Fresh Tomato (Lycopersicon esculentum Mill.) in the Diet Improves the Features of the Metabolic Syndrome: A Randomized Study in Postmenopausal Women
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Experimental Diets
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. The Nutrients of Control and Tomato Diet
3.2. Baseline Characteristics and Dietary Intake of Subjects
3.3. Glycemic-Control-Related Markers
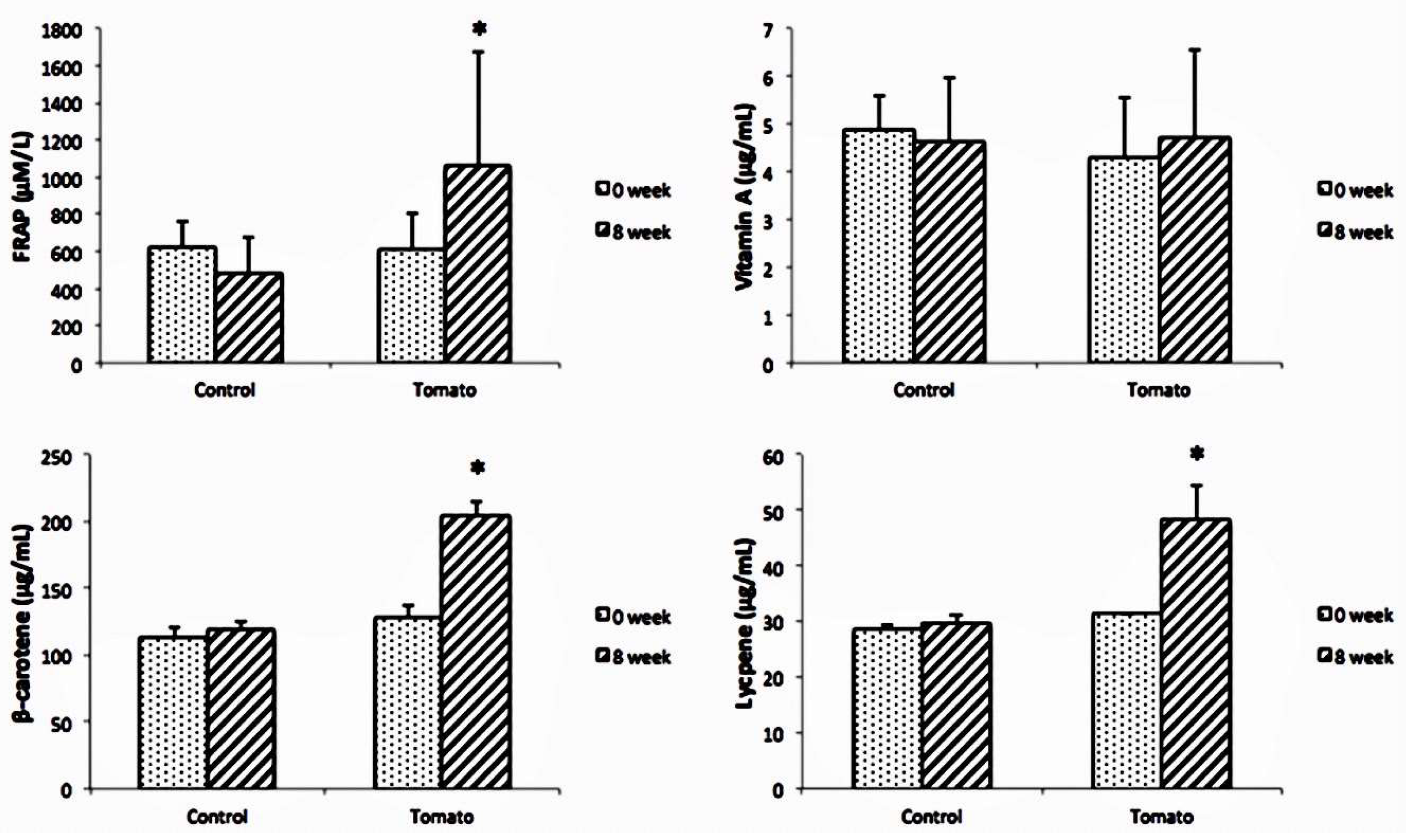
3.4. Oxidative-Stress-Related Markers
3.5. Anthropometric Measures, Blood Pressure, and Lipid Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalvand, S.; Niksima, S.H.; Meshkani, R.; Ghanei Gheshlagh, R.; Sadegh-Nejadi, S.; Kooti, W.; Parizad, N.; Zahednezhad, H.; Afrisham, R. Prevalence of Metabolic Syndrome among Iranian Population: A Systematic Review and Meta-analysis. Iran. J. Public Health 2017, 46, 456–467. [Google Scholar] [PubMed]
- Ness, A.R.; Powles, J.W. Fruit and vegetables, and cardiovascular disease: A review. Int. J. Epidemiol. 1997, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Liu, S.; Gaziano, J.M.; Buring, J.E. Dietary lycopene, tomato-based food products and cardiovascular disease in women. J. Nutr. 2003, 133, 2336–2341. [Google Scholar] [CrossRef] [PubMed]
- Nishi, S.K.; Khoury, N.; Valle Hita, C.; Zurbau, A.; Salas-Salvadó, J.; Babio, N. Vegetable and Fruit Intake Variety and Cardiovascular Health and Mortality: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2023, 15, 4913. [Google Scholar] [CrossRef] [PubMed]
- Cámara, M.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, R.M.; Domínguez, L.; Sesso, H.D. Scientific Evidence of the Beneficial Effects of Tomato Products on Cardiovascular Disease and Platelet Aggregation. Front. Nutr. 2022, 9, 849841. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Meyer, M.R. Postmenopausal hypertension: Mechanisms and therapy. Hypertension 2009, 54, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Nicolson, M.; Hirsch, J.; Heymsfield, S.B.; Gallagher, D.; Chu, F.; Leibel, R.L. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J. Clin. Endocrinol. Metab. 1996, 81, 3424–3427. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F.; Fortepiani, L.A. Novel mechanisms responsible for postmenopausal hypertension. Hypertension 2004, 43, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef]
- Rao, A.V.; Ray, M.R.; Rao, L.G. Lycopene. Adv. Food Nutr. Res. 2006, 51, 99–164. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Raka, M.A.; Khan, S.; Sarker, J.; Ahmed, N.; Nath, P.D.; Hasan, N.; Mohib, M.M.; Tisha, A.; Sagor, M.A.T. A clinical review of the effectiveness of tomato (Solanum lycopersicum) against cardiovascular dysfunction and related metabolic syndrome. J. Herbal Med. 2019, 16, 100235. [Google Scholar] [CrossRef]
- Wawrzyniak, A.; Hamułka, J.; Friberg, E.; Wolk, A. Dietary, anthropometric, and lifestyle correlates of serum carotenoids in postmenopausal women. Eur. J. Nutr. 2013, 52, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Basuny, A.M.; Gaafar, A.M.; Arafat, S.M. Tomato lycopene is a natural antioxidant and can alleviate hypercholesterolemia. Afr. J. Biotechnol. 2009, 8, 6627–6633. [Google Scholar]
- Lee, A.; Thurnham, D.I.; Chopra, M. Consumption of tomato products with olive oil but not sunflower oil increases the antioxidant activity of plasma. Free Radic. Biol. Med. 2000, 29, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Biasillo, G.; Leo, M.; Della Bona, R.; Biasucci, L.M. Inflammatory biomarkers and coronary heart disease: From bench to bedside and back. Intern. Emerg. Med. 2010, 5, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Markovits, N.; Ben Amotz, A.; Levy, Y. The effect of tomato-derived lycopene on low carotenoids and enhanced systemic inflammation and oxidation in severe obesity. Isr. Med. Assoc. J. 2009, 11, 598–601. [Google Scholar] [PubMed]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kushi, L.H.; Folsom, A.R.; Prineas, R.J.; Mink, P.J.; Wu, Y.; Bostick, R.M. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N. Engl. J. Med. 1996, 334, 1156–1162. [Google Scholar] [CrossRef]
- Mueller-Cunningham, W.M.; Quintana, R.; Kasim-Karakas, S.E. An ad libitum, very low-fat diet results in weight loss and changes in nutrient intakes in postmenopausal women. J. Am. Diet. Assoc. 2003, 103, 1600–1606. [Google Scholar] [CrossRef]
- Whigham, L.D.; Valentine, A.R.; Johnson, L.K.; Zhang, Z.; Atkinson, R.L.; Tanumihardjo, S.A. Increased vegetable and fruit consumption during weight loss effort correlates with increased weight and fat loss. Nutr. Diabetes 2012, 2, e48. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Agarwal, S. Role of antioxidant lycopene in cancer and heart disease. J. Am. Coll. Nutr. 2000, 19, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Nitta, A.; Imai, S.; Kajiyama, S.; Miyawaki, T.; Ozasa, N.; Kajiyama, S.; Hashimoto, Y.; Fukui, M. Tomato juice preload has a significant impact on postprandial glucose concentration in healthy women: A randomized cross-over trial. Asia Pac. J. Clin. Nutr. 2020, 29, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Rao, A.V. Tomato lycopene and low density lipoprotein oxidation: A human dietary intervention study. Lipids 1998, 33, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Catalano, A.; Simone, R.E.; Mele, M.C.; Cittadini, A. Effect of lycopene and tomato products on cholesterol metabolism. Ann. Nutr. Metab. 2012, 61, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.; Periago, M.J.; Böhm, V.; Berruezo, G.R. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. Br. J. Nutr. 2008, 99, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Hirai, S.; Takahashi, H.; Goto, T.; Ohyane, C.; Tsugane, T.; Konishi, C.; Fujii, T.; Inai, S.; Iijima, Y.; et al. 9-oxo-10(E),12(E)-Octadecadienoic acid derived from tomato is a potent PPAR α agonist to decrease triglyceride accumulation in mouse primary hepatocytes. Mol. Nutr. Food Res. 2011, 55, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Witztum, J.L. The oxidation hypothesis of atherosclerosis. Lancet 1994, 344, 793–795. [Google Scholar] [CrossRef]
- Boyd, N.F.; McGuire, V. Evidence of lipid peroxidation in premenopausal women with mammographic dysplasia. Cancer Lett. 1990, 50, 31–37. [Google Scholar] [CrossRef]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Chopra, M.; O’Neill, M.E.; Keogh, N.; Wortley, G.; Southon, S.; Thurnham, D.I. Influence of increased fruit and vegetable intake on plasma and lipoprotein carotenoids and LDL oxidation in smokers and nonsmokers. Clin. Chem. 2000, 46, 1818–1829. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Manson, J.E.; Gaziano, J.M.; Buring, J.E.; Sesso, H.D. Fruit and vegetable intake and the risk of hypertension in middle-aged and older women. Am. J. Hypertens. 2012, 25, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Bittner, V. Menopause, age, and cardiovascular risk: A complex relationship. J. Am. Coll. Cardiol. 2009, 54, 2374–2375. [Google Scholar] [CrossRef] [PubMed]
| Control | Tomato | |
|---|---|---|
| Energy (kcal) | 31.0 | 23.1 |
| Protein (g) | 3.1 | 0.9 |
| Carbohydrate (g) | 4.8 | 4.9 |
| Fat (g) | 0.5 | 0.2 |
| Fiber (g) | 0.6 | 0.6 |
| Dietary fiber (g) | 1.7 | 1.2 |
| Vitamin A (RE) | 0.0 | 84.2 |
| Vitamin C (mg) | 183.6 | 21 |
| Lycopene (mg) | 0.0 | 5.5 |
| Control, n = 23 | Tomato, n = 30 | p2 | |
|---|---|---|---|
| Age (y) | 59.2 ± 8.0 | 60.2 ± 5.9 | 0.637 |
| Height (cm) | 156.3 ± 5.7 | 155.6 ± 4.1 | 0.628 |
| Weight (kg) | 66.1 ± 8.4 | 63.9 ± 5.8 | 0.334 |
| BMI (kg/m2) | 27.0 ± 2.2 | 26.4 ± 2.1 | 0.375 |
| WC (cm) | 90.9 ± 5.5 | 89.0 ± 8.2 | 0.243 |
| HC (cm) | 120.4 ± 5.1 | 101.0 ± 3.8 | 0.315 |
| WHR | 0.9 ± 0.0 | 0.9 ± 0.1 | 0.603 |
| SBP (mmHg) | 140.5 ± 20.6 | 132.8 ± 18.0 | 0.195 |
| DBP (mmHg) | 79.8 ± 10.4 | 78.8 ± 8.3 | 0.722 |
| FSH (mIU/mL) | 57.9 ± 29.8 | 62.9 ± 23.3 | 0.129 |
| E2 (pg/mL) | 14.9 ± 8.1 | 11.4 ± 6.5 | 0.235 |
| Control, n = 23 | Tomato, n = 30 | p2 | |
|---|---|---|---|
| Energy (kcal) | |||
| 0 week | 1639.0 ± 61.7 | 1607.3 ± 72.7 | 0.173 |
| 4 weeks | 1606.9 ± 68.9 * | 1557.3 ± 66.4 * | 0.033 |
| 8 weeks | 1571.0 ± 64.9 * | 1522.2 ± 60.5 * | 0.024 |
| Protein (g) | |||
| 0 week | 60.9 ± 8.5 | 54.3 ± 11.2 | 0.061 |
| 4 weeks | 62.3 ± 10.8 | 56.1 ± 15.5 | 0.186 |
| 8 weeks | 65.8 ± 12.5 | 55.8 ± 12.7 | 0.021 |
| Fat (g) | |||
| 0 week | 59.9 ± 11.1 | 57.1 ± 8.2 | 0.382 |
| 4 weeks | 59.6 ± 10.7 | 54.5 ± 7.2 | 0.085 |
| 8 weeks | 55.3 ± 10.4 | 53.0 ± 7.4 * | 0.43 |
| Carbohydrate (g) | |||
| 0 week | 219.7 ± 25.3 | 223.7 ± 31.9 | 0.688 |
| 4 weeks | 211.0 ± 37.0 | 215.3 ± 30.4 | 0.697 |
| 8 weeks | 207.8 ± 30.5 | 210.1 ± 24.8 * | 0.8 |
| Fiber (g) | |||
| 0 week | 5.9 ± 2.5 | 5.9 ± 3.5 | 0.997 |
| 4 weeks | 5.9 ± 1.7 | 6.6 ± 2.5 | 0.332 |
| 8 weeks | 6.1 ± 1.8 | 6.7 ± 2.7 | 0.42 |
| Dietary fiber (g) | |||
| 0 week | 19.8 ± 7.6 | 18.8 ± 6.4 | 0.664 |
| 4 weeks | 22.0 ± 12.3 | 21.4 ± 6.8 | 0.859 |
| 8 weeks | 20.2 ± 5.4 | 20.5 ± 6.4 | 0.879 |
| Vitamin A (RE) | |||
| 0 week | 837.5 ± 712.0 | 1553.0 ± 2170.8 | 0.056 |
| 4 weeks | 684.8 ± 614.3 | 2005.6 ± 2782.1 | 0.02 |
| 8 weeks | 1014.9 ± 1204.9 | 2174.9 ± 2907.8 | 0.039 |
| Vitamin E (alpha-TE) | |||
| 0 week | 5.4 ± 1.5 | 5.0 ± 1.1 | 0.932 |
| 4 weeks | 5.8 ± 1.7 | 5.4 ± 1.2 | 0.668 |
| 8 weeks | 5.6 ± 1.6 | 5.6 ± 1.5 | 0.157 |
| Vitamin C (mg) | |||
| 0 week | 207.5 ± 192.6 | 198.5 ± 146.8 | <0.001 |
| 4 weeks | 535.1 ± 104.9 | 226.4 ± 109.9 | <0.001 |
| 8 weeks | 538.6 ± 113.5 | 258.1 ± 142.5 | <0.001 |
| Control, n = 23 | Tomato, n = 30 | p2 | |
|---|---|---|---|
| Blood glucose (mg/dL) | |||
| 0 week | 101.7 ± 33.1 | 103.1 ± 13.2 | 0.645 |
| 8 weeks | 103.0 ± 24.0 | 98.5 ± 13.3 * | 0.975 |
| Δ 0–8 weeks | 1.3 ± 10.6 | −5.2 ± 13.6 | 0.043 |
| Insulin (mU/dL) | |||
| 0 week | 10.7 ± 5.6 | 9.3 ± 4.2 | 0.092 |
| 8 weeks | 11.5 ± 4.7 | 8.4 ± 3.7 | 0.107 |
| Δ 0–8 weeks | 0.8 ± 5.6 | 0.6 ± 3.3 | 0.885 |
| HOMA-IR | |||
| 0 week | 2.9 ± 2.1 | 2.1 ± 1.3 | 0.138 |
| 8 weeks | 3.0 ± 1.7 | 2.4 ± 1.3 | 0.185 |
| QUICKI | |||
| 0 week | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.280 |
| 8 weeks | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.225 |
| Control, n = 23 | Tomato, n = 30 | p2 | |
|---|---|---|---|
| Weight (kg) | |||
| 4–0 week | −0.5 ± 0.7 | −0.7 ± 0.7 | 0.279 |
| 8–0 week | −0.7 ± 0.9 | −1.1 ± 1.0 | 0.284 |
| BMI (kg/m2) | |||
| 4–0 week | −0.2 ± 0.3 | −0.3 ± 0.3 | 0.085 |
| 8–0 week | −0.3 ± 0.4 | −0.4 ± 0.4 | 0.223 |
| Fat mass (kg) | |||
| 4–0 week | −0.2 ± 1.0 | −0.4 ± 1.2 | 0.498 |
| 8–0 week | −0.4 ± 1.1 | −0.8 ± 1.3 | 0.020 |
| Fat (%) | |||
| 4–0 week | −0.2 ± 1.4 | 0.1 ± 0.6 | 0.423 |
| 8–0 week | −0.2 ± 1.5 | −0.3 ± 0.8 | 0.008 |
| WC (cm) | |||
| 4–0 week | −1.3 ± 1.3 | −1.7 ± 1.4 | 0.727 |
| 8–0 week | −2.2 ± 2.5 | −2.9 ± 1.7 | 0.018 |
| HC (cm) | |||
| 4–0 week | −0.3 ± 1.6 | −1.3 ± 1.0 | 0.001 |
| 8–0 week | −1.5 ± 1.8 | −1.7 ± 1.1 | 0.010 |
| WHR | |||
| 4–0 week | 0.0 ± 0.2 | 0.0 ± 0.2 | 0.333 |
| 8–0 week | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.177 |
| Control, n = 23 | Tomato, n = 30 | p2 | |
|---|---|---|---|
| TC (mg/dL) | |||
| 0 week | 201.0 ± 36.8 | 216.4 ± 32.9 | 0.146 |
| 8 weeks | 197.9 ± 38.3 | 210.3 * ± 34.7 | 0.368 |
| Δ 0–8 weeks | −2.9 ± 19.2 | −6.1 ± 36.8 | 0.041 |
| TG (mg/dL) | |||
| 0 week | 109.4 ± 62.7 | 135.8 ± 50.1 | 0.128 |
| 8 weeks | 114.6 ± 75.1 | 113.6 * ± 49.4 | 0.293 |
| Δ 0–8 weeks | −4.5 ± 41.1 | −25.87 ± 49.4 | 0.023 |
| LDL-C (mg/dL) | |||
| 0 week | 136.1 ± 34.3 | 140 ± 31.6 | 0.573 |
| 8 weeks | 130.6 ± 34.4 | 141.8 ± 27.1 | 0.681 |
| Δ 0–8 weeks | −6.2 ± 18.4 | −2.7 ± 27.4 | 0.546 |
| HDL-C (mg/dL) | |||
| 0 week | 59.0 ± 14.7 | 63.2 ± 14.0 | 0.223 |
| 8 weeks | 57.6 ± 13.1 | 64.4 ± 13.6 | 0.311 |
| Δ 0–8 weeks | −2.5 ± 14.1 | 0.83 ± 14.9 | 0.019 |
| SBP (mmHg) | |||
| 0 week | 135.3 ± 21.5 | 133.4 ± 17.7 | 0.195 |
| 8 weeks | 135.6 ± 18.8 | 128.9 * ± 13.1 | 0.103 |
| Δ 0–8 weeks | −0.3 ± 19.8 | −5.5 ± 14.2 | 0.042 |
| DBP (mmHg) | |||
| 0 week | 77.3 ± 10.5 | 78.6 ± 8.1 | 0.722 |
| 8 weeks | 77.7 ± 10.3 | 77.2 ± 6.8 | 0.283 |
| Δ 0–8 weeks | 0.4 ± 10.4 | −1.2 ± 7.2 | 0.764 |
| IL-6 (pg/mL) | |||
| 0 week | 431.3 ± 322.6 | 433.85 ± 297.3 | 0.544 |
| 8 weeks | 61.7 * ± 72.6 | 11.92 * ± 29.4 | 0.088 |
| Δ 0–8 weeks | −429.2 ± 247.0 | −421.9 ± 284 | 0.449 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Chien, Y.-W. Fresh Tomato (Lycopersicon esculentum Mill.) in the Diet Improves the Features of the Metabolic Syndrome: A Randomized Study in Postmenopausal Women. Biology 2024, 13, 588. https://doi.org/10.3390/biology13080588
Chen C-Y, Chien Y-W. Fresh Tomato (Lycopersicon esculentum Mill.) in the Diet Improves the Features of the Metabolic Syndrome: A Randomized Study in Postmenopausal Women. Biology. 2024; 13(8):588. https://doi.org/10.3390/biology13080588
Chicago/Turabian StyleChen, Chein-Yin, and Yi-Wen Chien. 2024. "Fresh Tomato (Lycopersicon esculentum Mill.) in the Diet Improves the Features of the Metabolic Syndrome: A Randomized Study in Postmenopausal Women" Biology 13, no. 8: 588. https://doi.org/10.3390/biology13080588
APA StyleChen, C.-Y., & Chien, Y.-W. (2024). Fresh Tomato (Lycopersicon esculentum Mill.) in the Diet Improves the Features of the Metabolic Syndrome: A Randomized Study in Postmenopausal Women. Biology, 13(8), 588. https://doi.org/10.3390/biology13080588







