Effects of Ammonia Concentration on Sperm Vitality, Motility Rates, and Morphology in Three Marine Bivalve Species: A Comparative Study of the Noble Scallop Mimachlamys nobilis, Chinese Pearl Oyster Pinctada fucata martensii, and Small Rock Oyster Saccostrea mordax
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Trait Data Collection
2.3. Sperm Collection
2.4. Experimental Setup with Different Concentrations of Ammonia Solution on Sperm
2.5. The Impact of Different Concentrations of Ammonia Water on the Vitality of Sperm from M. nobiliss, P. fucata martensii, and S. mordax
2.6. The Impact of Different Concentrations of Ammonia Water on Curvilinear Velocity, Average Path Velocity, and Straight-Line Velocity of Sperm from M. nobiliss, P. fucata martensii, and S. mordax
2.7. Observation of Sperm Morphology under Different Concentrations of Ammonia Water
2.8. Quality Evaluation of Sperm Vitality
2.9. Data Analysis
3. Results
3.1. Impact of Different Ammonia Concentrations on the Vitality of Sperm from M. nobiliss, P. fucata martensii, and S. mordax
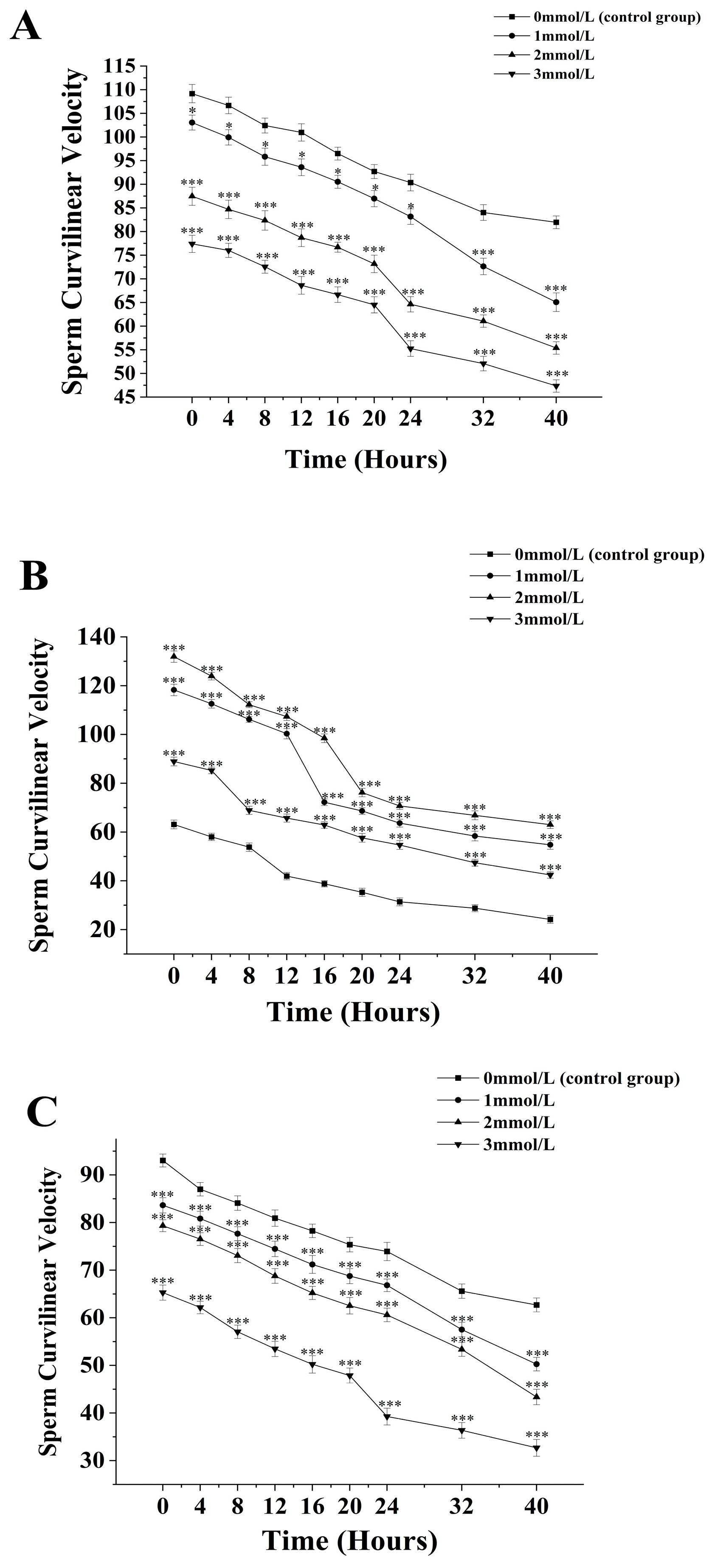
3.2. The Effects of Different Concentrations of Ammonia Water on the Curvilinear Velocity of Sperm from M. nobiliss, P. fucata Martensii, and S. mordax
3.3. The Effects of Different Concentrations of Ammonia Water on the Average Path Velocity of Sperm from M. nobiliss, P. fucata Martensii, and S. mordax
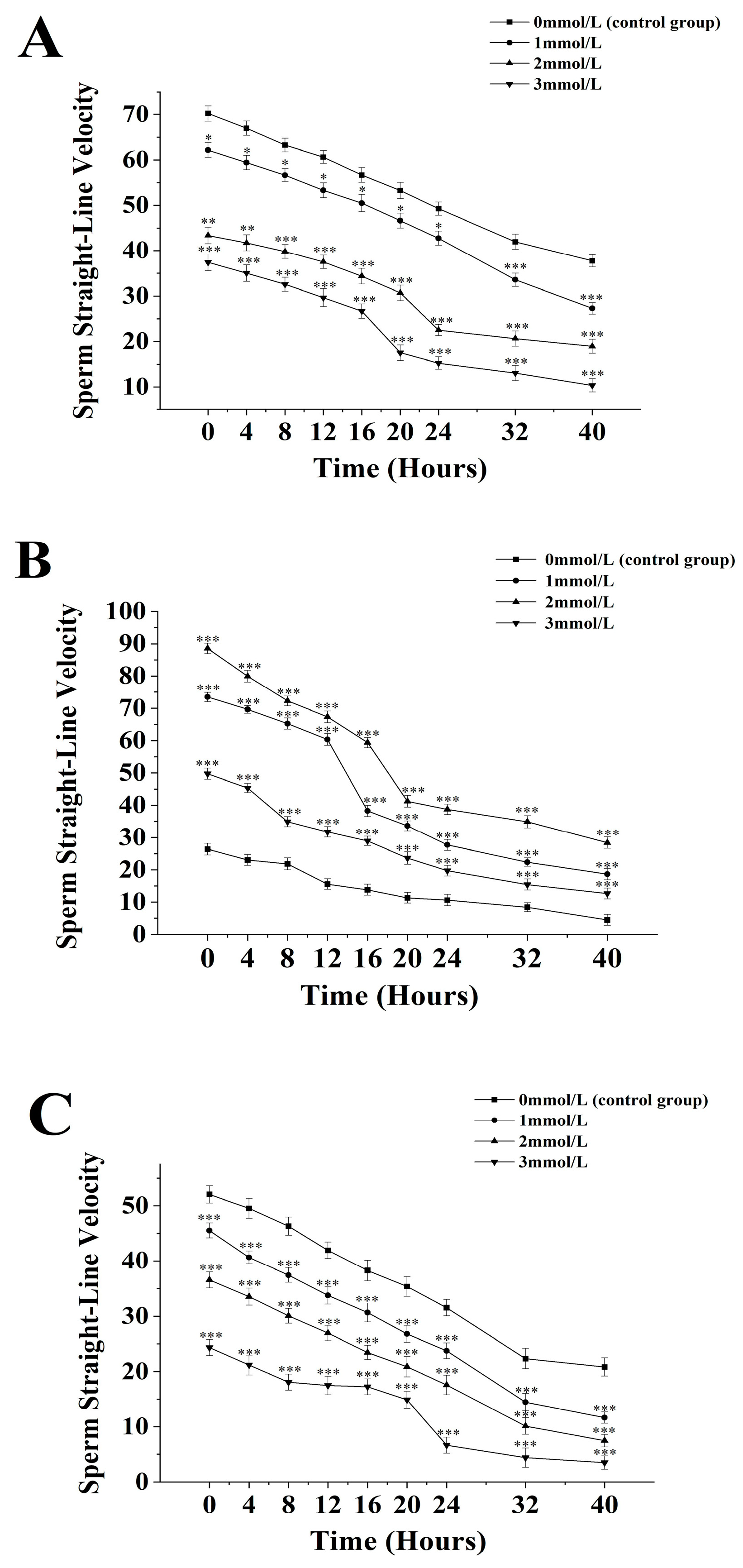
3.4. The Effects of Different Concentrations of Ammonia Water on the Straight-Line Velocity of Sperm from M. nobiliss, P. fucata martensii, and S. mordax
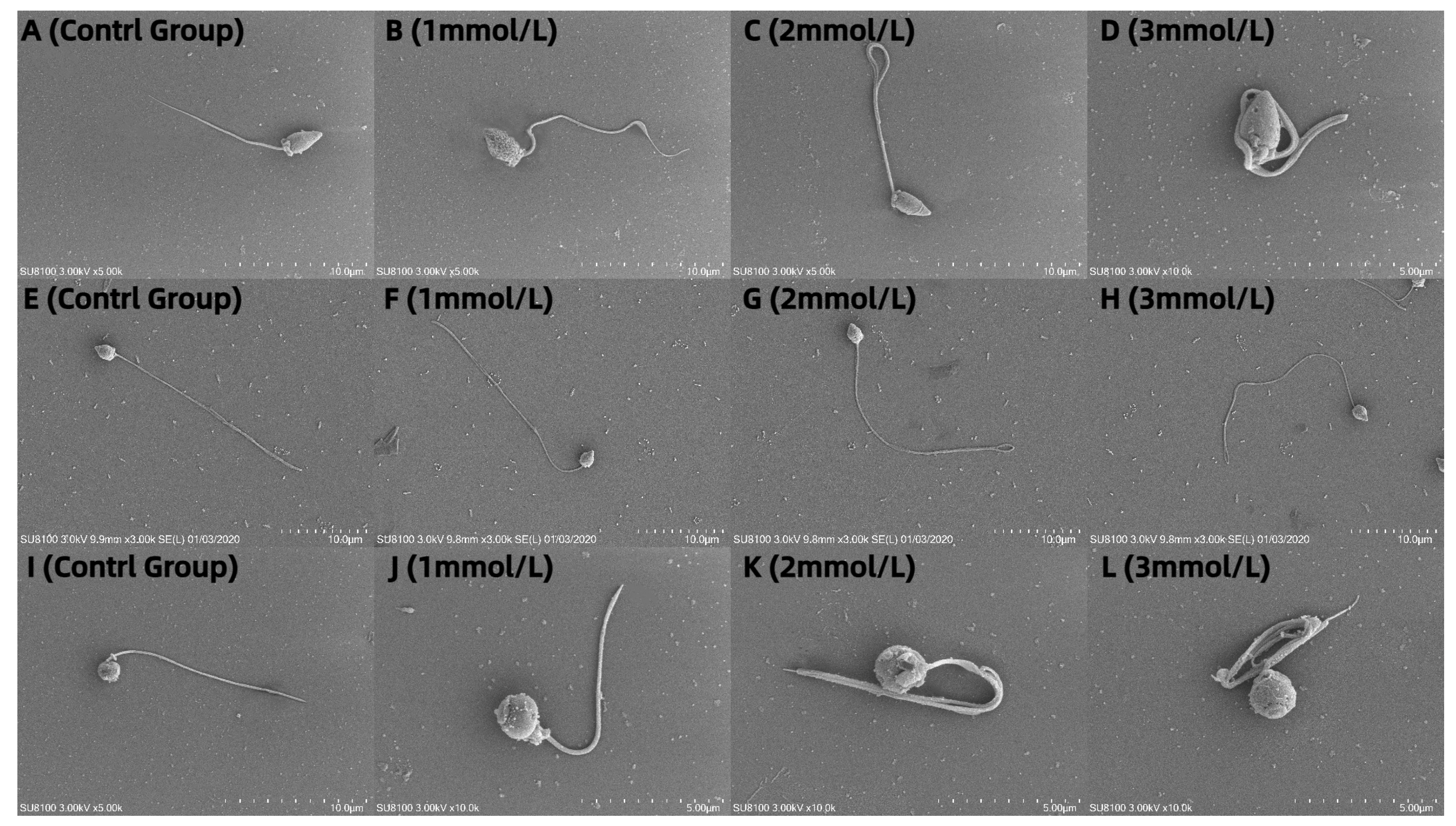
3.5. Observation of the Effects of Different Concentrations of Ammonia Water on the Morphology of Sperm from M. nobiliss, P. fucata Martensii, and S. mordax with Distinctive Biting or Tooth-like Structures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foote, M.; Edie, S.M.; Jablonski, D. Ecological structure of diversity-dependent diversification in phanerozoic marine bivalves. Biol. Lett. 2024, 20, 20230475. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wei, H.; Zhang, L.; Liu, L.; Li, Y.; Shu, Y.; Yang, Y.; Zhao, B.; Xing, Q.; Zeng, Q.; et al. K252a inhibition of sperm viability for efficient crossbreeding of hermaphroditic bivalves. Aquaculture 2023, 577, 739930. [Google Scholar] [CrossRef]
- Tan, K.; Xu, P.; Huang, L.; Luo, C.; Huang, J.; Fazhan, H.; Kwan, K.Y. Effects of bivalve aquaculture on plankton and benthic community. Sci. Total Environ. 2024, 914, 169892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, C.; Sun, Y.; Zhang, H.; Ye, T.; Liu, F.; Ma, H.; Li, S.; Zheng, H. Selective breeding in the noble scallop (Chlamys nobilis) for low-temperature resistance to reduce overwintering losses. Aquaculture 2024, 586, 740737. [Google Scholar] [CrossRef]
- Ye, T.; Tan, K.; Zhang, H.; Zheng, H. Potential causative factors of noble scallop Chlamys nobilis mass mortality in nan’ao island, shantou, china in 2017. Sci. Total Environ. 2021, 751, 142268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, Y.; Cao, W.; Zhou, L.; Zhang, C.; Qin, X.; Zheng, H.; Lin, H.; Gao, J. Novel insight into the role of processing stages in nutritional components changes and characteristic flavors formation of noble scallop Chlamys nobilis adductors. Food Chem. 2022, 378, 132049. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Deng, Z.; Qin, J.G.; Wang, A.; Gu, Z.; Ma, Z. Noble scallop, Chlamys nobilis, sperm motility duration in the post-activation phase. Anim. Reprod. Sci. 2018, 196, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, Y.; Gu, Z.; Li, S.; Shi, Y.; Guo, X. Development of expressed sequence tags from the pearl oyster, Pinctada martensii Dunker. Mar. Biotechnol. 2011, 13, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Deng, Z.; Ma, S.; Sun, D.; Liu, Y.; Yang, C.; Wu, Q.; Yu, G. A new record of Pinctada fucata (Bivalvia: Pterioida: Pteriidae) in mischief reef: A potential invasive species in the nansha islands, china. Diversity 2023, 15, 578. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, B.; Gu, Z.; Li, M.; Yang, S.; Wang, A.; Liu, C. Comparison in growth, feeding, and metabolism between a fast-growing selective strain and a cultured population of pearl oyster (Pinctada fucata martensii). Front. Mar. Sci. 2021, 8, 770702. [Google Scholar] [CrossRef]
- Hideo, A.; Akira, K.; Mitsuyoshi, N.; Kiyoshi, I.; Masahiro, H.; Takayuki, K.; Yuya, T.; Hiromi, O. Investigation of Methods for Mass Cryopreservation of Akoya Pearl Oyster Sperm. Nippon. Suisan Gakkaishi (Jpn. J. Fish. Sci.) 2007, 73, 1049–1056. [Google Scholar] [CrossRef]
- Zongmei, C.; Lisha, H.; Cui, L.; Zhen, Z.; Ximing, G.; Haiyan, W. Identification of Saccostrea mordax and a new species Saccostrea mordoides sp. Nov. (Bivalvia: Ostreidae) from china. J. Shellfish. Res. 2021, 40, 63–75. [Google Scholar] [CrossRef]
- Amaral, V.; Simone, L. Comparative anatomy of five species of saccostrea dollfus and dautzenberg, 1920 (Bivalvia: Ostreidae) from the pacific ocean. Nautilus 2016, 130, 53–71. [Google Scholar]
- Ardura, A.; Gonzalez-Sanz, A.; Clusa, L.; Planes, S.; Garcia-Vazquez, E. Beware of oysters. Rapid advance of non-native species in tropical pacific islands. Mar. Environ. Res. 2021, 170, 105436. [Google Scholar] [CrossRef] [PubMed]
- Nichols, Z.G.; Rikard, S.; Alavi, S.; Walton, W.C.; Butts, I. Regulation of sperm motility in eastern oyster (Crassostrea virginica) spawning naturally in seawater with low salinity. PLoS ONE 2021, 16, e0243569. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Zidni, I.; Lee, Y.H.; Lee, H.B.; Lim, H.K. Effect of long-term storage on the quality of cryopreserved sperm of the giant grouper, epinephelus lanceolatus. Aquaculture 2022, 555, 738154. [Google Scholar] [CrossRef]
- Nynca, J.; Judycka, S.; Liszewska, E.; Dobosz, S.; Krzyś, M.; Ciereszko, A. Effect of double freezing fish semen on sperm motility and fertility. Aquaculture 2021, 530, 735782. [Google Scholar] [CrossRef]
- Hassan, M.M.; Qin, J.G.; Li, X. Sperm cryopreservation in oysters: A review of its current status and potentials for future application in aquaculture. Aquaculture 2015, 438, 24–32. [Google Scholar] [CrossRef]
- Sandoval-Vargas, L.; Dumorné, K.; Contreras, P.; Farías, J.G.; Figueroa, E.; Risopatrón, J.; Valdebenito, I. Cryopreservation of coho salmon sperm (Oncorhynchus kisutch): Effect on sperm function, oxidative stress and fertilizing capacity. Aquaculture 2021, 533, 736151. [Google Scholar] [CrossRef]
- Altieri, K.E.; Spence, K.A.M.; Smith, S. Air-Sea Ammonia Fluxes Calculated from High-Resolution Summertime Observations Across the Atlantic Southern Ocean. Geophys. Res. Lett. 2021, 48, e2020GL091963. [Google Scholar] [CrossRef]
- Sintes, E.; De Corte, D.; Haberleitner, E.; Herndl, G.J. Geographic Distribution of Archaeal Ammonia Oxidizing Ecotypes in the Atlantic Ocean. Front. Microbiol. 2016, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; An, J.; Park, E.; Kim, J.; Cho, M.; Han, S.; Lee, J. Technical–Economic Analysis for Ammonia Ocean Transportation Using an Ammonia-Fueled Carrier. Sustainability 2024, 16, 827. [Google Scholar] [CrossRef]
- Zheng, X.; Fu, Z.; Lin, S.; Yang, R.; Wang, A.; Gu, Z.; Ma, Z. Which is the major trigger in aquatic environment for pearl oyster Pinctada fucata martensii sperm from gonad: Ammonia ion or pH? Aquaculture 2020, 520, 734673. [Google Scholar] [CrossRef]
- Dietrich, G.J.; Nynca, J.; Szczepkowski, M.; Dobosz, S.; Szczepkowska, B.; Ciereszko, A. The effect of cryopreservation of semen from whitefish (Coregonus lavaretus) and northern pike (Esox lucius) using a glucose-methanol extender on sperm motility parameters and fertilizing ability. Aquaculture 2016, 464, 60–64. [Google Scholar] [CrossRef]
- Sanchez, M.P.; Franca, T.S.; Gonzalez-Lopez, W.A.; Morini, M.; Asturiano, J.F.; Perez, L. Effect of seawater temperature and ph on the sperm motility of the european eel. Fish. Physiol. Biochem. 2024. [Google Scholar] [CrossRef] [PubMed]
- Rusco, G.; Di Iorio, M.; Esposito, S.; Antenucci, E.; Roncarati, A.; Iaffaldano, N. The use of ovarian fluid as natural fertilization medium for cryopreserved semen in mediterranean brown trout: The effects on sperm swimming performance. Vet. Sci. 2023, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Demoy-Schneider, M.; Schmitt, N.; Le Pennec, G.; Suquet, M.; Cosson, J. Quality assessment of cryopreserved black-lip pearl oyster Pinctada Margaritifera spermatozoa. Aquaculture 2018, 497, 278–286. [Google Scholar] [CrossRef]
- Adams, S.L.; Smith, J.F.; Taylor, J.; McGowan, L.T. Cryopreservation of Greenshell™ Mussel (Perna canaliculus) Sperm; Springer: New York, NY, USA, 2014; pp. 329–336. [Google Scholar] [CrossRef]
- Kvarnemo, C.; Green, L.; Svensson, O.; Lindström, K.; Schöld, S.; Griful-Dones, M.; Havenhand, J.N.; Leder, E.H. Molecular, behavioural and morphological comparisons of sperm adaptations in a fish with alternative reproductive tactics. Evol. Appl. 2022, 16, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Suquet, M.; Pouvreau, S.; Queau, I.; Boulais, M.; Le Grand, J.; Ratiskol, D.; Cosson, J. Biological characteristics of sperm in European flat oyster (Ostrea edulis). Aquat. Living Resour. 2018, 31, 20. [Google Scholar] [CrossRef]
- Vidal, E.; de Bastos, D.N.; Oliveira, E.A.; Butzge, A.J.; Mewes, J.K.; Baumgartner, G.; Sanches, P.V.; Bombardelli, R.A. Effect of ammonia on the artificial fertilization of oocytes and early development of jundia (Rhamdia quelen, Siluriformes, Heptapteridae). Semin. Agrar. 2013, 34, 2447–2456. [Google Scholar] [CrossRef]
- Dubé, F.; Eckberg, W.R. Intracellular pH Increase Driven by an Na+/H+ Exchanger upon Activation of Surf Clam Oocytes. Dev. Biol. 1997, 190, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, R.; Osanai, K. Meiosis reinitiation from the first prophase is dependent on the levels of intracellular Ca2+ and pH in oocytes of the bivalves Mactra chinensis and Limaria hakodatensis. Dev. Biol. 1994, 166, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Doree, M.; Sano, K.; Kanatani, H. Ammonia and other weak bases applied at any time of the hormone-dependent period inhibit 1-methyladenine-induced meiosis reinitiation of starfish oocytes. Dev. Biol. 1982, 90, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kabutomori, J.; Beloto-Silva, O.; Geyer, R.; Musa-Aziz, R. Rana catesbeiana Oocytes: An Alternative To The Xenopus laevis Heterologous Expression System For Ammonia Transporters. FASEB J. 2015, 29, 809–822. [Google Scholar] [CrossRef]
- Aramli, M.S.; Nazari, R.M.; Kalbassi, M.R.; Aramli, S. Semen of beluga, Huso huso: Ionic content and osmolality of seminal plasma and their physiological correlation with sperm motility indices. Fish. Aquac. J. 2013, 4, 1. [Google Scholar] [CrossRef]
- Bernardes, J.J.; Jimenez, J.E.; Bombardelli, R.A.; Nuner, A. Changes in external osmolality and ionic composition affect Megaleporinus obtusidens sperm motility. Anim. Reprod. Sci. 2018, 190, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.H. Chapter 56 measurement of membrane potential and Na+ and H+ transport in isolated sea urchin sperm flagella and their membrane vesicles. In Methods in Cell Biology; Dentler, W., Witman, G., Eds.; Academic Press: New York, NY, USA, 1995; pp. 401–406. [Google Scholar] [CrossRef]
- Bae, J.; Kwon, H.J.; Kim, S.; Ma, L.; Im, H.; Kim, E.; Kim, M.O.; Kwon, W. Corrigendum to “inhalation of ammonium sulfate and ammonium nitrate adversely affect sperm function” [reproductive toxicology, 96, (2020) 424–431]. Reprod. Toxicol. 2021, 102, 128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, W.; Liu, X.; Zhang, P.; Hao, Y.; Li, L.; Chen, L.; Shen, W.; Tang, X.; Min, L.; et al. Hydrogen sulfide and/or ammonia reduces spermatozoa motility through ampk/akt related pathways. Sci. Rep. 2016, 6, 37884. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, M.T.; Galindo, B.E.; de De La Torre, L.; Zapata, O.; Rodríguez, E.; Florman, H.M.; Darszon, A. A sustained increase in intracellular Ca2+ is required for the acrosome reaction in sea urchin sperm. Dev. Biol. 2001, 236, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Ryu, Y.; Matsubara, T. Features of sperm motility and circadian rhythm in japanese anchovy (Engraulis japonicus). Fish. Aquac. J. 2017, 8, 1000203. [Google Scholar] [CrossRef]
- Cosson, J.; Groison, A.L.; Suquet, M.; Fauvel, C.; Dreanno, C.; Billard, R. Studying sperm motility in marine fish: An overview on the state of the art. J. Appl. Ichthyol. 2008, 24, 460–486. [Google Scholar] [CrossRef]
- Boulais, M.; Demoy-Schneider, M.; Alavi, S.M.H.; Cosson, J. Spermatozoa motility in bivalves: Signaling, flagellar beating behavior, and energetics. Theriogenology 2019, 136, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Foldvik, A.; Holthe, E.; Bremset, G.; Solem, O. Effects of episodic exposure to high-ph water on survival of atlantic salmon eggs and juveniles: Results from laboratory and field studies. Environ. Toxicol. Chem. 2022, 41, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Boulais, M.; Suquet, M.; Arsenault-Pernet, E.J.; Malo, F.; Queau, I.; Pignet, P.; Ratiskol, D.; Le Grand, J.; Huber, M.; Cosson, J. Ph controls spermatozoa motility in the pacific oyster (Crassostrea gigas). Biol. Open 2018, 7, bio031427. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Kawamoto, T.; Isowa, K.; Aoki, H.; Hayashi, M.; Narita, T.; Komaru, A. Motility of spermatozoa obtained from testes of japanese pearl oyster Pinctada fucata martensii. Fish. Sci. 2007, 73, 107–111. [Google Scholar] [CrossRef]
- Nakajima, A.; Morita, M.; Takemura, A.; Kamimura, S.; Okuno, M. Increase in intracellular ph induces phosphorylation of axonemal proteins for activation of flagellar motility in starfish sperm. J. Exp. Biol. 2005, 208 Pt 23, 4411–4418. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, H.; Tateno, H. Prevention of high-temperature-induced chromosome damage in mouse spermatozoa freeze-dried using Ca2+ chelator-containing buffer alkalinized with naoh or koh. Cryobiology 2017, 79, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Dahui, Y.; Shigui, J.; Tianfeng, S.; Jingchun, C. The Role of Major Seawater Ions in Sperm Activation of Hepu Pearl Oyster. Acta Oceanol. Sin. 1999, 18, 5–10. [Google Scholar] [CrossRef]
- Zheng, X.; Gu, Z.; Huang, Z.; Ding, H.; Vasquez, H.E.; Liu, Y.; Shi, Y.; Wang, A. The effects of cryoprotectants on sperm motility of the chinese pearl oyster, Pinctada fucata martensii. Cryobiology 2018, 82, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Darszon, A.; Nishigaki, T.; Beltran, C.; Treviño, C.L. Calcium channels in the development, maturation, and function of spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar] [CrossRef] [PubMed]
- Reunov, A.; Vekhova, E.; Zakharov, E.; Reunova, Y.; Alexandrova, Y.; Sharina, S.; Adrianov, A. Variation of sperm morphology in pacific oyster precludes its use as a species marker but enables intraspecific geo-authentification and aquatic monitoring. Helgol. Mar. Res. 2018, 72, 8. [Google Scholar] [CrossRef]
- Chen, J.; Deng, Z.; Wei, H.; Zhao, W.; Chen, M.; Yu, G.; Sun, J.; Yu, D.; Li, Y.; Wang, Y.; et al. Spermatozoa morphology and embryo development of four species of bivalves from beibu gulf. Turk. J. Fish. Quat. Sci. 2021, 21, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Robinson, N.; Qin, J. Sperm cryopreservation in marine mollusk: A review. Aquac. Int. 2015, 23, 1505–1524. [Google Scholar] [CrossRef]
- De Castro, F.; Stefàno, E.; Migoni, D.; Iaconisi, G.N.; Muscella, A.; Marsigliante, S.; Benedetti, M.; Fanizzi, F.P. Synthesis and evaluation of the cytotoxic activity of water-soluble cationic organometallic complexes of the type [Pt(η1-C2H4OMe)(L)(Phen)]+ (L = NH3, DMSO; Phen = 1,10-Phenanthroline). Pharmaceutics 2021, 13, 642. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, M.C.; Morini, M.; Penaranda, D.S.; Gallego, V.; Asturiano, J.F.; Perez, L. Role of potassium and ph on the initiation of sperm motility in the european eel. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2017, 203, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.E.; Casciotti, K.L. Enrichment and characterization of ammonia-oxidizing archaea from the open ocean: Phylogeny, physiology and stable isotope fractionation. Int. Soc. Microb. Ecol. J. 2011, 5, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Masserini, R.T.; Abbot, W.; Hunt, H.R.; Friden, E.; Heil, C.A.; Klass, S.M. An improved reverse flow injection analysis (rFIA) technique for determination of nanomolar concentrations of ammonium in natural waters with automatic background fluorescence detection: Ammonification during a Karenia brevis bloom in Tampa Bay. Mar. Chem. 2022, 245, 104158. [Google Scholar] [CrossRef]
- Rolton, A.; Soudant, P.; Vignier, J.; Pierce, R.; Henry, M.; Shumway, S.E.; Bricelj, V.M.; Volety, A.K. Susceptibility of gametes and embryos of the eastern oyster, crassostrea virginica, to karenia brevis and its toxins). Pharmaceutics 2015, 13, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Kawamoto, T.; Isowa, K.; Aoki, H.; Hayashi, M.; Ohta, H.; Komaru, A. Effects of cryopreservation on sperm structure in japanese pearl osyter Pinctada fucata martensii. Fish. Sci. 2008, 74, 1069–1074. [Google Scholar] [CrossRef]
- Suminto, S.; Nuriman, A.A.; Chilmawati, D. The addition of ammonia solution with different concentrations in the sperms on the percentage ovulation egg of abalone, Haliotis Asinina. Saintek Perikan. Indones. J. Fish. Sci. Technol. 2010, 5, 31–37. [Google Scholar]


| Species | Shell Length (mm) | Wet Weight (g) | Gonad Weight (g) |
|---|---|---|---|
| M. nobiliss 1 | 53.86 | 51.96 | 13.57 |
| M. nobiliss 2 | 57.64 | 58.64 | 12.35 |
| M. nobiliss 3 | 61.31 | 61.31 | 13.61 |
| P. fucata martensii 1 | 63.90 | 27..37 | 6.7 |
| P. fucata martensii 2 | 59.75 | 25.84 | 7.3 |
| P. fucata martensii 3 | 60.59 | 27.09 | 8.6 |
| S. mordax 1 | 65.18 | 84.46 | 17.53 |
| S. mordax 2 | 62.43 | 87.63 | 19.83 |
| S. mordax 3 | 67.22 | 85.27 | 22.60 |
| Species | Concentration (mmol/L)-pH |
|---|---|
| M. nobiliss | 0-(7.80~8.20) |
| M. nobiliss | 1-(8.47~8.52) |
| M. nobiliss | 2-(9.05~9.12) |
| M. nobiliss | 3-(9.29~9.63) |
| P. fucata martensii | 0-(7.80~8.20) |
| P. fucata martensii | 1-(8.47~8.52) |
| P. fucata martensii | 2-(9.05~9.12) |
| P. fucata martensii | 3-(9.29~9.63) |
| S. mordax | 0-(7.80~8.20) |
| S. mordax | 1-(8.47~8.52) |
| S. mordax | 2-(9.05~9.12) |
| S. mordax | 3-(9.29~9.63) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wu, J.; Yang, R.; Fu, Z.; Yu, G.; Ma, Z. Effects of Ammonia Concentration on Sperm Vitality, Motility Rates, and Morphology in Three Marine Bivalve Species: A Comparative Study of the Noble Scallop Mimachlamys nobilis, Chinese Pearl Oyster Pinctada fucata martensii, and Small Rock Oyster Saccostrea mordax. Biology 2024, 13, 589. https://doi.org/10.3390/biology13080589
Li M, Wu J, Yang R, Fu Z, Yu G, Ma Z. Effects of Ammonia Concentration on Sperm Vitality, Motility Rates, and Morphology in Three Marine Bivalve Species: A Comparative Study of the Noble Scallop Mimachlamys nobilis, Chinese Pearl Oyster Pinctada fucata martensii, and Small Rock Oyster Saccostrea mordax. Biology. 2024; 13(8):589. https://doi.org/10.3390/biology13080589
Chicago/Turabian StyleLi, Minghao, Jiong Wu, Rui Yang, Zhengyi Fu, Gang Yu, and Zhenhua Ma. 2024. "Effects of Ammonia Concentration on Sperm Vitality, Motility Rates, and Morphology in Three Marine Bivalve Species: A Comparative Study of the Noble Scallop Mimachlamys nobilis, Chinese Pearl Oyster Pinctada fucata martensii, and Small Rock Oyster Saccostrea mordax" Biology 13, no. 8: 589. https://doi.org/10.3390/biology13080589
APA StyleLi, M., Wu, J., Yang, R., Fu, Z., Yu, G., & Ma, Z. (2024). Effects of Ammonia Concentration on Sperm Vitality, Motility Rates, and Morphology in Three Marine Bivalve Species: A Comparative Study of the Noble Scallop Mimachlamys nobilis, Chinese Pearl Oyster Pinctada fucata martensii, and Small Rock Oyster Saccostrea mordax. Biology, 13(8), 589. https://doi.org/10.3390/biology13080589








