Neutralizing Oxidized Phosphatidylcholine Reduces Airway Inflammation and Hyperreactivity in a Murine Model of Allergic Asthma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Animals
2.3. Murine Model of Allergic Asthma and E06 mAb Treatment
2.4. Real-Time PCR for Anti-Oxidant Genes
2.5. Lung Function Assessment
2.6. Bronchoalveolar Lavage Fluid (BALF) Collection
2.7. OxPC Quantification in BALF
2.8. Flow Cytometry Immunophenotyping of Inflammatory Cells in BALF
2.9. BALF Cytokine Analysis
2.10. Statistical Analysis
3. Results
3.1. HDM Challenge Induces Lung Inflammation and Inhibits Expression of Anti-Oxidant Genes
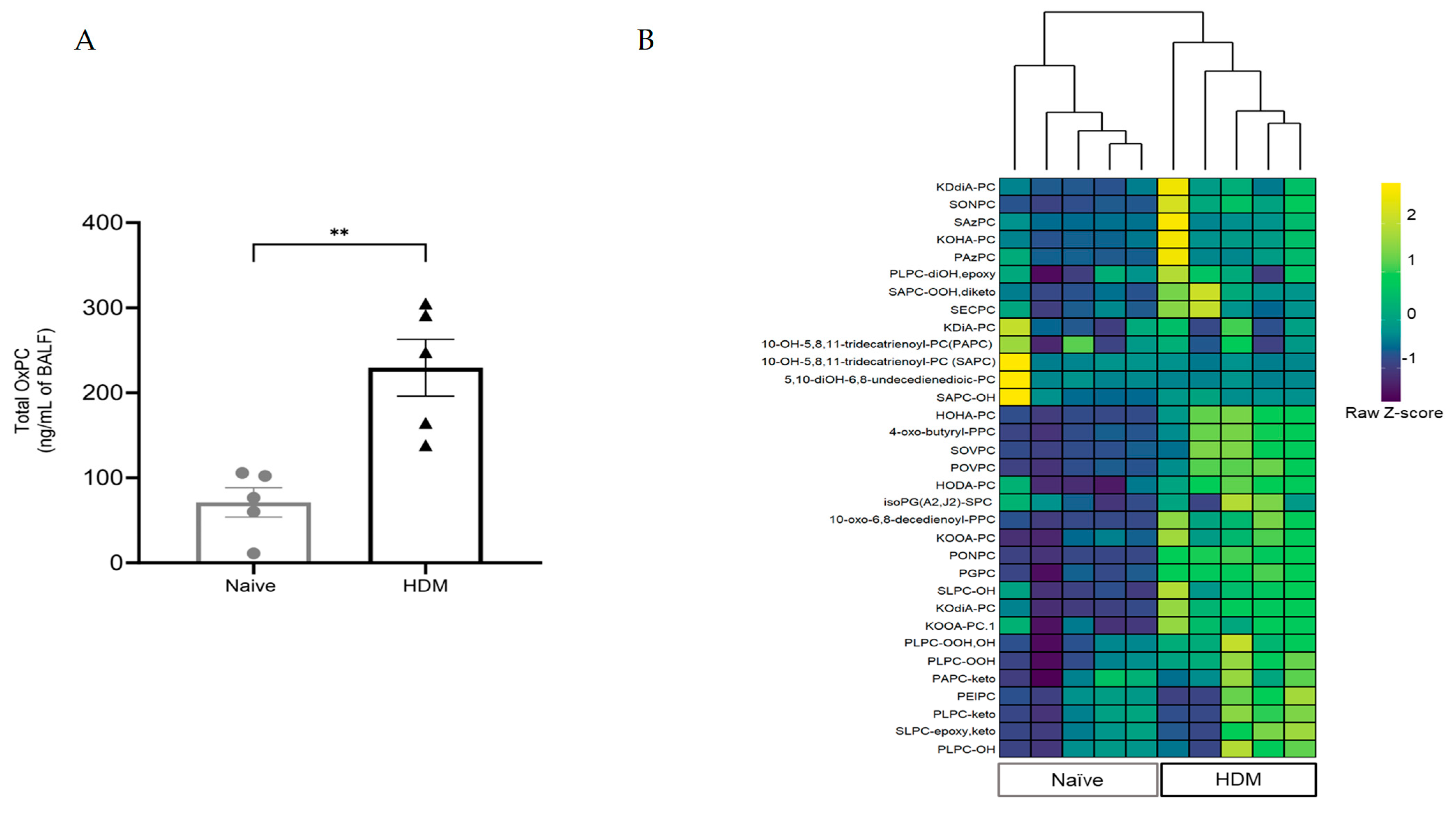
3.2. HDM Challenge Induces OxPC Formation in the Lung
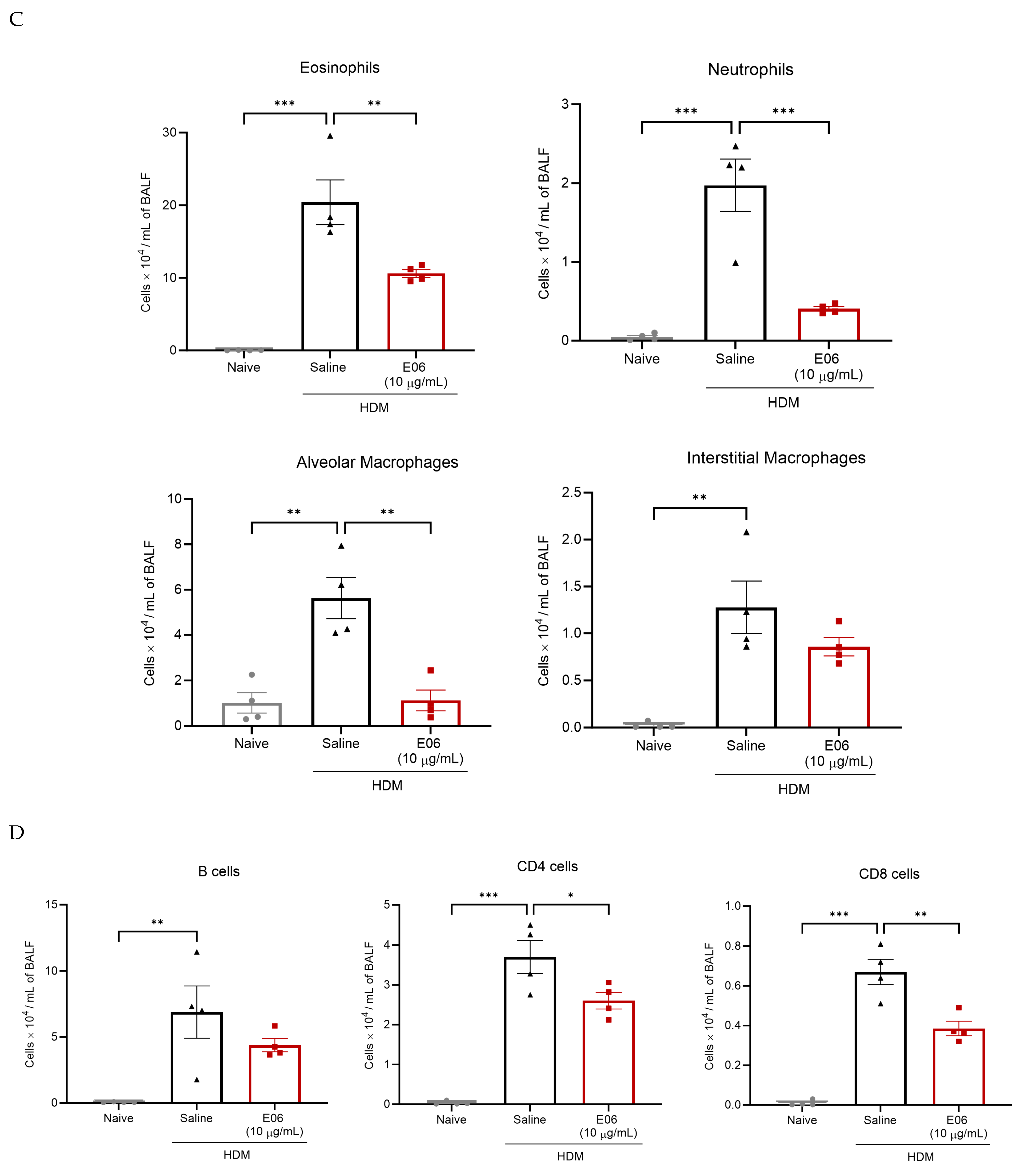
3.3. E06 mAb Treatment Reduces HDM-Induced Infiltration of Lung Inflammatory Cells
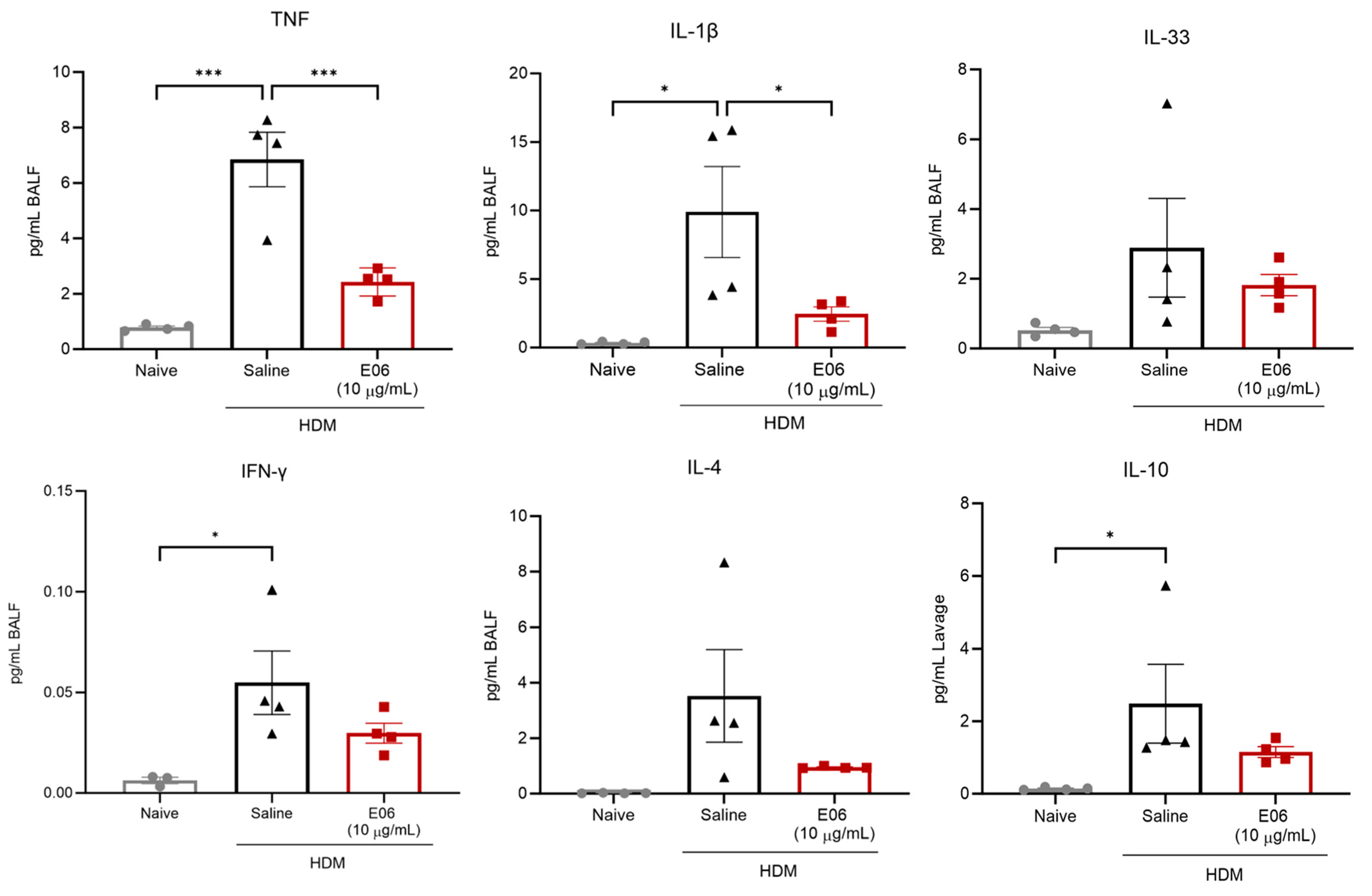
3.4. Impact of E06 mAb Treatment on HDM-Induced BALF Cytokines and Chemokines
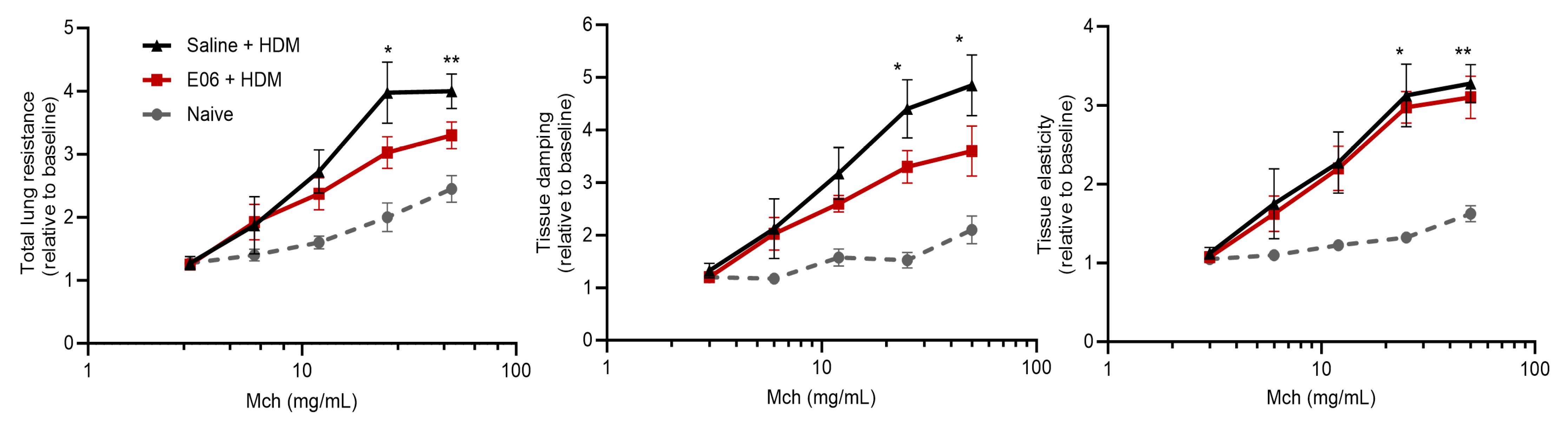
3.5. Impact of E06 mAb Treatment on HDM-Induced Lung Airway Hyperreactivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Erle, D.J.; Sheppard, D. The cell biology of asthma. J. Cell Biol. 2014, 205, 621–631. [Google Scholar] [CrossRef]
- Busse, W.W.; Pedersen, S.; Pauwels, R.A.; Tan, W.C.; Chen, Y.Z.; Lamm, C.J.; O’Byrne, P.M.; Group, S.I. The Inhaled Steroid Treatment As Regular Therapy in Early Asthma (START) study 5-year follow-up: Effectiveness of early intervention with budesonide in mild persistent asthma. J. Allergy Clin. Immunol. 2008, 121, 1167–1174. [Google Scholar] [CrossRef]
- Barisione, G.; Baroffio, M.; Crimi, E.; Brusasco, V. Beta-Adrenergic Agonists. Pharmaceuticals 2010, 3, 1016–1044. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.K.; Penn, R.B.; Hanania, N.A.; Dickey, B.F.; Bond, R.A. New perspectives regarding β(2) -adrenoceptor ligands in the treatment of asthma. Br. J. Pharmacol. 2011, 163, 18–28. [Google Scholar] [CrossRef]
- Billington, C.K.; Penn, R.B.; Hall, I.P. beta2 Agonists. Handb. Exp. Pharmacol. 2017, 237, 23–40. [Google Scholar] [CrossRef]
- To, T.; Stanojevic, S.; Moores, G.; Gershon, A.S.; Bateman, E.D.; Cruz, A.A.; Boulet, L.P. Global asthma prevalence in adults: Findings from the cross-sectional world health survey. BMC Public Health 2012, 12, 204, Erratum in BMC Public Health 2021, 21, 1809. [Google Scholar]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Scelo, G.; Torres-Duque, C.A.; Maspero, J.; Tran, T.N.; Murray, R.; Martin, N.; Menzies-Gow, A.N.; Hew, M.; Peters, M.J.; Gibson, P.G.; et al. Analysis of comorbidities and multimorbidity in adult patients in the International Severe Asthma Registry. Ann. Allergy Asthma Immunol. 2023, 132, 42–53. [Google Scholar] [CrossRef]
- Ray, A.; Raundhal, M.; Oriss, T.B.; Ray, P.; Wenzel, S.E. Current concepts of severe asthma. J. Clin. Investig. 2016, 126, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Qualls, C.; Seagrave, J.; McDonald, J.; Shohreh, R.; Chiavaroli, A.; Schuyler, M. Effect of allergen inhalation on airway oxidant stress, using exhaled breath condensate 8-isoprostane, in mild asthma. J. Asthma 2013, 50, 449–456. [Google Scholar] [CrossRef]
- van der Vliet, A.; Janssen-Heininger, Y.M.W.; Anathy, V. Oxidative stress in chronic lung disease: From mitochondrial dysfunction to dysregulated redox signaling. Mol. Aspects Med. 2018, 63, 59–69. [Google Scholar] [CrossRef]
- Lewis, B.W.; Ford, M.L.; Rogers, L.K.; Britt, R.D., Jr. Oxidative Stress Promotes Corticosteroid Insensitivity in Asthma and COPD. Antioxidants 2021, 10, 1335. [Google Scholar] [CrossRef]
- To, T.; Terebessy, E.; Zhu, J.; Zhang, K.; Lakey, P.S.; Shiraiwa, M.; Hatzopoulou, M.; Minet, L.; Weichenthal, S.; Dell, S.; et al. Does early life exposure to exogenous sources of reactive oxygen species (ROS) increase the risk of respiratory and allergic diseases in children? A longitudinal cohort study. Environ. Health 2022, 21, 90. [Google Scholar] [CrossRef]
- Vasconcelos, L.H.C.; Ferreira, S.R.D.; Silva, M.D.C.C.; Ferreira, P.B.; de Souza, I.L.L.; Cavalcante, F.A.; da Silva, B.A. Uncovering the Role of Oxidative Imbalance in the Development and Progression of Bronchial Asthma. Oxidative Med. Cell. Longev. 2021, 2021, 6692110. [Google Scholar] [CrossRef]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, O. Oxidative stress in asthma. World Allergy Organ. J. 2011, 4, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Erzurum, S.C. New Insights in Oxidant Biology in Asthma. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S1), S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Masood, A.; Siddiqui, N. Oxidant--antioxidant imbalance in asthma: Scientific evidence, epidemiological data and possible therapeutic options. Ther. Adv. Respir. Dis. 2008, 2, 215–235. [Google Scholar] [CrossRef]
- Vincenzo, S.D.; Ferrante, G.; Ferraro, M.; Cascio, C.; Malizia, V.; Licari, A.; La Grutta, S.; Pace, E. Oxidative Stress, Environmental Pollution, and Lifestyle as Determinants of Asthma in Children. Biology 2023, 12, 133. [Google Scholar] [CrossRef]
- Holguin, F. Oxidative stress in airway diseases. Ann. Am. Thorac. Soc. 2013, 10, S150–S157. [Google Scholar] [CrossRef]
- Pascoe, C.D.; Vaghasiya, J.; Halayko, A.J. Oxidation specific epitopes in asthma: New possibilities for treatment. Int. J. Biochem. Cell Biol. 2020, 129, 105864. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, C.D.; Roy, N.; Turner-Brannen, E.; Schultz, A.; Vaghasiya, J.; Ravandi, A.; Halayko, A.J.; West, A.R. Oxidized phosphatidylcholines induce multiple functional defects in airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L703–L717. [Google Scholar] [CrossRef]
- Vaghasiya, J.; Dalvand, A.; Sikarwar, A.; Mangat, D.; Ragheb, M.; Kowatsch, K.; Pandey, D.; Hosseini, S.M.; Hackett, T.L.; Karimi-Abdolrezaee, S.; et al. Oxidized Phosphatidylcholines Trigger TRPA1 and Ryanodine Receptor Dependent Airway Smooth Muscle Contraction. Am. J. Respir. Cell Mol. Biol. 2023, 69, 649–665. [Google Scholar] [CrossRef]
- Pascoe, C.D.; Jha, A.; Ryu, M.H.; Ragheb, M.; Vaghasiya, J.; Basu, S.; Stelmack, G.L.; Srinathan, S.; Kidane, B.; Kindrachuk, J.; et al. Allergen inhalation generates pro-inflammatory oxidised phosphatidylcholine associated with airway dysfunction. Eur. Respir. J. 2021, 57, 2000839. [Google Scholar] [CrossRef]
- Binder, C.J.; Papac-Milicevic, N.; Witztum, J.L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 2016, 16, 485–497. [Google Scholar] [CrossRef]
- Lee, S.; Birukov, K.G.; Romanoski, C.E.; Springstead, J.R.; Lusis, A.J.; Berliner, J.A. Role of phospholipid oxidation products in atherosclerosis. Circ. Res. 2012, 111, 778–799. [Google Scholar] [CrossRef] [PubMed]
- Oehler, B.; Kistner, K.; Martin, C.; Schiller, J.; Mayer, R.; Mohammadi, M.; Sauer, R.; Filipovic, M.; Nieto, F.; Kloka, J.; et al. Inflammatory pain control by blocking oxidized phospholipid-mediated TRP channel activation. Sci. Rep. 2017, 7, 5447. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Neely, G.G.; Yaghubian-Malhami, R.; Perkmann, T.; van Loo, G.; Ermolaeva, M.; Veldhuizen, R.; Leung, Y.H.; Wang, H.; et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008, 133, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Karki, P.; Kim, J.; Son, S.; Berdyshev, E.; Bochkov, V.N.; Birukova, A.A.; Birukov, K.G. Elevated truncated oxidized phospholipids as a factor exacerbating ALI in the aging lungs. FASEB J. 2019, 33, 3887–3900. [Google Scholar] [CrossRef]
- Usui, K.; Hulleman, J.D.; Paulsson, J.F.; Siegel, S.J.; Powers, E.T.; Kelly, J.W. Site-specific modification of Alzheimer’s peptides by cholesterol oxidation products enhances aggregation energetics and neurotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 18563–18568. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Pascoe, C.D.; Edel, A.L.; El-Gammal, A.; Ravandi, A.; Gauvreau, G.M.; O’Byrne, P.; Halayko, A.J. Peroxidation of Phosphatidylcholine: A Signature for Allergen-Induced Airway Responses and Allergen Specificity. In D98. Insights into Environmental Exposures in Asthma, Copd, and Constrictive Bronchiolitis, Proceedings of the American Thoracic Society 2017 International Conference, Washington, DC, USA, 19–24 May 2017; ATS: New York, NY, USA, 2017; p. A7338. [Google Scholar]
- Liu, B.; Tai, Y.; Caceres, A.I.; Achanta, S.; Balakrishna, S.; Shao, X.; Fang, J.; Jordt, S.E. Oxidized Phospholipid OxPAPC Activates TRPA1 and Contributes to Chronic Inflammatory Pain in Mice. PLoS ONE 2016, 11, e0165200. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W.; Hörkkö, S.; Miller, E.; Steinbrecher, U.P.; Powell, H.C.; Curtiss, L.K.; Witztum, J.L. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J. Clin. Investig. 1996, 98, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Friedman, P.; Horkko, S.; Steinberg, D.; Witztum, J.L.; Dennis, E.A. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J. Biol. Chem. 2002, 277, 7010–7020. [Google Scholar] [CrossRef] [PubMed]
- Hörkkö, S.; Bird, D.A.; Miller, E.; Itabe, H.; Leitinger, N.; Subbanagounder, G.; Berliner, J.A.; Friedman, P.; Dennis, E.A.; Curtiss, L.K.; et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Investig. 1999, 103, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Yeang, C.; Hasanally, D.; Que, X.; Hung, M.-Y.; Stamenkovic, A.; Chan, D.; Chaudhary, R.; Margulets, V.; Edel, A.L.; Hoshijima, M.; et al. Reduction of myocardial ischaemia–reperfusion injury by inactivating oxidized phospholipids. Cardiovasc. Res. 2019, 115, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Oehler, B.; Kloka, J.; Martin, C.; Brack, A.; Blum, R.; Rittner, H. Antinociception by the anti-oxidized phospholipid antibody E06. Br. J. Pharmacol. 2018, 175, 2940–2955. [Google Scholar] [CrossRef] [PubMed]
- Que, X.; Hung, M.Y.; Yeang, C.; Gonen, A.; Prohaska, T.A.; Sun, X.; Diehl, C.; Maatta, A.; Gaddis, D.E.; Bowden, K.; et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018, 558, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Sun, X.; Witztum, J.; Glass, C. Oxidized Phospholipid Neutralizing Antibody Ameliorates Hyperoxia-Induced Lung Injury. In B59. Acute Lung Injury and Ards: Cells, Tissues, and Animal Models, Proceedings of the American Thoracic Society 2022 International Conference, San Francisco, CA, USA, 13–18 May 2022; ATS: New York, NY, USA, 2022; p. A5648. [Google Scholar] [CrossRef]
- Jha, A.; Ryu, M.H.; Oo, O.; Bews, H.J.; Carlson, J.C.; Schwartz, J.; Basu, S.; Wong, C.S.; Halayko, A.J. Prophylactic benefits of systemically delivered simvastatin treatment in a house dust mite challenged murine model of allergic asthma. Br. J. Pharmacol. 2018, 175, 1004–1016. [Google Scholar] [CrossRef]
- Pascoe, C.D.; Jha, A.; Basu, S.; Mahood, T.; Lee, A.; Hinshaw, S.; Falsafi, R.; Hancock, R.E.W.; Mookherjee, N.; Halayko, A.J.; et al. The importance of reporting house dust mite endotoxin abundance: Impact on the lung transcriptome. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 318, L1229–L1236. [Google Scholar] [CrossRef]
- Jha, A. Effect of Simvastatin on Airway Inflammation, Remodelling and Hyperreactivity in a House Dust Mite Challenged Murine Model of Allergic Asthma. Ph.D. Thesis, University of Manitoba, Winnipeg, MB, Canada, 2018. [Google Scholar]
- Bates, J.H.; Irvin, C.G.; Farré, R.; Hantos, Z. Oscillation mechanics of the respiratory system. Compr. Physiol. 2011, 1, 1233–1272. [Google Scholar] [CrossRef]
- Addison, K.J.; Morse, J.; Robichaud, A.; Daines, M.O.; Ledford, J.G. A Novel in vivo System to Test Bronchodilators. J. Infect. Pulm. Dis. 2017, 3. [Google Scholar] [CrossRef]
- McGovern, T.K.; Robichaud, A.; Fereydoonzad, L.; Schuessler, T.F.; Martin, J.G. Evaluation of respiratory system mechanics in mice using the forced oscillation technique. J. Vis. Exp. 2013, 75, e50172. [Google Scholar] [CrossRef]
- Raemdonck, K.; Baker, K.; Dale, N.; Dubuis, E.; Shala, F.; Belvisi, M.G.; Birrell, M.A. CD4+ and CD8+ T cells play a central role in a HDM driven model of allergic asthma. Respir. Res. 2016, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Lopuhaä, C.E.; Out, T.A.; Jansen, H.M.; Aalberse, R.C.; van der Zee, J.S. Allergen-induced bronchial inflammation in house dust mite-allergic patients with or without asthma. Clin. Exp. Allergy 2002, 32, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Lukawska, J.J.; Livieratos, L.; Sawyer, B.M.; Lee, T.; O’Doherty, M.; Blower, P.J.; Kofi, M.; Ballinger, J.R.; Corrigan, C.J.; Gnanasegaran, G.; et al. Imaging Inflammation in Asthma: Real Time, Differential Tracking of Human Neutrophil and Eosinophil Migration in Allergen Challenged, Atopic Asthmatics In Vivo. eBioMedicine 2014, 1, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Netto, K.G.; Zhou, L.; Liu, X.; Wang, M.; Zhang, G.; Foster, P.S.; Li, F.; Yang, M. Single-cell transcriptomic analysis reveals the immune landscape of lung in steroid-resistant asthma exacerbation. Proc. Natl. Acad. Sci. USA 2021, 118, e2005590118. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; Shamsuddin, M.; Sporn, P.H.; Denenberg, M.; Anderson, J. Reduced superoxide dismutase in lung cells of patients with asthma. Free Radic. Biol. Med. 1997, 22, 1301–1307. [Google Scholar] [CrossRef]
- Comhair, S.A.; Erzurum, S.C. Redox control of asthma: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal 2010, 12, 93–124. [Google Scholar] [CrossRef]
- Dong, Y.; Yong, V.W. Oxidized phospholipids as novel mediators of neurodegeneration. Trends Neurosci. 2022, 45, 419–429. [Google Scholar] [CrossRef]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Hoftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative damage in multiple sclerosis lesions. Brain 2011, 134, 1914–1924. [Google Scholar] [CrossRef]
- Solati, Z.; Edel, A.L.; Shang, Y.; O, K.; Ravandi, A. Oxidized phosphatidylcholines are produced in renal ischemia reperfusion injury. PLoS ONE 2018, 13, e0195172. [Google Scholar] [CrossRef]
- Ravandi, A.; Leibundgut, G.; Hung, M.Y.; Patel, M.; Hutchins, P.M.; Murphy, R.C.; Prasad, A.; Mahmud, E.; Miller, Y.I.; Dennis, E.A.; et al. Release and capture of bioactive oxidized phospholipids and oxidized cholesteryl esters during percutaneous coronary and peripheral arterial interventions in humans. J. Am. Coll. Cardiol. 2014, 63, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.I.; Choi, S.H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; D’Mello, C.; Pinsky, W.; Lozinski, B.M.; Kaushik, D.K.; Ghorbani, S.; Moezzi, D.; Brown, D.; Melo, F.C.; Zandee, S.; et al. Oxidized phosphatidylcholines found in multiple sclerosis lesions mediate neurodegeneration and are neutralized by microglia. Nat. Neurosci. 2021, 24, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Testai, F.D.; Dawson, S.; Kilkus, J.; Dawson, G. Oxidized phosphatidylcholine formation and action in oligodendrocytes. J. Neurochem. 2009, 110, 1388–1399. [Google Scholar] [CrossRef]
- Dunigan-Russell, K.; Yaeger, M.J.; Hodge, M.X.; Kilburg-Basnyat, B.; Reece, S.W.; Birukova, A.; Guttenberg, M.A.; Novak, C.; Chung, S.; Ehrmann, B.M.; et al. Scavenger receptor BI attenuates oxidized phospholipid-induced pulmonary inflammation. Toxicol. Appl. Pharmacol. 2023, 462, 116381. [Google Scholar] [CrossRef]
- Thayaparan, D.; Shen, P.; Stämpfli, M.R.; Morissette, M.C. Induction of pulmonary antibodies against oxidized lipids in mice exposed to cigarette smoke. Respir. Res. 2016, 17, 97. [Google Scholar] [CrossRef]
- Ozaras, R.; Tahan, V.; Turkmen, S.; Talay, F.; Besirli, K.; Aydin, S.; Uzun, H.; Cetinkaya, A. Changes in malondialdehyde levels in bronchoalveolar fluid and serum by the treatment of asthma with inhaled steroid and beta2-agonist. Respirology 2000, 5, 289–292. [Google Scholar] [CrossRef]
- Handa, J.T.; Tagami, M.; Ebrahimi, K.; Leibundgut, G.; Janiak, A.; Witztum, J.L.; Tsimikas, S. Lipoprotein(A) with An Intact Lysine Binding Site Protects the Retina From an Age-Related Macular Degeneration Phenotype in Mice (An American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2015, 113, T5. [Google Scholar]
- Buga, G.M.; Frank, J.S.; Mottino, G.A.; Hakhamian, A.; Narasimha, A.; Watson, A.D.; Yekta, B.; Navab, M.; Reddy, S.T.; Anantharamaiah, G.M.; et al. D-4F reduces EO6 immunoreactivity, SREBP-1c mRNA levels, and renal inflammation in LDL receptor-null mice fed a Western diet. J. Lipid Res. 2008, 49, 192–205. [Google Scholar] [CrossRef]
- Stamenkovic, A.; Pierce, G.N.N.; Witztum, J.L.; Tsimikas, S.; Ravandi, A. Abstract 12366: Intracoronary Delivery of E06 Antibody Directed to Oxidized Phospholipids Decreases Infarct Size in a Porcine Model of Ischemia/Reperfusion Injury. Circulation 2021, 144, A12366. [Google Scholar] [CrossRef]
- Gowdy, K.M.; Kilburg-Basnyat, B.; Hodge, M.X.; Reece, S.W.; Yermalitsk, V.; Davies, S.S.; Manke, J.; Armstrong, M.L.; Reisdorph, N.; Tighe, R.M.; et al. Novel Mechanisms of Ozone-Induced Pulmonary Inflammation and Resolution, and the Potential Protective Role of Scavenger Receptor BI. Res. Rep. Health Eff. Inst. 2021, 2021, 1–49. [Google Scholar] [PubMed]
- Wyatt, T.A.; Kharbanda, K.K.; McCaskill, M.L.; Tuma, D.J.; Yanov, D.; DeVasure, J.; Sisson, J.H. Malondialdehyde-acetaldehyde-adducted protein inhalation causes lung injury. Alcohol 2012, 46, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Lv, Z.; Li, Y.; Chen, Y.; Huang, K.; Corrigan, C.J.; Ying, S. Elevated Expression of IL-33 and TSLP in the Airways of Human Asthmatics In Vivo: A Potential Biomarker of Severe Refractory Disease. J. Immunol. 2018, 200, 2253–2262. [Google Scholar] [CrossRef]
- Préfontaine, D.; Lajoie-Kadoch, S.; Foley, S.; Audusseau, S.; Olivenstein, R.; Halayko, A.J.; Lemière, C.; Martin, J.G.; Hamid, Q. Increased expression of IL-33 in severe asthma: Evidence of expression by airway smooth muscle cells. J. Immunol. 2009, 183, 5094–5103. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.; Hemshekhar, M.; Lloyd, D.; Mookherjee, N. IFNγ-mediated IL-33 production is dependent on the aryl hydrocarbon receptor in human bronchial epithelial cells. Cytokine 2023, 172, 156414. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Coomes, S.M.; Kannan, Y.; Pelly, V.S.; Entwistle, L.J.; Guidi, R.; Perez-Lloret, J.; Nikolov, N.; Müller, W.; Wilson, M.S. CD4(+) Th2 cells are directly regulated by IL-10 during allergic airway inflammation. Mucosal Immunol. 2017, 10, 150–161. [Google Scholar] [CrossRef]
- Hohlfeld, J.M.; Ahlf, K.; Enhorning, G.; Balke, K.; Erpenbeck, V.J.; Petschallies, J.; Hoymann, H.G.; Fabel, H.; Krug, N. Dysfunction of Pulmonary Surfactant in Asthmatics after Segmental Allergen Challenge. Am. J. Respir. Crit. Care Med. 1999, 159, 1803–1809. [Google Scholar] [CrossRef]
- Lewis, B.W.; Ford, M.L.; Khan, A.Q.; Walum, J.; Britt, R.D., Jr. Chronic Allergen Challenge Induces Corticosteroid Insensitivity With Persistent Airway Remodeling and Type 2 Inflammation. Front. Pharmacol. 2022, 13, 855247. [Google Scholar] [CrossRef] [PubMed]
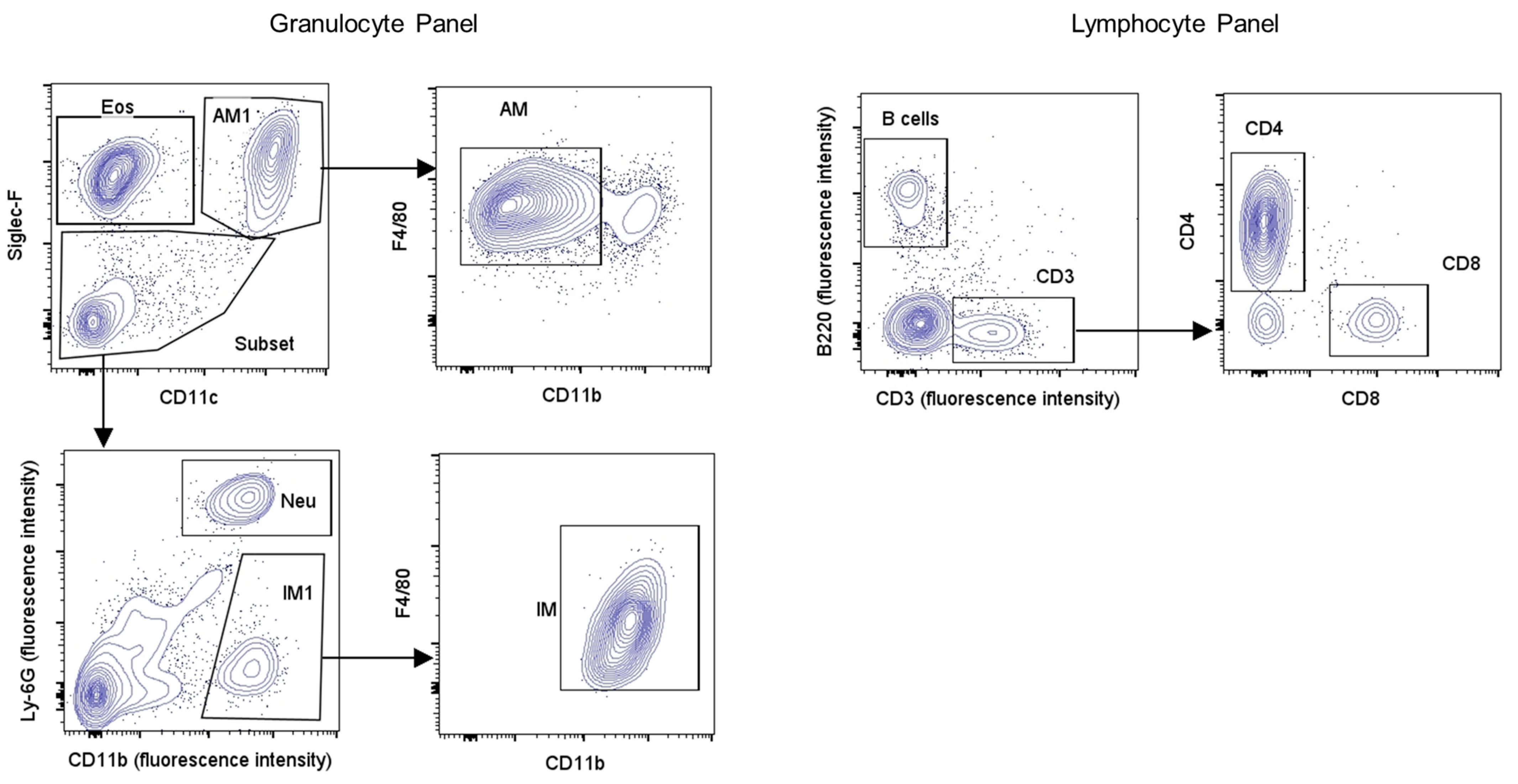




| Panel | Antibodies | Concentration (ng/µL) | Source |
|---|---|---|---|
| Compensation | PE anti-mouse CD4 | 2.0 | BioLegend, San Diego, CA, USA |
| APC anti-mouse CD4 | 2.0 | ||
| FITC anti-mouse CD4 | 5.0 | ||
| APC/Cy7 anti-mouse CD4 | 2.0 | ||
| PerCP/Cy5.5 anti-mouse CD4 | 2.0 | ||
| PE/Cy7 anti-mouse CD4 | 2.0 | ||
| Granulocytes | PE/Cy7 anti-mouse/human CD11b | 5.0 | |
| PE anti-mouse CD170 (Siglec-F) | 5.0 | ||
| FITC anti-mouse CD11c | 12.5 | ||
| PerCP/Cy5.5 anti-mouse F4/80 | 5.0 | ||
| APC anti-mouse Ly-6G | 5.0 | ||
| Lymphocytes | PE anti-mouse CD3 | 5.0 | |
| FITC anti-mouse CD8a | 12.5 | ||
| PerCP/Cy5.5 anti-mouse CD4 | 5.0 | ||
| APC anti-mouse/human CD45R/B220 | 5.0 | ||
| PE/Cyanine7 anti-mouse CD335 (NKp46) | 5.0 | ||
| Fc blocker | Anti-mouse CD16/CD32 | 5.0 | Life Technologies Corp, Carlsbad, CA, USA |
| Gene | Forward | Reverse |
|---|---|---|
| SOD1 | ACAATGGTGGTCCATGAGAAA (Sense) | GTTTACTGCGCAATCCCAATC (AntiSense) |
| SOD2 | GCAAGGAACAACAGGCCTTA (Sense) | CCCAGTTGATTACATTCCAAATAGC (AntiSense) |
| HO-1 | CTAGCCTGGTGCAAGATACTG (Sense) | CAACAGGAAGCTGAGAGTGAG (AntiSense) |
| NFE2L2 (NRF2) | CCATTCCCGAATTACAGTGTCT (Sense) | AGCGAGGAGATCGATGAGTAA (AntiSense) |
| Panel | Cell Types | Cell Surface Markers |
|---|---|---|
| Granulocytes | Eosinophils (Eos) | CD11clow Siglec-F+ |
| Neutrophils (Neu) | CD11clow Siglec-F− CD11bhigh Ly-6G+ | |
| Alveolar Macrophages (AM) | CD11chigh Siglec-F+ CD11bint F4/80+ | |
| Interstitial Macrophages (IM) | CD11clow Siglec-F− CD11bhigh F4/80+ | |
| Lymphocytes | B cells | B220+ |
| CD3 cells | CD3+ | |
| CD4 cells | CD3+ CD4+ | |
| CD8 cells | CD3+ CD8+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaghasiya, J.; Jha, A.; Basu, S.; Bagan, A.; Jengsuksavat, S.K.; Ravandi, A.; Pascoe, C.D.; Halayko, A.J., on behalf of the Canadian Respiratory Research Network. Neutralizing Oxidized Phosphatidylcholine Reduces Airway Inflammation and Hyperreactivity in a Murine Model of Allergic Asthma. Biology 2024, 13, 627. https://doi.org/10.3390/biology13080627
Vaghasiya J, Jha A, Basu S, Bagan A, Jengsuksavat SK, Ravandi A, Pascoe CD, Halayko AJ on behalf of the Canadian Respiratory Research Network. Neutralizing Oxidized Phosphatidylcholine Reduces Airway Inflammation and Hyperreactivity in a Murine Model of Allergic Asthma. Biology. 2024; 13(8):627. https://doi.org/10.3390/biology13080627
Chicago/Turabian StyleVaghasiya, Jignesh, Aruni Jha, Sujata Basu, Alaina Bagan, Siwon K. Jengsuksavat, Amir Ravandi, Christopher D. Pascoe, and Andrew J. Halayko on behalf of the Canadian Respiratory Research Network. 2024. "Neutralizing Oxidized Phosphatidylcholine Reduces Airway Inflammation and Hyperreactivity in a Murine Model of Allergic Asthma" Biology 13, no. 8: 627. https://doi.org/10.3390/biology13080627
APA StyleVaghasiya, J., Jha, A., Basu, S., Bagan, A., Jengsuksavat, S. K., Ravandi, A., Pascoe, C. D., & Halayko, A. J., on behalf of the Canadian Respiratory Research Network. (2024). Neutralizing Oxidized Phosphatidylcholine Reduces Airway Inflammation and Hyperreactivity in a Murine Model of Allergic Asthma. Biology, 13(8), 627. https://doi.org/10.3390/biology13080627







