapy for breast cancer. Clin. Genet. 2014, 86, 62–67. [Google Scholar] [CrossRef]
- Dumbrava, E.I.; Meric-Bernstam, F. Personalized cancer therapy- leveraging a knowledge base for clinical decision-making. Cold Spring Harb. Mol. Case Stud. 2018, 4, a001578. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.J.; Baselga, J. Targeted therapies for breast cancer. J. Clin. Investig. 2011, 121, 3797–3803. [Google Scholar] [CrossRef] [PubMed]
- Masoud, V.; Pages, G. Targeted therapies in breast cancer: New challenges to fight against resistance. World J. Clin. Oncol. 2017, 8, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Mendes, D.; Alves, C.; Afonso, N.; Cardoso, F.; Passos-Coelho, J.L.; Costa, L.; Andrade, S.; Batel-Marques, F. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer—A systematic review. Breast Cancer Res. 2015, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Massague, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.L.; Joshi, R.; Thakuri, P.S.; Tavana, H. Liquid-based three-dimensional tumor models for cancer research and drug discovery. Exp. Biol. Med. 2016, 241, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Urzì, O.; Gasparro, R.; Costanzo, E.; De Luca, A.; Giavaresi, G.; Fontana, S.; Alessandro, R. Three-Dimensional Cell Cultures: The Bridge between In Vitro and In Vivo Models. Int. J. Mol. Sci. 2023, 24, 12046. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Bissell, M.J. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin. Cancer Biol. 2008, 18, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Goliwas, K.F.; Richter, J.R.; Pruitt, H.C.; Araysi, L.M.; Anderson, N.R.; Samant, R.S.; Lobo-Ruppert, S.M.; Berry, J.L.; Frost, A.R. Methods to Evaluate Cell Growth, Viability, and Response to Treatment in a Tissue Engineered Breast Cancer Model. Sci. Rep. 2017, 7, 14167. [Google Scholar] [CrossRef]
- Singha, B.; Laski, J.; Valdés, Y.R.; Liu, E.; Dimattia, G.E.; Shepherd, T.G. Inhibiting ULK1 kinase decreases autophagy and cell viability in high-grade serous ovarian cancer spheroids. Am. J. Cancer Res. 2020, 10, 1384–1399. [Google Scholar] [PubMed]
- Vidi, P.A.; Bissell, M.J.; Lelievre, S.A. Three-dimensional culture of human breast epithelial cells: The how and the why. Methods Mol. Biol. 2013, 945, 193–219. [Google Scholar]
- Timmins, N.E.; Harding, F.J.; Smart, C.; Brown, M.A.; Nielsen, L.K. Method for the generation and cultivation of functional three-dimensional mammary constructs without exogenous extracellular matrix. Cell Tissue Res. 2005, 320, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Groebe, K.; Mueller-Klieser, W. Distributions of oxygen, nutrient, and metabolic waste concentrations in multicellular spheroids and their dependence on spheroid parameters. Eur. Biophys. J. 1991, 19, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control Release 2012, 164, 192–204. [Google Scholar] [CrossRef] [PubMed]
- LaBarbera, D.V.; Reid, B.G.; Yoo, B.H. The multicellular tumor spheroid model for high-throughput cancer drug discovery. Expert. Opin. Drug Discov. 2012, 7, 819–830. [Google Scholar] [CrossRef]
- Utama, R.H.; Atapattu, L.; O’Mahony, A.P.; Fife, C.M.; Baek, J.; Allard, T.; O’Mahony, K.J.; Ribeiro, J.C.C.; Gaus, K.; Kavallaris, M.; et al. A 3D Bioprinter Specifically Designed for the High-Throughput Production of Matrix-Embedded Multicellular Spheroids. IScience 2020, 23, 101621. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Pijuan-Galito, S.; Rho, H.S.; Vasilevich, A.S.; Eren, A.D.; Ge, L.; Habibović, P.; Alexander, M.R.; de Boer, J.; Carlier, A.; et al. High-Throughput Methods in the Discovery and Study of Biomaterials and Materiobiology. Chem. Rev. 2021, 121, 4561–4677. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschlager, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Langthasa, J.; Sarkar, P.; Narayanan, S.; Bhagat, R.; Vadaparty, A.; Bhat, R. Extracellular matrix mediates moruloid-blastuloid morphodynamics in malignant ovarian spheroids. Life Sci. Alliance 2021, 4, e202000942. [Google Scholar] [CrossRef] [PubMed]
- Kunjithapatham, R.; Karthikeyan, S.; Geschwind, J.F.; Kieserman, E.; Lin, M.; Fu, D.X.; Ganapathy-Kanniappan, S. Reversal of anchorage-independent multicellular spheroid into a monolayer mimics a metastatic model. Sci. Rep. 2014, 4, 6816. [Google Scholar] [CrossRef]
- de Barros, A.P.; Takiya, C.M.; Garzoni, L.R.; Leal-Ferreira, M.L.; Dutra, H.S.; Chiarini, L.B.; Meirelles, M.N.; Borojevic, R.; Rossi, M.I. Osteoblasts and bone marrow mesenchymal stromal cells control hematopoietic stem cell migration and proliferation in 3D in vitro model. PLoS ONE 2010, 5, e9093. [Google Scholar] [CrossRef] [PubMed]
- Garzoni, L.R.; Adesse, D.; Soares, M.J.; Rossi, M.I.; Borojevic, R.; de Meirelles Mde, N. Fibrosis and hypertrophy induced by Trypanosoma cruzi in a three-dimensional cardiomyocyte-culture system. J. Infect. Dis. 2008, 197, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Garzoni, L.R.; Rossi, M.I.; de Barros, A.P.; Guarani, V.; Keramidas, M.; Balottin, L.B.; Adesse, D.; Takiya, C.M.; Manso, P.P.; Otazu, I.B.; et al. Dissecting coronary angiogenesis: 3D co-culture of cardiomyocytes with endothelial or mesenchymal cells. Exp. Cell Res. 2009, 315, 3406–3418. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.I.; Barros, A.P.; Baptista, L.S.; Garzoni, L.R.; Meirelles, M.N.; Takiya, C.M.; Pascarelli, B.M.; Dutra, H.S.; Borojevic, R. Multicellular spheroids of bone marrow stromal cells: A three-dimensional in vitro culture system for the study of hematopoietic cell migration. Braz. J. Med. Biol. Res. 2005, 38, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Ferrão, P.M.; Nisimura, L.M.; Moreira, O.C.; Land, M.G.; Pereira, M.C.; de Mendonça-Lima, L.; Araujo-Jorge, T.C.; Waghabi, M.C.; Garzoni, L.R. Inhibition of TGF-β pathway reverts extracellular matrix remodeling in T. cruzi-infected cardiac spheroids. Exp. Cell Res. 2018, 362, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Nisimura, L.M.; Ferrão, P.M.; Nogueira, A.D.R.; Waghabi, M.C.; Meuser-Batista, M.; Moreira, O.C.; Urbina, J.A.; Garzoni, L.R. Effect of Posaconazole in an in vitro model of cardiac fibrosis induced by Trypanosoma cruzi. Mol. Biochem. Parasitol. 2020, 238, 111283. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Aya, L.F.; Gonzalez-Angulo, A.M. Adjuvant systemic therapies in breast cancer. Surg. Clin. N. Am. 2013, 93, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and triple-negative breast cancer: Challenges and treatment options. Drug Deliv. Transl. Res. 2018, 8, 1483–1507. [Google Scholar] [CrossRef]
- Parker, A.L.; Benguigui, M.; Fornetti, J.; Goddard, E.; Lucotti, S.; Insua-Rodríguez, J.; Wiegmans, A.P.; Early Career Leadership Council of the Metastasis Research Society. Current challenges in metastasis research future innovation for clinical translation. Clin. Exp. Metastasis 2022, 39, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.N.; Mei, Y.; Zhang, J. Cancer metastasis: Issues and challenges. Chin. J. Cancer 2017, 36, 38. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Emerman, J.T.; Enami, J.; Pitelka, D.R.; Nandi, S. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc. Natl. Acad. Sci. USA 1977, 74, 4466–4470. [Google Scholar] [CrossRef]
- Halfter, K.; Ditsch, N.; Kolberg, H.C.; Fischer, H.; Hauzenberger, T.; von Koch, F.E.; Bauerfeind, I.; von Minckwitz, G.; Funke, I.; Crispin, A.; et al. Prospective cohort study using the breast cancer spheroid model as a predictor for response to neoadjuvant therapy—The SpheroNEO study. BMC Cancer 2015, 15, 519. [Google Scholar] [CrossRef] [PubMed]
- Halfter, K.; Hoffmann, O.; Ditsch, N.; Ahne, M.; Arnold, F.; Paepke, S.; Grab, D.; Bauerfeind, I.; Mayer, B. Testing chemotherapy efficacy in HER2 negative breast cancer using patient-derived spheroids. J. Transl. Med. 2016, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Song, J.M. 3D bioprinted drug-resistant breast cancer spheroids for quantitative in situ evaluation of drug resistance. Acta Biomater. 2022, 138, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Bahcecioglu, G.; Basara, G.; Ellis, B.W.; Ren, X.; Zorlutuna, P. Breast cancer models: Engineering the tumor microenvironment. Acta Biomater. 2020, 106, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.P.; Gopsill, J.A.; Gomm, J.J.; Jones, J.L.; Grose, R.P. A 3D in vitro model of the human breast duct: A method to unravel myoepithelial-luminal interactions in the progression of breast cancer. Breast Cancer Res. 2017, 19, 50. [Google Scholar] [CrossRef]
- Nath, S.; Devi, G.R. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Weiswald, L.B.; Bellet, D.; Dangles-Marie, V. Spherical cancer models in tumor biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yu, P.; Tang, J. Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model. Onco Targets Ther. 2020, 13, 5395–5405. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Guan, X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Bouchalova, P.; Bouchal, P. Current methods for studying metastatic potential of tumor cells. Cancer Cell Int. 2022, 22, 394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, J. Advances in human organoids-on-chips in biomedical research. Life Med. 2023, 2, lnad007. [Google Scholar] [CrossRef]
- Kunz-Schughart, L.A.; Freyer, J.P.; Hofstaedter, F.; Ebner, R. The use of 3-D cultures for high-throughput screening: The multicellular spheroid model. J. Biomol. Screen. 2004, 9, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hansen, R.K.; Radisky, D.; Yoneda, T.; Barcellos-Hoff, M.H.; Petersen, O.W.; Turley, E.A.; Bissell, M.J. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J. Natl. Cancer Inst. 2002, 94, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Chandramouly, G.; Abad, P.C.; Knowles, D.W.; Lelièvre, S.A. The control of tissue architecture over nuclear organization is crucial for epithelial cell fate. J. Cell Sci. 2007, 120, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Halaoui, R.; Rejon, C.; Chatterjee, S.J.; Szymborski, J.; Meterissian, S.; Muller, W.J.; Omeroglu, A.; McCaffrey, L. Progressive polarity loss and luminal collapse disrupt tissue organization in carcinoma. Genes. Dev. 2017, 31, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Helfman, D.M. Up-regulated fibronectin in 3D culture facilitates spreading of triple negative breast cancer cells on 2D through integrin β-5 and Src. Sci. Rep. 2019, 9, 19950. [Google Scholar] [CrossRef] [PubMed]
- Streuli, C.H.; Bailey, N.; Bissell, M.J. Control of mammary epithelial differentiation: Basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J. Cell Biol. 1991, 115, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Correa de Sampaio, P.; Auslaender, D.; Krubasik, D.; Failla, A.V.; Skepper, J.N.; Murphy, G.; English, W.R. A heterogeneous in vitro three dimensional model of tumour-stroma interactions regulating sprouting angiogenesis. PLoS ONE 2012, 7, e30753. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Bilder, D. Polarity determination in breast tissue: Desmosomal adhesion, myoepithelial cells, and laminin 1. Breast Cancer Res. 2003, 5, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Kunz-Schughart, L.A.; Kreutz, M.; Knuechel, R. Multicellular spheroids: A three-dimensional in vitro culture system to study tumour biology. Int. J. Exp. Pathol. 1998, 79, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, S.M.; Mladovan, A.; Garbovesky, C.; Baldi, A.; Lüthy, I.A. Three novel hormone-responsive cell lines derived from primary human breast carcinomas: Functional characterization. J. Cell Physiol. 2004, 199, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Halfter, K.; Mayer, B. Bringing 3D tumor models to the clinic—Predictive value for personalized medicine. Biotechnol. J. 2017, 12, 1600295. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Weinberg, R.A. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget 2016, 7, 45745–45756. [Google Scholar] [CrossRef] [PubMed]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Evaluation of chemotherapeutics in a three-dimensional breast cancer model. J. Cancer Res. Clin. Oncol. 2015, 141, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Farach-Carson, M.C.; Jia, X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol. Adv. 2014, 32, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, W.; Zhang, X.Z.; Hou, L.; Lu, Y.J.; Chen, P.P.; Zhang, C.; Feng, D.; Kong, L.; Wang, X.L. Development of three-dimensional breast cancer cell culture drug resistance model. Sheng Li Xue Bao 2016, 68, 179–184. [Google Scholar] [PubMed]
- Yildiz-Ozturk, E.; Gulce-Iz, S.; Anil, M.; Yesil-Celiktas, O. Cytotoxic responses of carnosic acid and doxorubicin on breast cancer cells in butterfly-shaped microchips in comparison to 2D and 3D culture. Cytotechnology 2017, 69, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer 2018, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lv, Q.; Meng, W.; Tan, Q.; Zhang, S.; Mo, X.; Yang, X. Comparison of mammosphere formation from breast cancer cell lines and primary breast tumors. J. Thorac. Dis. 2014, 6, 829–837. [Google Scholar] [PubMed]
- Yilmazer, A. Evaluation of cancer stemness in breast cancer and glioblastoma spheroids in vitro. 3 Biotech 2018, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef]
- Koleini, N.; Kardami, E. Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity. Oncotarget 2017, 8, 46663–46680. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Y.; Liang, K.; Liu, B.; Fan, Z. Differential responses to doxorubicin-induced phosphorylation and activation of Akt in human breast cancer cells. Breast Cancer Res. 2005, 7, R589–R597. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Watson, M.B.; O’Kane, S.L.; Drew, P.J.; Lind, M.J.; Cawkwell, L. The analysis of doxorubicin resistance in human breast cancer cells using antibody microarrays. Mol. Cancer Ther. 2006, 5, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Rivankar, S. An overview of doxorubicin formulations in cancer therapy. J. Cancer Res. Ther. 2014, 10, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Vyas, D.; Laput, G.; Vyas, A.K. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. Onco Targets Ther. 2014, 12, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.F.; Tan, W.; Tian, K.; Yu, H.; Qiang, W.A.; Wang, Y.T. Combined effects of furanodiene and doxorubicin on the migration and invasion of MDA-MB-231 breast cancer cells in vitro. Oncol. Rep. 2017, 37, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Wang, L.; Agyin, J.; Tang, Y.; Lin, S.; Yeh, I.T.; De, K.; Sun, L.Z. Doxorubicin in combination with a small TGFbeta inhibitor: A potential novel therapy for metastatic breast cancer in mouse models. PLoS ONE 2010, 5, e10365. [Google Scholar] [CrossRef]
- Liu, C.L.; Chen, M.J.; Lin, J.C.; Lin, C.H.; Huang, W.C.; Cheng, S.P.; Chen, S.N.; Chang, Y.C. Doxorubicin Promotes Migration and Invasion of Breast Cancer Cells through the Upregulation of the RhoA/MLC Pathway. J. Breast Cancer 2019, 22, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Xu, J.D.; Wang, W.J.; Cao, X.X.; Chen, Q.; Tang, F.; Chen, Z.Q.; Liu, X.P.; Xu, Z.D. Twist1-mediated adriamycin-induced epithelial-mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin. Cancer Res. 2009, 15, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ma, Z.; Liu, Y.; Kan, X.; Wang, C.; Su, B.; Li, Y.; Zhang, Y.; Wang, P.; Luo, Y.; et al. Low doses of paclitaxel enhance liver metastasis of breast cancer cells in the mouse model. FEBS J. 2016, 283, 2836–2852. [Google Scholar] [CrossRef] [PubMed]


| Antibody | Manufacturer | Catalog Number | Antibody Dilution |
|---|---|---|---|
| Anti-E-cadherin antibody, rabbit monoclonal | Sigma-Aldrich | SAB5500022 | 1:100 |
| Anti-vimentin antibody, mouse monoclonal | Sigma-Aldrich | V5255 | 1:200 |
| Anti-fibronectin antibody, rabbit polyclonal | Sigma-Aldrich | F3648 | 1:200 |
| Anti-laminin antibody, rabbit polyclonal | Sigma-Aldrich | L9393 | 1:50 |
| Anti-Ki-67 antibody, rabbit recombinant monoclonal | Abcam | ab16667 | 1:100 |
| Donkey anti-mouse IgG secondary antibody (Alexa Fluor™ 594) | Thermo Scientific | A21203 | 1:800 |
| Donkey anti-rabbit IgG secondary antibody (Alexa Fluor™ 488) | Thermo Scientific | A21206 | 1:800 |
| Antibody | Manufacturer | Catalog Number | Antibody Dilution |
|---|---|---|---|
| Anti-E-cadherin antibody, rabbit monoclonal | Sigma-Aldrich | SAB5500022 | 1:2000 |
| Anti-vimentin antibody, mouse monoclonal | Sigma-Aldrich | V5255 | 1:3000 |
| Anti-GAPDH antibody, mouse monoclonal | Fitzgerald | 10R-G109a | 1:80,000 |
| Goat anti-mouse IgG secondary antibody (HRP) | R&D Systems | HAF007 | 1:1000 |
| Goat anti-rabbit IgG secondary antibody (HRP) | R&D Systems | HAF008 | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, L.L.; Vianna, M.M.; da Silva, D.M.; Gonzaga, B.M.d.S.; Ferreira, R.R.; Monteiro, A.C.; Bonomo, A.C.; Manso, P.P.d.A.; de Carvalho, M.A.; Vargas, F.R.; et al. Spheroid Model of Mammary Tumor Cells: Epithelial–Mesenchymal Transition and Doxorubicin Response. Biology 2024, 13, 463. https://doi.org/10.3390/biology13070463
Coelho LL, Vianna MM, da Silva DM, Gonzaga BMdS, Ferreira RR, Monteiro AC, Bonomo AC, Manso PPdA, de Carvalho MA, Vargas FR, et al. Spheroid Model of Mammary Tumor Cells: Epithelial–Mesenchymal Transition and Doxorubicin Response. Biology. 2024; 13(7):463. https://doi.org/10.3390/biology13070463
Chicago/Turabian StyleCoelho, Laura Lacerda, Matheus Menezes Vianna, Debora Moraes da Silva, Beatriz Matheus de Souza Gonzaga, Roberto Rodrigues Ferreira, Ana Carolina Monteiro, Adriana Cesar Bonomo, Pedro Paulo de Abreu Manso, Marcelo Alex de Carvalho, Fernando Regla Vargas, and et al. 2024. "Spheroid Model of Mammary Tumor Cells: Epithelial–Mesenchymal Transition and Doxorubicin Response" Biology 13, no. 7: 463. https://doi.org/10.3390/biology13070463





