Handedness in Animals and Plants
Abstract
:Simple Summary
Abstract
1. The Asymmetric Nature of the Universe
“Life as manifested to us is a function of the asymmetry of the universe and of the consequences of this fact”.Pasteur, 1860
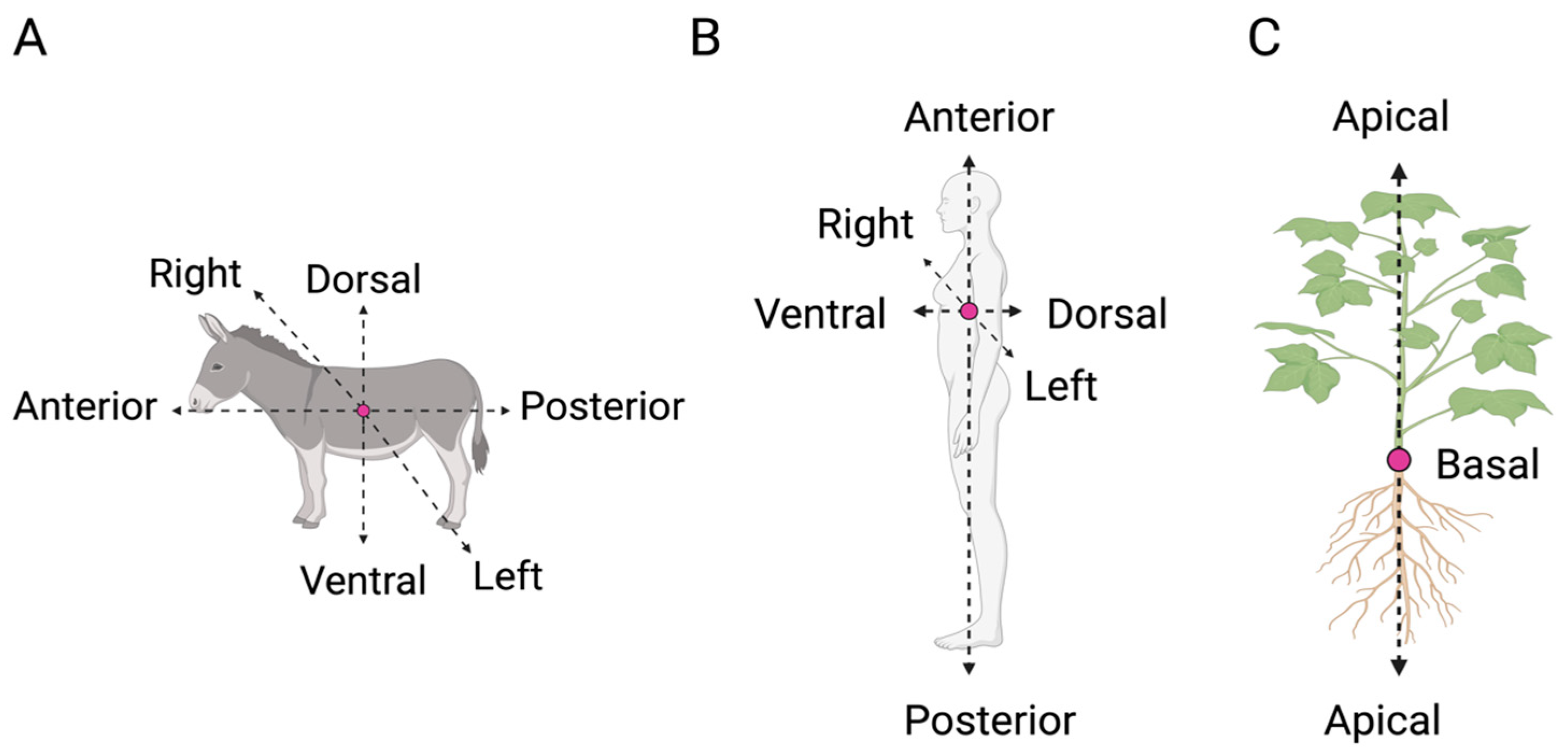
2. Handedness: One Term, Multiple Meanings
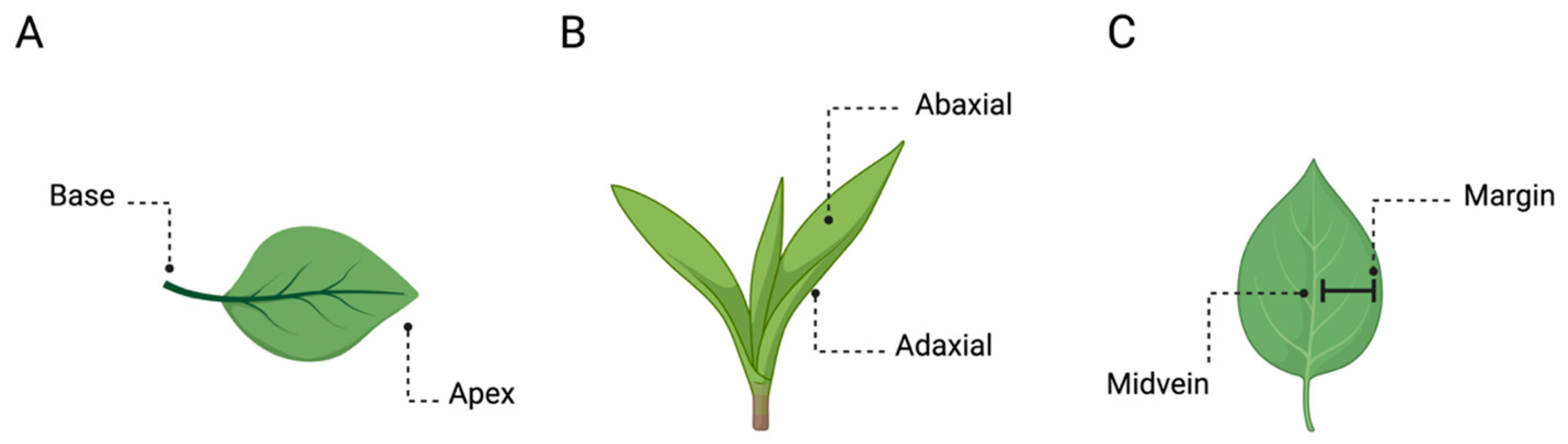
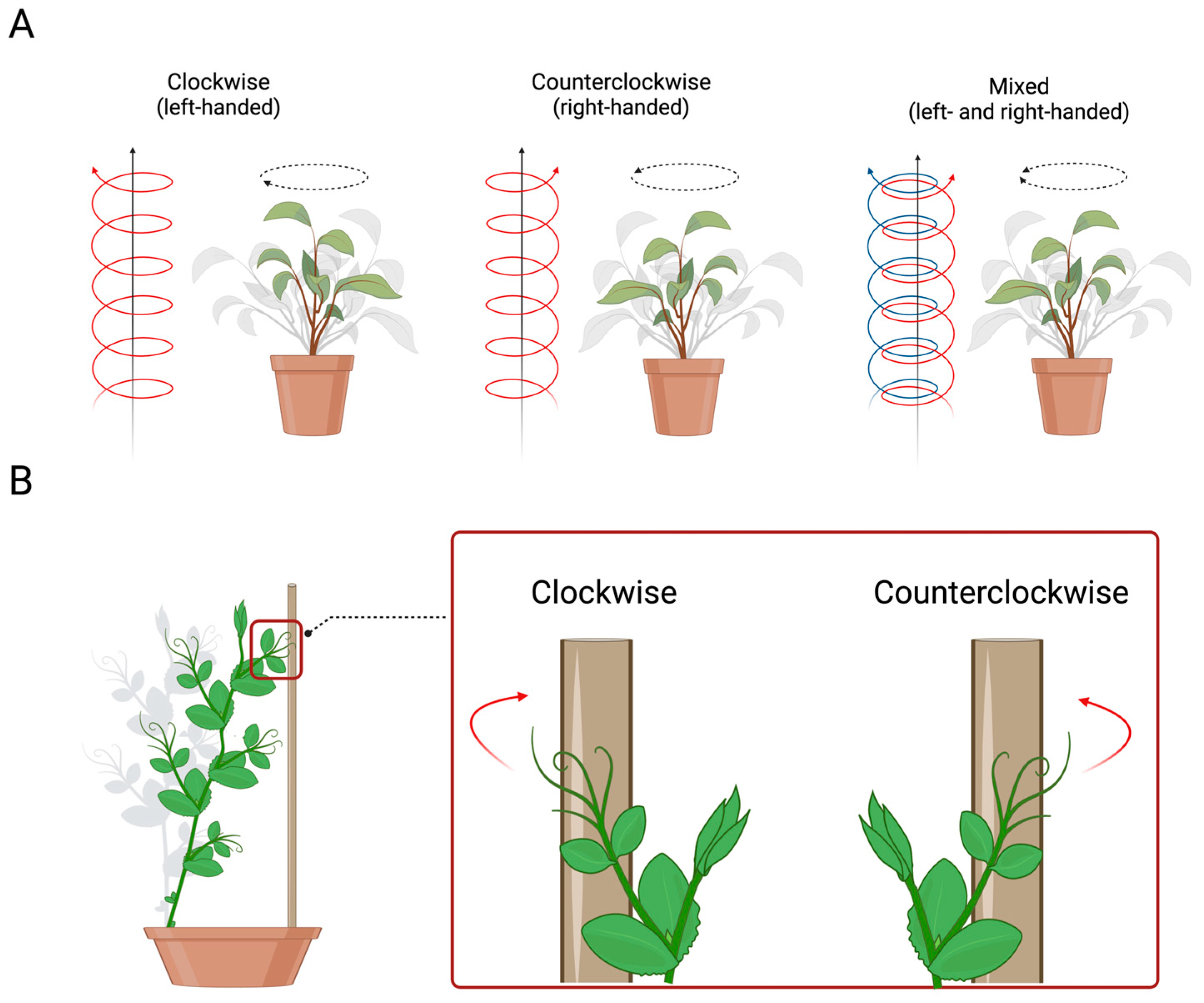
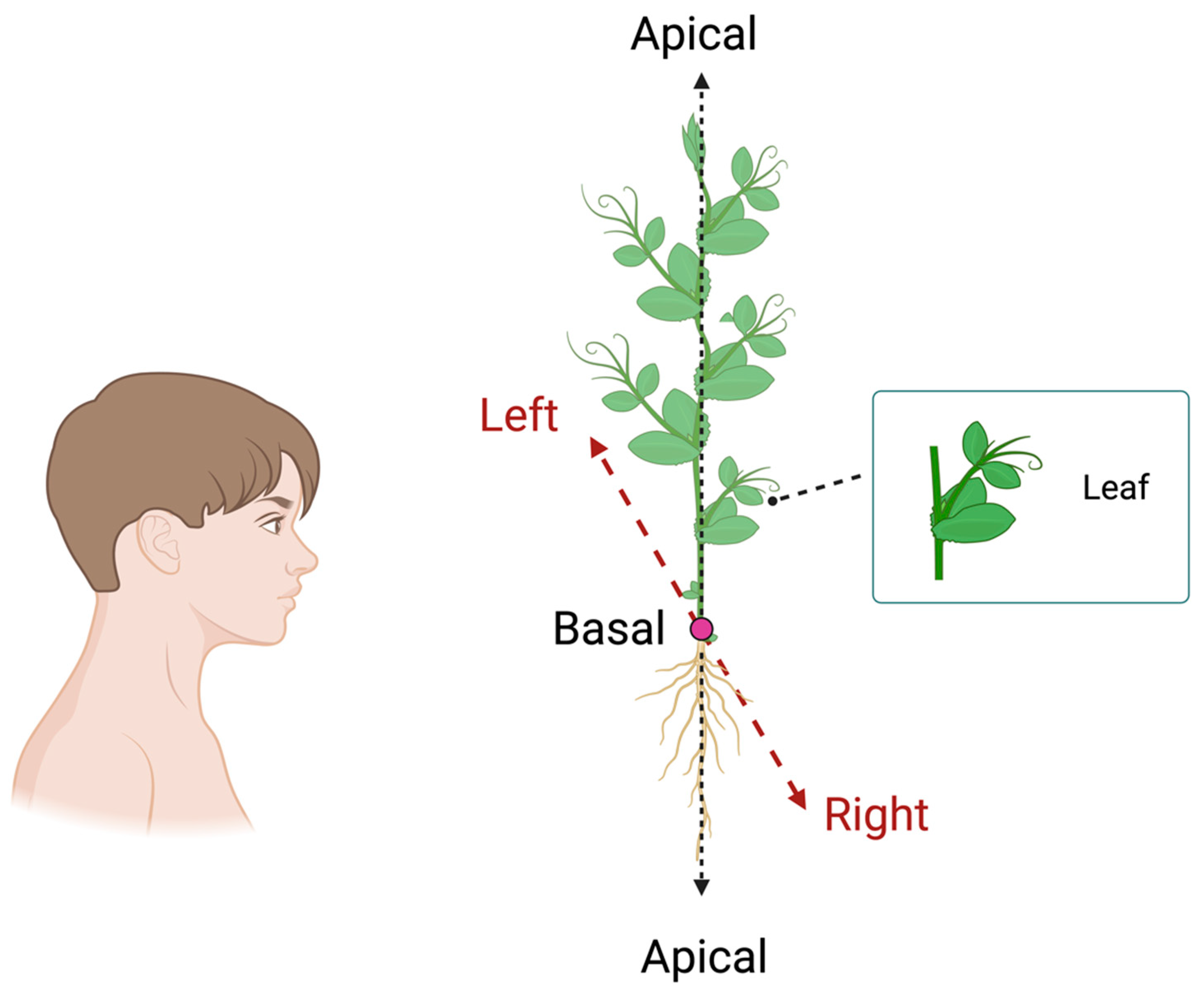
3. Handedness in Plants
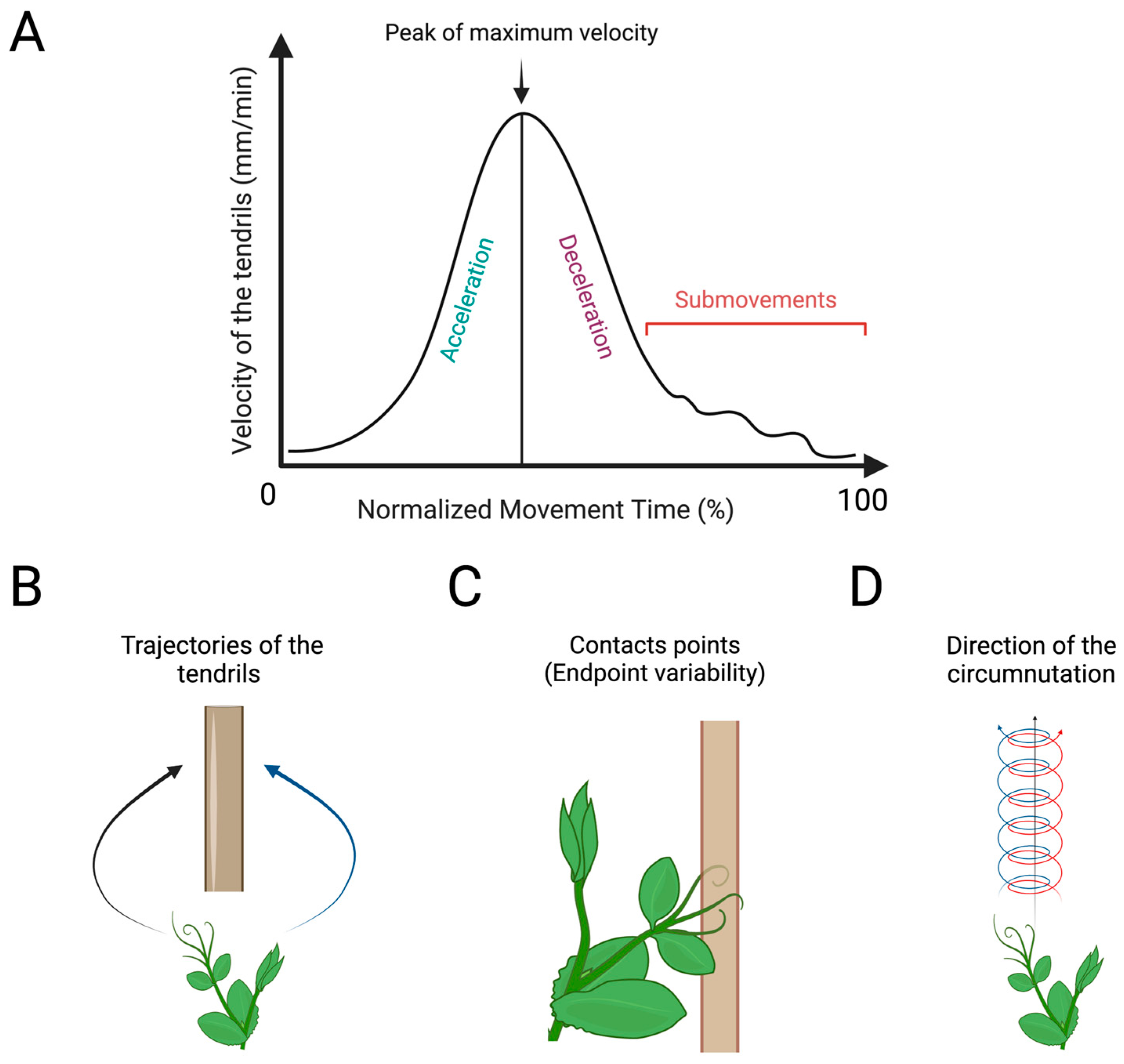
4. Toward a Comparative Study of Handedness in Animals and Plants?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inaki, M.; Liu, J.; Matsuno, K. Cell chirality: Its origin and roles in left–right asymmetric development. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150403. [Google Scholar] [CrossRef]
- Hegstrom, R.A.; Kondepudi, D.K. The handedness of the universe. Sci. Am. 1990, 262, 108–115. Available online: https://www.jstor.org/stable/24996649 (accessed on 1 February 2024). [CrossRef]
- Naganathan, S.R.; Fürthauer, S.; Nishikawa, M.; Jülicher, F.; Grill, S.W. Active torque generation by the actomyosin cell cortex drives left–right symmetry breaking. eLife 2014, 3, e04165. [Google Scholar] [CrossRef]
- Chen, T.H.; Hsu, J.J.; Zhao, X.; Guo, C.; Wong, M.N.; Huang, Y.; Li, Z.; Garfinkel, A.; Ho, C.M.; Tintut, Y.; et al. Left-right symmetry breaking in tissue morphogenesis via cytoskeletal mechanics novelty and significance. Circul. Res. 2012, 110, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Q.; Ronaldson, K.; Park, M.; Taylor, G.; Zhang, Y.; Gimble, J.M.; Vunjak-Novakovic, G. Micropatterned mammalian cells exhibit phenotype-specific left–right asymmetry. Proc. Natl Acad. Sci. USA 2011, 108, 12295–12300. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Maeda, R.; Ando, T.; Okumura, T.; Nakazawa, N.; Hatori, R.; Nakamura, M.; Hozumi, S.; Fujiwara, H.; Matsuno, K. Chirality in planar cell shape contributes to left–right asymmetric epithelial morphogenesis. Science 2011, 333, 339–341. [Google Scholar] [CrossRef]
- Lengyel, J.A.; Iwaki, D.D. It takes guts: The Drosophila hindgut as a model system for organogenesis. Dev. Biol. 2002, 243, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, H.; Kondo, S. Rotating pigment cells exhibit an intrinsic chirality. Genes Cells 2015, 20, 29–35. [Google Scholar] [CrossRef]
- Voss, J.; Bischof, H.J. Eye movements of laterally eyed birds are not independent. J. Exp. Biol. 2009, 212, 1568–1575. [Google Scholar] [CrossRef]
- Hudson, A. Development of symmetry in plants. Annu. Rev. Plant Biol. 2000, 51, 349–370. [Google Scholar] [CrossRef]
- Jürgens, G. Axis formation in plant embryogenesis: Cues and clues. Cell 1995, 81, 467–470. [Google Scholar] [CrossRef]
- Jeong, S.; Eilbert, E.; Bolbol, A.; Lukowitz, W. Going mainstream: How is the body axis of plants first initiated in the embryo? Dev. Biol. 2016, 419, 78–84. [Google Scholar] [CrossRef]
- Berger, F.; Brownlee, C. Establishment of the apical-basal axis in multicellular plant embryos. Biol. Cell 1995, 84, 7–11. [Google Scholar] [CrossRef]
- Sachs, T. Cell polarity and tissue patterning in plants. Development 1991, 113, 83–93. [Google Scholar] [CrossRef]
- Dahdul, W.M.; Cui, H.; Mabee, P.M.; Mungall, C.J.; Osumi-Sutherland, D.; Walls, R.L.; Haendel, M.A. Nose to tail, roots to shoots: Spatial descriptors for phenotypic diversity in the Biological Spatial Ontology. J. Biomed. Semant. 2014, 5, 34. [Google Scholar] [CrossRef]
- Hofmeister, W. Allgemeine Morphologie der Gewächse. In Handbuch der Physiologischen Botanik; Engelmann: Leipzig, Germany, 1868; Volume 1, pp. 405–664. [Google Scholar]
- Barth, K.A.; Miklosi, A.; Watkins, J.; Bianco, I.H.; Wilson, S.W.; Andrew, R.J. Fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr. Biol. 2005, 15, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Dadda, M.; Domenichini, A.; Piffer, L.; Argenton, F.; Bisazza, A. Early differences in epithalamic left–right asymmetry influence lateralization and personality of adult zebrafish. Behav. Brain Res. 2010, 206, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Braybrook, S.A.; Kuhlemeier, C. How a plant builds leaves. Plant Cell 2010, 22, 1006–1018. [Google Scholar] [CrossRef]
- Endress, P.K.; Doyle, J.A. Floral phyllotaxis in basal angiosperms: Development and evolution. Curr. Opin. Plant Biol. 2007, 10, 52–57. [Google Scholar] [CrossRef]
- Isparta, S.; Töre-Yargın, G.; Wagner, S.C.; Mundorf, A.; Cinar Kul, B.; Da Graça Pereira, G.; Güntürkün, O.; Ocklenburg, S.; Freund, N.; Demirbas, Y.S. Measuring paw preferences in dogs, cats and rats: Design requirements and innovations in methodology. Laterality 2024, 1–37. [Google Scholar] [CrossRef] [PubMed]
- McManus, I.C. The history and geography of human handedness. In Language Lateralization and Psychosis; Cambridge University Press: Cambridge, UK, 2009; pp. 37–57. [Google Scholar]
- McManus, I.C. The incidence of left-handedness: A meta-analysis. arXiv 2019. [Google Scholar] [CrossRef]
- Nelson, E.L.; Karimi, A. Systematic Review: The Development of Behavioral Laterality Across the First Year of Life in Nonhuman Primates. Symmetry 2023, 15, 1335. [Google Scholar] [CrossRef]
- Papadatou-Pastou, M.; Ntolka, E.; Schmitz, J.; Martin, M.; Munafò, M.R.; Ocklenburg, S.; Paracchini, S. Human handedness: A meta-analysis. Psychol. Bull. 2020, 146, 481. [Google Scholar] [CrossRef]
- Roth, E.D. ‘Handedness’ in snakes? Lateralization of coiling behaviour in a cottonmouth, Agkistrodon piscivorus leucostoma, population. Anim. Behav. 2003, 66, 337–341. [Google Scholar] [CrossRef]
- Quaranta, A.; Siniscalchi, M.; Vallortigara, G. Asymmetric tail-wagging responses by dogs to different emotive stimuli. Curr. Biol. 2007, 17, R199–R201. [Google Scholar] [CrossRef] [PubMed]
- Giljov, A.; De Silva, S.; Karenina, K. Context-dependent lateralization of trunk movements in wild Asian elephants. Biol. Commun. 2017, 2, 82–92. [Google Scholar] [CrossRef]
- Haakonsson, J.E.; Semple, S. Lateralisation of trunk movements in captive Asian elephants (Elephas maximus). Laterality 2009, 14, 413–422. [Google Scholar] [CrossRef]
- Versace, E.; Vallortigara, G. Forelimb preferences in human beings and other species: Multiple models for testing hypotheses on lateralization. Front. Psychol. 2015, 6, 129626. [Google Scholar] [CrossRef] [PubMed]
- Marcori, A.J.; Okazaki, V.H.A. A historical, systematic review of handedness origins. Laterality 2020, 25, 87–108. [Google Scholar] [CrossRef]
- Warren, J.M. Handedness and laterality in humans and other animals. Physiol. Psychol. 1980, 8, 351–359. [Google Scholar] [CrossRef]
- Annett, M. Left, Right, Hand and Brain: The Right Shift Theory; Lawrence Erlbaum Associates: London, UK, 1985. [Google Scholar]
- Michel, G.F.; Babik, I.; Nelson, E.L.; Campbell, J.M.; Marcinowski, E.C. How the development of handedness could contribute to the development of language. Dev. Psychobiol. 2013, 55, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Knecht, S.; Dräger, B.; Deppe, M.; Bobe, L.; Lohmann, H.; Flöel, A.; Ringelstein, E.-B.; Henningsen, H. Handedness and hemispheric language dominance in healthy humans. Brain 2000, 123, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Nottebohm, F. Neural lateralization of vocal control in a passerine bird. I. Song. J. Exp. Zool. 1971, 177, 229–261. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J.; Anson, J.M. Lateralisation of function in the chicken fore-brain. Pharmacol. Biochem. Behav. 1979, 10, 679–686. [Google Scholar] [CrossRef]
- Andrew, R.J.; Mench, J.; Rainey, C. Right-left asymmetry of response to visual stimuli in the domestic chick. In Analysis of Visual Behaviour; The MIT Press: Cambridge, MA, USA, 1982; pp. 197–209. [Google Scholar]
- Boulinguez-Ambroise, G.; Aychet, J.; Pouydebat, E. Limb preference in animals: New insights into the evolution of manual laterality in hominids. Symmetry 2022, 14, 96. [Google Scholar] [CrossRef]
- Hopkins, W.D. Neuroanatomical asymmetries and handedness in chimpanzees (Pan troglodytes): A case for continuity in the evolution of hemispheric specialization. Ann. N. Y. Acad. Sci. 2013, 1288, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Laterality in animals. Int. J. Comp. Psychol. 1989, 3, 5–25. [Google Scholar] [CrossRef]
- Annett, J. Motor learning: A review. In Motor Behavior; Heuer, H., Kleinbeck, U., Schmidt, K.H., Eds.; Springer: Berlin/Heidelberg, Germany, 1985. [Google Scholar] [CrossRef]
- McManus, I.C. Handedness, language dominance and aphasia: A genetic model. Psychol. Med. Monogr. Suppl. 1985, 8, 3–40. [Google Scholar] [CrossRef]
- Rat-Fischer, L.; O’Regan, J.K.; Fagard, J. Handedness in infants’ tool use. Dev. Psychobiol. 2013, 55, 860–868. [Google Scholar] [CrossRef]
- Domellöf, E.; Johansson, A.M.; Rönnqvist, L. Handedness in preterm born children: A systematic review and a meta-analysis. Neuropsychologia 2011, 49, 2299–2310. [Google Scholar] [CrossRef]
- Raymond, M.; Pontier, D. Is there geographical variation in human handedness? Laterality 2004, 9, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Russell, J.L.; Lambeth, S.; Schapiro, S.J. Handedness and neuroanatomical asymmetries in captive chimpanzees: A summary of 15 years of research. Int. J. Primatol. 2007, 5, 146–181. [Google Scholar] [CrossRef]
- Crow, T. Directional asymmetry is the key to the origin of modern Homo sapiens (the Broca-Annett axiom): A reply to Rogers’ review of The Speciation of Modern Homo Sapiens. Later. Asymmetries Body Brain Cognit. 2004, 9, 233–242. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Cantalupo, C. Handedness in chimpanzees (Pan troglodytes) is associated with asymmetries of the primary motor cortex but not with homologous language areas. Behav. Neurosci. 2004, 118, 1176. [Google Scholar] [CrossRef] [PubMed]
- Meunier, H.; Vauclair, J.; Fagard, J. Human infants and baboons show the same pattern of handedness for a communicative gesture. PLoS ONE 2012, 7, e33959. [Google Scholar] [CrossRef] [PubMed]
- Dahmen, R.; Fagard, J. The effect of explicit cultural bias on lateral preferences in Tunisia. Cortex 2005, 41, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Kushner, H.I. Deficit or creativity: Cesare Lombroso, Robert Hertz, and the meanings of left-handedness. Laterality 2013, 18, 416–436. [Google Scholar] [CrossRef]
- Faurie, C.; Raymond, M. Handedness, homicide and negative frequency-dependent selection. Proc. R. Soc. B Biol. Sci. 2005, 272, 25–28. [Google Scholar] [CrossRef]
- Kaplan, G.; Rogers, L.J. Brain Size Associated with Foot Preferences in Australian Parrots. Symmetry 2021, 13, 867. [Google Scholar] [CrossRef]
- Giljov, A.; Karenina, K.; Ingram, J.; Malashichev, Y. Parallel emergence of true handedness in the evolution of marsupials and placentals. Curr. Biol. 2015, 25, 1878–1884. [Google Scholar] [CrossRef]
- Caspar, K.R.; Pallasdies, F.; Mader, L.; Sartorelli, H.; Begall, S. The evolution and biological correlates of hand preferences in anthropoid primates. eLife 2022, 11, e77875. [Google Scholar] [CrossRef]
- Vallortigara, G.; Rogers, L. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005, 28, 595–596. [Google Scholar] [CrossRef]
- Watson, S.L.; Hanbury, D.B. Prosimian Primates as Models of Laterality; Elsevier: Amsterdam, The Netherlands, 2007; Volume 5, pp. 228–250. [Google Scholar] [CrossRef]
- Bisazza, A.; Sovrano, V.A.; Vallortigara, G. Consistency among different tasks of left–right asymmetries in lines of fish originally selected for opposite direction of lateralization in a detour task. Neuropsychologia 2001, 39, 1077–1085. [Google Scholar] [CrossRef]
- Fine, M.L.; Friel, J.P.; McElroy, D.; King, C.B.; Loesser, K.E.; Newton, S. Pectoral spine locking and sound production in the channel catfish Ictalurus punctatus. Copeia 1994, 1997, 777–790. [Google Scholar] [CrossRef]
- Shine, R.; Olsson, M.M.; LeMaster, M.P.; Moore, I.T.; Mason, R.T. Are snakes right–handed? Asymmetry in hemipenis size and usage in gartersnakes (Thamnophis sirtalis). Behav. Ecol. 2000, 11, 411–415. [Google Scholar] [CrossRef]
- Darwin, C.; Darwin, F. The Power of Movement in Plants; John Murray: London, UK, 1880. [Google Scholar] [CrossRef]
- Darwin, C. The Movements and Habits of Climbing Plants; John Murray: London, UK, 1875. [Google Scholar]
- Gianoli, E. The behavioural ecology of climbing plants. AoB Plants 2015, 7, plv013. [Google Scholar] [CrossRef]
- Edwards, W.; Moles, A.T.; Franks, P. The global trend in plant twining direction. Glob. Ecol. Biogeogr. 2007, 16, 95–800. [Google Scholar] [CrossRef]
- Gardner, M. The Ambidextrous Universe; Penguin: Southbank, VIC, Australia, 1964. [Google Scholar]
- Jackson, D.B. A Glossary of Botanical Terms, 4th ed.; Duckworth: London, UK, 1953. [Google Scholar]
- Jaffe, M.J. Physiological Studies on Pea Tendrils VIII. The Relationship of Circumnutation to Contact Coiling—With a Description of a Laboratory Intervalometer Using Integrated Digital Circuits. Physiol. Plant. 1972, 26, 73–80. [Google Scholar] [CrossRef]
- Kihara, H. Right– and Left–Handedness in Plants—A review; ISO 690; Kihara Institute of Biological Research: Yokohama, Japan, 1973. [Google Scholar]
- Smyth, D.R. Helical growth in plant organs: Mechanisms and significance. Development 2016, 143, 3272–3282. [Google Scholar] [CrossRef]
- Bahadur, B.; Krishnamurthy, K.V.; Ghose, M.; Adams, S.J. (Eds.) Asymmetry in Plants: Biology of Handedness; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Davis, T.A.; Davis, B. Association of coconut foliar spirality with latitude. Math. Model. 1987, 8, 730–733. [Google Scholar] [CrossRef]
- Hashimoto, T. Molecular genetic analysis of left–right handedness in plants. Philos. Philos. Trans. R. Soc. 2002, 357, 799–808. [Google Scholar] [CrossRef]
- Minorsky, P.V. Latitudinal differences in coconut foliar spiral direction: A re-evaluation and hypothesis. Ann. Bot. 1998, 82, 133–140. [Google Scholar] [CrossRef]
- Minorsky, P.V.; Bronstein, N.B. Natural experiments indicate that geomagnetic variations cause spatial and temporal variations in coconut palm asymmetry. Plant Physiol. 2006, 142, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Atamian, H.S.; Creux, N.M.; Brown, R.I.; Garner, A.G.; Blackman, B.K.; Harmer, S.L. Circadian regulation of sunflower heliotropism, floral orientation, and pollinator visits. Science 2016, 353, 587–590. [Google Scholar] [CrossRef]
- Bose, J.C.; Guha, S.C. The dia-heliotropic attitude of leaves as determined by transmitted nervous excitation. Proc. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character 1922, 93, 153–178. [Google Scholar] [CrossRef]
- Schuster, J.; Engelmann, W. Circumnutations of Arabidopsis thaliana seedlings. Biol. Rhythm. Res. 1997, 28, 422–440. [Google Scholar] [CrossRef]
- Millet, B.; Melin, D.; Badot, P.M. Circumnutation in Phaseolus vulgaris. I. Growth, osmotic potential and cell ultrastructure in the free-moving part of the shoot. Physiol. Plant. 1988, 72, 133–138. [Google Scholar] [CrossRef]
- Guerra, S.; Bruno, G.; Spoto, A.; Panzeri, A.; Wang, Q.; Bonato, B.; Simonetti, V.; Castiello, U. Ascent and attachment in pea plants: A matter of iteration. Plants 2024, 13, 1389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guerra, S.; Bonato, B.; Simonetti, V.; Bulgheroni, M.; Castiello, U. Decision–making underlying support searching in pea plants. Plants 2023, 12, 1597. [Google Scholar] [CrossRef]
- Silk, W.K.; Holbrook, N.M. The importance of frictional interactions in maintaining the stability of the twining habit. Am. J. Bot. 2005, 92, 1820–1826. [Google Scholar] [CrossRef]
- Lüttge, U.; Souza, G.M. The Golden Section and beauty in nature: The perfection of symmetry and the charm of asymmetry. Prog. Biophys. Mol. Biol. 2019, 146, 98–103. [Google Scholar] [CrossRef]
- Souza, G.M.; Viana, J.D.O.F.; de Oliveira, R.F. Asymmetrical leaves induced by water deficit show asymmetric photosynthesis in common bean. Braz. J. Plant Physiol. 2005, 17, 223–227. [Google Scholar] [CrossRef]
- Bonato, B.; Simonetti, V.; Bulgheroni, M.; Wang, Q.; Guerra, S.; Quaggiotti, S.; Ruperti, B.; Castiello, U. Evidence of motor intentions in plants: A kinematical study. J. Comp. Psychol. 2023, 137, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Castiello, U. (Re) claiming plants in comparative psychology. J. Comp. Psychol. 2021, 135, 127. [Google Scholar] [CrossRef]
- Ceccarini, F.; Guerra, S.; Peressotti, A.; Peressotti, F.; Bulgheroni, M.; Baccinelli, W.; Bonato, B.; Castiello, U. On-line control of movement in plants. Biochem. Biophys. Res. Commun. 2020, 564, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Bonato, B.; Wang, Q.; Ceccarini, F.; Peressotti, A.; Peressotti, F.; Bacinelli, W.; Bulgheroni, M.; Castiello, U. The coding of object thickness in plants: When roots matter. J. Comp. Psychol. 2021, 135, 495–504. [Google Scholar] [CrossRef]
- Guerra, S.; Peressotti, A.; Peressotti, F.; Bulgheroni, M.; Bacinelli, W.; D’Amico, E.; Gómez, A.; Massaccesi, S.; Ceccarini, F.; Castiello, U. Flexible control of movement in plants. Sci. Rep. 2019, 9, 16570. [Google Scholar] [CrossRef] [PubMed]
- Falik, O.; Reides, P.; Gersani, M.; Novoplansky, A. Root navigation by self inhibition. Plant Cell Environ. 2005, 28, 562–569. [Google Scholar] [CrossRef]
- Gilroy, S.; Trewavas, T. Agency, teleonomy and signal transduction in plant systems. Biol. J. Linn. Soc. 2023, 139, 514–529. [Google Scholar] [CrossRef]
- Novoplansky, A. Picking battles wisely: Plant behaviour under competition. Plant Cell Environ. 2009, 32, 726–741. [Google Scholar] [CrossRef]
- Parise, A.G.; De Toledo, G.R.A.; de Carvalho Oliveira, T.F.; Souza, G.M.; Castiello, U.; Gagliano, M.; Marder, M. Do plants pay attention? A possible phenomenological–empirical approach. Prog. Biophys. Mol. Biol. 2022, 173, 11–23. [Google Scholar] [CrossRef]
- Parise, A.G.; Marder, M. Extended plant cognition: A critical consideration of the concept. Theor. Exp. Plant Physiol. 2023. [Google Scholar] [CrossRef]
- Parise, A.G.; Reissig, G.N.; Basso, L.F.; Senko, L.G.S.; Oliveira, T.F.D.C.; De Toledo, G.R.A.; Ferreira, A.S.; Souza, G.M. Detection of different hosts from a distance alters the behaviour and bioelectrical activity of Cuscuta racemosa. Front. Plant Sci. 2021, 12, 594195. [Google Scholar] [CrossRef] [PubMed]
- Saito, K. A study on diameter-dependent support selection of the tendrils of Cayratia japonica. Sci. Rep. 2022, 12, 4461. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Barbariol, T.; Susto, G.A.; Bonato, B.; Guerra, S.; Castiello, U. Classifying circumnutation in pea plants via supervised machine learning. Plants 2023, 12, 965. [Google Scholar] [CrossRef] [PubMed]
- Parma, V.; Brasselet, R.; Zoia, S.; Bulgheroni, M.; Castiello, U. The origin of human handedness and its role in pre-birth motor control. Sci. Rep. 2017, 7, 16804. [Google Scholar] [CrossRef] [PubMed]
- Fagard, J. The nature and nurture of human infant hand preference. Ann. N. Y. Acad. Sci. 2013, 1288, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.F.A.; Smitsman, W. Action planning in young children’s tool use. Dev. Sci. 2006, 9, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Flindall, J.W.; Gonzalez, C.L. On the evolution of handedness: Evidence for feeding biases. PLoS ONE 2013, 8, e78967. [Google Scholar] [CrossRef]
- Leconte, P.; Fagard, J. Which factors affect hand selection in children’s grasping in hemispace? Combined effects of task demand and motor dominance. Brain Cognit. 2006, 60, 88–93. [Google Scholar] [CrossRef]
- Morange–Majoux, F.; Peze, A.; Bloch, H. Organisation of left and right hand movement in a prehension task: A longitudinal study from 20 to 32 weeks. Later. Asymmetries Body Brain Cognit. 2000, 5, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Rönnqvist, L.; Domellöf, E. Quantitative Assessment of Right and Left Reaching Movements in Infants: A Longitudinal Study from 6 to 36 Months. Dev. Psychobiol. 2006, 48, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Sacrey, L.A.R.; Arnold, B.; Whishaw, I.Q.; Gonzalez, C. Precocious hand use preference in reach-to-eat behavior versus manual construction in 1- to 5-year old children. Dev. Psychobiol. 2012, 55, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, J.; Richie, D.; Chaudhari, A.M.; Tudella, E.; Spees, C.K.; Heathcock, J.C. Task-Related Differences in End-Point Kinematics in School-Age Children with Typical Development. Behav. Sci. 2023, 13, 528. [Google Scholar] [CrossRef]
- Begliomini, C.; Nelini, C.; Caria, A.; Grodd, W.; Castiello, U. Cortical activations in humans grasp–related areas depend on hand used and handedness. PLoS ONE 2008, 3, e3388. [Google Scholar] [CrossRef]
- Clark, L.D.E.; Iskandarani, M.; Riggs, S.L. The effect of movement direction, hand dominance, and hemispace on reaching movement kinematics in virtual reality. In Proceedings of the 2023 CHI Conference on Human Factors in Computing Systems, Hamburg, Germany, 23–28 April 2023; pp. 1–18. [Google Scholar]
- Gonzalez, C.L.; Whitwell, R.L.; Morrissey, B.; Ganel, T.; Goodale, M.A. Left handedness does not extend to visually guided precision grasping. Exp. Brain Res. 2007, 182, 275–279. [Google Scholar] [CrossRef]
- Nelson, E.L.; Berthier, N.E.; Konidaris, G.D. Handedness and reach-to-place kinematics in adults: Left-handers are not reversed right-handers. J. Mot. Behav. 2018, 50, 381–391. [Google Scholar] [CrossRef]
- Stins, J.F.; Kadar, E.E.; Costall, A. A kinematic analysis of hand selection in a reaching task. Laterality 2001, 6, 347–367. [Google Scholar] [CrossRef]
- Grosskopf, A.; Kuhtz-Buschbeck, J.P. Grasping with the left and right hand: A kinematic study. Exp. Brain Res. 2006, 168, 230–240. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Cantalupo, C.; Wesley, M.J.; Hostetter, A.B.; Pilcher, D.L. Grip morphology and hand use in chimpanzees (Pan troglodytes): Evidence of a left hemisphere specialization in motor skill. J. Exp. Psychol. Gen. 2002, 131, 412. [Google Scholar] [CrossRef]
- Nelson, E.L.; Konidaris, G.D.; Berthier, N.E.; Braun, M.C.; Novak, M.F.; Suomi, S.J.; Novak, M.A. Kinematics of reaching and implications for handedness in rhesus monkey infants. Dev. Psychol. 2012, 54, 460–467. [Google Scholar] [CrossRef]
- Castiello, U.; Dadda, M. A review and consideration on the kinematics of reach-to-grasp movements in macaque monkeys. J. Neurosci. 2019, 121, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, V.; Bulgheroni, M.; Guerra, S.; Peressotti, A.; Peressotti, F.; Baccinelli, W.; Ceccarini, F.; Bonato, B.; Wang, Q.; Castiello, U. Can plants move like animals? A three-dimensional stereovision analysis of movement in plants. Animals 2021, 11, 1854. [Google Scholar] [CrossRef] [PubMed]
- MacNeilage, P.F.; Studdert-Kennedy, M.G.; Lindblom, B. Primate handedness reconsidered. Behav. Brain Sci. 1987, 10, 247–263. [Google Scholar] [CrossRef]
- MacNeilage, P.F. Present status of the postural origins theory. In The Evolution of Hemispheric Specialization in Primates; Hopkins, W.D., Ed.; Elsevier: Oxford, UK, 2007; pp. 59–91. [Google Scholar] [CrossRef]
- Goble, D.J.; Brown, S.H. The biological and behavioral basis of upper limb asymmetries in sensorimotor performance. Neurosci. Biobehav. Rev. 2008, 32, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Bryden, P.J. The influence of M. P. Bryden’s work on lateralization of motor skill: Is the preferred hand selected for and better at tasks requiring a high degree of skill? Laterality 2016, 21, 312–328. [Google Scholar] [CrossRef]
- Palmer, R.D. Development of a differentiated handedness. Psychol. Bull. 1964, 62, 257–272. [Google Scholar] [CrossRef]
- Vallortigara, G.; Bisazza, A. How ancient is brain lateralization. In Comparative Vertebrate Lateralization; Cambridge University Press: Cambridge, UK, 2002; pp. 9–69. [Google Scholar]
- Andrew, R.J. Lateralization of emotional and cognitive function in higher vertebrates, with special reference to the domestic chick. In Advances in Vertebrate Neuroethology; Springer: Boston, MA, USA, 1983; pp. 477–509. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, S.; Castiello, U.; Bonato, B.; Dadda, M. Handedness in Animals and Plants. Biology 2024, 13, 502. https://doi.org/10.3390/biology13070502
Guerra S, Castiello U, Bonato B, Dadda M. Handedness in Animals and Plants. Biology. 2024; 13(7):502. https://doi.org/10.3390/biology13070502
Chicago/Turabian StyleGuerra, Silvia, Umberto Castiello, Bianca Bonato, and Marco Dadda. 2024. "Handedness in Animals and Plants" Biology 13, no. 7: 502. https://doi.org/10.3390/biology13070502




