Prolonged Cold Exposure Negatively Impacts Atlantic Salmon (Salmo salar) Liver Metabolism and Function
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocol and Sampling
2.3. Lipid Class and Fatty Acid Analyses
2.4. Analysis of mRNA Expression of Winter Syndrome-Related Genes
2.4.1. RNA Preparation
2.4.2. cDNA Synthesis
2.4.3. Quantitative Real-Time Polymerase Chain Reaction (qPCR) Conditions
2.4.4. Primer Design and Quality Control (QC) Testing
2.4.5. Normalizer Gene Selection
2.4.6. Experimental qPCR Analyses
2.5. Statistical Analyses
3. Results
3.1. Lipid Analyses
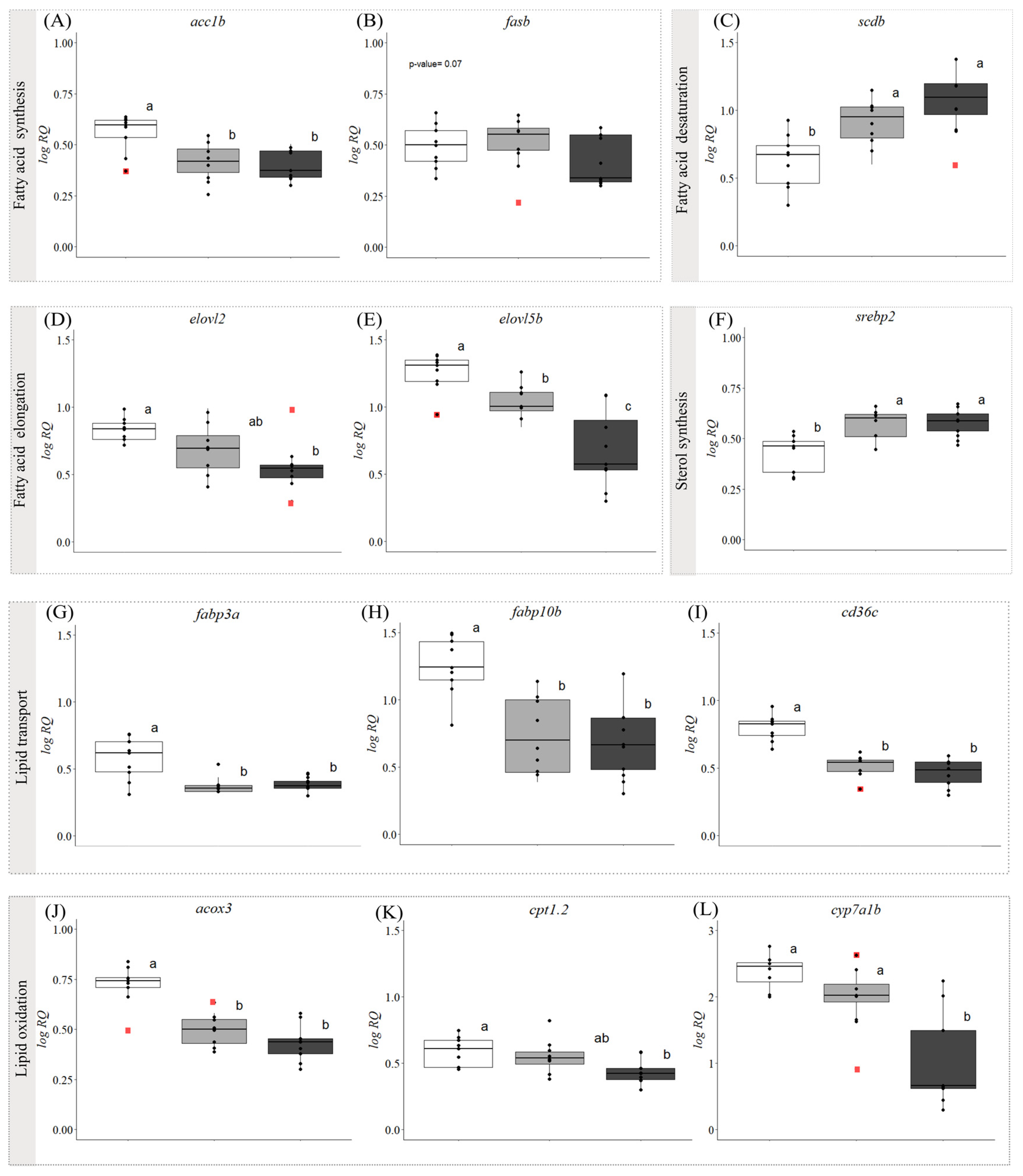
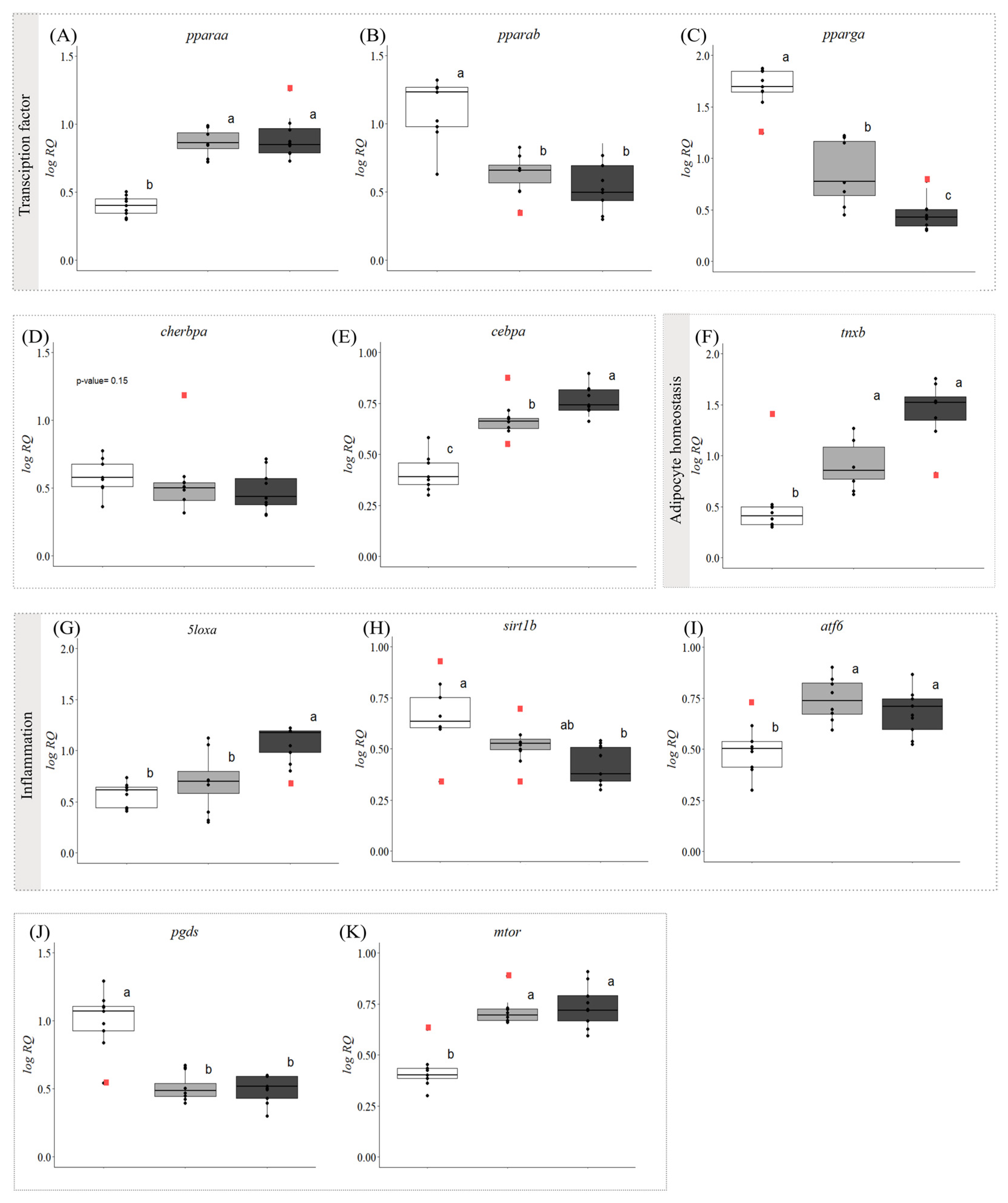
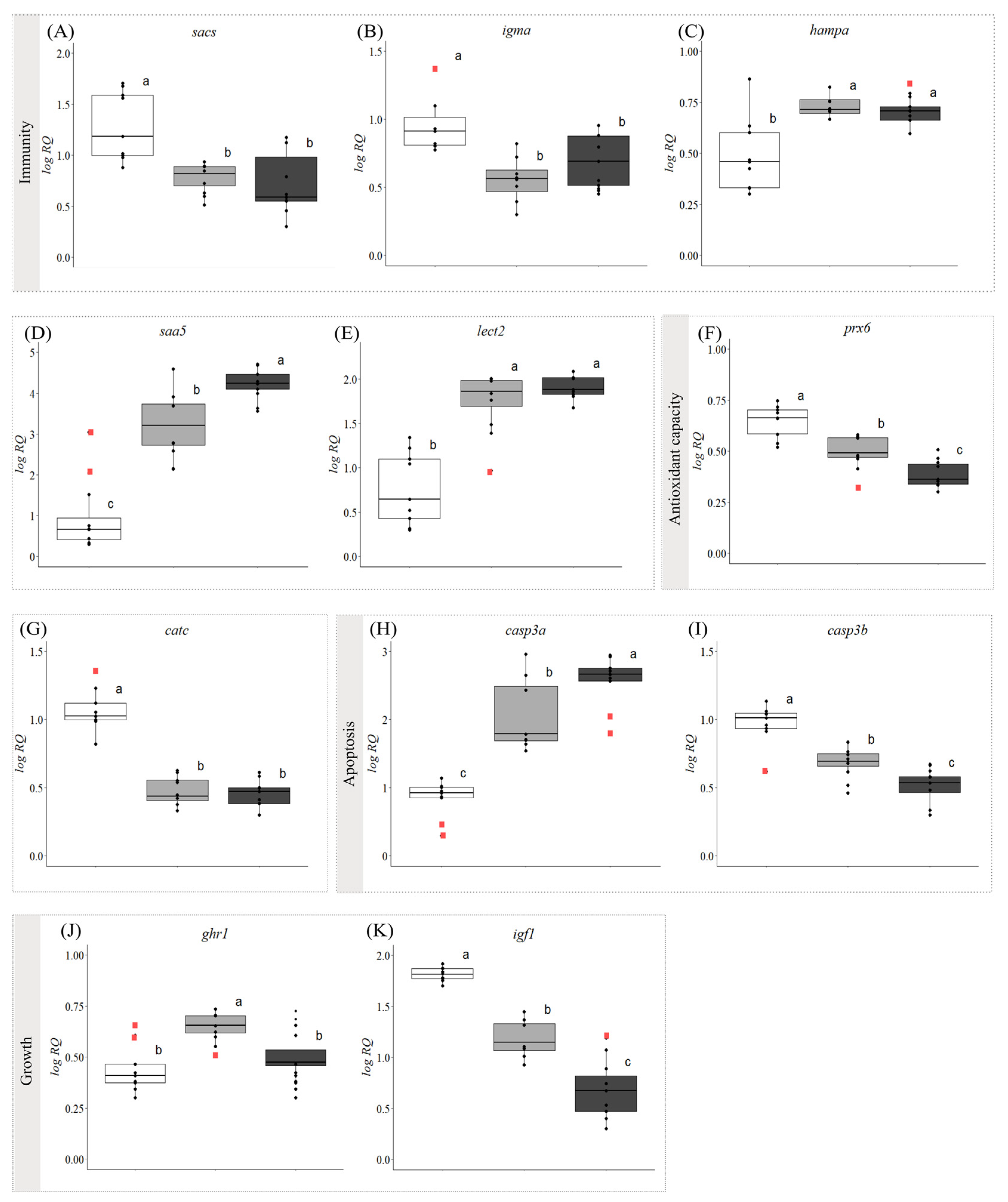
3.2. Gene Expression
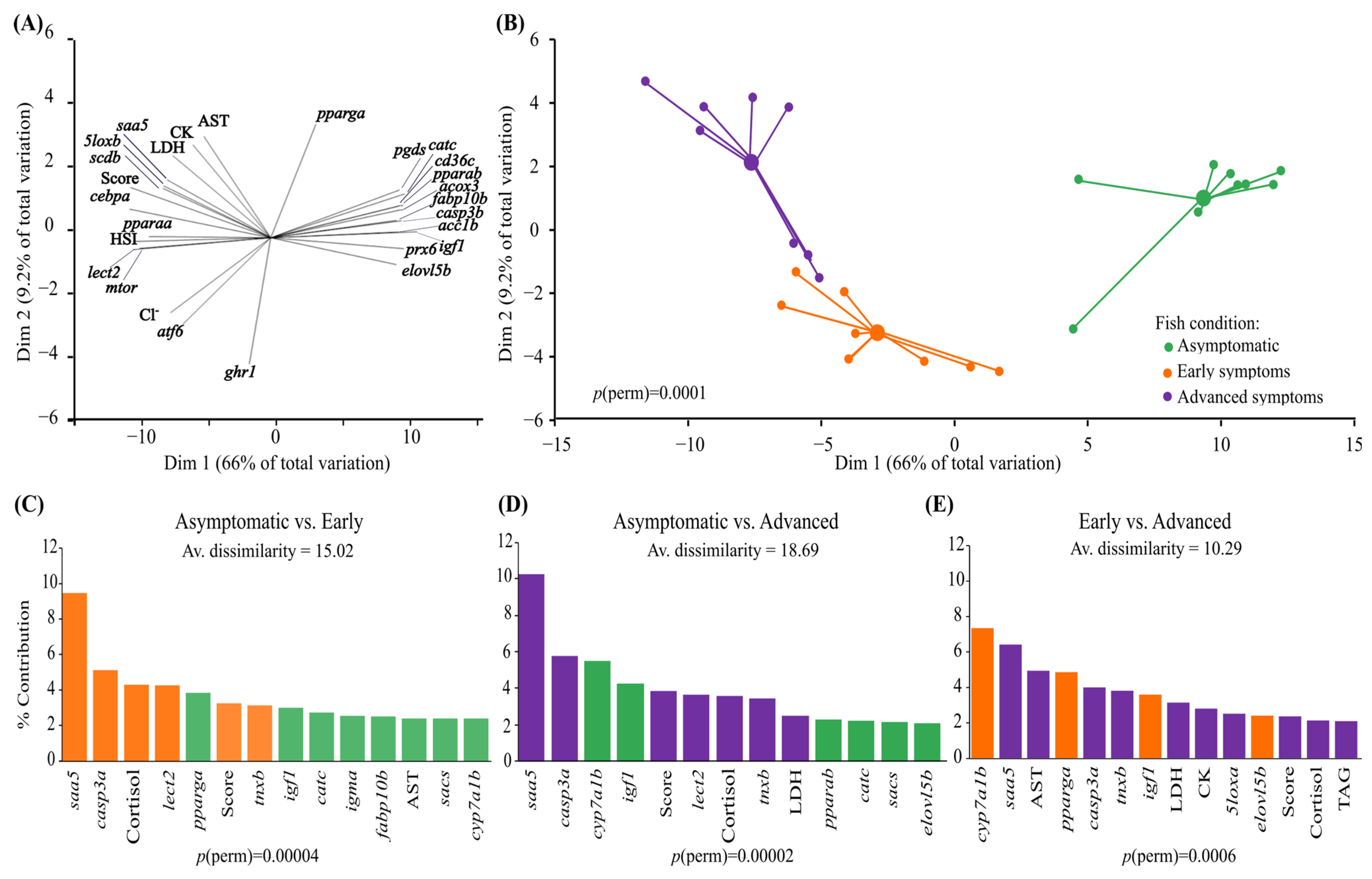
3.3. Multivariate Approach
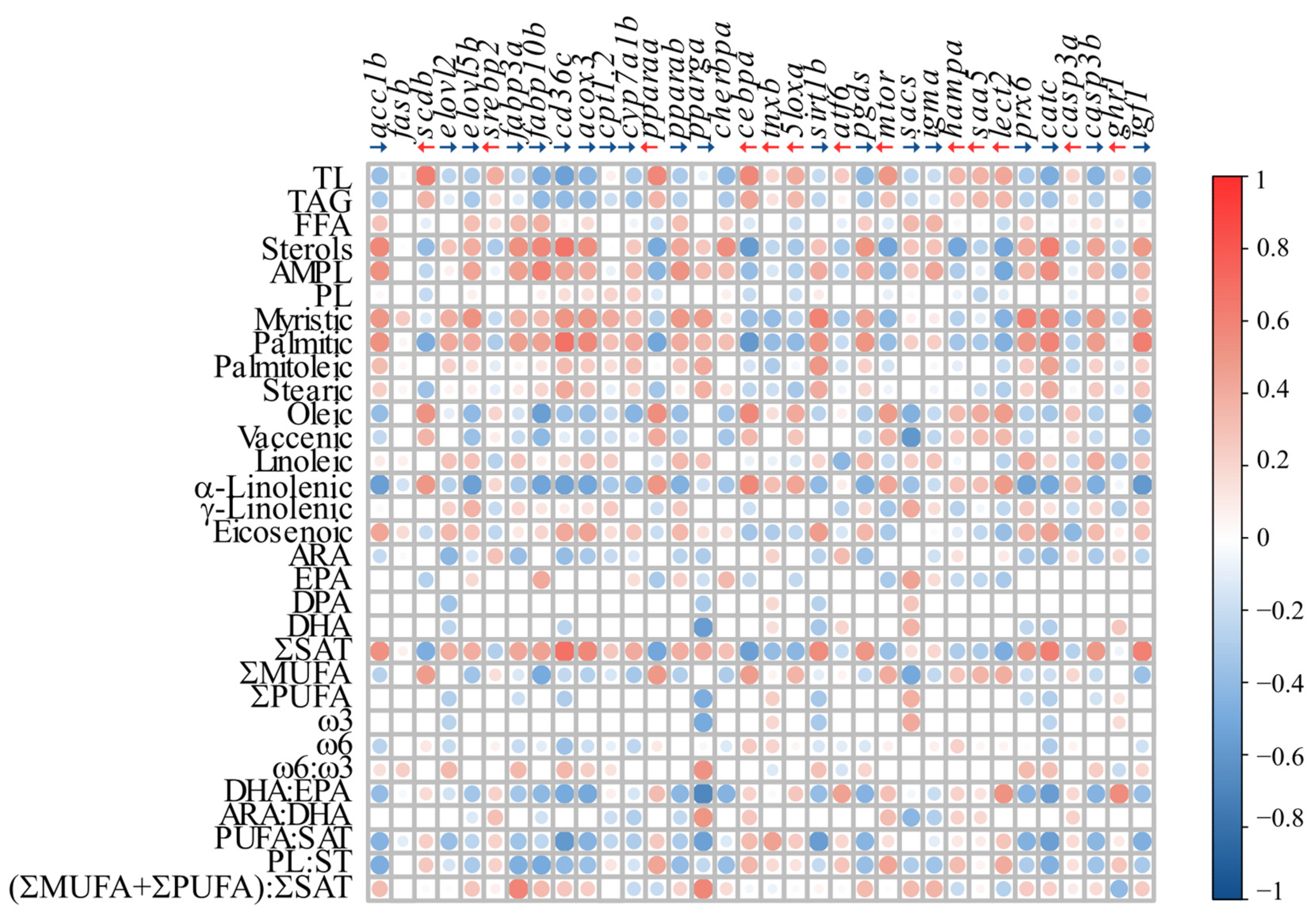
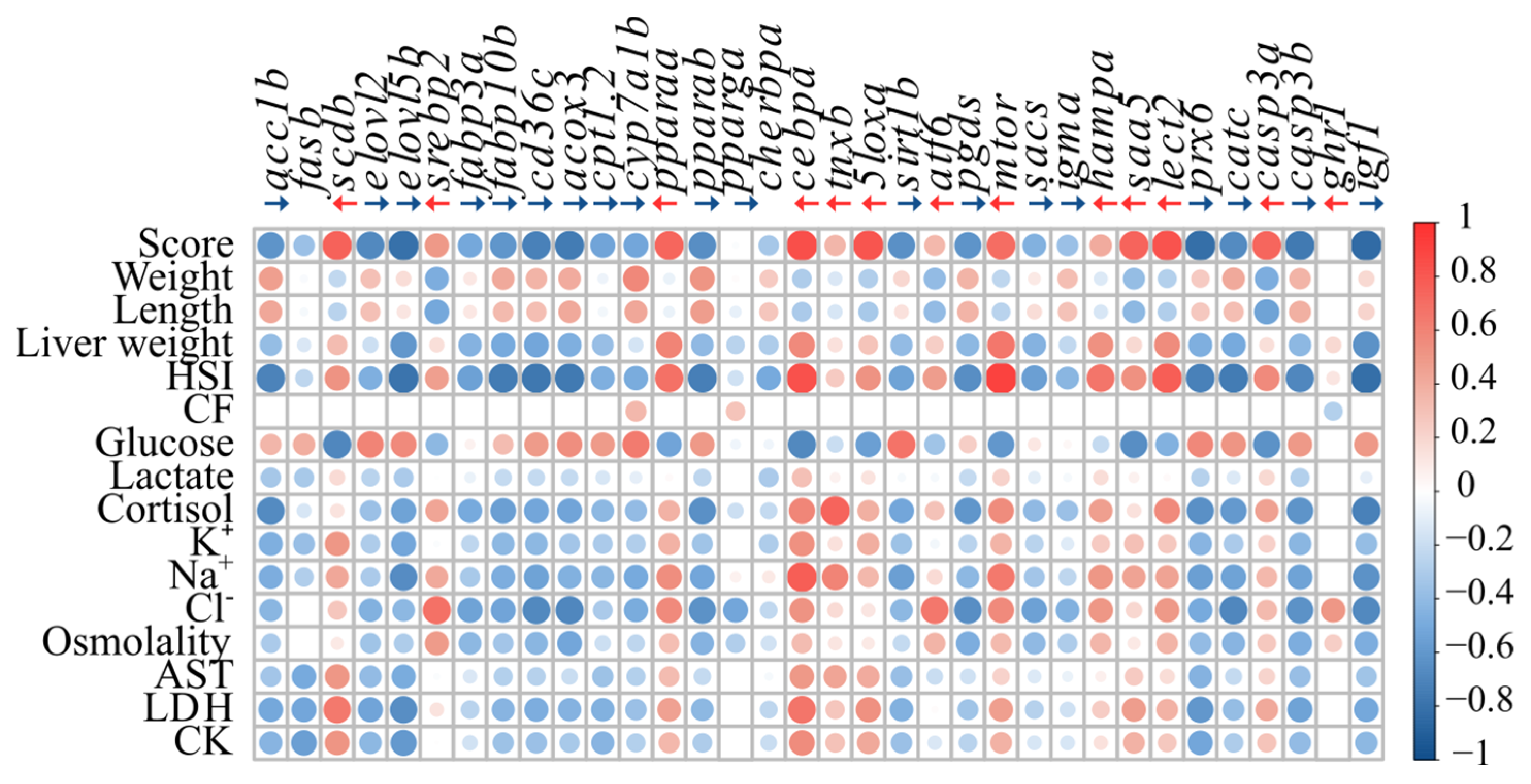
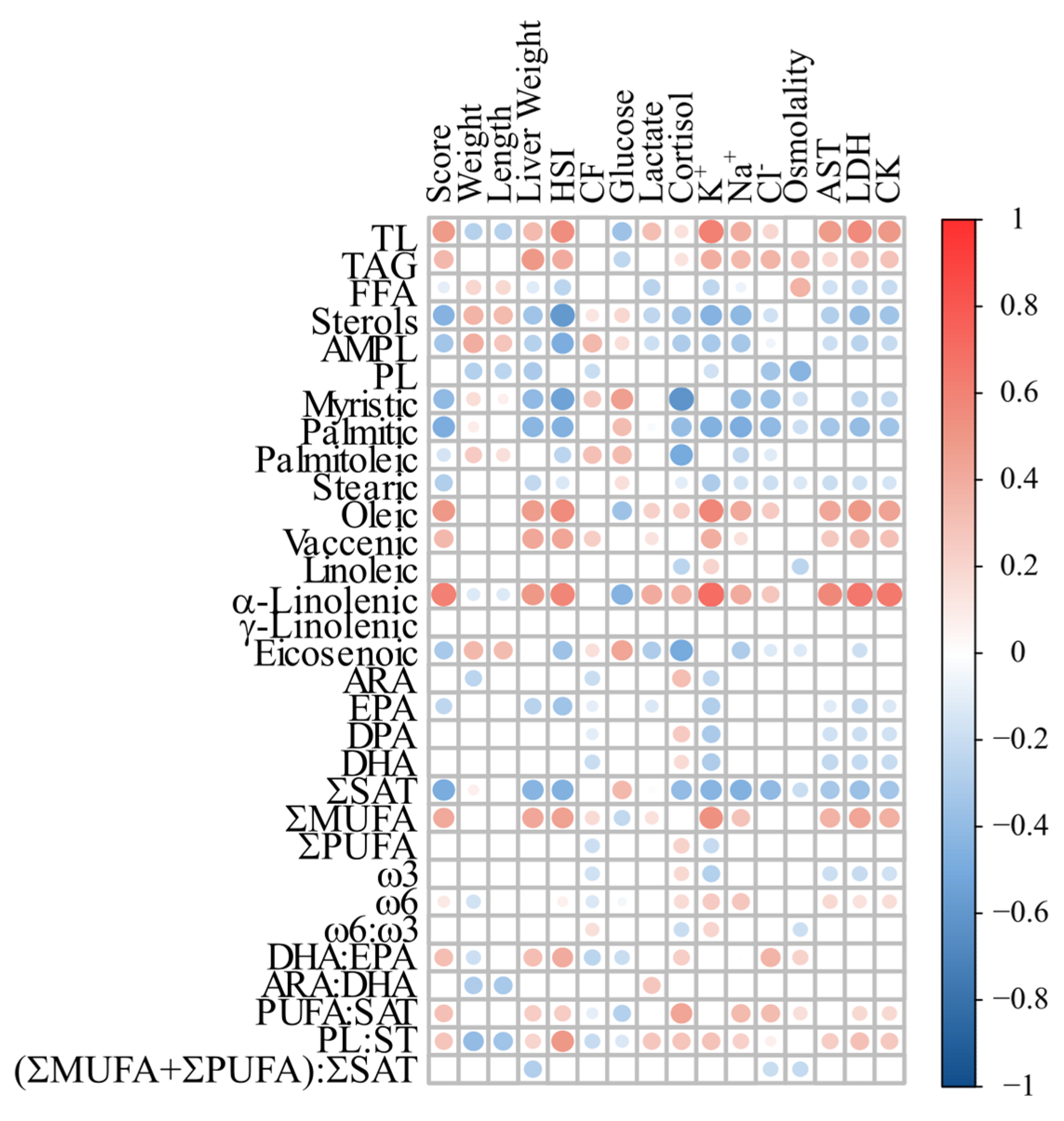
3.4. Correlation Analyses
4. Discussion
4.1. Cold Exposure and Hepatic Steatosis (Fatty Liver)
4.1.1. Increases in Liver Lipids and Triacylglycerols
4.1.2. Changes in Liver Lipid Classes and Fatty Acids
4.2. Evidence of Steatohepatitis (Fatty Liver Disease) in Symptomatic Fish
4.3. Proposed Biomarkers of FLD in Atlantic Salmon
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO FAOSTAT Statistical Database. Available online: https://www.fao.org/fishery/statistics-query/en/global_production/global_production_quantity (accessed on 14 August 2023).
- Calado, R.; Mota, V.C.; Madeira, D.; Leal, M. Summer Is Coming! Tackling Ocean Warming in Atlantic Salmon Cage Farming. Animals 2021, 11, 1800. [Google Scholar] [CrossRef]
- Hvas, M.; Folkedal, O.; Imsland, A.; Oppedal, F. The Effect of Thermal Acclimation on Aerobic Scope & Critical Swimming Speed in Atlantic Salmon, Salmo Salar. J. Exp. Biol. 2017, 220, 2757–2764. [Google Scholar] [CrossRef]
- Tromp, J.J.; Jones, P.L.; Brown, M.S.; Donald, J.A.; Biro, P.A.; Afonso, L.O.B. Chronic Exposure to Increased Water Temperature Reveals Few Impacts on Stress Physiology and Growth Responses in Juvenile Atlantic Salmon. Aquaculture 2018, 495, 196–204. [Google Scholar] [CrossRef]
- Gamperl, A.K.; Ajiboye, O.O.; Zanuzzo, F.S.; Sandrelli, R.M.; Peroni, E.; Ellen de Fátima, C.P.; Beemelmanns, A. The Impacts of Increasing Temperature and Moderate Hypoxia on the Production Characteristics, Cardiac Morphology and Haematology of Atlantic Salmon (Salmo salar). Aquaculture 2020, 519, 734874. [Google Scholar] [CrossRef]
- Zanuzzo, F.S.; Beemelmanns, A.; Hall, J.R.; Rise, M.L.; Gamperl, A.K. The Innate Immune Response of Atlantic Salmon (Salmo salar) Is Not Negatively Affected by High Temperature and Moderate Hypoxia. Front. Immunol. 2020, 11, 1009. [Google Scholar] [CrossRef]
- Bartlett, C.B.; Garber, A.F.; Gonen, S.; Benfey, T.J. Acute Critical Thermal Maximum Does Not Predict Chronic Incremental Thermal Maximum in Atlantic Salmon (Salmo salar). Comp. Biochem. Physiol. A 2022, 266, 111143. [Google Scholar] [CrossRef]
- Ignatz, E.H.; Rise, M.L.; Gamperl, A.K. Impact of Stress Phenotype, Elevated Temperature, and Bacterin Exposure on Male Atlantic Salmon (Salmo salar) Growth, Stress, and Immune Biomarker Gene Expression. Physiol. Genom. 2023, 55, 587–605. [Google Scholar] [CrossRef]
- Beemelmanns, A.; Zanuzzo, F.S.; Xue, X.; Sandrelli, R.M.; Rise, M.L.; Gamperl, A.K. The Transcriptomic Responses of Atlantic Salmon (Salmo salar) to High Temperature Stress Alone, and in Combination with Moderate Hypoxia. BMC Genom. 2021, 22, 261. [Google Scholar] [CrossRef]
- Ignatz, E.H.; Sandrelli, R.M.; Vadboncoeur, É.; Zanuzzo, F.S.; Perry, G.M.L.; Rise, M.L.; Gamperl, A.K. The Atlantic Salmon’s (Salmo salar) Incremental Thermal Maximum Is a More Relevant and Sensitive Indicator of Family-Based Differences in Upper Temperature Tolerance than Its Critical Thermal Maximum. Aquaculture 2023, 574, 739628. [Google Scholar] [CrossRef]
- Ćirić, J. 500 Tonnes of Salmon Die in Arnarlax Fish Farms. Available online: https://www.icelandreview.com/economy/500-tonnes-of-salmon-die-in-arnarlax-fish-farms/ (accessed on 13 September 2023).
- Pennell, J. Cold Winter Killing Salmon at Fish Farms. Available online: https://www.saltwire.com/newfoundland-labrador/business/cold-winter-killing-salmon-at-fish-farms-90564/ (accessed on 13 September 2023).
- TheFishSite. Arctic Fish Reports 2000 Tonne Salmon Mortality. Available online: https://thefishsite.com/articles/arctic-fish-reports-2-000-tonne-salmon-mortality (accessed on 13 September 2023).
- Willick, F. Cold Water Kills 10,000 Salmon at Cooke Fish Farm near Liverpool. Available online: https://www.cbc.ca/news/canada/nova-scotia/cooke-aquaculture-fish-kill-liverpool-1.5062704 (accessed on 13 September 2023).
- Mugaas, P. Warm Water Blamed for Petuna Salmon Die-Off. Available online: https://www.fishfarmingexpert.com/australia-petuna-salmon-die-off/warm-water-blamed-for-petuna-salmon-die-off/1332390 (accessed on 13 September 2023).
- Mugaas, P. Storms Cause Salmon Deaths in Newfoundland. Available online: https://www.fishfarmingexpert.com/cooke-aquaculture-fish-deaths-gerry-byrne/storms-cause-salmon-deaths-in-newfoundland/1376414 (accessed on 13 September 2023).
- Saunders, R.L.; Muise, B.C.; Henderson, E.B. Mortality of Salmonid Cultured at Low Temperature in Sea Water. Aquaculture 1975, 5, 243–252. [Google Scholar] [CrossRef]
- Donaldson, M.R.; Cooke, S.J.; Patterson, D.A.; Macdonald, J.S. Cold Shock and Fish. J. Fish Biol. 2008, 73, 1491–1530. [Google Scholar] [CrossRef]
- Reid, C.H.; Patrick, P.H.; Rytwinski, T.; Taylor, J.J.; Willmore, W.G.; Reesor, B.; Cooke, S.J. An Updated Review of Cold Shock and Cold Stress in Fish. J. Fish Biol. 2022, 100, 1102–1137. [Google Scholar] [CrossRef]
- Vadboncoeur, É.; Nelson, C.; Clow, K.A.; Sandrelli, R.M.; Brauner, C.J.; Swanson, A.K.; Gamperl, A.K. ‘Cold Shock’ Has Few Physiological Effects on Cultured Atlantic Salmon (Salmo salar) Acclimated to Low Temperatures. Aquaculture 2023, 577, 739900. [Google Scholar] [CrossRef]
- Vadboncoeur, É.; Nelson, C.; Hall, J.R.; Clow, K.A.; Sandrelli, R.M.; Brauner, C.J.; Swanson, A.K.; Gamperl, A.K. Lowering Temperature to 1 °C Results in Physiological Changes, Stress and Mortality in Cultured Atlantic Salmon (Salmo salar). Aquaculture 2023, 568, 739313. [Google Scholar] [CrossRef]
- Vadboncoeur, É. Low Temperatures Typical of Winter Cage-Site Conditions in Atlantic Canada Impact the Growth, Physiology, Health and Welfare of Atlantic Salmon (Salmo salar). Master’s Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, 2023. Available online: https://research.library.mun.ca/view/creator_az/Vadboncoeur=3A=C9mile=3A=3A.html (accessed on 21 August 2023).
- Sandrelli, R.M. Biologgers as a Tool to Investigate the Environmental Tolerances of Fishes. Master’s Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, 2024. [Google Scholar]
- Gallardo, M.Á.; Sala-Rabanal, M.; Ibarz, A.; Padrós, F.; Blasco, J.; Fernández-Borràs, J.; Sánchez, J. Functional Alterations Associated with “Winter Syndrome” in Gilthead Sea Bream (Sparus Aurata). Aquaculture 2003, 223, 15–27. [Google Scholar] [CrossRef]
- Ibarz, A.; Padrós, F.; Gallardo, M.Á.; Fernández-Borràs, J.; Blasco, J.; Tort, L. Low-Temperature Challenges to Gilthead Sea Bream Culture: Review of Cold-Induced Alterations and “Winter Syndrome”. Rev. Fish Biol. Fish 2010, 20, 539–556. [Google Scholar] [CrossRef]
- Ibarz, A.; Blasco, J.; Sala-Rabanal, M.; Gallardo, Á.; Redondo, A.; Fernández-Borràs, J. Metabolic Rate and Tissue Reserves in Gilthead Sea Bream (Sparus aurata) under Thermal Fluctuations and Fasting and Their Capacity for Recovery. Can. J. Fish. Aquat. Sci. 2007, 64, 1034–1042. [Google Scholar] [CrossRef]
- Jiao, S.; Nie, M.; Song, H.; Xu, D.; You, F. Physiological Responses to Cold and Starvation Stresses in the Liver of Yellow Drum (Nibea albiflora) Revealed by LC-MS Metabolomics. Sci. Total Environ. 2020, 715, 136940. [Google Scholar] [CrossRef]
- Song, H.; Xu, D.; Tian, L.; Chen, R.; Wang, L.; Tan, P.; You, Q. Overwinter Mortality in Yellow Drum (Nibea albiflora): Insights from Growth and Immune Responses to Cold and Starvation Stress. Fish Shellfish Immunol. 2019, 92, 341–347. [Google Scholar] [CrossRef]
- Dessen, J.E.; Østbye, T.K.; Ruyter, B.; Bou, M.; Thomassen, M.S.; Rørvik, K.A. Sudden Increased Mortality in Large Seemingly Healthy Farmed Atlantic Salmon (Salmo salar L.) Was Associated with Environmental and Dietary Changes. J. Appl. Aquac. 2021, 33, 165–182. [Google Scholar] [CrossRef]
- Noble, C.; Gismervik, K.; Iversen, M.H.; Kolarevic, J.; Nilsson, J.; Stien, L.H.; Turnbull, J.F. Welfare Indicators for Farmed Atlantic Salmon: Tools for Assessing Fish Welfare; Nord universitet: Bodø, Norway, 2018; p. 351. [Google Scholar]
- Katan, T.; Caballero-Solares, A.; Taylor, R.G.; Rise, M.L.; Parrish, C.C. Effect of Plant-Based Diets with Varying Ratios of Ω6 to Ω3 Fatty Acids on Growth Performance, Tissue Composition, Fatty Acid Biosynthesis and Lipid-Related Gene Expression in Atlantic Salmon (Salmo salar). Comp. Biochem. Physiol. D 2019, 30, 290–304. [Google Scholar] [CrossRef]
- Xu, Q.; Feng, C.Y.; Hori, T.S.; Plouffe, D.A.; Buchanan, J.T.; Rise, M.L. Family-Specific Differences in Growth Rate and Hepatic Gene Expression in Juvenile Triploid Growth Hormone (GH) Transgenic Atlantic Salmon (Salmo salar). Comp. Biochem. Physiol. D 2013, 8, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Emam, M.; Eslamloo, K.; Caballero-Solares, A.; Lorenz, E.K.; Xue, X.; Umasuthan, N.; Gnanagobal, H.; Santander, J.; Taylor, R.G.; Balder, R.; et al. Nutritional Immunomodulation of Atlantic Salmon Response to Renibacterium salmoninarum Bacterin. Front. Mol. Biosci. 2022, 9, 931548. [Google Scholar] [CrossRef]
- Caballero-Solares, A.; Hall, J.R.; Xue, X.; Eslamloo, K.; Taylor, R.G.; Parrish, C.C.; Rise, M.L. The Dietary Replacement of Marine Ingredients by Terrestrial Animal and Plant Alternatives Modulates the Antiviral Immune Response of Atlantic Salmon (Salmo salar). Fish Shellfish Immunol. 2017, 64, 24–38. [Google Scholar] [CrossRef]
- Emam, M.; Katan, T.; Caballero-Solares, A.; Taylor, R.G.; Parrish, K.S.; Rise, M.L.; Parrish, C.C. Interaction between ω 6 and ω 3 Fatty Acids of Different Chain Lengths Regulates Atlantic Salmon Hepatic Gene Expression and Muscle Fatty Acid Profiles. Phil. Trans. R. Soc. B 2020, 375, 20190648. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hixson, S.M.; Hori, T.S.; Booman, M.; Parrish, C.C.; Anderson, D.M.; Rise, M.L. Atlantic Salmon (Salmo salar) Liver Transcriptome Response to Diets Containing Camelina sativa Products. Comp. Biochem. Physiol D 2015, 14, 1–15. [Google Scholar] [CrossRef]
- Caballero-Solares, A.; Xue, X.; Parrish, C.C.; Foroutani, M.B.; Taylor, R.G.; Rise, M.L. Changes in the Liver Transcriptome of Farmed Atlantic Salmon (Salmo salar) Fed Experimental Diets Based on Terrestrial Alternatives to Fish Meal and Fish Oil. BMC Genom. 2018, 19, 796. [Google Scholar] [CrossRef]
- Colombo, S.M.; Emam, M.; Peterson, B.C.; Hall, J.R.; Burr, G.; Zhang, Z.; Rise, M.L. Freshwater, Landlocked Grand Lake Strain of Atlantic Salmon (Salmo salar L.) as a Potential Genetic Source of Long Chain Polyunsaturated Fatty Acids Synthesis. Front Mar. Sci. 2021, 8, 641824. [Google Scholar] [CrossRef]
- Eslamloo, K.; Caballero-Solares, A.; Inkpen, S.M.; Emam, M.; Kumar, S.; Bouniot, C.; Avendaño-Herrera, R.; Jakob, E.; Rise, M.L. Transcriptomic Profiling of the Adaptive and Innate Immune Responses of Atlantic Salmon to Renibacterium salmoninarum Infection. Front Immunol. 2020, 11, 567838. [Google Scholar] [CrossRef]
- Hixson, S.M.; Parrish, C.C.; Xue, X.; Wells, J.S.; Collins, S.A.; Anderson, D.M.; Rise, M.L. Growth Performance, Tissue Composition, and Gene Expression Responses in Atlantic Salmon (Salmo salar) Fed Varying Levels of Different Lipid Sources. Aquaculture 2017, 467, 76–88. [Google Scholar] [CrossRef]
- Umasuthan, N.; Xue, X.; Caballero-Solares, A.; Kumar, S.; Westcott, J.D.; Chen, Z.; Fast, M.D.; Skugor, S.; Nowak, B.F.; Taylor, R.G.; et al. Transcriptomic Profiling in Fins of Atlantic Salmon Parasitized with Sea Lice: Evidence for an Early Imbalance Between Chalimus-Induced Immunomodulation and the Host’s Defense Response. Int. J. Mol. Sci. 2020, 21, 2417. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Caballero-Solares, A.; Hall, J.R.; Umasuthan, N.; Kumar, S.; Jakob, E.; Skugor, S.; Hawes, C.; Santander, J.; Taylor, R.G.; et al. Transcriptome Profiling of Atlantic Salmon (Salmo salar) Parr with Higher and Lower Pathogen Loads Following Piscirickettsia salmonis Infection. Front. Immunol. 2021, 12, 789465. [Google Scholar] [CrossRef] [PubMed]
- Koressaar, T.; Remm, M. Enhancements and Modifications of Primer Design Program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3-New Capabilities and Interfaces. Nucleic Acids Res. 2012, 40, 1–12. [Google Scholar] [CrossRef]
- Kõressaar, T.; Lepamets, M.; Kaplinski, L.; Raime, K.; Andreson, R.; Remm, M. Primer3-Masker: Integrating Masking of Template Sequence with Primer Design Software. Bioinformatics 2018, 34, 1937–1938. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; de Preter, K.; Pattyn, F.; Poppe, B.; van Roy, N.; de Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome. Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. QBase Relative Quantification Framework and Software for Management and Automated Analysis of Real-Time Quantitative PCR Data. Genome. Biol. 2008, 8, R19. [Google Scholar] [CrossRef]
- Régnier, M.; Carbinatti, T.; Parlati, L.; Benhamed, F.; Postic, C. The Role of ChREBP in Carbohydrate Sensing and NAFLD Development. Nat. Rev. Endocrinol. 2023, 19, 336–349. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, Z.; Yang, Y.; Wang, J.; Yang, T.; Chen, X.; Liang, L.; Mu, W. Histology, Physiology, and Glucose and Lipid Metabolism of Lateolabrax maculatus under Low Temperature Stress. J. Therm. Biol. 2022, 104, 103161. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Y.; Miao, X.M.; Tao, Y.X.; Xie, L.; Li, Y. A Model Construction of Starvation Induces Hepatic Steatosis and Transcriptome Analysis in Zebrafish Larvae. Biology 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Solares, A.; Xue, X.; Cleveland, B.M.; Foroutani, M.B.; Parrish, C.C.; Taylor, R.G.; Rise, M.L. Diet-Induced Physiological Responses in the Liver of Atlantic Salmon (Salmo salar) Inferred Using Multiplex PCR Platforms. Mar. Biotechnol. 2020, 22, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Takami, T.; Shintani, H.; Sasai, N.; Matsumoto, T.; Yamamoto, N.; Sakaida, I. Seasonal Variations in Photoperiod Affect Hepatic Metabolism of Medaka (Oryzias latipes). FEBS Open Bio. 2021, 11, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Parlati, L.; Régnier, M.; Guillou, H.; Postic, C. New Targets for NAFLD. J. Hepatol. 2021, 3, 100346. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, Y.; Terai, S.; Sakaida, I.; Nishina, H. The Expanding Role of Fish Models in Understanding Non-Alcoholic Fatty Liver Disease. DMM 2014, 7, 409. [Google Scholar] [CrossRef]
- Komstra, L. R Package “Outliers”: Tests for Outliers; (Version 0.15), The R Project for Statistical Computing: Vienna, Austria, 2022; pp. 1–15. [Google Scholar] [CrossRef]
- De Mendiburu, F. R Package “Agricolae”: Statistical Procedures for Agricultural Research; (Version 1.3), The R Project for Statistical Computing: Vienna, Austria, 2023. [Google Scholar] [CrossRef]
- Konietschke, F.; Placzek, M.; Schaarschmidt, F.; Hothorn, L.A. Nparcomp: An R Software Package for Nonparametric Multiple Comparisons and Simultaneous Confidence Intervals. J. Stat. Softw. 2015, 64, 1–17. [Google Scholar] [CrossRef]
- Beemelmanns, A.; Ribas, L.; Anastasiadi, D.; Moraleda-Prados, J.; Zanuzzo, F.S.; Rise, M.L.; Gamperl, A.K. DNA Methylation Dynamics in Atlantic Salmon (Salmo salar) Challenged with High Temperature and Moderate Hypoxia. Front. Mar. Sci. 2021, 7, 604878. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix; (Version 0.92), The R Project for Statistical Computing: Vienna, Austria, 2022. [Google Scholar] [CrossRef]
- Ibarz, A.; Blasco, J.; Gallardo, M.A.; Fernández-Borràs, J. Energy Reserves and Metabolic Status Affect the Acclimation of Gilthead Sea Bream (Sparus aurata) to Cold. Comp. Biochem. Physiol. A 2010, 155, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Ibarz, A.; Beltrán, M.; Fernández-Borràs, J.; Gallardo, M.A.; Sánchez, J.; Blasco, J. Alterations in Lipid Metabolism and Use of Energy Depots of Gilthead Sea Bream (Sparus aurata) at Low Temperatures. Aquaculture 2007, 262, 470–480. [Google Scholar] [CrossRef]
- Chen, W.H.; Sun, L.T.; Tsai, C.L.; Song, Y.L.; Chang, C.F. Cold-Stress Induced the Modulation of Catecholamines, Cortisol, Immunoglobulin M, and Leukocyte Phagocytosis in Tilapia. Gen. Comp. Endocrinol. 2002, 126, 90–100. [Google Scholar] [CrossRef]
- Melis, R.; Sanna, R.; Braca, A.; Bonaglini, E.; Cappuccinelli, R.; Slawski, H.; Roggio, T.; Uzzau, S.; Anedda, R. Molecular Details on Gilthead Sea Bream (Sparus aurata) Sensitivity to Low Water Temperatures from 1H NMR Metabolomics. Comp. Biochem. Physiol. A 2017, 204, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F.W. Steatohepatitis: A Tale of Two “Hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Ibarz, A.; Blasco, J.; Beltrán, M.; Gallardo, M.A.; Sánchez, J.; Sala, R.; Fernández-Borràs, J. Cold-Induced Alterations on Proximate Composition and Fatty Acid Profiles of Several Tissues in Gilthead Sea Bream (Sparus aurata). Aquaculture 2005, 249, 477–486. [Google Scholar] [CrossRef]
- Rossi, A.; Bacchetta, C.; Cazenave, J. Effect of Thermal Stress on Metabolic and Oxidative Stress Biomarkers of Hoplosternum littorale (Teleostei, Callichthyidae). Ecol. Indic. 2017, 79, 361–370. [Google Scholar] [CrossRef]
- Liu, A.; Pirozzi, I.; Codabaccus, B.M.; Stephens, F.; Francis, D.S.; Sammut, J.; Booth, M.A. Effects of Dietary Choline on Liver Lipid Composition, Liver Histology and Plasma Biochemistry of Juvenile Yellowtail Kingfish (Seriola lalandi). Br. J. Nutr. 2021, 125, 1344–1358. [Google Scholar] [CrossRef] [PubMed]
- Sissener, N.H.; Liland, N.S.; Holen, E.; Stubhaug, I.; Torstensen, B.E.; Rosenlund, G. Phytosterols Are Not Involved in the Development of Fatty Liver in Plant Oil Fed Atlantic Salmon (Salmo salar) at High or Low Water Temperature. Aquaculture 2017, 480, 123–134. [Google Scholar] [CrossRef]
- Ruyter, B.; Moya-Falcón, C.; Rosenlund, G.; Vegusdal, A. Fat Content and Morphology of Liver and Intestine of Atlantic Salmon (Salmo salar): Effects of Temperature and Dietary Soybean Oil. Aquaculture 2006, 252, 441–452. [Google Scholar] [CrossRef]
- Pafili, K.; Roden, M. Nonalcoholic Fatty Liver Disease (NAFLD) from Pathogenesis to Treatment Concepts in Humans. Mol. Metab. 2021, 50, 101122. [Google Scholar] [CrossRef]
- Ibarz, A.; Martín-Pérez, M.; Blasco, J.; Bellido, D.; De Oliveira, E.; Fernández-Borràs, J. Gilthead Sea Bream Liver Proteome Altered at Low Temperatures by Oxidative Stress. Proteomics 2010, 10, 963–975. [Google Scholar] [CrossRef]
- Mininni, A.N.; Milan, M.; Ferraresso, S.; Petochi, T.; Di Marco, P.; Marino, G.; Livi, S.; Romualdi, C.; Bargelloni, L.; Patarnello, T. Liver Transcriptome Analysis in Gilthead Sea Bream upon Exposure to Low Temperature. BMC Genom. 2014, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; González-Rodríguez, Á.; García-Monzón, C.; Valverde, Á.M. Understanding Lipotoxicity in NAFLD Pathogenesis: Is CD36 a Key Driver? Cell Death Dis. 2020, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Espe, M.; Xie, S.; Chen, S.; Pedro, A.; Holen, E. Development of a Fatty Liver Model Using Oleic Acid in Primary Liver Cells Isolated from Atlantic Salmon and the Prevention of Lipid Accumulation Using Metformin. Aquac. Nutr. 2019, 25, 737–746. [Google Scholar] [CrossRef]
- Zhong, S.; Zhao, L.; Wang, Y.; Zhang, C.; Liu, J.; Wang, P.; Zhou, W.; Yang, P.; Varghese, Z.; Moorhead, J.F.; et al. Cluster of Differentiation 36 Deficiency Aggravates Macrophage Infiltration and Hepatic Inflammation by Upregulating Monocyte Chemotactic Protein-1 Expression of Hepatocytes Through Histone Deacetylase 2-Dependent Pathway. Antioxid. Redox Signal. 2017, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Mera, P.; Casas, J.; Salvador, J.; Rodríguez, A.; Alonso, S.; Sebastián, D.; Soler-Vázquez, M.C.; Montironi, C.; Recalde, S.; et al. Liver CPT1A Gene Therapy Reduces Diet-Induced Hepatic Steatosis in Mice and Highlights Potential Lipid Biomarkers for Human NAFLD. FASEB J. 2020, 34, 11816–11837. [Google Scholar] [CrossRef] [PubMed]
- Haunerland, N.H.; Spener, F. Fatty Acid-Binding Proteins—Insights from Genetic Manipulations. Prog. Lipid Res. 2004, 43, 328–349. [Google Scholar] [CrossRef]
- 80. Vanden Berghe, W.; Vermeulen, L.; Delerive, P.; De Bosscher, K.; Staels, B.; Haegeman, G. A Paradigm for Gene Regulation: Inflammation, NF-κB and PPAR. In Peroxisomal Disorders and Regulation of Genes, Advances in Experimental Medicine and Biology; Roels, F., Baes, M., De Bie, S., Eds.; Springer: Boston, MA, USA, 2003; Volume 544. [Google Scholar] [CrossRef]
- Chasapi, A.; Balampanis, K.; Tanoglidi, A.; Kourea, E.; Lambrou, G.I.; Lambadiari, V.; Kalfarentzos, F.; Hatziagelaki, E.; Melachrinou, M.; Sotiropoulou-Bonikou, G. NF-ΚB; the Critical Link between Immune and Metabolic Pathways: Could NF-ΚB Be Used as a Novel Diagnostic and Prognostic Biomarker for Non-Alcoholic Steatohepatitis? OBM Hepatol. Gastroenterol. 2019, 3, 1–30. [Google Scholar] [CrossRef]
- Madureira, T.V.; Pinheiro, I.; Malhão, F.; Lopes, C.; Urbatzka, R.; Castro, L.F.C.; Rocha, E. Cross-Interference of Two Model Peroxisome Proliferators in Peroxisomal and Estrogenic Pathways in Brown Trout Hepatocytes. Aquat. Toxicol. 2017, 187, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Eslamloo, K.; Kumar, S.; Xue, X.; Parrish, K.S.; Purcell, S.L.; Fast, M.D.; Rise, M.L. Global Gene Expression Responses of Atlantic Salmon Skin to Moritella viscosa. Sci. Rep. 2022, 12, 4622. [Google Scholar] [CrossRef]
- Jia, R.; Cao, L.P.; Du, J.L.; He, Q.; Gu, Z.Y.; Jeney, G.; Xu, P.; Yin, G.J. Effects of High-Fat Diet on Steatosis, Endoplasmic Reticulum Stress and Autophagy in Liver of Tilapia (Oreochromis niloticus). Front. Mar. Sci. 2020, 7, 363. [Google Scholar] [CrossRef]
- Xu, R.; Wang, T.; Ding, F.-F.; Zhou, N.-N.; Qiao, F.; Chen, L.-Q.; Du, Z.-Y.; Zhang, M.-L. Lactobacillus plantarum Ameliorates High-Carbohydrate Diet-Induced Hepatic Lipid Accumulation and Oxidative Stress by Upregulating Uridine Synthesis. Antioxidants 2022, 11, 1238. [Google Scholar] [CrossRef] [PubMed]
- Sen Özdemir, N.; Parrish, C.C.; Parzanini, C.; Mercier, A.; Pernet, F. Neutral and Polar Lipid Fatty Acids in Five Families of Demersal and Pelagic Fish from the Deep Northwest Atlantic. ICES J. Mar. Sci. 2019, 76, 1807–1815. [Google Scholar] [CrossRef]
- Liu, C.; Ge, J.; Zhou, Y.; Thirumurugan, R.; Gao, Q.; Dong, S. Effects of Decreasing Temperature on Phospholipid Fatty Acid Composition of Different Tissues and Hematology in Atlantic Salmon (Salmo salar). Aquaculture 2020, 515, 734587. [Google Scholar] [CrossRef]
- Morais, S.; Monroig, O.; Zheng, X.; Leaver, M.J.; Tocher, D.R. Highly Unsaturated Fatty Acid Synthesis in Atlantic Salmon: Characterization of ELOVL5- and ELOVL2-like Elongases. Mar. Biotechnol. 2009, 11, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.A.; Hammer, R.E.; Horton, J.D. Deletion of ELOVL5 Leads to Fatty Liver through Activation of SREBP-1c in Mice. J. Lipid Res. 2009, 50, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Gillis, R.C.; Daley, B.J.; Enderson, B.L.; Karlstad, M.D. Inhibition of 5-Lipoxygenase Induces Cell Death in Anti-Inflammatory Fatty Acid-Treated HL-60 Cells. J. Parenter Enteral. Nutr. 2004, 28, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Simões, I.C.M.; Fontes, A.; Pinton, P.; Zischka, H.; Wieckowski, M.R. Mitochondria in Non-Alcoholic Fatty Liver Disease. Int. J. Biochem. Cell Biol. 2017, 95, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Besse-Patin, A.; Léveillé, M.; Oropeza, D.; Nguyen, B.N.; Prat, A. Estrogen Signals Through Peroxisome Proliferator-Activated Receptor−γ Coactivator 1α to Reduce Oxidative Damage Associated with Diet-Induced Fatty Liver Disease. Gastroenterology 2017, 152, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Kosmalski, M.; Frankowski, R.; Ziółkowska, S.; Różycka-Kosmalska, M.; Pietras, T. What’s New in the Treatment of Non-Alcoholic Fatty Liver Disease (NAFLD). J. Clin. Med. 2023, 12, 1852. [Google Scholar] [CrossRef]
- Xue, M.; Yao, T.; Xue, M.; Francis, F.; Qin, Y.; Jia, M.; Li, J.; Gu, X. Mechanism Analysis of Metabolic Fatty Liver on Largemouth Bass (Micropterus salmoides) Based on Integrated Lipidomics and Proteomics. Metabolites 2022, 12, 759. [Google Scholar] [CrossRef]
- Deng, C.J.; Lo, T.H.; Chan, K.Y.; Li, X.; Wu, M.Y.; Xiang, Z.; Wong, C.M. Role of B Lymphocytes in the Pathogenesis of NAFLD: A 2022 Update. Int. J. Mol. Sci. 2022, 23, 12376. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Kojima, F.; Yang, L.; Crofford, L.J. Sequential Induction of Pro-and Anti-Inflammatory Prostaglandins and Peroxisome Proliferators Activated Receptor-Gamma during Normal Wound Healing: A Time Course Study. Prostaglandins Leukot Essent Fat. Acids 2007, 76, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Sun, W.; Sun, F.; Yin, G.; Liang, P.; Chen, S.; Liu, X.; Jiang, T.; Zhang, F. Biological Mechanisms and Related Natural Inhibitors of CD36 in Nonalcoholic Fatty Liver. Drug Des. Devel. Ther. 2022, 16, 3829–3845. [Google Scholar] [CrossRef] [PubMed]
- Hammes, T.O.; Pedroso, G.L.; Hartmann, C.R.; Escobar, T.D.C.; Fracasso, L.B.; Da Rosa, D.P.; Marroni, N.P.; Porawski, M.; Da Silveira, T.R. The Effect of Taurine on Hepatic Steatosis Induced by Thioacetamide in Zebrafish (Danio rerio). Dig. Dis. Sci. 2012, 57, 675–682. [Google Scholar] [CrossRef]
- Howarth, D.L.; Lindtner, C.; Vacaru, A.M.; Sachidanandam, R.; Tsedensodnom, O.; Vasilkova, T.; Buettner, C.; Sadler, K.C. Activating Transcription Factor 6 Is Necessary and Sufficient for Alcoholic Fatty Liver Disease in Zebrafish. PLoS Genet. 2014, 10, 1004335. [Google Scholar] [CrossRef] [PubMed]
- Cinaroglu, A.; Gao, C.; Imrie, D.; Sadler, K.C. Activating Transcription Factor 6 Plays Protective and Pathological Roles in Steatosis Due to Endoplasmic Reticulum Stress in Zebrafish. Hepatology 2011, 54, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hu, Y.; Peng, J. Targeting Programmed Cell Death in Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD): A Promising New Therapy. Cell. Mol. Biol. Lett. 2021, 26, 17. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, Y.; Su, N.; Wang, A.; Tan, X.; Sun, Z.; Zou, C.; Liu, Q.; Ye, C. Effects of Replacing Fish Meal with Rendered Animal Protein Blend on Growth Performance, Hepatic Steatosis and Immune Status in Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Aquaculture 2019, 511, 734203. [Google Scholar] [CrossRef]
- Takle, H.; Andersen, Ø. Caspases and Apoptosis in Fish. J. Fish Biol. 2007, 71, 326–349. [Google Scholar] [CrossRef]
- Spead, O.; Verreet, T.; Donelson, C.J.; Poulain, F.E. Characterization of the Caspase Family in Zebrafish. PLoS ONE 2018, 13, 0197966. [Google Scholar] [CrossRef]
- Krasnov, A.; Timmerhaus, G.; Schiøtz, B.L.; Torgersen, J.; Afanasyev, S.; Iliev, D.; Jørgensen, J.; Takle, H.; Jørgensen, S.M. Genomic Survey of Early Responses to Viruses in Atlantic Salmon, Salmo salar L. Mol. Immunol. 2011, 49, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.M.; Günther, O.P.; Li, S.; Kaukinen, K.H.; Ming, T.J. Molecular Indices of Viral Disease Development in Wild Migrating Salmon. Conserv. Physiol. 2017, 5, cox036. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.R.; Lehnert, S.J.; Gonzalez, E.; Hanlon, J.M.; Kumar, S.; Morris, C.J.; Rise, M.L. Snow Crab (Chionoecetes opilio) Hemocytes and Hepatopancreas Transcriptomes: Identification, Validation, and Application of Immune-Relevant Biomarkers of Exposure to Noise. Front. Mar. Sci. 2023, 10, 1198036. [Google Scholar] [CrossRef]
- Álvarez, C.A.; Santana, P.A.; Salinas-Parra, N.; Beltrán, D.; Guzmán, F.; Vega, B.; Acosta, F.; Mercado, L. Immune Modulation Ability of Hepcidin from Teleost Fish. Animals 2022, 12, 1586. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-Z.; Ma, Z.-Y.; Wu, C.-S.; Wang, J.; Zhang, Y.-A.; Zhang, X.-J. LECT2 Is a Novel Antibacterial Protein in Vertebrates. J. Immunol. 2022, 208, 2037–2053. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Z.; Fan, W.; Huang, Y.; Niu, J.; Luo, G.; Liu, X.; Huang, Y.; Jian, J. LECT2 Protects Nile Tilapia (Oreochromis niloticus) Against Streptococcus agalatiae Infection. Front. Immunol. 2021, 12, 667781. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.B.; Lunde, H.; Jensen, L.; Whitehead, A.S.; Robertsen, B. Serum Amyloid A Transcription in Atlantic Salmon (Salmo salar L.) Hepatocytes Is Enhanced by Stimulation with Macrophage Factors, Recombinant Human IL-1β, IL-6 and TNFα or Bacterial Lipopolysaccharide. Dev. Comp. Immunol. 2000, 24, 553–563. [Google Scholar] [CrossRef]
- Buks, R.; Alnabulsi, A.; Zindrili, R.; Alnabulsi, A.; Wang, A.; Wang, T.; Martin, S.A.M. Catch of the Day: New Serum Amyloid A (SAA) Antibody Is a Valuable Tool to Study Fish Health in Salmonids. Cells 2023, 12, 2097. [Google Scholar] [CrossRef]
- Cristin, L.; Montini, A.; Martinino, A.; Scarano Pereira, J.P.; Giovinazzo, F.; Agnes, S. The Role of Growth Hormone and Insulin Growth Factor 1 in the Development of Non-Alcoholic Steato-Hepatitis: A Systematic Review. Cells 2023, 12, 517. [Google Scholar] [CrossRef]
- Kopchick, J.J.; Berryman, D.E.; Puri, V.; Lee, K.Y.; Jorgensen, J.O.L. The Effects of Growth Hormone on Adipose Tissue: Old Observations, New Mechanisms. Nat. Rev. Endocrinol. 2020, 16, 135–146. [Google Scholar] [CrossRef]
- Gui, R.; Li, W.; Li, Z.; Wang, H.; Wu, Y.; Jiao, W.; Zhao, G.; Shen, Y.; Wang, L.; Zhang, J.; et al. Effects and Potential Mechanisms of IGF1/IGF1R in the Liver Fibrosis: A Review. Int. J. Biol. Macromol. 2023, 251, 126263. [Google Scholar] [CrossRef]
- Wu, Y.; Li, R.; Wu, X.; Guo, W.; Zhong, W.; Li, Y.; Song, Y.; Tao, B.; Chen, J.; Han, D.; et al. Overexpression of Growth Hormone Improved Hepatic Glucose Catabolism and Relieved Liver Lipid Deposition in Common Carp (Cyprinus carpio L.) Fed a High-Starch Diet. Front. Endocrinol. 2022, 13, 1038479. [Google Scholar] [CrossRef]
- Zeng, N.; Bao, J.; Shu, T.T.; Shi, C.; Zhai, G.; Jin, X.; He, J.; Lou, Q.; Yin, Z. Sexual Dimorphic Effects of Igf1 Deficiency on Metabolism in Zebrafish. Front. Endocrinol. 2022, 13, 879962. [Google Scholar] [CrossRef]
- Vilarinho, S.; Sari, S.; Mazzacuva, F.; Bilgüvar, K.; Esendagli-Yilmaz, G.; Jain, D.; Akyol, G.; Dalgiç, B.; Günel, M.; Clayton, P.T.; et al. ACOX2 Deficiency: A Disorder of Bile Acid Synthesis with Transaminase Elevation, Liver Fibrosis, Ataxia, and Cognitive Impairment. Proc. Natl. Acad. Sci. USA 2016, 113, 11289–11293. [Google Scholar] [CrossRef]
- Andújar-Vera, F.; Ferrer-Millán, M.; García-Fontana, C.; García-Fontana, B.; González-Salvatierra, S.; Sanabria-de la Torre, R.; Martínez-Heredia, L.; Riquelme-Gallego, B.; Muñoz-Torres, M. Analysis of the Genetic Relationship between Atherosclerosis and Non-Alcoholic Fatty Liver Disease through Biological Interaction Networks. Int. J. Mol. Sci. 2023, 24, 4124. [Google Scholar] [CrossRef]
- Kanda, T.; Matsuoka, S.; Yamazaki, M.; Shibata, T.; Nirei, K.; Takahashi, H.; Kaneko, T.; Fujisawa, M.; Higuchi, T.; Nakamura, H.; et al. Apoptosis and Non-Alcoholic Fatty Liver Diseases. World J. Gastroenterol. 2018, 24, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Takata, N.; Ishii, K.; Takayama, H.; Nagashimada, M.; Kamoshita, K.; Tanaka, T.; Kikuchi, A.; Takeshita, Y.; Matsumoto, Y.; Ota, T.; et al. LECT2 as a Hepatokine Links Liver Steatosis to Inflammation via Activating Tissue Macrophages in NASH. Sci. Rep. 2021, 11, 555. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, D.; Hu, Y.; Li, W.; Liu, F.; Zhu, X.; Li, X.; Zhang, H.; Bai, H.; Yang, Q.; et al. Serum Amyloid A1 Exacerbates Hepatic Steatosis via TLR4-Mediated NF-ΚB Signaling Pathway. Mol. Metab. 2022, 59, 101462. [Google Scholar] [CrossRef]
- Shieh, Y.S.; Chang, Y.S.; Hong, J.R.; Chen, L.J.; Jou, L.K.; Hsu, C.C.; Her, G.M. Increase of Hepatic Fat Accumulation by Liver Specific Expression of Hepatitis B Virus X Protein in Zebrafish. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 721–730. [Google Scholar] [CrossRef]
- Castellano, M.; Silva-Álvarez, V.; Aversa-Marnai, M.; Lamas-Bervejillo, M.; Quartiani, I.; Perretta, A.; Villarino, A.; Ferreira, A.M. Serum Amyloid A Is a Positive Acute Phase Protein in Russian Sturgeon Challenged with Aeromonas Hydrophila. Sci. Rep. 2020, 10, 22162. [Google Scholar] [CrossRef]
- Sissener, N.H.; Torstensen, B.E.; Owen, M.A.G.; Liland, N.S.; Stubhaug, I.; Rosenlund, G. Temperature Modulates Liver Lipid Accumulation in Atlantic Salmon (Salmo salar L.) Fed Low Dietary Levels of Long-Chain n-3 Fatty Acids. Aquac. Nutr. 2016, 23, 865–878. [Google Scholar] [CrossRef]
- Teodósio, R.; Aragão, C.; Colen, R.; Carrilho, R.; Dias, J.; Engrola, S. A Nutritional Strategy to Promote Gilthead Seabream Performance under Low Temperatures. Aquaculture 2021, 537, 736494. [Google Scholar] [CrossRef]
- Canadian Council of Animal Care. Guidelines on the Care and Use of Fish in Research, Teaching and Testing; Canadian Council of Animal Care, Ed.; Canadian Council of Animal Care: Ottawa, ON, Canada, 2005; ISBN 0-919087-43-4. [Google Scholar]
| Asymptomatic | Early | Advanced | p-Value | |
|---|---|---|---|---|
| Total Lipids (mg·g−1 wet mass) | 24.82 ± 4.03 | 35.00 ± 3.50 | 38.09 ± 4.70 | 0.062 |
| Triacylglycerols | 17.50 ± 3.50 | 27.24 ± 5.91 | 27.74 ± 5.47 | 0.086 |
| Free FAs | 6.56 ± 1.21 | 6.06 ± 1.27 | 5.87 ± 0.64 | 0.878 |
| Sterols | 9.50 ± 0.75 | 6.32 ± 1.12 | 6.08 ± 0.54 | 0.066 |
| Acetone Mobile Polar Lipids | 6.89 ± 1.04 | 4.21 ± 1.30 | 4.07 ± 0.80 | 0.100 |
| Phospholipids | 59.18 ± 4.73 | 55.72 ± 7.07 | 55.12 ± 6.12 | 0.859 |
| Myristic Acid (14:0) | 1.75 ± 0.14 a | 1.29 ± 0.08 b | 1.33 ± 0.09 b | 0.006 |
| Palmitic Acid (16:0) | 20.32 ± 0.70 a | 16.65 ± 1.11 b | 16.36 ± 0.89 b | 0.013 |
| Palmitoleic Acid (16:1ꞷ7) | 3.41 ± 0.26 | 3.02 ± 0.19 | 3.08 ± 0.19 | 0.374 |
| Stearic Acid (18:0) | 5.72 ± 0.25 | 5.26 ± 0.22 | 5.33 ± 0.29 | 0.432 |
| Oleic Acid (18:1ꞷ9) | 26.30 ± 1.43 | 28.73 ± 1.85 | 31.32 ± 1.44 | 0.086 |
| Vaccenic Acid (18:1ꞷ7) | 3.33 ± 0.14 | 3.50 ± 0.10 | 3.59 ± 0.12 | 0.346 |
| Linoleic Acid (18:2ꞷ6) | 7.68 ± 0.42 | 6.75 ± 0.35 | 6.94 ± 0.22 | 0.165 |
| α-Linolenic Acid (18:3ꞷ3) | 1.33 ± 0.10 | 1.21 ± 0.09 | 1.14 ± 0.07 | 0.176 |
| γ-Linolenic Acid (18:3ꞷ6) | 0.35 ± 0.04 b | 0.63 ± 0.12 a | 0.78 ± 0.07 a | 0.026 |
| Eicosenoic Acid (20:1ꞷ9) | 1.11 ± 0.12 | 1.04 ± 0.11 | 0.87 ± 0.06 | 0.183 |
| Eicosatetreanoic Acid (20:4ꞷ6) | 2.17 ± 0.23 | 2.58 ± 0.21 | 2.58 ± 0.20 | 0.462 |
| Eicosapenteanoic Acid (20:5ꞷ3) | 4.31 ± 0.34 | 4.12 ± 0.28 | 3.98 ± 0.35 | 0.339 |
| Docosapentaenoic Acid (22:5ꞷ6) | 1.37 ± 0.15 | 1.54 ± 0.13 | 1.45 ± 0.16 | 0.748 |
| Docosahexaenoic Acid (22:6ꞷ3) | 13.48 ± 1.49 | 15.79 ± 0.82 | 13.75 ± 1.05 | 0.399 |
| ΣSAT | 28.54 ± 0.85 a | 23.80 ± 1.29 b | 23.61 ± 1.13 b | 0.011 |
| ΣMUFA | 36.61 ± 1.72 | 38.89 ± 1.83 | 41.07 ± 1.86 | 0.242 |
| ΣPUFA | 34.44 ± 1.72 | 36.95 ± 0.94 | 34.99 ± 1.56 | 0.549 |
| Σꞷ3 | 21.66 ± 1.87 | 24.17 ± 1.03 | 21.78 ± 1.50 | 0.480 |
| Σꞷ6 | 11.99 ± 0.44 | 11.98 ± 0.20 | 12.33 ± 0.19 | 0.575 |
| ꞷ6:ꞷ3 | 0.65 ± 0.06 | 0.50 ± 0.03 | 0.59 ± 0.04 | 0.462 |
| DHA:EPA | 3.06 ± 0.22 b | 3.90 ± 0.16 a | 3.49 ± 0.15 ab | 0.014 |
| ARA:DHA | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.19 ± 0.01 | 0.227 |
| PUFA:SAT | 1.25 ± 0.08 | 1.58 ± 0.08 | 1.51 ± 0.09 | 0.153 |
| Phospholipids:Sterols | 6.87 ± 0.89 | 10.03 ± 1.04 | 9.34 ± 1.59 | 0.217 |
| (ΣMUFA + ΣPUFA):ΣSAT | 2.54 ± 0.15 b | 3.27 ± 0.23 a | 3.32 ± 0.22 a | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas, I.; Caballero-Solares, A.; Vadboncoeur, É.; Sandrelli, R.M.; Hall, J.R.; Clow, K.A.; Parrish, C.C.; Rise, M.L.; Swanson, A.K.; Gamperl, A.K. Prolonged Cold Exposure Negatively Impacts Atlantic Salmon (Salmo salar) Liver Metabolism and Function. Biology 2024, 13, 494. https://doi.org/10.3390/biology13070494
Rojas I, Caballero-Solares A, Vadboncoeur É, Sandrelli RM, Hall JR, Clow KA, Parrish CC, Rise ML, Swanson AK, Gamperl AK. Prolonged Cold Exposure Negatively Impacts Atlantic Salmon (Salmo salar) Liver Metabolism and Function. Biology. 2024; 13(7):494. https://doi.org/10.3390/biology13070494
Chicago/Turabian StyleRojas, Isis, Albert Caballero-Solares, Émile Vadboncoeur, Rebeccah M. Sandrelli, Jennifer R. Hall, Kathy A. Clow, Christopher C. Parrish, Matthew L. Rise, Andrew K. Swanson, and Anthony K. Gamperl. 2024. "Prolonged Cold Exposure Negatively Impacts Atlantic Salmon (Salmo salar) Liver Metabolism and Function" Biology 13, no. 7: 494. https://doi.org/10.3390/biology13070494
APA StyleRojas, I., Caballero-Solares, A., Vadboncoeur, É., Sandrelli, R. M., Hall, J. R., Clow, K. A., Parrish, C. C., Rise, M. L., Swanson, A. K., & Gamperl, A. K. (2024). Prolonged Cold Exposure Negatively Impacts Atlantic Salmon (Salmo salar) Liver Metabolism and Function. Biology, 13(7), 494. https://doi.org/10.3390/biology13070494










