Soil Microbial Community Structure and Carbon Stocks Following Fertilization with Organic Fertilizers and Biological Inputs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Sampling
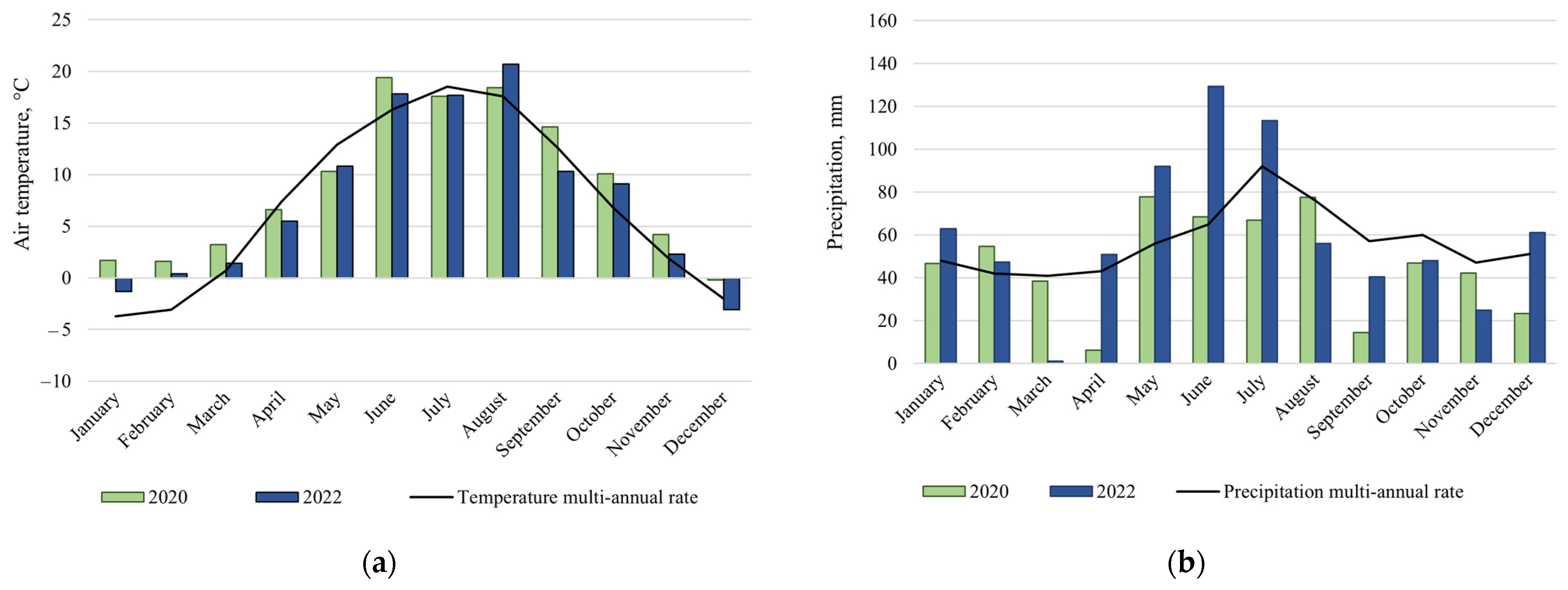
2.2. Climate Conditions
2.3. Soil Agrochemical Analysis
2.4. Soil DNA Extraction and Microbiomic Analysis
2.5. Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
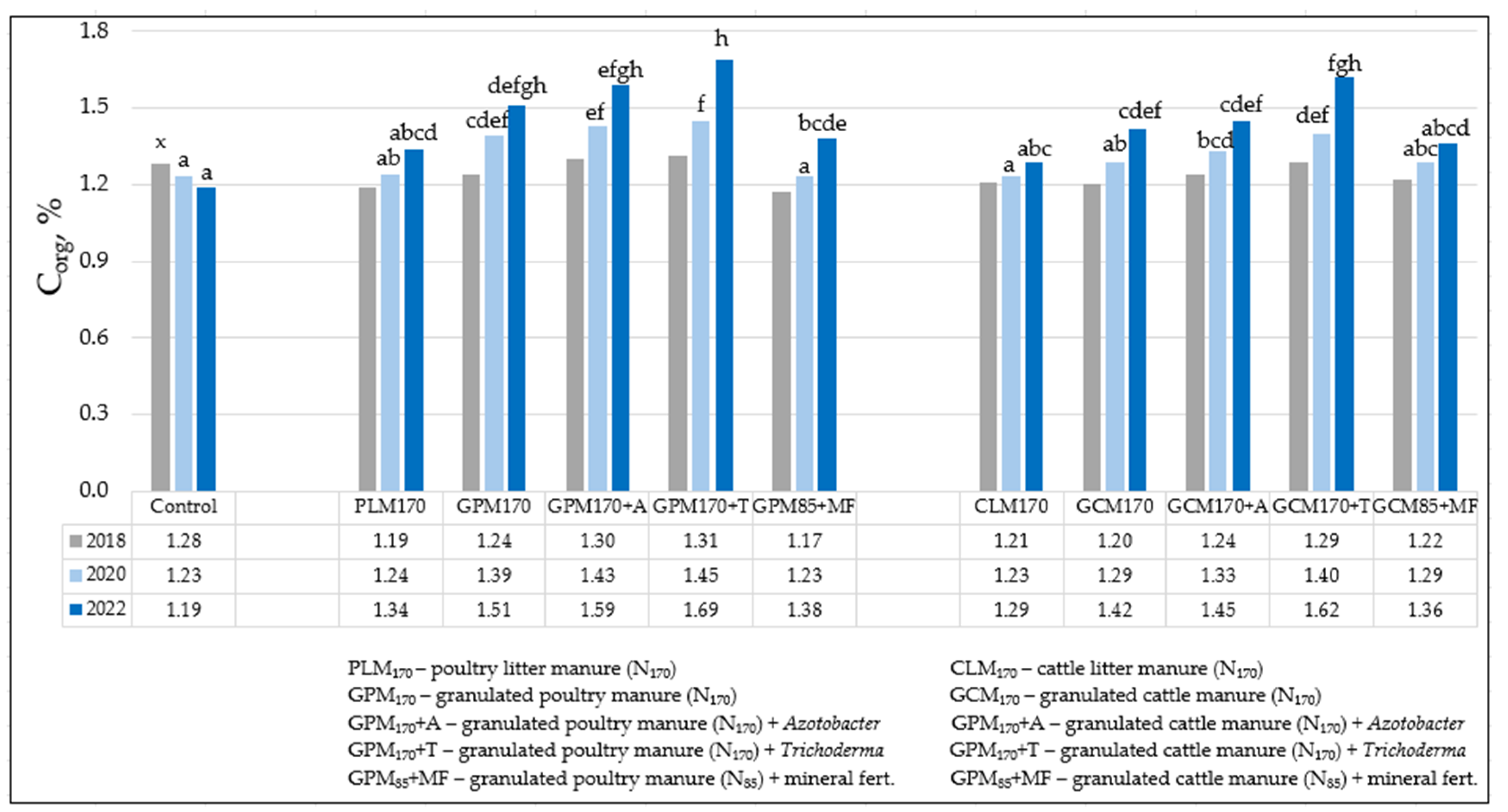
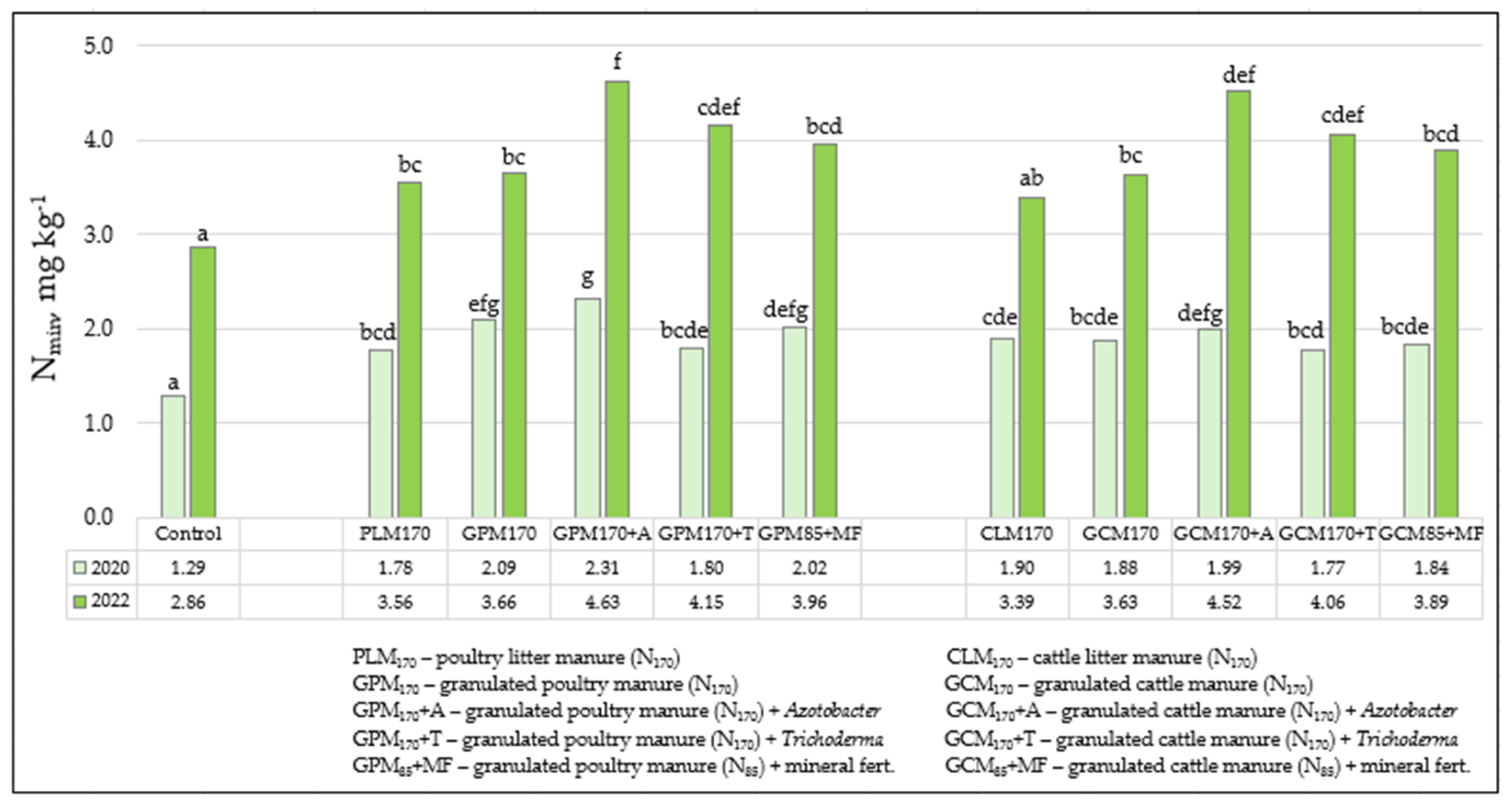
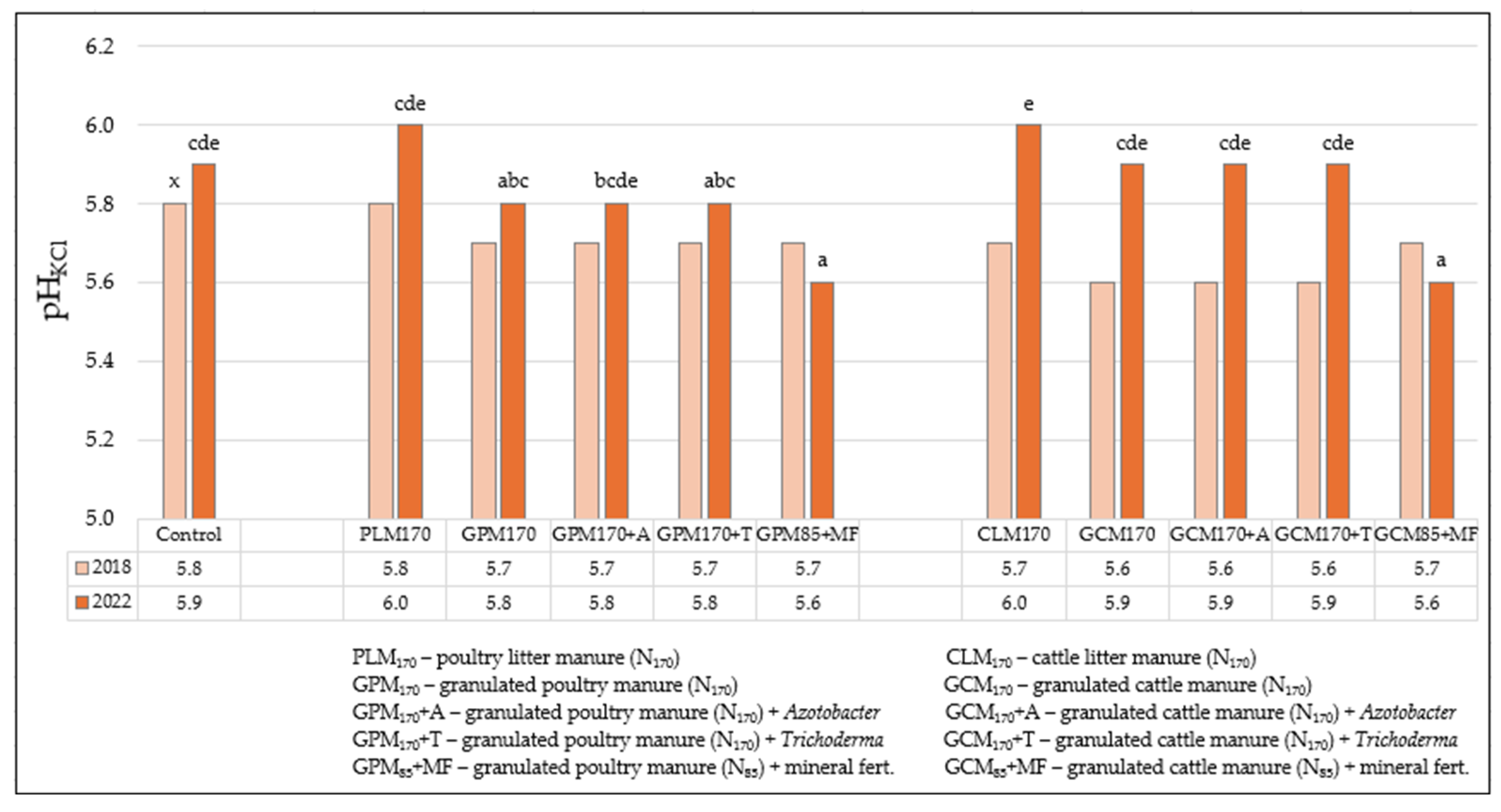
3.1. Soil Agrochemical Analysis
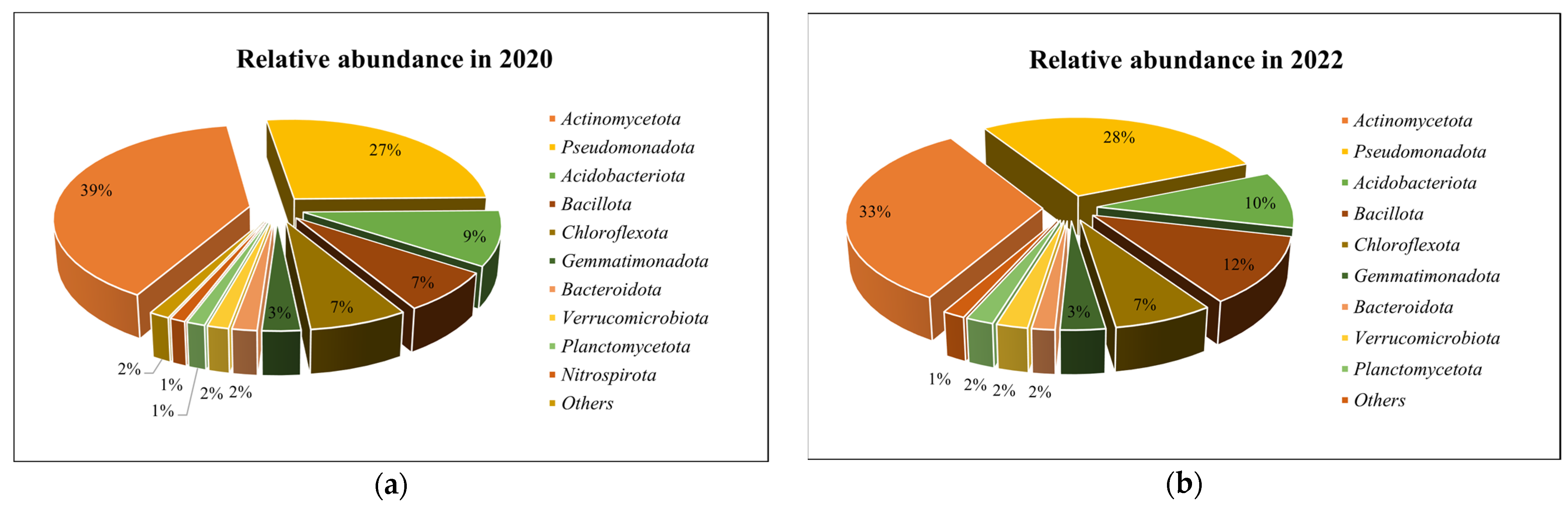
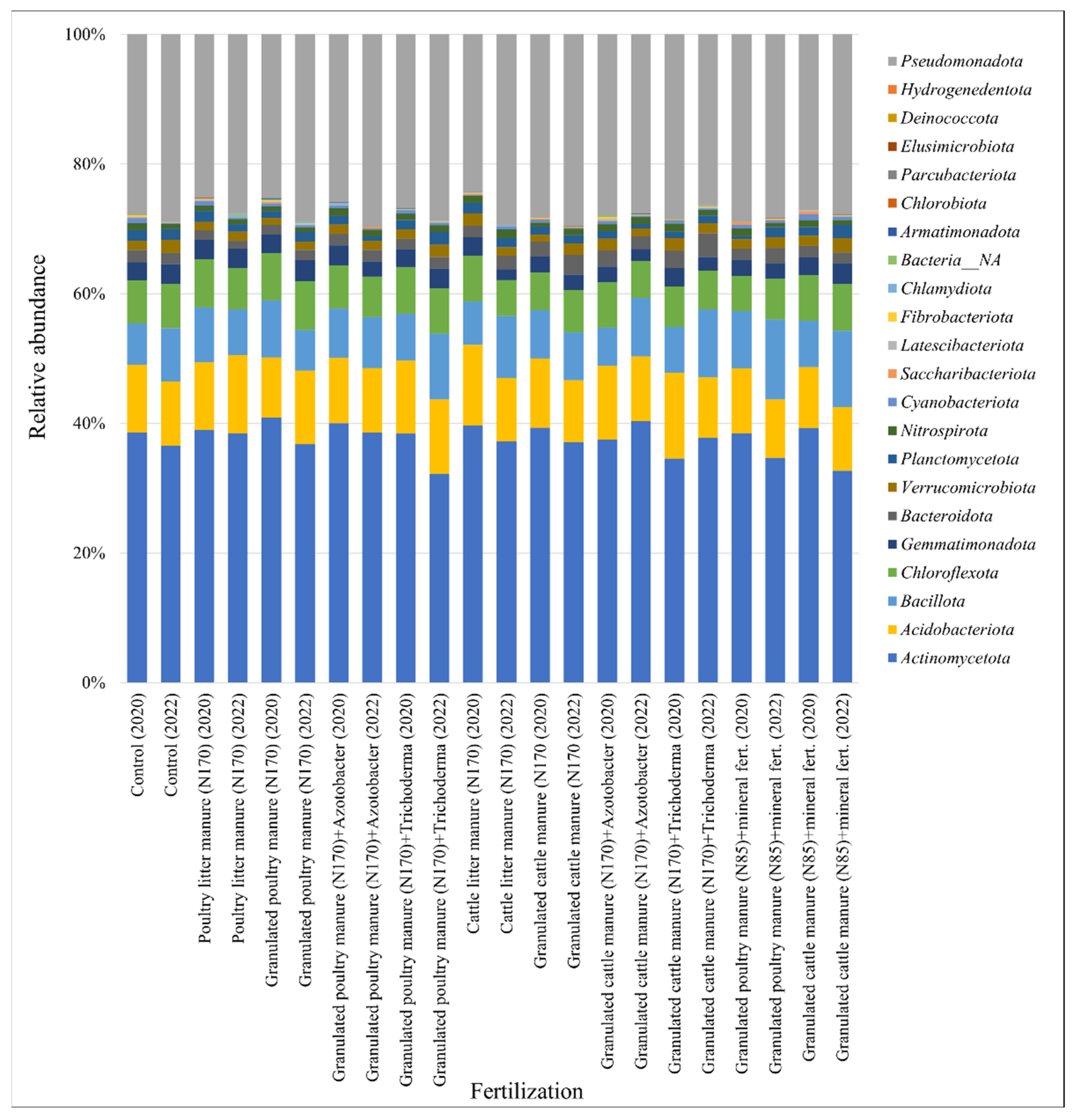
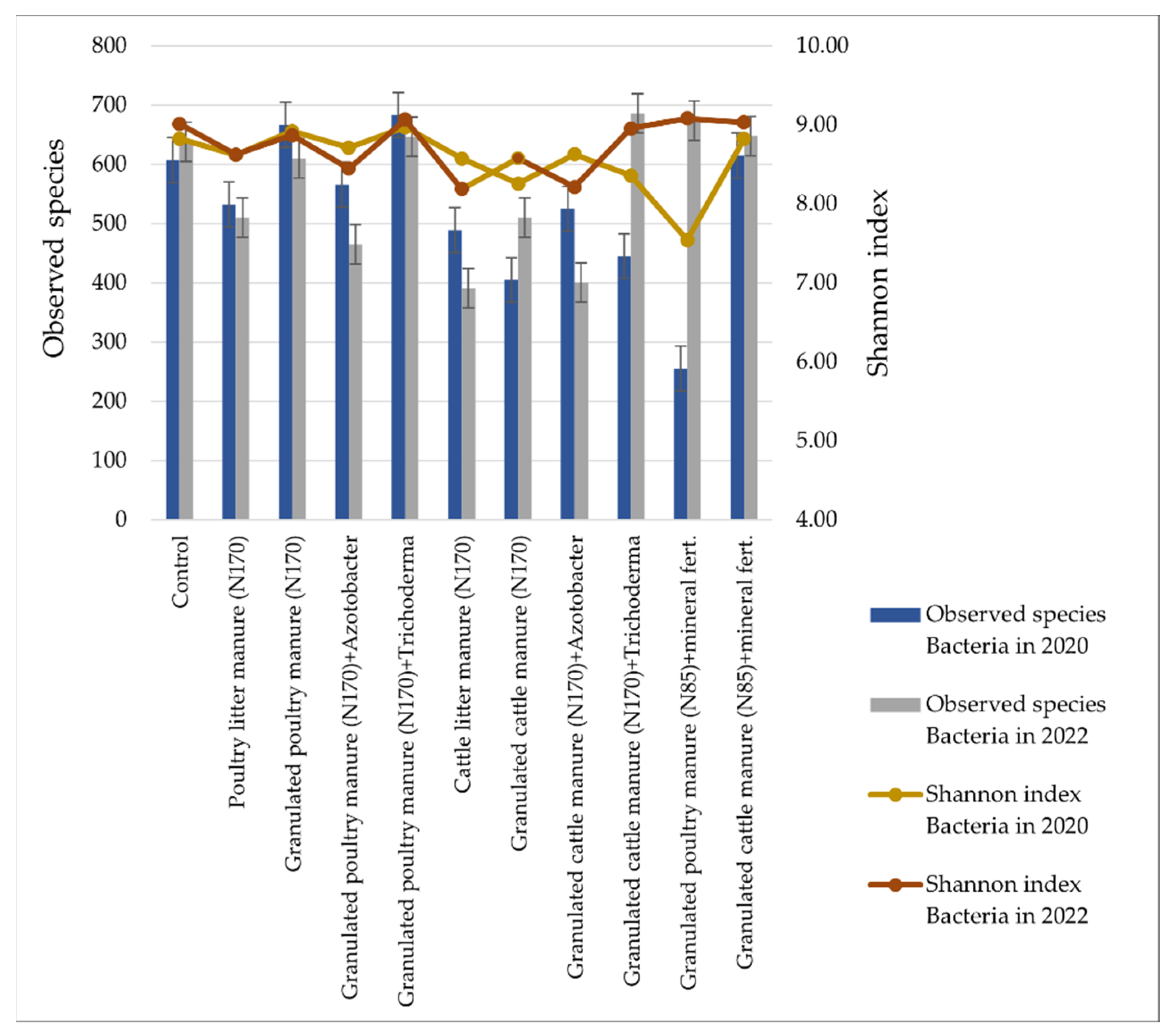
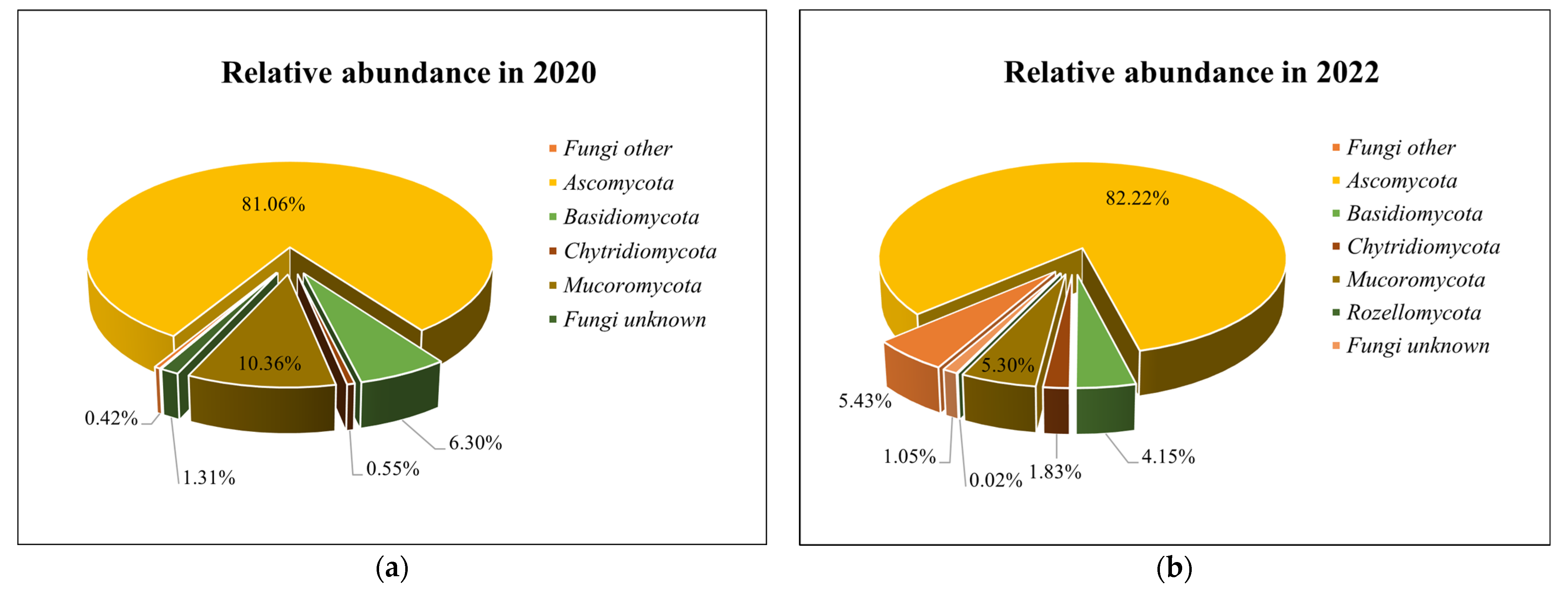
3.2. Soil Microbiomic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rehman, A.B. Importance of agriculture in life. Int. Rev. Agric. World 2022, 22, 59615. [Google Scholar]
- Robertson, G.P.; Gross, K.L.; Hamilton, S.K.; Landis, D.A.; Schmidt, T.M.; Snapp, S.S.; Swinton, S.M. Farming for Ecosystem Services: An Ecological Approach to Production Agriculture. BioScience 2014, 64, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Tahat, M.M.; Alananbeh, K.M.; Othman, Y.A.; Leskovar, D.I. Soil Health and Sustainable Agriculture. Sustainability 2020, 12, 4859. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Pal, R.; Chakraborty, A.; Chakrabarti, K. Microbial Biomass and Activity in a Laterite Soil Amended with Municipal Solid Waste Compost. J. Agron. Crop Sci. 2001, 187, 207–211. [Google Scholar] [CrossRef]
- Mangalassery, S.; Kalaivanan, D.; Philip, P.S. Effect of inorganic fertilisers and organic amendments on soil aggregation and biochemical characteristics in a weathered tropical soil. Soil Tillage Res. 2019, 187, 144–151. [Google Scholar] [CrossRef]
- Ojo, J.A.; Olowoake, A.A.; Obembe, A. Efficacy of organomineral fertilizer and un-amended compost on the growth and yield of watermelon (Citrullus lanatus Thumb) in Ilorin Southern Guinea Savanna zone of Nigeria. Int. J. Recycl. Org. Waste Agric. 2014, 3, 121–125. [Google Scholar] [CrossRef]
- Savci, S. Investigation of Effect of Chemical Fertilizers on Environment. APCBEE Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Budiono, R.; Adinurani, P.G. Efficiency analysis of production factors utilization in upland rice farming. In Proceedings of the International Conference on Natural Resources and Life Sciences (NRLS-2016), Surabaya, Indonesia, 20–21 October 2016; KnE Life Sciences: Dubai, United Arab Emirates, 2017; pp. 180–187. [Google Scholar]
- Abdulkareem, M.A.; Aday, S.H.; Muhsin, S.J. Effect of the manure levels, depth and application methods using subsoil laying machine on the soil salinity and soil pH. Thi-Qar Univ. J. Agric. Res. 2018, 7, 155–172. [Google Scholar]
- Zhao, X.; Huang, J.; Lu, J.; Sun, Y. Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotoxicol. Environ. Saf. 2019, 170, 218–226. [Google Scholar] [CrossRef]
- Ailincai, D.; Ailincai, C.; Zbant, M.; Mercus, A.; Topa, D. Influence of organo-mineral fertilization on wheat and maize crops and the evolution of soil fertility under long-term experiments in the Moldavian Plain. Cercet. Agron. Mold. XLI 2008, 3, 33–42. [Google Scholar]
- Lal, R. Intensive agriculture, and the soil carbon pool. J. Crop Improv. 2013, 27, 735–751. [Google Scholar] [CrossRef]
- Murphy, B.W. Impact of soil organic matter on soil properties—A review with emphasis on Australian soils. Soil Res. 2015, 53, 605–635. [Google Scholar] [CrossRef]
- Olaniyi, J.O.; Ogunbiyi, E.M.; Alagbe, D.D. Effects of organo-mineral fertilizers on growth, yield and mineral nutrients uptake in cucumber. J. Anim. Plant Sci. 2009, 5, 437–442. [Google Scholar]
- Akyol, C.; Ince, O.; Ince, B. Crop-based composting of lignocellulosic digestates: Focus on bacterial and fungal diversity. Bioresour. Technol. 2019, 288, 121549. [Google Scholar] [CrossRef]
- Dinca, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and Soil Microbial Community: A Review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Singh, R. (Ed.) Organic Fertilizers Types: Production and Environmental Impact; Nova Science Publisher: New York, NY, USA, 2012; pp. 1–261. [Google Scholar]
- Mo, Z.; Li, W.; Pan, S.; Fitzgerald, T.L.; Xiao, F.; Tang, Y.; Wang, Y.; Duan, M.; Tian, H.; Tang, X. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice 2015, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, H.; Wang, J.; Wang, Y.; Pan, S.; Tian, H.; Duan, M.; Wang, S.; Tang, X.; Mo, Z. Responses of plant growth, physiological, gas exchange parameters of super and non-super rice to rhizosphere temperature at the tillering stage. Sci. Rep. 2019, 9, 10618. [Google Scholar] [CrossRef]
- Sikora, J.; Niemiec, M.; Szelag-Sikora, A.; Grodek-Szostak, Z.A.; Kubon, M.; Komorowska, M. The Impact of a Controlled-Release Fertilizer on Greenhouse Gas Emissions and the Efficiency of the Production of Chinese Cabbage. Energies 2020, 13, 2063. [Google Scholar] [CrossRef]
- Jensen, L.S.; Oelofse, M.; Hoeve, M.; Bruun, S. Environmental Impact Assessment on the Production and Use of Biobased Fertilizers. Biorefinery Inorg. Recover. Miner. Nutr. Biomass Org. Waste 2020, 1, 329–362. [Google Scholar]
- Purbajanti, E.D.; Kusmiyati, F.; Slamet, W.; Adinurani, P.G. Chlorophyll, crop growth rate and forage yield of Brachiaria (Brachiaria brizantha Stapf) as the result of goat manure in various nitrogen dosage. AIP Conf. Proc. 2016, 1755, 130013. [Google Scholar]
- Pocius, A.; Jotautiene, E.; Pekarskas, J.; Palsauskas, M. Investigation of physical-mechanical properties of experimental organic granular fertilizers. In Proceedings of the Engineering for Rural Development: 15th International Scientific Conference Proceedings, Jelgava, Latvia, 25–27 May 2016; pp. 1115–1120. [Google Scholar]
- Jasinskas, A.; Pekarskas, J.; Kucinskas, V.; Aboltins, A. Investigation of natural magnesium mineral fertilizer granulation and determination of granule qualitative indicators. In Proceedings of the Engineering for Rural Development: 15th International Scientific Conference Proceedings, Jelgava, Latvia, 25–27 May 2016; pp. 647–652. [Google Scholar]
- Česonienė, L.; Rutkovienė, V. Lysimetric research of nutrient losses from organic fertilizers. Agron. Res. 2009, 7, 224–232. [Google Scholar]
- Mieldažys, R.; Jotautienė, E.; Jasinskas, A.; Pekarskas, J.; Zinkevičienė, R. Investigation of physical-mechanical properties and impact on soil of granulated manure compost fertilizers. J. Environ. Eng. Landsc. Manag. 2019, 27, 153–162. [Google Scholar] [CrossRef]
- Gannes, V.; Eudoxie, G.; Bekele, I.; Hickey, W.J. Relations of microbiome characteristics to edaphic properties of tropical soils from Trinidad. Front. Microbiol. 2015, 6, 1045. [Google Scholar] [CrossRef] [PubMed]
- Schlatter, D.C.; Kahl, K.; Carlson, B.; Huggins, D.R.; Paulitz, T. Fungal community composition and diversity vary with soil depth and landscape position in a no-till wheat-based cropping system. FEMS Microbiol. Ecol. 2018, 94, fiy098. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Yoo, G.; Yun, S.T.; Jun, S.C.; Chung, H. Bacterial and fungal community composition across the soil depth profiles in a fallow field. J. Ecol. Environ. 2017, 41, 34. [Google Scholar] [CrossRef]
- Frey, B.; Walthert, L.; Perz-Mon, C.; Stierli, B.; Kochli, R.; Dharmarajan, A.; Brunner, I. Deep Soil Layers of Drought-Exposed Forests Harbor Poorly Known Bacterial and Fungal Communities. Front. Microbiol. 2021, 12, 674160. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Bengtson, P. Microbial regulation of global biogeochemical cycles. Front. Microbiol. 2014, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- d’Entremont, T.W.; Kivlin, S.N. Specificity in plant-mycorrhizal fungal relationships: Prevalence, parameterization, and prospects. Front. Plant Sci. 2023, 14, 1260286. [Google Scholar] [CrossRef] [PubMed]
- Patoine, G.; Eisenhauer, N.; Cesarz, S.; Phillips, H.R.P.; Xu, X.; Zhang, L.; Guerra, C.A. Drivers and trends of global soil microbial carbon over two decades. Nat. Commun. 2022, 13, 4195. [Google Scholar] [CrossRef]
- Bastida, F.; Eldridge, D.J.; García, C.; Png, G.K.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil microbial diversity–biomass relationships are driven by soil carbon content across global biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Shinfuku, M.; Junier, P.; Poirier, S.; Verrecchia, E.; Sebag, D.; DeAngelis, K.M. Direct evidence for the role of microbial community composition in the formation of soil organic matter composition and persistence. ISME Commun. 2021, 1, 64. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.N.; Parra-Saldívar, R. Soil carbon sequestration—An interplay between soil microbial community and soil organic matter dynamics. Sci. Total Environ. 2022, 815, 152928. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 2008, 163, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Prasun, K.; Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma Research in the Genome Era. Annu. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar]
- López-Bucioa, J.; Pelagio-Floresa, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Chennappa, G.; Naik, M.K.; Amaresh, Y.S.; Nagaraja, H.; Sreenivasa, M.Y. Azotobacter: A Potential Biofertilizer and Bioinoculants for Sustainable Agriculture. In Microorganisms for Green Revolution, Microorganisms for Sustainability; Panpatte, D.G., Jhala, Y., Vyas, R., Shelat, H., Eds.; Springer Nature: Singapore, 2017; Volume 6, pp. 87–106. [Google Scholar]
- Sagar, A.; Debbarma, V.; Abraham, T.; Shukla, P.K.; Ramteke, P.W. Functional Diversity of Soil Bacteria from Organic Agro Ecosystem. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3500–3518. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Ahmad, P. (Eds.) Plant Microbiome: Stress Response. In Microorganisms for Sustainability; Springer Nature: Singapore, 2018; Volume 5. [Google Scholar]
- Lithuanian Hydrometeorological Service Database. Available online: http://www.meteo.lt/en/ (accessed on 11 November 2023).
- LHS/ME. Comparative evaluation of climate changes in Lithuania 1961–1990 and 1991–2020 standard climate norms. 2021, 18. (In Lituanian). Available online: https://www.meteo.lt/app/uploads/2023/11/Lietuvos_klimato_pokyciu_vertinimas_lyginant_klimato_normas.pdf (accessed on 16 July 2024).
- ISO 10390:2021; Soil Quality—Determination of pH. ISO: Geneva, Switzerland, 2005. Available online: https://www.iso.org/standard/75243.html (accessed on 16 July 2024).
- ISO 10694:1995; Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). ISO: Geneva, Switzerland, 1995. Available online: https://www.iso.org/standard/18782.html (accessed on 16 July 2024).
- ISO 14256-2:2005; Soil Quality—Determination of Nitrate, Nitrite and Ammonium in Field-Moist Soils by Extraction with Potassium Chloride Solution. ISO: Geneva, Switzerland, 2005. Available online: https://www.iso.org/standard/32399.html (accessed on 16 July 2024).
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xiao, Y.; Wang, Y.F.; Yang, K.J. Trichoderma affects the physiochemical characteristics and bacterial community composition of saline-alkaline maize rhizosphere soils in the cold region of Heilongjiang Province. Plant Soil 2019, 436, 211–227. [Google Scholar] [CrossRef]
- Kizilkaya, R. Nitrogen fixation capacity of Azotobacter spp. Strains isolated from soils in different ecosystems and relationship between them and the microbiological properties of soils. J. Environ. Biol. 2009, 30, 73–82. [Google Scholar] [PubMed]
- Dar, S.A.; Bhat, R.A.; Dervash, M.A.; Dar, Z.A.; Dar, G.H. Azotobacter as biofertilizer for sustainable soil and plant health under saline environmental conditions. In Microbiota and Biofertilizers; Hakeem, K.R., Dar, G.H., Mehmood, M.A., Bhat, R.A., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Joshi, K.K.; Kumar, V.; Dubey, R.C.; Maheshwari, D.K.; Bajpai, V.K.; Kang, S.C. Effect of Chemical Fertilizer adaptive Variants, Pseudomonas aeruginosa GRC 2 and Azotobacter chroococcum AC 1, on Macrophominaphaseolina Causing Charcoal Rot of Brassica juncea. Korean J. Environ. Agric. 2006, 25, 228–235. [Google Scholar] [CrossRef]
- Buryak, S.M.; Chernikova, O.V.; Mazhayskiy, Y.A. The influence of granulated and rotted turkey manure on the productivity of perennial grasses. IOP Conf. Ser. Earth Environ. Sci. 2023, 1212, 012015. [Google Scholar] [CrossRef]
- Magagula, N.E.M.; Ossom, E.M.; Rhykerd, R.L.; Rhykerd, L. Effects of chicken manure on soil properties under sweetpotato (Ipomoea batatas (L.) Lam.) culture in Swaziland. Am. -Eurasian J. Agron. 2010, 3, 36–43. [Google Scholar]
- Raksarikorn, C.; Pumisak, I.; Ruankuan, I. Influence of organic granular fertilizer, chemical and granular organic fertilizer with hormone mixed formula (HO), and chemical fertilizer to the yield and starch content of Cassava. Rev. Gestão Soc. E Ambient. 2024, 18, e05410. [Google Scholar] [CrossRef]
- Kavaliauskaitė, D.; Karklelienė, R.; Jankauskienė, J. Impact of an organic fertiliser on the yield of white cabbage (Brassica oleracea var. capitata) and the soil productivity. Hortic. Sci. 2023, 50, 290–296. [Google Scholar]
- Tripolskaja, L.; Romanovkaja, D.; Šlepetienė, A.; Verbylienė, I. Comparison of the efficiency of green manure and mineral fertilizers for winter rye and barley yields in a sandy loam soil. Žemės Ūkio Moksl. 2012, 19, 27–35. [Google Scholar]
- Martens, D.A.; Reedy, T.R.; Lewis, D.T. Soil organic carbon content and composition of 130-year crop, pasture, and forest land-use managements. Glob. Chang. Biol. 2004, 10, 65–78. [Google Scholar] [CrossRef]
- Wang, R.; Guo, S.; Li, N.; Li, R.; Zhang, Y.; Jiang, J.; Wang, Z.; Liu, Q.; Wu, D.; Sun, Q.; et al. Phosphorus Accumulation and Sorption in Calcareous Soil under Long-Term Fertilization. PLoS ONE 2015, 10, e0135160. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, W.; Reszka, R. Influence of liming and mineral fertilization on the content of mineral nitrogen in soil. J. Elem. 2008, 13, 301–308. [Google Scholar]
- Liu, H.B.; Wu, W.L.; Zhang, J. Regional differentiation of non-point source pollution of agriculture-derived nitrate nitrogen in groundwater in northern China. Agric. Ecosyst. Environ. 2005, 107, 211–220. [Google Scholar] [CrossRef]
- Kyllmar, K.; Carlsson, C.; Gustafson, A.; Ulen, B.; Jahnsson, H. Nutrient discharge from small agricultural catchments in Sweden: Characteristics and trends. Agric. Ecosyst. Environ. 2006, 115, 15–26. [Google Scholar] [CrossRef]
- Žičkienė, L. Mineral Nitrogen Fluxes in Different Soils. Ph.D. Thesis, Lithuanian Research Centre for Agriculture and Forestry, Dotnuva, Lithuania, 2016; 138p. [Google Scholar]
- Rutkowska, A.; Fotyma, M. Mineral nitrogen as a universal soil test to predict plant n requirements and ground watter pollution—Casestudy for Poland. In Principles, Application and Assessment in Soil Science; Burcu Özkaraova Güngör, E., Ed.; Intech Open: London, UK, 2011; pp. 333–350. [Google Scholar]
- Jabloun, M.; Schelde, K.; Tao, T.; Olesen, E.J. Effect of temperature and precipitation on nitrate leaching from organic cereal cropping systems in Denmark. Eur. J. Agron. 2015, 62, 55–64. [Google Scholar] [CrossRef]
- Kuśmierz, S.; Skowrońska, M.; Tkaczyk, P.; Lipiński, W.; Mielniczuk, J. soil organic carbon and mineral nitrogen contents in soils as affected by their pH, texture and fertilization. Agronomy 2023, 13, 267. [Google Scholar] [CrossRef]
- Plošek, L.; Elbl, J.; Lošák, T.; Kužel, S.; Kintl, A.; Juřička, D.; Kynický, J.; Martensson, A.; Brtnický, M. Leaching of mineral nitrogen in the soil influenced by addition of compost and N-mineral fertilizer. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 607–614. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Liu, R.; Zhang, A.; Yang, Z. Effect of Nitrate Leaching Caused by Swine Manure Application in Fields of the Yellow River Irrigation Zone of Ningxia, China. Sci. Rep. 2017, 7, 13693. [Google Scholar] [CrossRef] [PubMed]
- Elbl, J.; Šimečkova, J.; Škarpa, P.; Kintl, A.; Brtnicky, M.; Vaverkova, M.D. Comparison of the Agricultural Use of Products from Organic Waste Processing with Conventional Mineral Fertilizer: Potential Effects on Mineral Nitrogen Leaching and Soil Quality. Agronomy 2020, 10, 226. [Google Scholar] [CrossRef]
- Kukreja, K.; Suneja, S.; Goyal, S.; Narula, N. Phytohormone production by Azotobacter–a review. Agric. Rev. 2004, 25, 70–75. [Google Scholar]
- Kyaw, E.P.; Soe, M.M.; Yu, S.S.; Latt, Z.K.; Lynn, T.M. Study on plant growth promoting activities of azotobacter isolates for sustainable agriculture in Myanmar. J. Biotechnol. Biores. 2019, 1, 1–6. [Google Scholar]
- Hakeem, K.R.; Akhtar, M.S.; Abdullah, S.N.A. (Eds.) Plant, Soil and Microbes: Implications in Crop Science; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1. [Google Scholar]
- Shokri, D.; Emtiazi, G. Indole-3-acetic acid (IAA) production in symbiotic and non-symbiotic nitrogen fixing bacteria and its optimization by Taguchi design. Curr. Microbiol. 2010, 61, 217–225. [Google Scholar] [CrossRef]
- Gauri, S.S.; Mandal, S.M.; Pati, B.R. Impact of Azotobacter exopolysaccharides on sustainable agriculture. Appl. Microbiol. Biotechnol. 2012, 95, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Halim, A.M.; Shivanand, P.; Krishnamoorthy, S.; Taha, H. A review on the biological properties of Trichoderma spp. as a prospective biocontrol agent and biofertilizer. J. Appl. Biol. Biotechnol. 2023, 11, 34–46. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Vaish, A.; Dwivedi, S.; Chakrabarty, D.; Singh, N.; Tripathi, R.D. Biological removal of arsenic pollution by soil fungi. Sci. Total Environ. 2011, 409, 2430–2442. [Google Scholar] [CrossRef]
- Mastouri, F.; Bjorkman, T.; Harman, G.E. Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathology 2010, 100, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Stepantsova, L.V.; Matsnev, I.N.; Palchikov, E.V.; Volkov, S.A.; Moskaleva, E.V. Effect of granular fertilizer of disinfected chicken dung application on crop productivity and soil fertility. IOP Conf. Ser. Earth Environ. Sci. 2021, 845, 012035. [Google Scholar] [CrossRef]
- Schlegel, A.J.; Assefa, Y.; Bond, H.D.; Haag, L.A.; Stone, L.R. Changes in soil nutrients after 10 years of cattle manure and swine effluent application. Soil Tillage Res. 2017, 172, 48–58. [Google Scholar] [CrossRef]
- Mažeika, R.; Arbačiauskas, J.; Masevičienė, A.; Narutytė, I.; Šumskis, D.; Žičkienė, L.; Rainys, K.; Drapanauskaite, D.; Staugaitis, G.; Baltrusaitis, J. Nutrient Dynamics and Plant Response in Soil to Organic Chicken Manure Based Fertilizers. Waste Biomass Valorization 2021, 12, 371–382. [Google Scholar] [CrossRef]
- Slessarev, E.W.; Lin, Y.; Bingham, N.L.; Johnson, J.E.; Dai, Y.; Schimel, J.P.; Chadwick, O.A. Water balance creates a threshold in soil pH at the global scale. Nature 2016, 540, 567–569. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Wu, W.; Liu, H. Factors affecting variations of soil pH in different horizons in hilly regions. PLoS ONE 2019, 14, e0218563. [Google Scholar] [CrossRef]
- Hong, S.; Gan, P.; Chen, A. Environmental controls on soil pH in planted forest and its response to nitrogen deposition. Environ. Res. 2019, 172, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Mažvila, J. (Ed.) Lietuvos Dirvožemių Makromorfologinė Diagnostika: Monografija; Lietuvos Žemdirbystės Institutas: Kėdainiai, Lithuania, 2006; 283p. [Google Scholar]
- Poveda, J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biol. Control 2021, 159, 104634. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Meireles, C.I.R.; Gomes, C.J.P.; Ribeiro, N.M.C.A. The Impact of Climate Change on Forest Development: A Sustainable Approach to Management Models Applied to Mediterranean-Type Climate Regions. Plants 2022, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Klavina, D.; Tedersoo, L.; Agan, A.; Adamson, K.; Bitenieks, K.; Gaitnieks, T.; Drenkhan, R. Soil fungal communities in young Norway spruce-dominant stands: Footprints of former land use and selective thinning. Eur. J. For. Res. 2022, 141, 503–516. [Google Scholar] [CrossRef]
- Frac, M.; Hannula, S.E.; Bełka, M.; Jedryczka, M. Fungal biodiversity and their role in soil health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef]
- Furtak, K.; Gajda, A.M. Activity and Variety of Soil Microorganisms Depending on the Diversity of the Soil Tillage System. In Sustainability of Agroecosystems; de Oliveira, A.B., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Ge, Y.; Zhang, J.; Zhang, L.; Yang, M.; He, J. Long-term fertilization regimes affect bacterial community structure and diversity of an agricultural soil in northern China. J. Soils Sediments 2008, 8, 43–50. [Google Scholar] [CrossRef]
- Liang, R.; Hou, R.; Li, J.; Lyu, Y.; Hang, S.; Gong, H.; Ouyang, Z. Effects of Different Fertilizers on Rhizosphere Bacterial Communities of Winter Wheat in the North China Plain. Agronomy 2020, 10, 93. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, T.; Li, J.; Lu, Q.; Fang, Z.; Huang, Q.; Zhang, R.; Li, R.; Shen, B.; Shen, Q. Effects of organic–inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice–wheat cropping system. Appl. Soil Ecol. 2016, 99, 1–12. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Y.; Gao, J.; Peng, F.; Gao, P. Long-term combined application of manure and chemical fertilizer sustained higher nutrient status and rhizospheric bacterial diversity in reddish paddy soil of Central South China. Sci. Rep. 2018, 8, 16554. [Google Scholar] [CrossRef]
- Rieke, E.L.; Soupir, M.L.; Moorman, T.B.; Yang, F.; Howe, A.C. Temporal Dynamics of Bacterial Communities in Soil and Leachate Water After Swine Manure Application. Front. Microbiol. 2018, 9, 3197. [Google Scholar] [CrossRef] [PubMed]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. Organic Amendments: Microbial Community Structure, Activity and Abundance of Agriculturally Relevant Microbes Are Driven by Long-Term Fertilization Strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef] [PubMed]
- Cinnadurai, C.; Gopalaswamy, G.; Balachandar, D. Diversity of cultivable Azotobacter in the semi-arid alfisol receiving long-term organic and inorganic nutrient amendments. Ann. Microbiol. 2013, 63, 1397–1404. [Google Scholar] [CrossRef]
- Adediran, J.A.; De Baets, N.; Mnkeni, P.N.S.; Kiekens, L.; Muyima, N.Y.O.; Thys, A. Organic Waste Materials for Soil Fertility Improvement in the Border Region of the Eastern Cape, South Africa. Biol. Agric. Hortic. 2003, 20, 283–300. [Google Scholar] [CrossRef]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; Bruggen, A. Mineral and Organic Fertilizers Distinctly Affect Fungal Communities in the Crop Rhizosphere. J. Fungi 2022, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Dsouza, M.; Gilbert, J.A.; Guo, X.; Wang, D.; Guo, Z.; Ni, Y.; Chu, H. Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter. Environ. Microbiol. 2016, 18, 5137–5150. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Jiang, X.; Guan, D.; Zhao, B.; Ma, M.; Zhou, B.; Cao, F.; Yang, X.; Li, L.; Li, J. Influence of inorganic fertilizer and organic manure application on fungal communities in a long-term field experiment of Chinese Mollisols. Appl. Soil Ecol. 2017, 111, 114–122. [Google Scholar] [CrossRef]
- Ye, G.; Lin, Y.; Luo, J.; Di, H.J.; Lindsey, S.; Liu, D.; Fan, J.; Ding, W. Responses of soil fungal diversity and community composition to long-term fertilization: Field experiment in an acidic Ultisol and literature synthesis. Appl. Soil Ecol. 2020, 145, 103305. [Google Scholar] [CrossRef]
- Xiang, X.; Liu, J.; Zhang, J.; Li, D.; Xu, C.; Kuzyakov, Y. Divergence in fungal abundance and community structure between soils under long-term mineral and organic fertilization. Soil Tillage Res. 2020, 196, 104491. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil Biol. Biochem. 2015, 83, 29–39. [Google Scholar] [CrossRef]
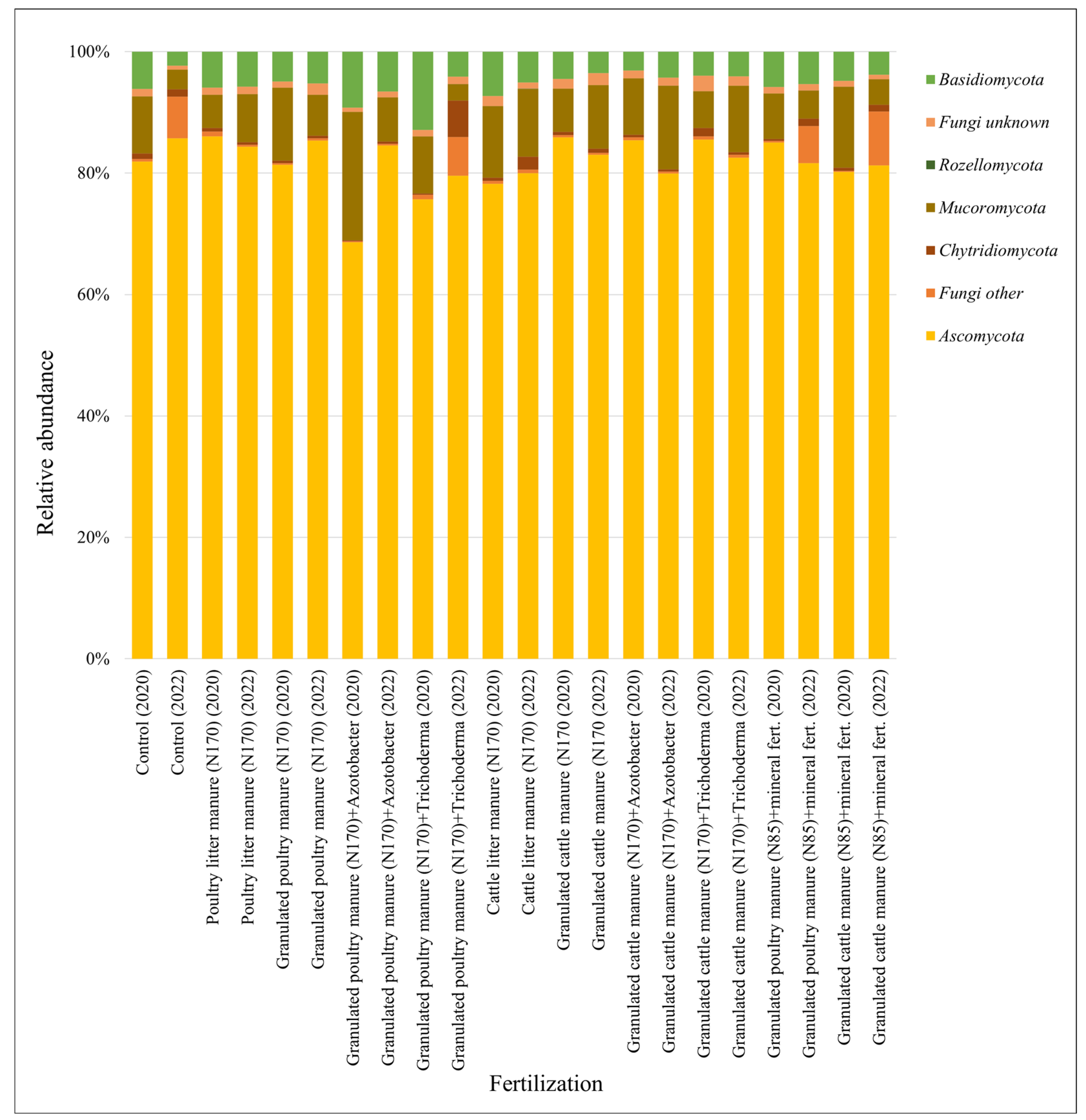
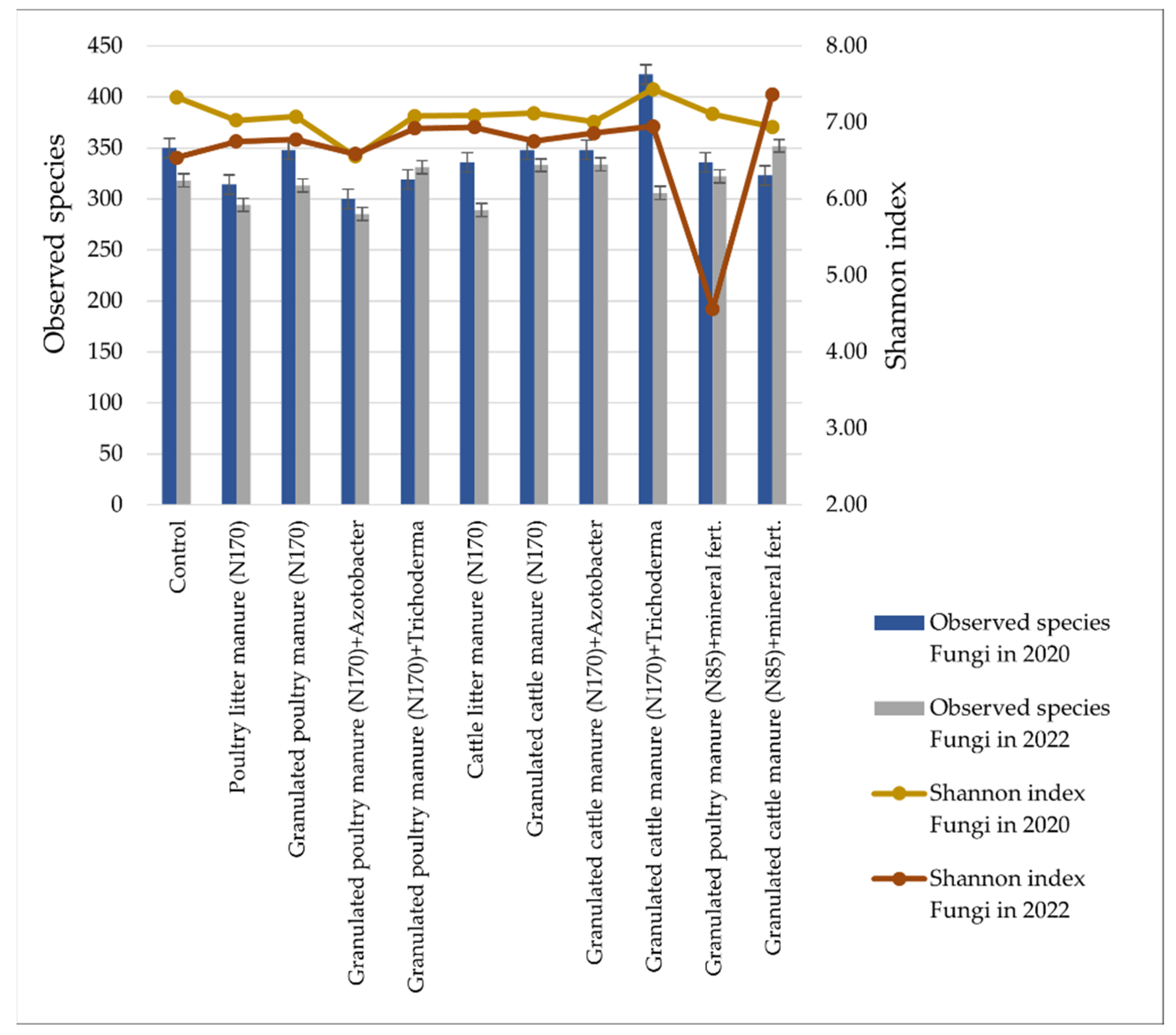
| Treatment | Description |
|---|---|
| C | control (without any fertilizer—N0P0K0) |
| PLM170 | poultry litter manure (N170) 3 |
| GPM170 | granulated poultry manure (N170) 3 |
| GPM170 + A | granulated poultry manure (N170) + bio-input No. 1 3 |
| GPM170 + T | granulated poultry manure (N170) + bio-input No. 2 3 |
| CLM170 | cattle litter manure (N170) 3 |
| GCM170 | granulated cattle manure (N170) 3 |
| GCM170 + A | granulated cattle manure (N170) + bio-input No. 1 3 |
| GCM170 + T | granulated cattle manure (N170) + bio-input No. 2 3 |
| GPM85 + MF | granulated poultry manure (N85) 2 + mineral fertilizers (N60) 1 |
| GCM85 + MF | granulated cattle manure (N85) 2 + mineral fertilizers (N60) 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivojienė, D.; Masevičienė, A.; Žičkienė, L.; Ražukas, A.; Kačergius, A. Soil Microbial Community Structure and Carbon Stocks Following Fertilization with Organic Fertilizers and Biological Inputs. Biology 2024, 13, 534. https://doi.org/10.3390/biology13070534
Sivojienė D, Masevičienė A, Žičkienė L, Ražukas A, Kačergius A. Soil Microbial Community Structure and Carbon Stocks Following Fertilization with Organic Fertilizers and Biological Inputs. Biology. 2024; 13(7):534. https://doi.org/10.3390/biology13070534
Chicago/Turabian StyleSivojienė, Diana, Aistė Masevičienė, Lina Žičkienė, Almantas Ražukas, and Audrius Kačergius. 2024. "Soil Microbial Community Structure and Carbon Stocks Following Fertilization with Organic Fertilizers and Biological Inputs" Biology 13, no. 7: 534. https://doi.org/10.3390/biology13070534
APA StyleSivojienė, D., Masevičienė, A., Žičkienė, L., Ražukas, A., & Kačergius, A. (2024). Soil Microbial Community Structure and Carbon Stocks Following Fertilization with Organic Fertilizers and Biological Inputs. Biology, 13(7), 534. https://doi.org/10.3390/biology13070534







