Activation of P2X7 Receptor Mediates the Abnormal Ovulation Induced by Chronic Restraint Stress and Chronic Cold Stress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Grouping
2.2. Vaginal Smear
2.3. Superovulation
2.4. Hematoxylin–Eosin (H&E) Staining
2.5. Sirius Red Staining
2.6. Granulosa Cell Culture and Treatment
2.7. Cumulus–Oocyte Complex (COC) Culture and Treatment
2.8. Immunohistochemistry
2.9. Corticosterone and Progesterone Determination by ELISA
2.10. RNA Extraction and Real-Time PCR
2.11. Western Blot
2.12. Statistical Analysis
3. Results
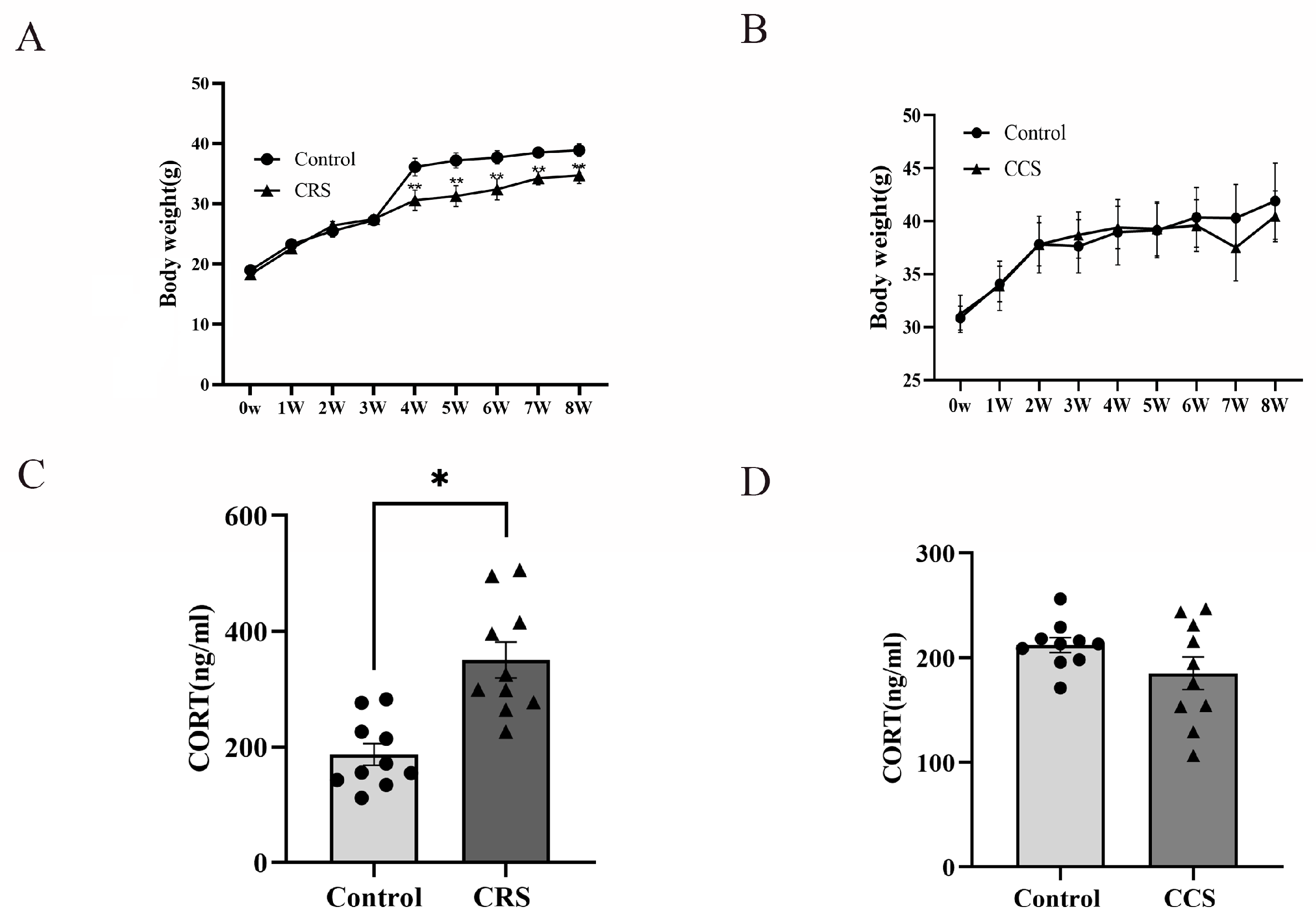
3.1. The Effects of Chronic Stress on Body Weight, Serum Corticosterone Concentration and Estrous Cycle in Mice
3.2. The Corpus Luteum Number and Progesterone Were Decreased in the Chronic Stress Model
3.3. The Expression of P2X7R Was Increased in Ovaries of Chronic Stress Mice and P2X7R Antagonist Partially Rescued the Ovulation Rate of Chronic Stress Mice
3.4. P2X7R Signaling Was Involved in Cumulus Expansion by Regulating NPPC Expression
3.5. Ovarian Fibrosis Was Increased in Chronic Stress Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar] [PubMed]
- Schneiderman, N.; Ironson, G.; Siegel, S.D. Stress and health: Psychological, behavioral, and biological determinants. Annu. Rev. Clin. Psychol. 2005, 1, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Golbidi, S.; Frisbee, J.C.; Laher, I. Chronic stress impacts the cardiovascular system: Animal models and clinical outcomes. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1476–H1498. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Steptoe, A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 2018, 15, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Rashidy-Pour, A. Association between chronic stress and Alzheimer’s disease: Therapeutic effects of Saffron. Biomed. Pharmacother. = Biomed. Pharmacother. 2021, 133, 110995. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, Y.; Wang, Y.; Liu, L.; Zhang, X.; Li, B.; Cui, R. The Effects of Psychological Stress on Depression. Curr. Neuropharmacol. 2015, 13, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Liu, Y.; Cao, C.; Zeng, Y.; Pan, Y.; Liu, X.; Peng, Y.; Wu, F. Chronic Stress: Impacts on Tumor Microenvironment and Implications for Anti-Cancer Treatments. Front. Cell Dev. Biol. 2021, 9, 777018. [Google Scholar] [CrossRef] [PubMed]
- Oyola, M.G.; Handa, R.J. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: Sex differences in regulation of stress responsivity. Stress (Amst. Neth.) 2017, 20, 476–494. [Google Scholar] [CrossRef]
- Heck, A.L.; Handa, R.J. Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: An important role for gonadal hormones. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 44, 45–58. [Google Scholar] [CrossRef]
- Xiang, Y.; Jiang, L.; Gou, J.; Sun, Y.; Zhang, D.; Xin, X.; Song, Z.; Huang, J. Chronic unpredictable mild stress-induced mouse ovarian insufficiency by interrupting lipid homeostasis in the ovary. Front. Cell Dev. Biol. 2022, 10, 933674. [Google Scholar] [CrossRef]
- Wu, L.M.; Liu, Y.S.; Tong, X.H.; Shen, N.; Jin, R.T.; Han, H.; Hu, M.H.; Wang, W.; Zhou, G.X. Inhibition of follicular development induced by chronic unpredictable stress is associated with growth and differentiation factor 9 and gonadotropin in mice. Biol. Reprod. 2012, 86, 121. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, M.; Arancibia, S.; Fiedler, J.L.; Lara, H.E. Chronic intermittent cold stress activates ovarian sympathetic nerves and modifies ovarian follicular development in the rat. Biol. Reprod. 2003, 68, 2038–2043. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.M.; Hu, M.H.; Tong, X.H.; Han, H.; Shen, N.; Jin, R.T.; Wang, W.; Zhou, G.X.; He, G.P.; Liu, Y.S. Chronic unpredictable stress decreases expression of brain-derived neurotrophic factor (BDNF) in mouse ovaries: Relationship to oocytes developmental potential. PLoS ONE 2012, 7, e52331. [Google Scholar] [CrossRef]
- Smith, J.; Ferguson, D.; Jauregui, G.; Panarace, M.; Medina, M.; Lehnert, S.; Hill, J.R. Short-term maternal psychological stress in the post-conception period in ewes affects fetal growth and gestation length. Reproduction (Camb. Engl.) 2008, 136, 259–265. [Google Scholar] [CrossRef]
- Wiebold, J.L.; Stanfield, P.H.; Becker, W.C.; Hillers, J.K. The effect of restraint stress in early pregnancy in mice. J. Reprod. Fertil. 1986, 78, 185–192. [Google Scholar] [CrossRef]
- Sugino, N.; Nakamura, Y.; Okuno, N.; Shimamura, K.; Teyama, T.; Ishimatsu, M.; Kato, H. Effects of restraint stress on luteal function in rats during mid-pregnancy. J. Reprod. Fertil. 1994, 101, 23–26. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Tian, Z.; Wu, Y.; Wang, Y.; Fang, Y.; Lin, L.; Han, Y.; Wu, S.; Haq, I.; et al. Effects of chronic heat stress on granulosa cell apoptosis and follicular atresia in mouse ovary. J. Anim. Sci. Biotechnol. 2016, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, M.R.; Soch, A.; Ziko, I.; De Luca, S.N.; Spencer, S.J.; Sominsky, L. Chronic predator stress in female mice reduces primordial follicle numbers: Implications for the role of ghrelin. J. Endocrinol. 2019, 241, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Mao, H.; Cui, Z.; Yao, H.; Shi, R.; Gao, Y. Scream Sound-induced Chronic Psychological Stress Results in Diminished Ovarian Reserve in Adult Female Rat. Endocrinology 2022, 163, bqac042. [Google Scholar] [CrossRef]
- Casillas, F.; Flores-González, A.; Juárez-Rojas, L.; López, A.; Betancourt, M.; Casas, E.; Bahena, I.; Bonilla, E.; Retana-Márquez, S. Chronic stress decreases fertility parameters in female rats. Syst. Biol. Reprod. Med. 2023, 69, 234–244. [Google Scholar] [CrossRef]
- Sluyter, R. The P2X7 Receptor. Adv. Exp. Med. Biol. 2017, 1051, 17–53. [Google Scholar]
- Yue, N.; Huang, H.; Zhu, X.; Han, Q.; Wang, Y.; Li, B.; Liu, Q.; Wu, G.; Zhang, Y.; Yu, J. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J. Neuroinflammation 2017, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Ota, K.T.; Li, X.Y.; Sakaue, F.; Li, N.; Dutheil, S.; Banasr, M.; Duric, V.; Yamanashi, T.; Kaneko, K.; et al. Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biol. Psychiatry 2016, 80, 12–22. [Google Scholar] [CrossRef]
- Ribeiro, D.E.; Roncalho, A.L.; Glaser, T.; Ulrich, H.; Wegener, G.; Joca, S. P2X7 Receptor Signaling in Stress and Depression. Int. J. Mol. Sci. 2019, 20, 2778. [Google Scholar] [CrossRef]
- von Muecke-Heim, I.A.; Ries, C.; Urbina, L.; Deussing, J.M. P2X7R antagonists in chronic stress-based depression models: A review. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 1343–1358. [Google Scholar] [CrossRef]
- Huang, Z.; Tan, S. P2X7 Receptor as a Potential Target for Major Depressive Disorder. Curr. Drug Targets 2021, 22, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.S.; Casarotto, P.C.; Hiroaki-Sato, V.A.; Sartim, A.G.; Guimarães, F.S.; Joca, S.R. Antidepressant- and anticompulsive-like effects of purinergic receptor blockade: Involvement of nitric oxide. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2013, 23, 1769–1778. [Google Scholar] [CrossRef]
- Yamashita, Y.; Shimada, M. The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process. J. Reprod. Dev. 2012, 58, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Richani, D.; Gilchrist, R.B. The epidermal growth factor network: Role in oocyte growth, maturation and developmental competence. Hum. Reprod. Update 2018, 24, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Umehara, T.; Hoshino, Y. Roles of epidermal growth factor (EGF)-like factor in the ovulation process. Reprod. Med. Biol. 2016, 15, 201–216. [Google Scholar] [CrossRef]
- Zhang, M.; Su, Y.Q.; Sugiura, K.; Xia, G.; Eppig, J.J. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science (New York N.Y.) 2010, 330, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Shi, L.B.; Zhang, S.Y. Ovarian Fibrosis: A Phenomenon of Concern. Chin. Med. J. 2017, 130, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, E.; Szigeti, J.F.; Ujma, P.P.; Sexty, R.; Balog, P. Anxiety and depression among infertile women: A cross-sectional survey from Hungary. BMC Women’s Health 2017, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Rooney, K.L.; Domar, A.D. The relationship between stress and infertility. Dialogues Clin. Neurosci. 2018, 20, 41–47. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, F.; Zhang, Y.; Wang, W.; Cao, Q. Diminished ovarian reserve induced by chronic unpredictable stress in C57BL/6 mice. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2020, 36, 49–54. [Google Scholar] [CrossRef]
- Filatov, M.; Khramova, Y.; Parshina, E.; Bagaeva, T.; Semenova, M. Influence of gonadotropins on ovarian follicle growth and development in vivo and in vitro. Zygote (Camb. Engl.) 2017, 25, 235–243. [Google Scholar] [CrossRef]
- Joseph, D.N.; Whirledge, S. Stress and the HPA Axis: Balancing Homeostasis and Fertility. Int. J. Mol. Sci. 2017, 18, 2224. [Google Scholar] [CrossRef]
- Toufexis, D.; Rivarola, M.A.; Lara, H.; Viau, V. Stress and the reproductive axis. J. Neuroendocrinol. 2014, 26, 573–586. [Google Scholar] [CrossRef]
- Wei, Y.; Li, W.; Meng, X.; Zhang, L.; Shen, M.; Liu, H. Corticosterone Injection Impairs Follicular Development, Ovulation and Steroidogenesis Capacity in Mice Ovary. Animals 2019, 9, 1047. [Google Scholar] [CrossRef]
- Nair, B.B.; Khant Aung, Z.; Porteous, R.; Prescott, M.; Glendining, K.A.; Jenkins, D.E.; Augustine, R.A.; Silva, M.S.B.; Yip, S.H.; Bouwer, G.T.; et al. Impact of chronic variable stress on neuroendocrine hypothalamus and pituitary in male and female C57BL/6J mice. J. Neuroendocrinol. 2021, 33, e12972. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Sun, J.; Wang, Q.; Zhang, Q.; Wei, C.; Lai, D. Chronic restraint stress induces excessive activation of primordial follicles in mice ovaries. PLoS ONE 2018, 13, e0194894. [Google Scholar] [CrossRef]
- Matsuura, N.; Nagasawa, K.; Minagawa, Y.; Ito, S.; Sano, Y.; Yamada, Y.; Hattori, T.; Watanabe, S.; Murohara, T.; Nagata, K. Restraint stress exacerbates cardiac and adipose tissue pathology via β-adrenergic signaling in rats with metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1275–H1286. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Hall, D.; Subramanian, M. Sympathetic nervous system as a target for aging and obesity-related cardiovascular diseases. GeroScience 2019, 41, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.K.; Capitanio, J.P.; Tarara, R.P.; Mendoza, S.P.; Mason, W.A.; Cole, S.W. Social stress enhances sympathetic innervation of primate lymph nodes: Mechanisms and implications for viral pathogenesis. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 8857–8865. [Google Scholar] [CrossRef]
- Townsend, A.D.; Wilken, G.H.; Mitchell, K.K.; Martin, R.S.; Macarthur, H. Simultaneous analysis of vascular norepinephrine and ATP release using an integrated microfluidic system. J. Neurosci. Methods 2016, 266, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Wier, W.G.; Zang, W.J.; Lamont, C.; Raina, H. Sympathetic neurogenic Ca2+ signalling in rat arteries: ATP, noradrenaline and neuropeptide Y. Exp. Physiol. 2009, 94, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic signalling: From discovery to current developments. Exp. Physiol. 2014, 99, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Goonetilleke, L.; Ralevic, V.; Dunn, W.R. Influence of pressure on adenosine triphosphate function as a sympathetic neurotransmitter in small mesenteric arteries from the spontaneously hypertensive rat. J. Hypertens. 2013, 31, 312–320. [Google Scholar] [CrossRef]
- Haddock, R.E.; Hill, C.E. Sympathetic overdrive in obesity involves purinergic hyperactivity in the resistance vasculature. J. Physiol. 2011, 589 Pt 13, 3289–3307. [Google Scholar] [CrossRef]
- Kowalski, R.; Kreft, E.; Kasztan, M.; Jankowski, M.; Szczepanska-Konkel, M. Chronic renal denervation increases renal tubular response to P2X receptor agonists in rats: Implication for renal sympathetic nerve ablation. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2012, 27, 3443–3448. [Google Scholar] [CrossRef]
- Zamah, A.M.; Hsieh, M.; Chen, J.; Vigne, J.L.; Rosen, M.P.; Cedars, M.I.; Conti, M. Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin. Hum. Reprod. (Oxf. Engl.) 2010, 25, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Kiyosu, C.; Akiyama, K.; Kunieda, T. CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles. Mol. Reprod. Dev. 2012, 79, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kong, N.; Li, N.; Hao, X.; Wei, K.; Xiang, X.; Xia, G.; Zhang, M. Epidermal growth factor receptor signaling-dependent calcium elevation in cumulus cells is required for NPR2 inhibition and meiotic resumption in mouse oocytes. Endocrinology 2013, 154, 3401–3409. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M.; Di Vincenzo, A.; Pagano, C.; El Hadi, H.; Vettor, R. The P2X7 Receptor and NLRP3 Axis in Non-Alcoholic Fatty Liver Disease: A Brief Review. Cells 2020, 9, 1047. [Google Scholar] [CrossRef]
- Gonçalves, R.G.; Gabrich, L.; Rosário, A., Jr.; Takiya, C.M.; Ferreira, M.L.; Chiarini, L.B.; Persechini, P.M.; Coutinho-Silva, R.; Leite, M., Jr. The role of purinergic P2X7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int. 2006, 70, 1599–1606. [Google Scholar] [CrossRef]





| Gene | Sense and Antisense Primers |
|---|---|
| EGF | 5′-ACTCCCACCTACCCTCCTA-3′ 5′-CTGAACTGGCTCTGTCTGC-3′ |
| EGFR | 5′-ACCTCTCCCGGTCAGAGATG-3′ 5′-CTTGTGCCTTGGCAGACTTTC-3′ |
| EREG | 5′-TGCTTTGTCTAGGTTCCCACC-3′ 5′-GGCGGTACAGTTATCCTCGG-3′ |
| AREG | 5′-GCTGAGGACAATGCAGGGTAA-3′ 5′-GTGACAACTGGGCATCTGGA-3′ |
| BTC | 5′-CCTCACAGCACAGTTGATGG-3′ 5′-GGTGTTCTGGTTGTGTTCCC-3′ |
| NPPC | 5′-GGTCTGGGATGTTAGTGCAGCTA-3′ 5′-TAAAAGCCACATTGCGTTGGA-3′ |
| NPR2 | 5′-GCTGACCCGGCAAGTTCTGT-3′ 5′-ACAATACTCGGTGACAATGCAGAT-3′ |
| β-actin | 5′-CACGATGGAGGGGCCGGACTCATC-3′ 5′-TAAAGACCTCTATGCCAACACAGT-3′ |
| Group | n | Proestrus (d) | Estrus (d) | Late Estrus (d) | Estrus Interval (d) | Estrous Cycle (d) |
|---|---|---|---|---|---|---|
| Control | 10 | 1.05 ± 0.05 | 1.27 ± 0.09 | 1.92 ± 0.15 | 1.21 ± 0.08 | 4.54 ± 0.51 |
| CRS | 10 | 0.81 ± 0.04 | 1.21 ± 0.09 | 2.58 ± 0.15 * | 2.01 ± 0.09 * | 5.81 ± 0.79 * |
| Control | 10 | 1.09 ± 0.16 | 1.72 ± 0.23 | 1.36 ± 0.20 | 1.27 ± 0.19 | 5.36 ± 0.52 |
| CCS | 10 | 1.15 ± 0.10 | 1.38 ± 0.14 | 2.46 ± 0.29 ** | 1.76 ± 0.12 | 6.53 ± 0.63 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Wang, J.; Ma, Y.; Chai, D.; Han, S.; Xiao, C.; Huang, Y.; Wang, X.; Wang, J.; Wang, S.; et al. Activation of P2X7 Receptor Mediates the Abnormal Ovulation Induced by Chronic Restraint Stress and Chronic Cold Stress. Biology 2024, 13, 620. https://doi.org/10.3390/biology13080620
Fan X, Wang J, Ma Y, Chai D, Han S, Xiao C, Huang Y, Wang X, Wang J, Wang S, et al. Activation of P2X7 Receptor Mediates the Abnormal Ovulation Induced by Chronic Restraint Stress and Chronic Cold Stress. Biology. 2024; 13(8):620. https://doi.org/10.3390/biology13080620
Chicago/Turabian StyleFan, Xiang, Jing Wang, Yinyin Ma, Dandan Chai, Suo Han, Chuyu Xiao, Yingtong Huang, Xiaojie Wang, Jianming Wang, Shimeng Wang, and et al. 2024. "Activation of P2X7 Receptor Mediates the Abnormal Ovulation Induced by Chronic Restraint Stress and Chronic Cold Stress" Biology 13, no. 8: 620. https://doi.org/10.3390/biology13080620
APA StyleFan, X., Wang, J., Ma, Y., Chai, D., Han, S., Xiao, C., Huang, Y., Wang, X., Wang, J., Wang, S., Xiao, L., & Zhang, C. (2024). Activation of P2X7 Receptor Mediates the Abnormal Ovulation Induced by Chronic Restraint Stress and Chronic Cold Stress. Biology, 13(8), 620. https://doi.org/10.3390/biology13080620







