Effect of Dietary Supplementation with Organic Silicon on the Growth Performance, Blood Biochemistry, Digestive Enzymes, Morphohistology, Intestinal Microbiota and Stress Resistance in Juvenile Hybrid Tilapia (Oreochromis mossambicus × Oreochromis niloticus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Formulation and Preparation of Experimental Diets
2.3. Chemical Analysis of Diets
2.4. Experimental Design and Rearing Conditions
2.5. Sample Collection
2.6. Fish Growth Performance
2.7. Blood Biochemistry
2.8. Digestive Enzyme Activity
2.9. Intestine Histology
2.10. Gut Microbiota Metagenomics
2.11. Hypoxia Stress
2.12. Statistical Analysis
3. Results
3.1. Fish Growth Performance
3.2. Blood Biochemistry
3.3. Digestive Enzyme Activity
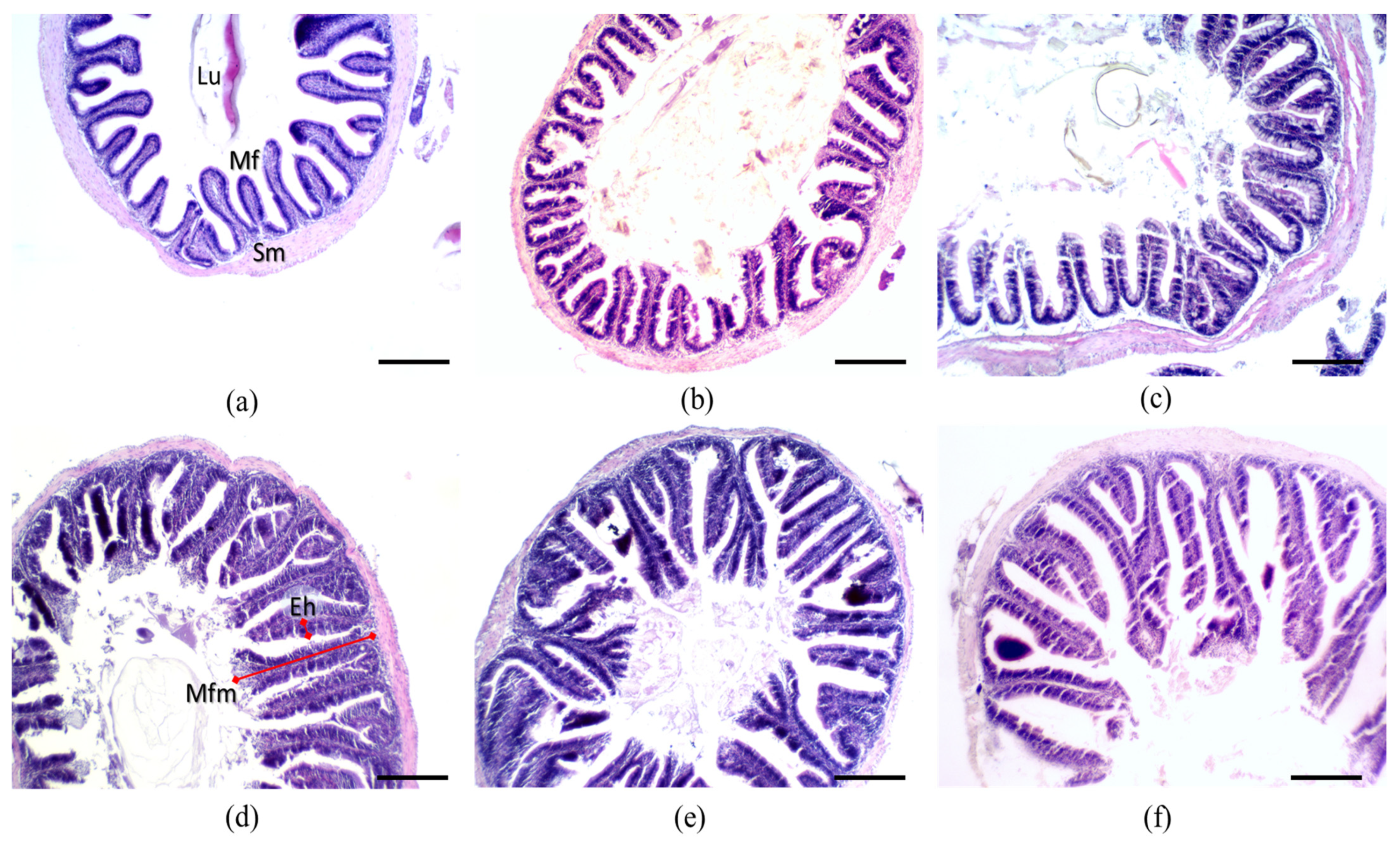
3.4. Intestinal Morphohistology
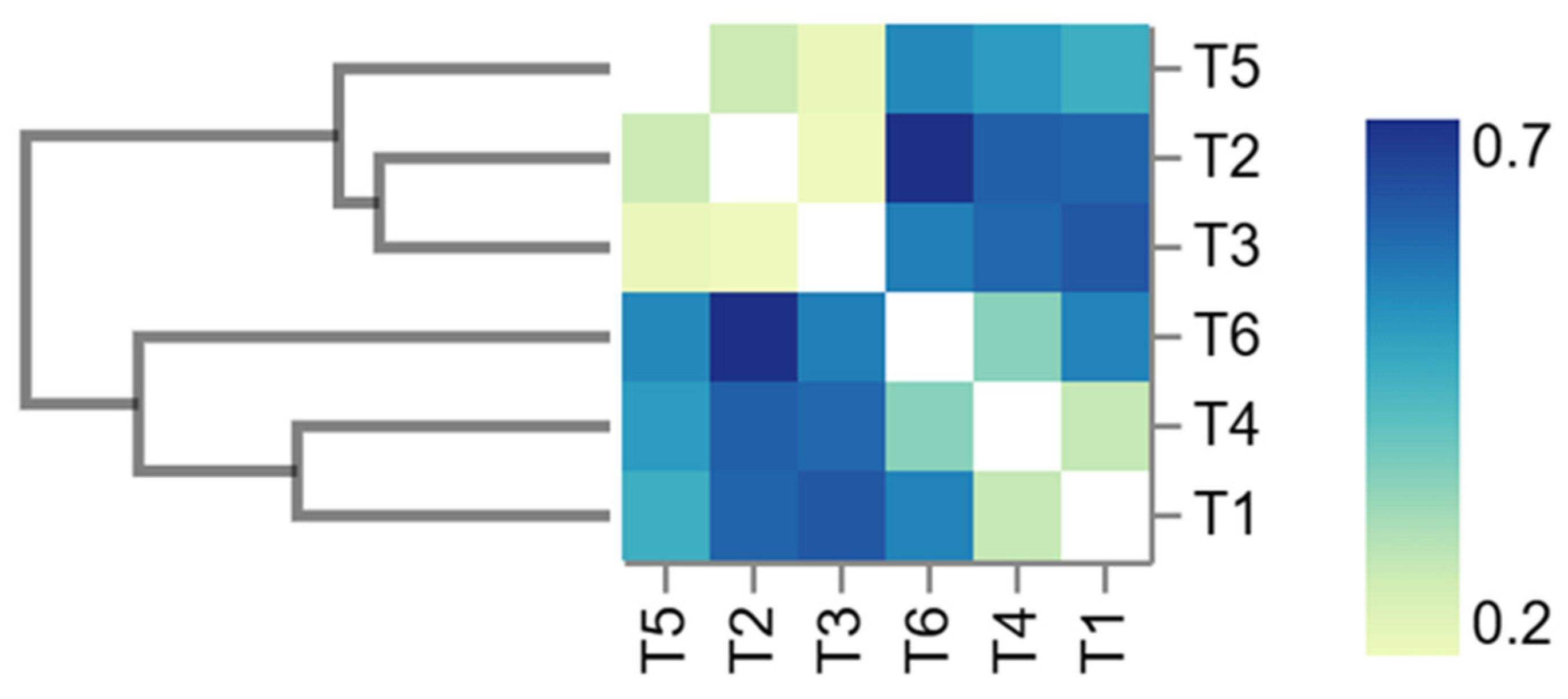
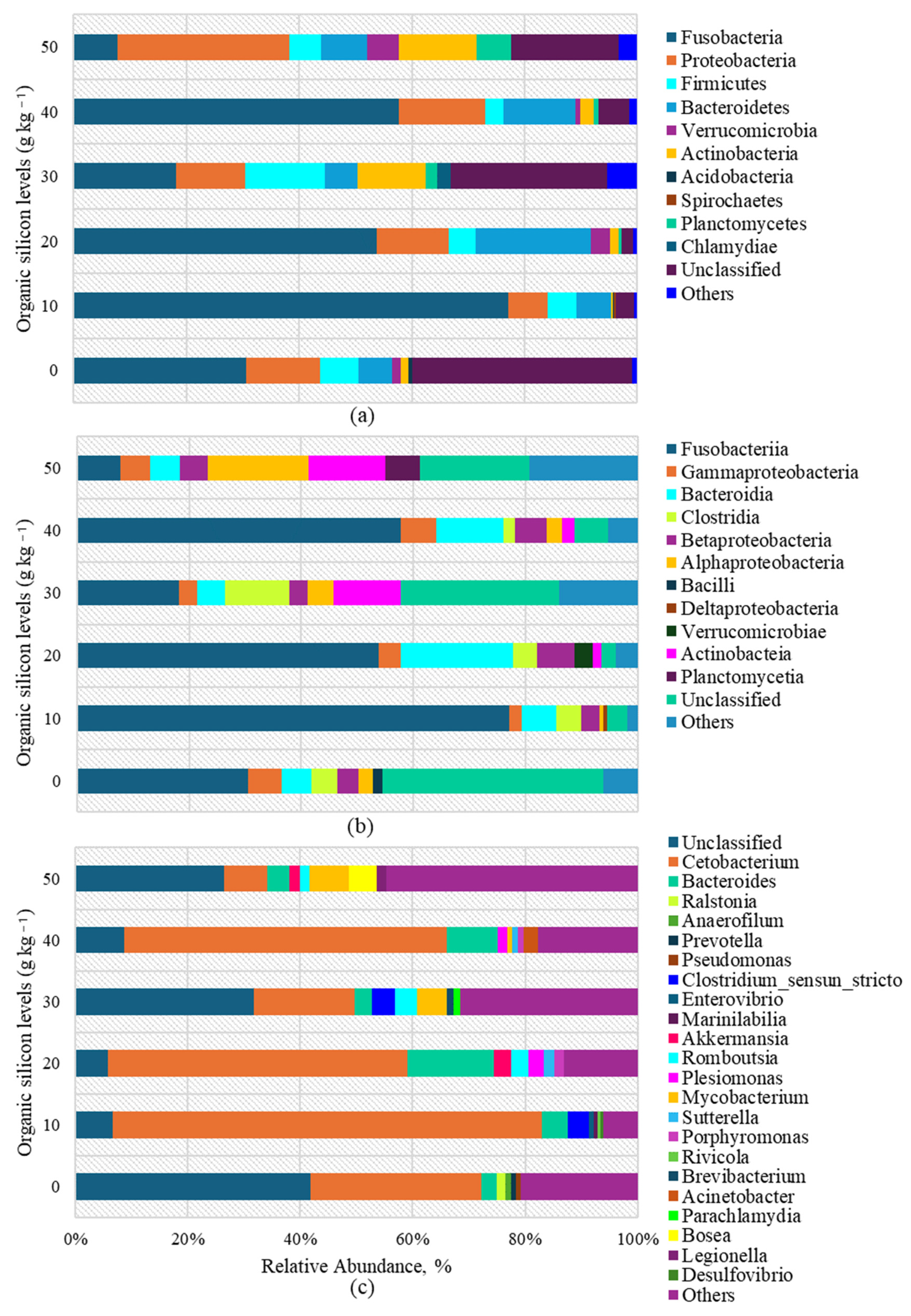
3.5. Gut Microbiome Metagenomics
3.6. Stress Resistance
4. Discussion
4.1. Fish Growth Performance
4.2. Blood Biochemistry
4.3. Digestive Enzyme Activity
4.4. Intestinal Morphohistology
4.5. Gut Microbiome Metagenomics
4.6. Hypoxia Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FishStatJ Tool for Fishery Statistics Analysis, Release 4.0.0. Universal Software for Fishery and Aquaculture Statistical Time Series. Global Capture and Aquaculture Production: Quantities 1950–2022; Aquaculture Values; Food and Agriculture Organization (FAO) Fisheries Department, Fishery Information, Data and Statistics Unit: Rome, Italy, 2024; Available online: https://www.fao.org/fishery/en/fishstat (accessed on 16 May 2024).
- Fatsi, P.S.K.; Hashem, S.; Kodama, A.; Appiah, E.K.; Saito, H.; Kawai, K. Population genetics and taxonomic signatures of wild Tilapia in Japan based on mitochondrial DNA control region analysis. Hydrobiologia 2020, 847, 1491–1504. [Google Scholar] [CrossRef]
- Zaki, M.F.; Said, M.M.; Tahoun, A.A.; Amer, M. Evaluation of Different Sex Reversal Treatments in Red Tilapia Hybrid. Egypt. J. Aquat. Biol. Fish. 2021, 25, 279–292. [Google Scholar] [CrossRef]
- Wang, M.; Lu, M. Tilapia polyculture: A global review. Aquac. Res. 2016, 47, 2363–2374. [Google Scholar] [CrossRef]
- Geletu, T.T.; Zhao, J. Genetic resources of Nile Tilapia (Oreochromis niloticus Linnaeus, 1758) in its native range and aquaculture. Hydrobiologia 2023, 850, 2425–2445. [Google Scholar] [CrossRef]
- Méndez-Martinez, Y.; Narváez-Narváez, R.I.; Angulo, C.; Cortés-Jacinto, E.; Botello Leon, A.; Verdecia, D.; Torres-Navarrete, Y.G. Chemical composition of Tithonia diversifolia (Hemsl.) and its effect on growth performance, feed efficiency and metabolic biochemistry of juvenile hybrid Tilapia, Oreochromis mossambicus × Oreochromis niloticus. Not. Bot. Horti Agrobo. 2023, 51, 13337. [Google Scholar] [CrossRef]
- Arumugam, M.; Jayaraman, S.; Sridhar, A.; Venkatasamy, V.; Brown, P.B.; Abdul Kari, Z.; Tellez-Isaias, G.; Ramasamy, T. Recent advances in Tilapia production for sustainable developments in Indian aquaculture and its economic benefits. Fishes 2023, 8, 176. [Google Scholar] [CrossRef]
- Setyawan, P.; Aththar, M.H.F.; Imron, I.; Gunadi, B.; Haryadi, J.; Bastiaansen, J.W.; Camara, M.D.; Komen, H. Genetic parameters and genotype by environment interaction in a unique Indonesian hybrid Tilapia strain selected for production in brackish water pond culture. Aquaculture 2022, 561, 738626. [Google Scholar] [CrossRef]
- Chabet dis, C.; Amira, D.; Rachida, I.; Wissam, C.; Bouchra, A.; Besma, L.B.H.; Amina, S. Effect of a Gradual Salinity Increase on Water Quality, Survival and Growth Performance of Hybrid Red Tilapia (Oreochromis mossambicus× O. niloticus). Inland. Water Biol. 2023, 16, 1112–1121. [Google Scholar] [CrossRef]
- Klahan, R.; Yuangsoi, B.; Whangchai, N.; Ramaraj, R.; Unpaprom, Y.; Khoo, K.S.; Deepanraj, B.; Pimpimol, T. Biorefining and biotechnology prospects of low-cost fish feed on Red Tilapia production with different feeding regime. Chemosphere 2023, 311, 137098. [Google Scholar] [CrossRef]
- Sotomayor, M.A.; Reyes, J.K.; Restrepo, L.; Domínguez-Borbor, C.; Maldonado, M.; Bayot, B. Efficacy assessment of commercially available natural products and antibiotics, commonly used for mitigation of pathogenic Vibrio outbreaks in Ecuadorian Penaeus (Litopenaeus) vannamei hatcheries. PLoS ONE 2019, 14, e0210478. [Google Scholar] [CrossRef]
- Méndez-Martínez, Y.; Vera-Veliz, A.R.; Cortés-Jacinto, E.; Cruz-Quintana, Y.; Botello-León, A.; Mendoza-Carranza, P.D.; Calvo, N.S. Growth Performance, Feed Utilisation, Digestive and Metabolic Enzyme Activity, and Liver Morphohistology in Hybrid Tilapia (Oreochromis mossambicus × Oreochromis niloticus) Juveniles Fed with the Inclusion of Chitosan in Their Diet. Fishes 2023, 8, 546. [Google Scholar] [CrossRef]
- Yang, H.T.; Zou, S.S.; Zhai, L.J.; Wang, Y.; Zhang, F.M.; An, L.G.; Yang, G.W. Pathogen invasion changes the intestinal microbiota composition and induces innate immune responses in the zebrafish intestine. Fish Shellfish Immunol. 2017, 71, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Genz, J.; Jyde, M.B.; Svendsen, J.C.; Steffensen, J.F.; Ramløv, H. Excess post-hypoxic oxygen consumption is independent from lactate accumulation in two cyprinid fishes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 165, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Urbich, A.V.; da Cruz, T.P.; Wernick, B.; Adeshina, I.; Sampaio, A.V.; Gonçalves, G.S.; Furuya, W.M. Effects of Replacing Inorganic with Organic Glycinates Trace Minerals on Growth Performance, Gut Function, and Minerals Loss of Juvenile Nile Tilapia. Aquac. Nutr. 2024, 1, 1422124. [Google Scholar] [CrossRef]
- Libanori, M.C.M.; Santos, G.G.; Pereira, S.A.; Lopes, G.R.; Owatari, M.S.; Soligo, T.A.; Yamashita, E.; Pereira, U.P.; Martins, M.L.; Mouriño, J.L.P. Dietary supplementation with benzoic organic acid improves the growth performance and survival of Nile tilapia (Oreochromis niloticus) after challenge with Streptococcus agalactiae (Group B). Aquaculture 2021, 545, 737204. [Google Scholar] [CrossRef]
- Sadowska, A.; Świderski, F. Sources, Bioavailability, and Safety of Silicon Derived from Foods and Other Sources Added for Nutritional Purposes in Food Supplements and Functional Foods. Appl. Sci. 2020, 10, 6255. [Google Scholar] [CrossRef]
- Zhong, T.; Xia, M.; Yao, Z.; Han, C. Chitosan/Silica nanocomposite preparation from shrimp shell and its adsorption performance for methylene blue. Sustainability 2022, 15, 47. [Google Scholar] [CrossRef]
- Ribault-Le, L. Silicio Orgánico de 5ª Generación y sus Aplicaciones en el Campo de la Salud; Francisco San Martín, Discovery De Salud nº 74: Madrid, Spain, 2005. [Google Scholar]
- Bashar, A.; Hasan, N.A.; Haque, M.M.; Rohani, M.F.; Hossain, M.S. Effects of dietary silica nanoparticle on growth performance, protein digestibility, hematology, digestive morphology, and muscle composition of Nile Tilapia, Oreochromis niloticus. Front. Mar. Sci. 2021, 8, 706179. [Google Scholar] [CrossRef]
- Farooq, M.A.; Dietz, K.J. Silicon as versatile player in plant and human biology: Overlooked and poorly understood. Front. Plant Sci. 2015, 6, 165767. [Google Scholar] [CrossRef]
- Zhang, Q.; Tao, Z.; Gao, Q.; Ma, Z. A review of the biogeochemical cycles of dissolved silicon in rivers. Adv. Earth Sci. 2015, 30, 50–59. [Google Scholar] [CrossRef]
- Sánchez-Muniz, F.J.; González-Muñoz, M.J.; Macho-González, A.B.J.; Garcimartín, A.; López-Oliva, E.; Santos-López, J.A.; Bastida, S. When silicon transmutes in gold. J. Negat. No Posit. Results 2020, 5, 202–211. [Google Scholar] [CrossRef]
- Garcimartín. A. Efectos Neuro y Hepatoprotector del Silicio y su Aplicación Como Ingrediente Funcional. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2016. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=119368 (accessed on 19 May 2024).
- Eleftheriadis, T.; Pissas, G.; Zarogiannis, S.; Liakopoulos, V.; Stefanidis, I. Crystalline silica activates the T-cell and the B-cell antigen receptor complexes and induces T-cell and B-cell proliferation. Autoimmunity 2019, 52, 136–143. [Google Scholar] [CrossRef]
- Zhang, R.; Bocharnikova, E.; Matichenkov, V. Microbial Growth in Shrimp Ponds as Influenced by Monosilicic and Polysilicic Acids. Silicon 2022, 14, 8887–8894. [Google Scholar] [CrossRef]
- Pieszka, M.; Bederska-Łojewska, D.; Szczurek, P.; Pieszka, M. The membrane interactions of nano-silica and its potential application in animal nutrition. Animals 2019, 9, 91041. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Shahin, K.; Mahmoud, S.M.; Altohamy, D.E.; Husseiny, W.A.; Mansour, D.A.; Shalaby, S.I.; Gaballa, M.M.S.; Shaalan, M.; Alkafafy, M.; et al. Silica nanoparticles are novel aqueous additive mitigating heavy metals toxicity and improving the health of African catfish, Clarias gariepinus. Aquat. Toxicol. 2022, 249, 106238. [Google Scholar] [CrossRef]
- Jarvie, H.P.; Al-Obaidi, H.; King, S.M.; Bowes, M.J.; Lawrence, M.J.; Drake, A.F.; Green, A.M.; Dobson, P.J. Fate of silica nanoparticles in simulated primary wastewater treatment. Environ. Sci. Technol. 2019, 43, 8622–8628. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Stass, A.; Horst, W.J. Apoplastic binding of aluminum is involved in silicon induced amelioration of aluminum toxicity in maize. Plant Physiol. 2004, 136, 3762–3770. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; AOAC: Gaithersburg, MD, USA, 2019; Available online: https://www.aoac.org/resources/official-methods-of-analysis-revisions-to-21st-edition/ (accessed on 25 January 2024).
- Ramanathan, G.; Ramalakshmi, P.; Gopperundevi, B.; Suresh, J.I. Production Characterization and Aqua Feed Supplementation of Astaxanthin from Halobacterium salinarium. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 56. Available online: https://www.ijcmas.com/vol-4-3/G (accessed on 2 April 2024).
- NRC. Nutrient Requirements of Fish and Shrimp; Animal Nutrition Series; National Research Council of the National Academies: Washington, DC, USA, 2011; pp. 70–71. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Bowers, G.N., Jr.; Horder, M.; Moss, D.W. Provisional recommendations on IFCC methods for the measurement of catalytic concentrations of enzymes. Part 2. IFCC method for aspartate aminotransferase. Clin. Chim. Acta 1976, 70, F19–F29. [Google Scholar] [CrossRef] [PubMed]
- Trinder, P. Determination of Glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Scheletter, G.; Nussel, E. Quantitative enzymatic Colorimetric determination of triglycerides in serum or plasma. Arbeitsmed Sozialmed Pr. 1975, 10, 25. [Google Scholar]
- Bernfeld, P. Amylase, alpha and beta. Methods Enzymol. 1955, 1, 149–151. Available online: https://doi.cir.nii.ac.jp/crid/1573668924362136704 (accessed on 5 May 2024).
- Walter, H.E. Proteases and their inhibitors. 2. 15. 2 Method with haemoglobin, casein, and azocoll as substrate. Methods Enzym. Anal. 1984, 270–277. Available online: https://cir.nii.ac.jp/crid/1571698599309827584 (accessed on 10 April 2024).
- Versaw, W.; Cuppett, S.L.; Winters, D.D.; Williams, L.E. An improved colorimetric assay for bacterial lipase in nonfat dry milk. J. Food Sci. 1989, 54, 232–254. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Giatsis, C.; Sipkema, D.; Smidt, H.; Verreth, J.; Verdegem, M. The Colonization dynamics of the gut microbiota in Tilapia larvae. PLoS ONE 2014, 9, e103641. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.R.; Cote, R.J.; Wu, L.; Liu, C.; Datar, R.; Shi, Y.; Liu, D.; Lim, H.; Taylor, C.R. DNA extraction from archival formalin-fixed, paraffin-embedded tissue sections based on the antigen retrieval principle: Heating under the influence of pH. J. Histochem. Cytochem. 2002, 50, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Pryer, K.M.; Miao, V.P.; Palmer, J.D.; Pryer, K.M.; Miao, V.P.; Palmer, J.D.; Miao, V.P.; Palmer, J.D.; Palmer, J.D. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J. Eukaryot. Microbiol. 1999, 46, 327–338. [Google Scholar] [CrossRef]
- Rudi, K.; Skulberg, O.M.; Larsen, F.; Jakobsen, K.S. Strain characterization and classification of oxyphotobacteria in clone cultures on the basis of 16S rRNA sequences from the variable regions V6, V7, and V8. Appl. Environ. Microbiol. 1997, 63, 2593–2599. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Ruangroupan, L.; Kitao, T.; Yoshida, T. Protective efficacy of Aeromonas hydrophila vaccines in Nile Tilapia. Vet. Immunol. Immunopathol. 1986, 12, 345–350. [Google Scholar] [CrossRef]
- Chepeleva, E.; Kozyr, K.; Zubarev, D.; Kudriashov, A.; Kretov, E.; Vasilieva, M.; Kashin, O.; Lotkov, A.; Slabodchikov, V.; Sergeevichev, D. Blood plasma cytokines releasing after implantation of self-expanding nitinol stents modified with silicon in experimental animals. AIP Conf. Proc. 2018, 2051, 020048. [Google Scholar] [CrossRef]
- Hasan, M.M.; Haque, M.M.; Hasan, N.A.; Bashar, A.; Ahammad, A.S.; Hossain, M.T. Assessing the impacts of zeolite on water quality, growth performance, heavy metal content and health condition of farmed Tilapia (Oreochromis niloticus). Aquac. Rep. 2023, 31, 101678. [Google Scholar] [CrossRef]
- Diab, A.M.; Eldeghaidy, E.E.; Abo-Raya, M.H.; Shukry, M.; Abdeen, A.; Ibrahim, S.F.; Fericean, L.; Abdo, M.; Khalafalla, M.M. Assessment of Growth-Related Parameters, Immune-Biochemical Profile, and Expression of Selected Genes of Red Tilapia Fed with Roselle Calyces (Hibiscus sabdariffa) Extract. Fishes 2023, 8, 172. [Google Scholar] [CrossRef]
- EL-Erian, M.A.; Ibrahim, M.S.; Salem, S.M.R.; Mohammady, E.Y.; El-Haroun, E.R.; Hassaan, M.S. Evaluation of Different Copper Sources in Nile Tilapia Diets: Growth, Body Indices, Hematological Assay, Plasma Metabolites, Immune, Anti-Oxidative Ability, and Intestinal Morphometric Measurements. Biol. Trace Elem. Res. 2023, 201, 4900–4911. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhamed, M.; Allm, S.M.; El-Deeb, K.; Metwalli, A.A.; Saleh, H.H.; Abdel-Aziz, M.F. Applying Nano-technology in Tilapia Nutrition: Influence of Iron and Zinc nanoparticles as dietary supplementary on biological performance and body composition of Oreochromis niloticus fry. Mediterr. Aquac. J. 2021, 8, 30–41. [Google Scholar] [CrossRef]
- Alandiyjany, M.N.; Kishawy, A.T.Y.; Hassan, A.A.; Eldoumani, H.; Elazab, S.T.; El-Mandrawy, S.A.M.; Saleh, A.A.; ElSawy, N.A.; Attia, Y.A.; Arisha, A.H.; et al. Nano-silica and magnetized-silica mitigated lead toxicity: Their efficacy on bioaccumulation risk, performance, and apoptotic targeted genes in Nile Tilapia (Oreochromis niloticus). Aquat. Toxicol. 2021, 242, 106054. [Google Scholar] [CrossRef]
- Shukry, M.; Abd El-Kader, M.F.; Hendam, B.M.; Dawood, M.A.; Farrag, F.A.; Aboelenin, S.M.; Abdel-Latif, H.M. Dietary Aspergillus oryzae modulates serum biochemical indices, immune responses, oxidative stress, and transcription of HSP70 and cytokine genes in Nile Tilapia exposed to salinity stress. Animals 2021, 11, 1621. [Google Scholar] [CrossRef]
- Lin, B.; Xi, Z.; Zhang, Y.; Zhang, H. Primary study on the hepatotoxicity and nephrotoxicity of rats induced by three kinds of nanomaterials. Wei Sheng Yan Jiu = J. Hyg. Res. 2008, 37, 651–653. Available online: https://europepmc.org/article/med/19238994 (accessed on 22 February 2024).
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Zhou, W.H.; Wu, C.C.; Limbu, S.M.; Li, R.X.; Chen, L.Q.; Qiao, F.; Luo, Y.; Zhang, M.Y.; Han, T.; Du, Z.Y. More simple more worse: Simple carbohydrate diets cause alterations in glucose and lipid metabolism in Nile Tilapia (Oreochromis niloticus). Aquaculture 2022, 550, 737857. [Google Scholar] [CrossRef]
- Martínez-Porchas, M.; Martínez-Cordova, L.R.; Ramos-Enriquez, R. Cortisol and glucose: Reliable indicator of fish stress? Pan-Am. J. Aquat. Sci. 2009, 4, 158–178. Available online: https://panamjas.org/pdf_artigos/panamjas_4(2)_158-178.pdf (accessed on 8 April 2024).
- Kurniaji, A. Blood glucose of Tilapia fish Oreochromis mossambica as a water bioindicator in the downstream of brantas waters, east java. AACL Bioflux 2021, 14, 2040–2049. Available online: http://bioflux.com.ro/docs/2021.2040-2049.pdf (accessed on 7 April 2024).
- Hernández-Martín, M.; Macho-González, A.; Garcimartín, A.; López-Oliva, M.E.; Bocanegra, A.; Redondo-Castillejo, R.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Silicon-enriched meat positively improves plasma lipidaemia and lipoproteinaemia, LDLr, and insulin capability and the signalling pathway induced by an atherogenic diet in late-stage type 2 diabetes mellitus rats. Food Funct. 2024, 15, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Martín, M.; Bocanegra, A.; Redondo-Castillejo, R.; Macho-González, A.; Sánchez-Muniz, F.J.; Benedí, J.; Bastida, S.; García-Fernández, R.A.; Garcimartín, A.; López-Oliva, M.E. Could Duodenal Molecular Mechanisms be Involved in the Hypocholesterolemic Effect of Silicon Used as Functional Ingredient in Late-Stage Type 2 Diabetes Mellitus? Mol. Nutr. Food Res. 2022, 66, 2200104. [Google Scholar] [CrossRef]
- Peluso, M.R.; Schneeman, B.O. A food-grade silicon dioxide is hypocholesterolemic in the diet of cholesterol-fed rats. J. Nutr. 1994, 124, 853–860. [Google Scholar] [CrossRef]
- Delgado, M.J.; Cerdá-Reverter, J.M.; Soengas, J.L. Hypothalamic Integration of Metabolic, Endocrine, and Circadian Signals in Fish: Involvement in the Control of Food Intake. Front. Neurosci. 2017, 11, 354. [Google Scholar] [CrossRef]
- Rønnestad, I.; Gomes, A.S.; Murashita, K.; Angotzi, R.; Jönsson, E.; Volkoff, H. Appetite-Controlling Endocrine Systems in Teleosts. Front. Endocrinol. 2017, 8, 73. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; Palma, J.; Shawer, E.E.; El-Haroun, E. The effect of dietary sericite on growth performance, digestive enzymes activity, gut microbiota and haematological parameters of Nile Tilapia, Oreochromis niloticus (L.) fingerlings. Anim. Feed. Sci. Technol. 2020, 262, 114400. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Nssar, K.M.; Mohammady, E.Y.; Amin, A.; Tayel, S.I.; El-Haroun, E.R. Nano-zeolite efficiency to mitigate the aflatoxin B1 (AFB1) toxicity: Effects on growth, digestive enzymes, antioxidant, DNA damage and bioaccumulation of AFB1 residues in Nile Tilapia (Oreochromis niloticus). Aquaculture 2020, 523, 735123. [Google Scholar] [CrossRef]
- Abdel Rahman, A.N.; Elsheshtawy, H.M.; Yassin, E.M.M.; Omran, A.; Hashem, M.A.; Eltanahy, A.; Khamis, T.; Ismail, S.H.; Yousef, M.; Mahboub, H.H. Hematological, immuno-antioxidant disruptions, and genes downregulation induced by Aeromonas veronii challenge in Clarias gariepinus: The ameliorative role of silica nanoparticles. Fish Shellfsh Immunol. 2023, 18, 108842. [Google Scholar] [CrossRef]
- Wu, T.; Sun, L.C.; Du, C.H.; Cai, Q.F.; Zhang, Q.B.; Su, W.J.; Cao, M.J. Identification of pepsinogens and pepsins from the stomach of European eel (Anguilla anguilla). Food Chem. 2009, 115, 137–142. [Google Scholar] [CrossRef]
- Fuentes-Quesada, J.P.; Viana, M.T.; Rombenso, A.N.; Guerrero-Rentería, Y.; Nomura-Solís, M.; Gomez-Calle, V.; Lazo, J.P.; Mata-Sotres, J.A. Enteritis induction by soybean meal in Totoaba macdonaldi diets: Effects on growth performance, digestive capacity, immune response and distal intestine integrity. Aquaculture 2018, 495, 78–89. [Google Scholar] [CrossRef]
- Dawood, M.A.; Moustafa, E.M.; Gewaily, M.S.; Abdo, S.E.; AbdEl-Kader, M.F.; SaadAllah, M.S.; Hamouda, A.H. Ameliorative effects of Lactobacillus plantarum L-137 on Nile Tilapia (Oreochromis niloticus) exposed to deltamethrin toxicity in rearing water. Aquat. Toxicol. 2020, 219, 105377. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Naby, A.S.; Al-Sagheer, A.A.; Negm, S.S.; Naiel, M.A.E. Dietary combination of chitosan nanoparticle and thymol affects feed utilization, digestive enzymes, antioxidant status, and intestinal morphology of Oreochromis niloticus. Aquaculture 2020, 515, 734577. [Google Scholar] [CrossRef]
- Cerezuela, R.; Fumanal, M.; Tapia-Paniagua, S.T.; Meseguer, J.; Morinigo, M.A.; Esteban, M.A. Histological alterations and microbial ecology of the intestine’ in gilthead seabream (Sparus aurata L.) fed dietary probiotics and microalgae. Cell Tissue Res. 2012, 350, 477–489. [Google Scholar] [CrossRef]
- Oguz, A.R.A. histological study of the kidney structure of Van fish (Alburnus tarichi) acclimated to highly alkaline water and freshwater. Mar. Freshw. Behav. Physiol. 2015, 48, 135–144. [Google Scholar] [CrossRef]
- Giorgini, E.; Randazzo, B.; Gioacchini, G.; Cardinaletti, G.; Vaccari, L.; Tibaldi, E.; Olivotto, I. New insights on the macromolecular building of rainbow trout (O. mykiss) intestine: FTIR Imaging and histological correlative study. Aquaculture 2018, 497, 1–9. [Google Scholar] [CrossRef]
- Martinez-Porchas, M.; Preciado-Álvarez, A.; Vargas-Albores, F.; Gracia-Valenzuela, M.H.; Cicala, F.; Martinez-Cordova, L.R.; Medina-Félix, D.; Garibay-Valdez, E. Microbiota plasticity in Tilapia gut revealed by meta-analysis evaluating the effect of probiotics, prebiotics, and biofloc. PeerJ 2023, 11, e16213. [Google Scholar] [CrossRef]
- Hennersdorf, P.; Kleinertz, S.; Theisen, S.; Abdul-Aziz, M.A.; Mrotzek, G.; Palm, H.W.; Saluz, H.P. Microbial diversity and parasitic load in tropical fish of different environmental conditions. PLoS ONE 2016, 11, e0151594. [Google Scholar] [CrossRef]
- Llewellyn, M.S.; McGinnity, P.; Dionne, M.; Letourneau, J.; Thonier, F.; Carvalho, G.R.; Creer, S.; Derome, N. The biogeography of the atlantic salmon (Salmo salar) gut microbiome. ISME J. 2016, 10, 1280–1284. [Google Scholar] [CrossRef]
- Kamble, M.T.; Chaiyapechara, S.; Salin, K.R.; Bunphimpapha, P.; Chavan, B.R.; Bhujel, R.C.; Medhe, S.V.; Kettawan, A.; Thiyajai, P.; Thompson, K.D.; et al. Guava and Star gooseberry leaf extracts improve growth performance, innate immunity, intestinal microbial community, and disease resistance in Nile Tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Aquacult. Rep. 2024, 35, 101947. [Google Scholar] [CrossRef]
- Wang, J.; Fan, H.; Han, Y.; Zhao, J.; Zhou, Z. Characterization of the microbial communities along the gastrointestinal tract of sheep by pyrosequencing analysis. Asian-Australas J. Anim. Sci. 2017, 30, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Van-Kessel, M.; Dutilh, B.E.; Neveling, K.; Kwint, M.P.; Veltman, J.A.; Flik, G.; Jetten, M.S.; Klaren, P.H.; Camp, H.J. Pyrosequencing of 16S rRNA gene amplicons to study the microbiota in the gastrointestinal tract of carp (Cyprinus carpio L.). AMB Express 2011, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.M.; Mohammed, H.H.; Arias, C.R. Characterization of the gut microbiota of three commercially valuable warmwater fish species. J. Appl. Microbiol. 2014, 116, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Ofek, T.; Lalzar, M.; Laviad-Shitrit, S.; Izhaki, I.; Halpern, M. Comparative study of intestinal microbiota composition of six edible fish species. Front. Microbiol. 2021, 604, 760266. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ma, D.Y.; Zhai, S.W. Revealing the difference of intestinal microbiota composition of cultured European eels (Anguilla anguilla) with different growth rates. Isr. J. Aquacult-Bamid. 2020, 72, 959575. [Google Scholar] [CrossRef]
- Birg, A.; Ritz, N.L.; Lin, H.C. The Unknown Effect of Antibiotic-Induced Dysbiosis on the Gut Microbiota. Microbiome Metabolome Diagn. Ther. Other Strateg. Appl. 2019, 20, 195–200. [Google Scholar] [CrossRef]
- Lowrey, L.; Woodhams, D.C.; Tacchi, L.; Salinas, I. Topographical mapping of the rainbow trout (Oncorhynchus mykiss) microbiome reveals a diverse bacterial community with antifungal properties in the skin. Appl. Environ. Microbiol. 2015, 81, 6915–6925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ding, L.; Yu, Y.; Kong, W.; Yin, Y.; Huang, Z.; Zhang, X.; Xu, Z. The Change of Teleost Skin Commensal Microbiota Is Associated with Skin Mucosal Transcriptomic Responses During Parasitic Infection by Ichthyophthirius multifillis. Front. Immunol. 2018, 9, 2972. [Google Scholar] [CrossRef]
- Wang, A.; Ran, C.; Ringø, E.; Aug, Z.Z. Progress in fish gastrointestinal microbiota research. Rev. Aquac. 2018, 10, 626–640. [Google Scholar] [CrossRef]
- Asha, A.A.; Haque, M.M.; Hossain, M.K.; Hasan, M.M.; Bashar, A.; Hasan, M.Z.; Shohan, M.H.; Farin, N.N.; Schneider, P.; Bablee, A.L. Effects of Commercial Probiotics on the Growth Performance, Intestinal Microbiota and Intestinal Histomorphology of Nile Tilapia (Oreochromis niloticus) Reared in Biofloc Technology (BFT). Biology 2024, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Terova, G.; Rimoldi, S.; Corà, S.; Bernardini, G.; Gornati, R.; Saroglia, M. Acute and chronic hypoxia affects HIF-1α mRNA levels in sea bass (Dicentrarchus labrax). Aquaculture 2008, 279, 150–159. [Google Scholar] [CrossRef]
- Herbert, N.A.; Steffensen, J.F. The response of Atlantic cod, Gadus morhua, to progressive hypoxia: Fish swimming speed and physiological stress. Mar. Biol. 2005, 147, 1403–1412. [Google Scholar] [CrossRef]
- Roesner, A.; Hankeln, T.; Burmester, T. Hypoxia induces a complex response of globin expression in zebrafish (Danio rerio). J. Exp. Biol. 2006, 209, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Bacca, H.; Huvet, A.; Fabioux, C.; Daniel, J.Y.; Delaporte, M.; Pouvreau, S.; Van Wormhoudt, A.; Moal, J. Molecular cloning and seasonal expression of oyster glycogen phosphorylase and glycogen synthase genes. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 140, 635–646. [Google Scholar] [CrossRef]
- Bartrons, R.; Caro, J. Hypoxia, glucose metabolism and the Warburg’s effect. J. Bioenerg. Biomembr. 2007, 39, 223–229. [Google Scholar] [CrossRef]
| Ingredients (g·kg−1) | Organic Silicon Levels (mg·kg−1) | Cost (USD·kg−1) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | ||
| Fish meal 1 | 245 | 245 | 245 | 245 | 245 | 245 | 1.91 |
| Soybean meal 2 | 260 | 260 | 260 | 260 | 260 | 260 | 0.64 |
| Wheat flour 3 | 210 | 210 | 210 | 210 | 210 | 210 | 0.50 |
| Corn meal 4 | 209.8 | 208.8 | 207.8 | 206.8 | 205.8 | 204.8 | 0.44 |
| Organic silicon 5 (mg·kg−1) | 0 | 10 | 20 | 30 | 40 | 50 | 22.50 |
| Vegetable oil 6 | 10 | 10 | 10 | 10 | 10 | 10 | 0.80 |
| Fish oil 7 | 15 | 15 | 15 | 15 | 15 | 15 | 1.20 |
| Gelatine 8 | 19 | 19 | 19 | 19 | 19 | 19 | 0.50 |
| Mineral premixes 9,10 | 10 | 10 | 10 | 10 | 10 | 10 | 4.75 |
| Vitamin premixes 11,12 | 20 | 20 | 20 | 20 | 20 | 20 | 4.75 |
| Ascorbic acid | 1 | 1 | 1 | 1 | 1 | 1 | 21.64 |
| Antioxidant BHT 13 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 13.50 |
| Real Proximal Composition (g·kg−1, as Fed Basis) 1 | Organic Silicon Levels (mg·kg−1) | |||||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | |
| Dry matter (DM) | 925.0 | 927.5 | 936.5 | 929.5 | 929.1 | 929.0 |
| Crude protein | 323.8 | 323.0 | 322.2 | 311.6 | 320.1 | 319.5 |
| Crude lipid (CL) | 57.5 | 58.2 | 57.9 | 57.5 | 57.1 | 56.8 |
| Crude fibre | 15.1 | 17.4 | 18.3 | 20.4 | 25.1 | 27.3 |
| Ash | 102.9 | 103.6 | 106.1 | 108.4 | 109.3 | 111.5 |
| Nitrogen-free extract (NFE) | 425.7 | 425.3 | 432.0 | 431.6 | 417.5 | 413.9 |
| Digestible energy (MJ kg−1 food) | 12.29 | 12.28 | 12.29 | 12.29 | 12.3 | 12.30 |
| PC ED−1 (mg PC MJ−1) | 26.35 | 26.29 | 26.22 | 25.35 | 26.03 | 25.97 |
| Calculated essential amino acids (%, as fed basis) | ||||||
| Threonine | 1.45 | 1.45 | 1.44 | 1.43 | 1.42 | 1.42 |
| Valine | 1.63 | 1.63 | 1.61 | 1.59 | 1.58 | 1.56 |
| Methionine | 0.73 | 0.73 | 0.73 | 0.73 | 0.73 | 0.73 |
| Isoleucine | 1.32 | 1.32 | 1.32 | 1.31 | 1.31 | 1.30 |
| Leucine | 2.37 | 2.37 | 2.36 | 2.35 | 2.34 | 2.33 |
| Lysine | 1.85 | 1.85 | 1.83 | 1.81 | 1.80 | 1.79 |
| Histidine | 0.85 | 0.85 | 0.84 | 0.84 | 0.84 | 0.84 |
| Arginine | 1.40 | 1.40 | 1.39 | 1.39 | 1.38 | 1.38 |
| Tryptophan | 0.33 | 0.33 | 0.33 | 0.32 | 0.32 | 0.32 |
| Phenylalanine | 1.21 | 1.21 | 1.20 | 1.19 | 1.18 | 1.18 |
| Productive Parameters | Organic Silicon Levels (mg·kg−1) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | ||
| IW (g) | 7.50 ± 3.35 a | 7.51 ± 3.53 a | 7.49 ± 3.14 a | 7.49 ± 3.09 a | 7.51 ± 3.47 a | 7.50 ± 3.79 a | 0.9999 |
| FW (g) | 25.05 ± 5.05 b | 25.57 ± 6.36 b | 26.38 ± 5.85 ab | 25.46 ± 6.79 b | 28.71 ± 6.94 ab | 29.56 ± 7.30 a | 0.0026 |
| FL (cm) | 11.94 ± 1.00 b | 11.93 ± 1.24 b | 12.25 ± 1.22 b | 11.93 ± 1.32 b | 12.19 ± 1.33 b | 13.39 ± 1.28 a | 0.0001 |
| WG (g) | 17.55 ± 6.95 b | 18.06 ± 0.89 b | 18.88 ± 0.12 ab | 17.97 ± 0.28 b | 21.20 ± 0.51 a | 22.06 ± 0.96 a | 0.0430 |
| SGR | 2.15 ± 0.13 c | 2.19 ± 0.17 c | 2.26 ± 0.17 b | 2.19 ± 0.09 c | 2.35 ± 0.19 a | 2.42 ± 0.10 a | 0.0335 |
| CF | 1.47 ± 0.08 b | 1.51 ± 0.03 b | 1.43 ± 0.04 b | 1.50 ± 0.05 b | 1.53 ± 0.02 b | 1.21 ± 0.02 a | 0.0001 |
| FCF | 1.48 ± 0.09 a | 1.44 ± 0.01 ab | 1.41 ± 0.07 abc | 1.46 ± 0.03 ab | 1.32 ± 0.03 bc | 1.27 ± 0.04 c | 0.0030 |
| SR (%) | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a | 0.4910 |
| Blood Biochemistry | Organic Silicon Levels (mg·kg−1) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | ||
| ALP (UL−1) | 41.50 ± 1.50 ab | 39.50 ± 0.05 b | 38.00 ± 3.00 b | 41.67 ± 0.57 ab | 45.00 ± 3.26 a | 42.50 ± 2.04 ab | 0.0460 |
| AST (UL−1) | 140.45 ± 4.25 a | 96.32 ± 3.22 b | 92.46 ± 2.46 bc | 107.30 ± 5.21 b | 86.45 ± 1.75 c | 89.94 ± 2.43 c | 0.0064 |
| Glucose (mg dL−1) | 73.00 ± 3.00 b | 67.33 ± 2.52 bc | 62.00 ± 4.00 c | 63.00 ± 4.00 c | 77.33 ± 0.58 a | 79.00 ± 1.00 a | 0.0001 |
| Cholesterol (mg dL−1) | 79.33 ± 4.51 a | 77.33 ± 2.52 a | 67.33 ± 1.53 b | 66.33 ± 1.53 b | 67.33 ± 2.52 b | 69.00 ± 5.57 b | 0.0186 |
| Triglycerides (mg dL−1) | 204.00 ± 4.00 a | 190.00 ± 5.00 b | 187.00 ± 3.00 b | 195.33 ± 0.58 ab | 193.33 ± 3.51 ab | 192.33 ± 5.86 b | 0.0044 |
| Enzyme Activity (U mg−1 of Protein) | Organic Silicon Levels (mg·kg−1) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | ||
| Proteases | 37.84 ± 1.80 a | 42.09 ± 2.80 a | 51.96 ± 3.10 b | 54.03 ± 2.70 b | 52.91 ± 2.60 b | 38.94 ± 2.20 a | 0.0001 |
| Lipases | 35.92 ± 2.70 ab | 34.01 ± 3.70 a | 39.62 ± 1.50 b | 41.39 ± 1.60 b | 38.84 ± 1.20 b | 38.62 ± 1.00 b | 0.0049 |
| Amylases | 71.00 ± 2.20 ab | 71.72 ± 2.20 ab | 66.89 ± 3.00 a | 68.34 ± 1.40 a | 75.45 ± 1.90 bc | 80.24 ± 2.00 c | 0.0002 |
| Intestinal Morphohistology | Organic Silicon Levels (mg·kg−1) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | ||
| SM (µm) | 16.89 ± 1.35 cd | 16.30 ± 0.06 d | 17.47 ± 0.89 bcd | 19.85 ± 0.49 ab | 19.45 ± 1.00 abc | 21.09 ± 1.64 a | 0.0006 |
| EH (µm) | 14.75 ± 1.50 b | 16.20 ± 0.05 ab | 17.75 ± 0.50 ab | 19.59 ± 0.37 a | 17.44 ± 2.10 ab | 20.09 ± 2.30 a | 0.0055 |
| MFM (µm) | 259.37 ± 2.00 e | 283.17 ± 2.14 d | 294.08 ± 2.68 c | 342.56 ± 2.41 a | 309.00 ± 0.08 b | 304.57 ± 1.5 b | 0.0001 |
| NMF | 37.36 ± 1.00 bc | 32.43 ± 0.64 d | 39.61 ± 1.07 bc | 35.32 ± 2.88 cd | 40.41 ± 1.00 ab | 44.52 ± 2.44 a | 0.0001 |
| Alpha Diversity | Organic Silicon Levels (mg·kg−1) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | ||
| OTUs | 384 ± 14.34 c | 387 ± 19.62 c | 433 ± 13.63 b | 457 ± 21.51 b | 403 ± 10.30 b | 533 ± 15.29 a | 0.037 |
| Shannon (H′) | 1.86 ± 0.34 b | 1.01 ± 0.48 c | 1.77 ± 0.23 b | 2.46 ± 0.28 a | 1.84 ± 0.36 b | 2.84 ± 0.51 a | 0.000 |
| Simpson (1-λ) | 0.07 ± 0.008 b | 0.06 ± 0.005 b | 0.07 ± 0.033 b | 0.09 ± 0.005 a | 0.08 ± 0.028 ab | 0.09 ± 0.009 a | 0.002 |
| Chao-1 | 397 ± 11.2 c | 405 ± 10.57 c | 468 ± 18.43 b | 482 ± 13.51 b | 489 ± 8.97 b | 542 ± 17.52 a | 0.007 |
| RMS | 110,335 | 269,430 | 294,902 | 147,553 | 187,822 | 172,741 | |
| Hypoxia Stress Response (%) | Organic Silicon Levels (mg·kg−1) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | ||
| Mortality rate | 77.78 d | 38.89 c | 33.33 bc | 38.89 c | 27.78 ab | 16.67 a | 0.000 |
| RPL | - | 50.00 c | 57.14 bc | 50.00 c | 64.29 ab | 78.57 a | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Martínez, Y.; Valensuela-Barros, H.A.; Cruz-Quintana, Y.; Botello-León, A.; Muñoz-Mestanza, R.D.; Orellana-Castro, G.L.; Angulo, C. Effect of Dietary Supplementation with Organic Silicon on the Growth Performance, Blood Biochemistry, Digestive Enzymes, Morphohistology, Intestinal Microbiota and Stress Resistance in Juvenile Hybrid Tilapia (Oreochromis mossambicus × Oreochromis niloticus). Biology 2024, 13, 531. https://doi.org/10.3390/biology13070531
Méndez-Martínez Y, Valensuela-Barros HA, Cruz-Quintana Y, Botello-León A, Muñoz-Mestanza RD, Orellana-Castro GL, Angulo C. Effect of Dietary Supplementation with Organic Silicon on the Growth Performance, Blood Biochemistry, Digestive Enzymes, Morphohistology, Intestinal Microbiota and Stress Resistance in Juvenile Hybrid Tilapia (Oreochromis mossambicus × Oreochromis niloticus). Biology. 2024; 13(7):531. https://doi.org/10.3390/biology13070531
Chicago/Turabian StyleMéndez-Martínez, Yuniel, Helen A. Valensuela-Barros, Yanis Cruz-Quintana, Aroldo Botello-León, Roberto D. Muñoz-Mestanza, Grace L. Orellana-Castro, and Carlos Angulo. 2024. "Effect of Dietary Supplementation with Organic Silicon on the Growth Performance, Blood Biochemistry, Digestive Enzymes, Morphohistology, Intestinal Microbiota and Stress Resistance in Juvenile Hybrid Tilapia (Oreochromis mossambicus × Oreochromis niloticus)" Biology 13, no. 7: 531. https://doi.org/10.3390/biology13070531
APA StyleMéndez-Martínez, Y., Valensuela-Barros, H. A., Cruz-Quintana, Y., Botello-León, A., Muñoz-Mestanza, R. D., Orellana-Castro, G. L., & Angulo, C. (2024). Effect of Dietary Supplementation with Organic Silicon on the Growth Performance, Blood Biochemistry, Digestive Enzymes, Morphohistology, Intestinal Microbiota and Stress Resistance in Juvenile Hybrid Tilapia (Oreochromis mossambicus × Oreochromis niloticus). Biology, 13(7), 531. https://doi.org/10.3390/biology13070531










