Effects of Ocean Acidification and Temperature Coupling on Photosynthetic Activity and Physiological Properties of Ulva fasciata and Sargassum horneri
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Experimental Condition
2.3. Rapid Light Curve and Fluorescence Induction Parameters
2.4. Relative Growth Rate
2.5. Dissolved Organic Carbon Release
2.6. Photosynthetic Pigment Content
2.7. Statistical Analysis
3. Results
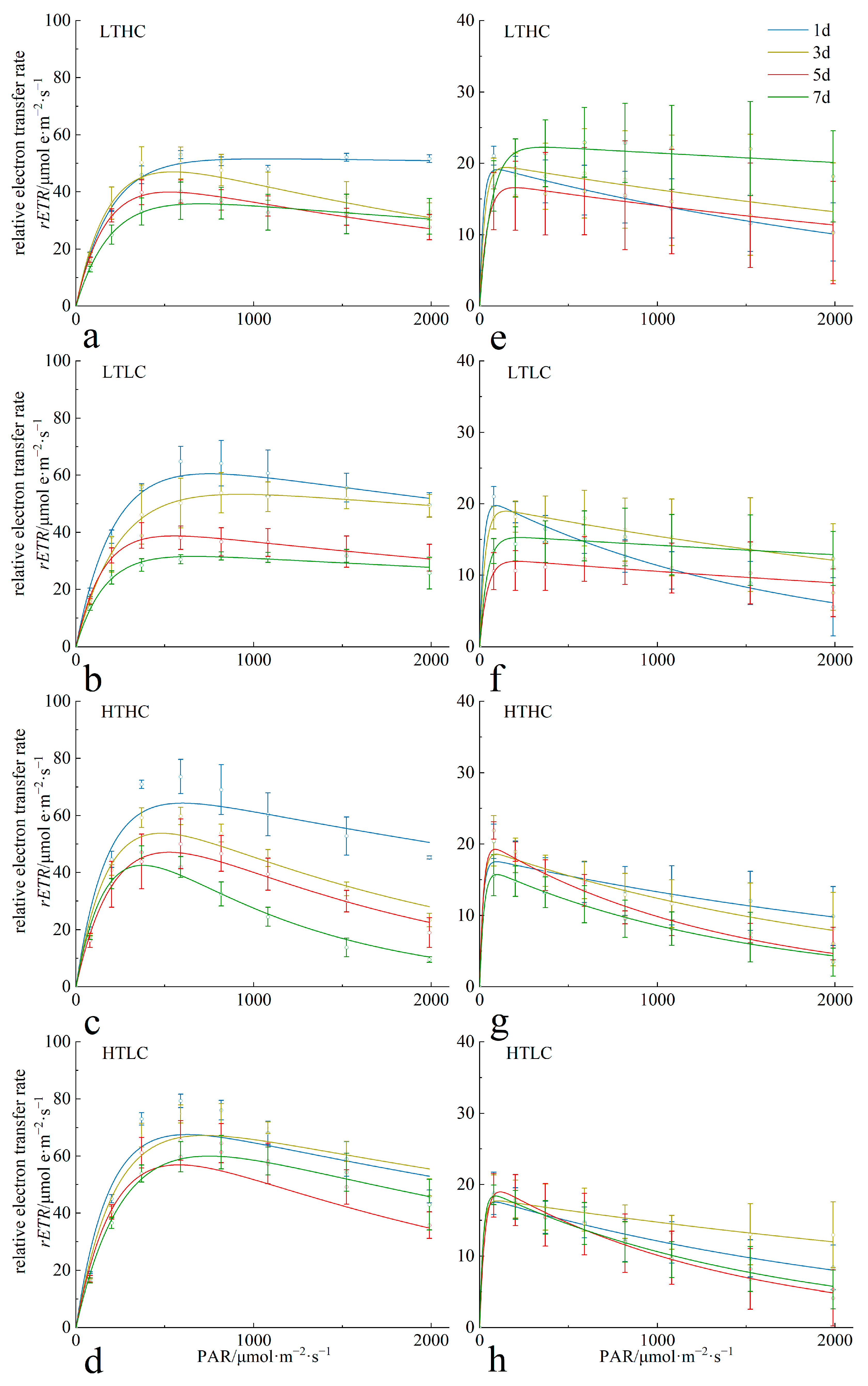
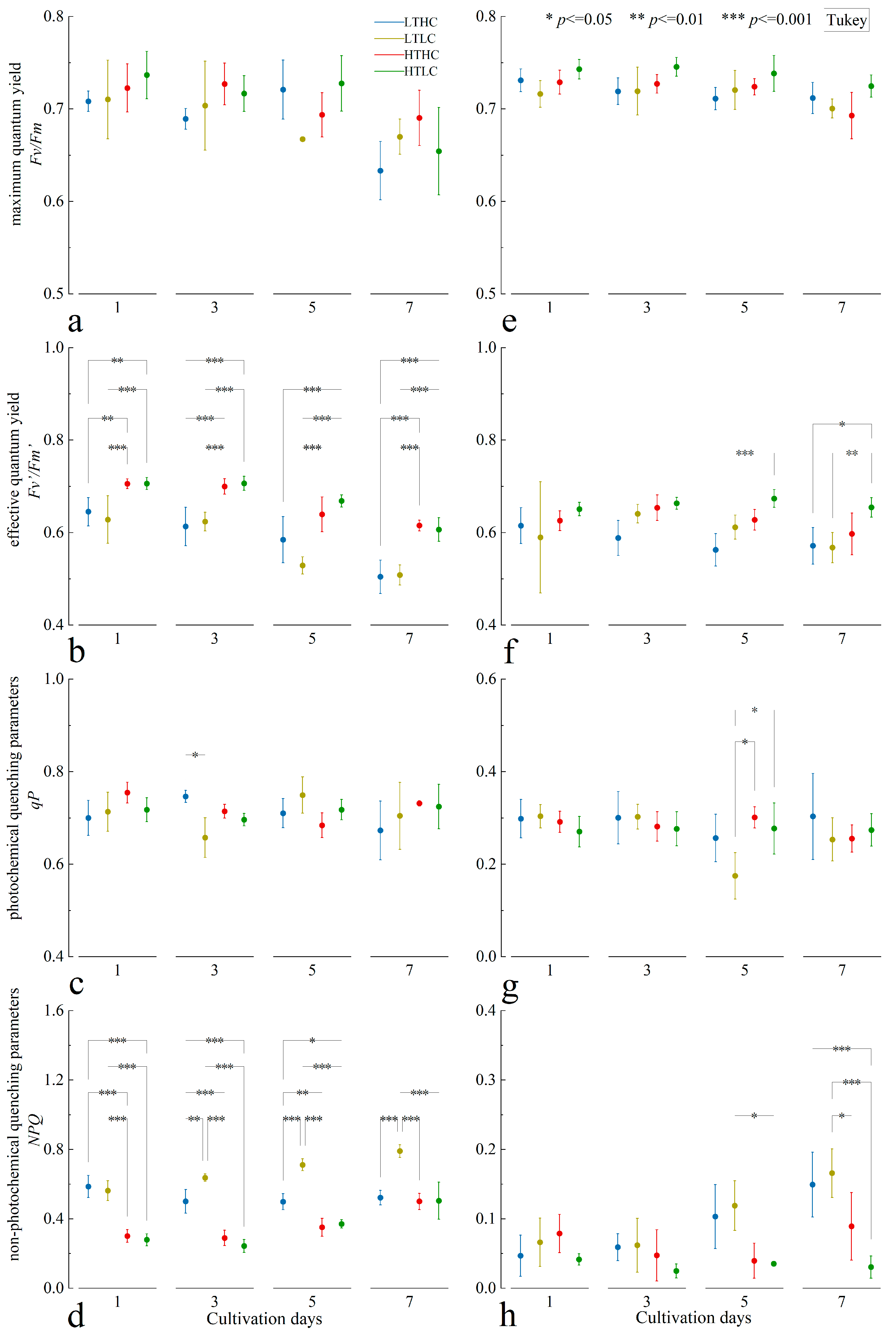
3.1. Changes in Chlorophyll Fluorescence Parameters
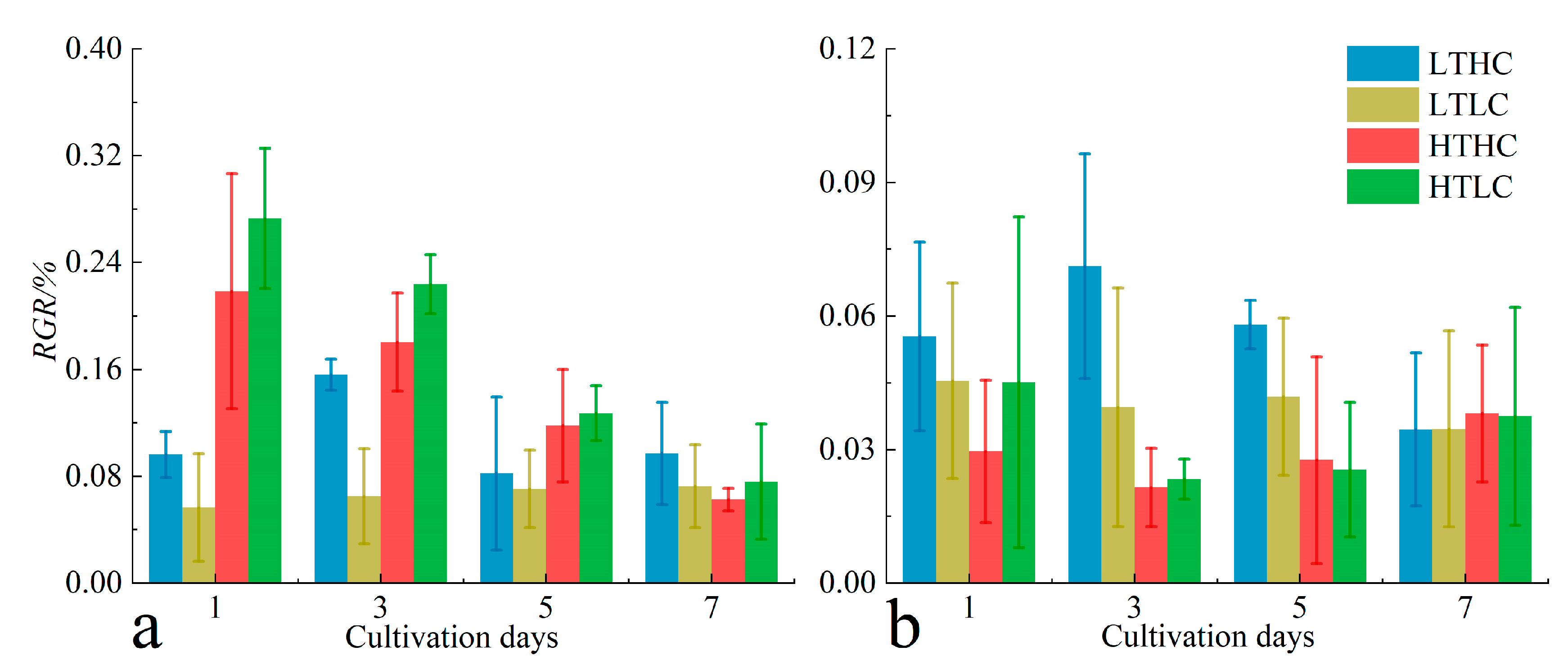
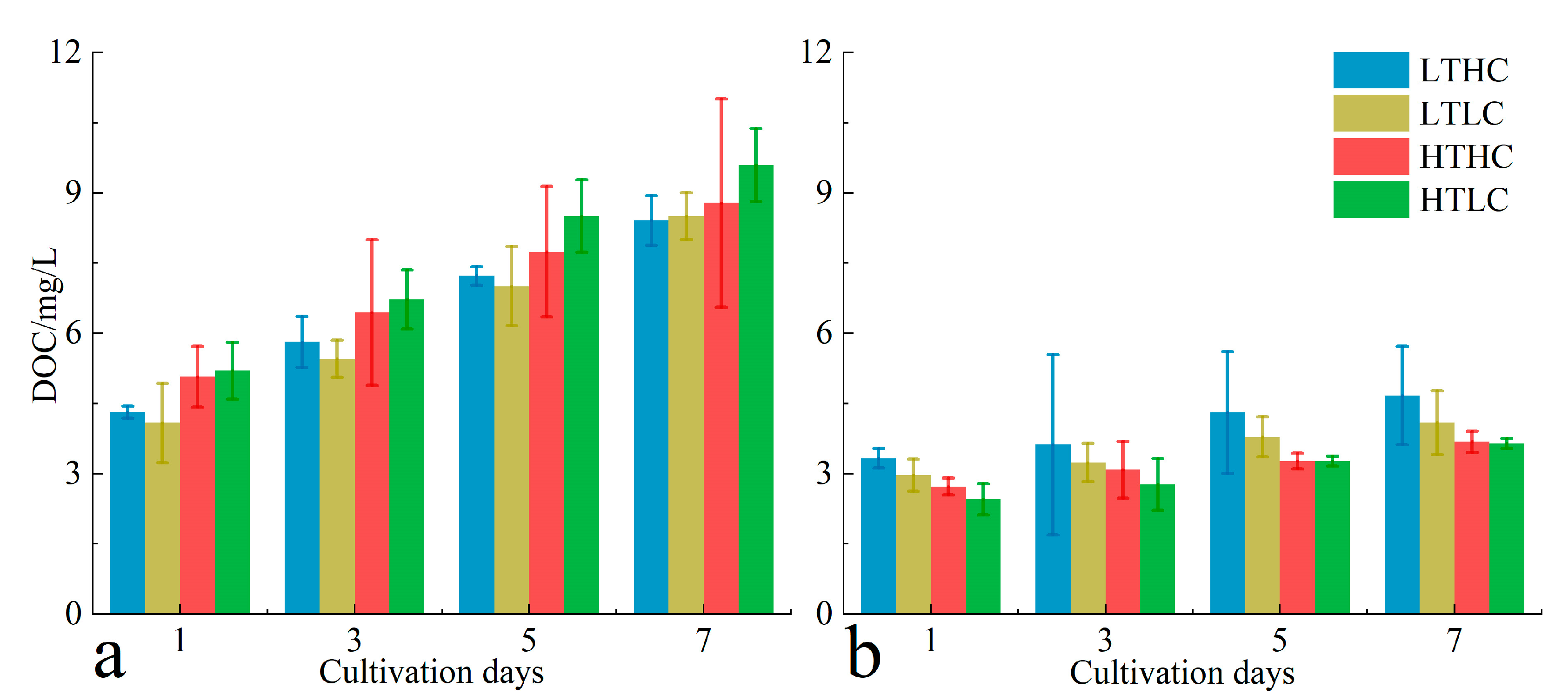
3.2. Changes in RGR and DOC Release
3.3. Changes in Photosynthetic Pigment Content
4. Discussion
4.1. Changes in Fluorescence Parameters and Growth of Macroalgae
4.2. Changes in Biochemical Characterization Parameters of Macroalgae
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gattuso, J.P.; Magnan, A.; Bille, R.; Cheung, W.W.L.; Howes, E.L.; Joos, F.; Allemand, D.; Bopp, L.; Cooley, S.R.; Eakin, C.M.; et al. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 2015, 349, aac4722. [Google Scholar] [CrossRef] [PubMed]
- Delille, B. CO2 in Seawater: Equilibrium, Kinetics, Isotopes. J. Mar. Syst. 2002, 36, 269–270. [Google Scholar] [CrossRef]
- Scheffer, M.; Brovkin, V.; Cox, P.M. Positive feedback between global warming and atmospheric CO2 concentration inferred from past climate change. Geophys. Res. Lett. 2006, 33, 10702-1–10702-4. [Google Scholar] [CrossRef]
- Feely, A.R.; Doney, C.S.; Cooley, R.S. Ocean Acidification: Present Conditions and Future Changes in a High-CO2 World. Oceanography 2009, 22, 36–47. [Google Scholar] [CrossRef]
- Gao, G.; Clare, A.S.; Rose, C.; Caldwell, G.S. Intrinsic and extrinsic control of reproduction in the green tide-forming alga, Ulva rigida. Environ. Exp. Botany 2017, 139, 14–22. [Google Scholar] [CrossRef]
- Wu, H.L.; Feng, J.C.; Li, X.S.; Zhao, C.Y.; Liu, Y.H.; Yu, J.T.; Xu, J.T. Effects of increased CO2 and temperature on the physiological characteristics of the golden tide blooming macroalgae Sargassum horneri in the Yellow Sea, China. Mar. Pollut. Bull. 2019, 146, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Zou, D.H. Do increased temperature and CO2 levels affect the growth, photosynthesis, and respiration of the marine macroalga Pyropia haitanensis (Rhodophyta)? An experimental study. Hydrobiologia 2015, 745, 285. [Google Scholar] [CrossRef]
- Suárez-Alvarez, S.; Gómez-Pinchetti, J.L.; García-Reina, G. Effects of increased CO2 levels on growth, photosynthesis, ammonium uptake and cell composition in the macroalga Hypnea spinella (Gigartinales, Rhodophyta). J. Appl. Phycol. 2012, 24, 815–823. [Google Scholar] [CrossRef]
- Mercado, J.M.; Javier, F.; Gordillo, L.; Niell, F.X.; Figueroa, F.L. Effects of different levels of CO2 on photosynthesis and cell components of the red alga Porphyra leucosticta. J. Appl. Phycol. 1999, 11, 455–461. [Google Scholar] [CrossRef]
- Coutinho, R.; Zingmark, R. Interactions of light and nitrogen on photosynthesis and growth of the marine macroalga Ulva curvata (Kützing) De Toni. J. Exp. Mar. Biol. Ecol. 1993, 167, 11–19. [Google Scholar] [CrossRef]
- Chakraborty, K.; Lipton, A.P.; Raj, R.P.; Vijayan, K.K. Antibacterial labdane diterpenoids of Ulva fasciata Delile from southwestern coast of the Indian Peninsula. Food Chem. 2010, 119, 1399–1408. [Google Scholar] [CrossRef]
- He, P.M.; Liu, Y.Y.; Zhang, J.W.; Wu, H.L.; Yu, K.F.; Huo, Y.Z.; Zhang, J.H. Research progress on the effects of macroalgae on carbon sink. J. Fish. Sci. China 2015, 22, 588–595. [Google Scholar]
- Li, M.Y.; Xu, K.; Tang, X.H.; Zhang, L.R.; Xu, Y.; Wang, W.L.; Ji, D.H.; Chen, D.S.; Xie, C.T. Effects of ocean acidification on the light and temperature adaptations of commercial seaweed Pyropia haitanensis. J. Appl. Oceanogr. 2021, 40, 388–394. [Google Scholar] [CrossRef]
- Xu, X.T.; Li, Y.H.; Wang, D.; Xu, N.J. Effects of simulated acid rain on the photosynthetic physiological characteristics in Ulva fasciata under salt stress. J. Fish. China 2016, 40, 731–739. [Google Scholar] [CrossRef]
- Huang, Y.H.; Yang, R.; Sun, Q.H. Effect of Temperature on Growth and Chlorophyll Fluorescence of Ulva fasciata. Biotechnol. Bull. 2016, 32, 99–105. [Google Scholar] [CrossRef]
- Zhang, T.; Shen, Z.; Xu, P.; Zhu, J.Y.; Lu, Q.Q.; Shen, Y.; Wang, Y.; Yao, C.Y.; Li, J.F.; Wang, Y.X.; et al. Analysis of photosynthetic pigments and chlorophyll fluorescence characteristics of different strains of Porphyra yezoensis. J. Appl. Phycol. 2012, 24, 881–886. [Google Scholar] [CrossRef]
- Russell, B.D.; Passarelli, C.A.; Connell, S.D. Forecasted CO2 modifies the influence of light in shaping subtidal habitat. J. Phycol. 2011, 47, 744–752. [Google Scholar] [CrossRef]
- Gordillo, F.J.L.; Aguilera, J.; Wiencke, C.; Jiménez, C. Ocean acidification modulates the response of two Arctic kelps to ultraviolet radiation. J. Plant Physiol. 2015, 173, 41–50. [Google Scholar] [CrossRef]
- Gordillo, F.J.L.; Carmona, R.; Viñegla, B.; Wiencke, C.; Jiménez, C. Effects of simultaneous increase in temperature and ocean acidification on biochemical composition and photosynthetic performance of common macroalgae from Kongsfjorden (Svalbard). Polar Biol. 2016, 39, 1993–2007. [Google Scholar] [CrossRef]
- Choi, C.G.; Kim, H.G.; Sohn, C.H. Transplantation of Young Fronds of Sargassum horneri for Construction of Seaweed Beds. Korean J. Fish. Aquat. Sci. 2003, 36, 469–473. [Google Scholar] [CrossRef][Green Version]
- Zhao, X. Photosyntheitc Physiology and Ecological Effects of Macroalgae in Gouqi Island; Shanghai Ocean University: Shanghai, China, 2022. [Google Scholar]
- Cai, W.J.; Hu, X.; Huang, W.J.; Murrell, M.C.; Lehrter, J.C.; Lohrenz, S.E.; Chou, W.C.; Zhai, W.D.; Hollibaugh, J.T.; Wang, Y.C.; et al. Acidiffcation of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 2011, 4, 766–770. [Google Scholar] [CrossRef]
- Kim, J.K.; Yarish, C.; Pereira, R. Tolerances to hypo-osmotic and temperature stresses in native and invasive species of Gracilaria (Rhodophyta). Phycologia 2016, 55, 257–264. [Google Scholar] [CrossRef]
- Fu, F.X.; Warner, M.E.; Zhang, Y.; Feng, Y.Y.; Hutchins, D.A. Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (cyanobacteria). J. Phycol. 2007, 43, 485–496. [Google Scholar] [CrossRef]
- Ji, Y.; Jiang, Y.; Jin, P.; Xia, J.R. Interactive effects of ocean acidification and nitrate on Ulva lactuca. J. Appl. Phycol. 2024. [Google Scholar] [CrossRef]
- Rautenberger, R.; Fernandez, P.A.; Strittmatter, M.; Heesch, S.; Cornwall, C.E.; Hurd, C.L.; Roleda, M.Y. Saturating light and not increased carbon dioxide under ocean acidification drives photosynthesis and growth in Ulva rigida (Chlorophyta). Ecol. Evol. 2015, 5, 874–888. [Google Scholar] [CrossRef] [PubMed]
- Bao, O.N.; Shi, D.G.; Guan, W.C.; Sun, M.; Zhang, P.; Peng, X.; Wang, T.G.; Chen, S.B.; Qiu, J.B. Effect of CO2 concentration and irradiance on the growth of Sargassum horneri (Phaeophyceae) in Nanji Archipelago. Acta Agric. Zhejiangensis 2014, 26, 649–655. [Google Scholar] [CrossRef]
- Pederson, M.F.; Paling, E.I.; Walker, D.I. Nitrogen uptake and allocation in the seagrass Amphibolis Antarctica. Aquat. Botany 1997, 56, 105–117. [Google Scholar] [CrossRef]
- Sun, J.Z.; Zhuang, D.G.; Sun, Q.H.; Pang, S.J. Artificial cultivation trials of Sargassum horneri at Nanji island of China. South China Fish. Sci. 2009, 5, 41–46. [Google Scholar] [CrossRef]
- Dai, W.; Wang, X.R.; Zhuang, M.M.; Sun, J.Y.; Shen, Y.F.; Xia, Z.Y.; Wu, T.J.; Jiang, R.T.; Li, A.Q.; Bi, F.L.; et al. Responses of photosynthesis-related genes in Sargassum horneri to high temperature stress. Mar. Pollut. Bull. 2024, 199, 115944. [Google Scholar] [CrossRef]
- Rogers, A.; Fischer, B.U.; Bryant, J.; Frehner, M.; Blum, H.; Raines, C.A.; Long, S.P. Acclimation of Photosynthesis to Elevated CO2 under Low-Nitrogen Nutrition Is Affected by the Capacity for Assimilate Utilization. Perennial Ryegrass under Free-Air CO2. Enrich. Plant Phys. 1998, 118, 683–689. [Google Scholar] [CrossRef]
- Chen, B.B.; Xia, J.R.; Zou, D.H.; Zhang, X. Responses to ocean acidification and diurnal temperature variation in a commercially farmed seaweed, Pyropia haitanensis (Rhodophyta). Eur. J. Phycol. 2019, 54, 184–192. [Google Scholar] [CrossRef]
- Barakat, K.M.; El-Sayed, H.S.; Khairy, H.M.; El-Sheikh, M.A.; Al-Rashed, S.A.; Arif, I.A.; Elshobary, M.E. Effects of ocean acidification on the growth and biochemical composition of a green alga (Ulva fasciata) and its associated microbiota. Saudi J. Biol. Sci. 2021, 28, 5106–5114. [Google Scholar] [CrossRef]
- Marañón, E.; Cermeño, P.; Fernãndez, E.; Rodríguez, J.; Zabala, L. Significance and Mechanisms of Photosynthetic Production of Dissolved Organic Carbon in a Coastal Eutrophic Ecosystem. Limnol. Oceanogr. 2004, 49, 1652–1666. [Google Scholar] [CrossRef]
- Luan, Q.; Lv, F.; Wu, H.Y.; Ding, G.; Zhan, D.M. Effects of culture conditions on nutrient composition of Sargassum horneri. Prog. Fish. Sci. 2019, 40, 123–130. [Google Scholar] [CrossRef]
- Fu, W.T.; Liu, J.; Zhang, J.L.; Feng, T.W.; Su, Y.M.; Zhu, X.L.; Li, Y.Y.; Liu, J.; Liu, Y.; Cao, S.Q.; et al. Effects of elevated atmospheric CO2 concentration on growth and pigment contents of macroalga Ulva pertusa. J. Dalian Ocean Univ. 2013, 28, 481–486. [Google Scholar] [CrossRef]
- Miller, S.M.; Wing, S.R.; Hurd, C.L. Photoacclimation of Ecklonia radiata (Laminariales, Heterokontophyta) in Doubtful Sound, Fjordland, Southern New Zealand. Phycologia 2006, 45, 44–52. [Google Scholar] [CrossRef]
- Mercado, J.M.; Gordillo, F.J.L.; Figueroa, F.L.; Niell, F.X. External carbonic anhydrase and affinity for inorganic carbon in intertidal macroalgae. J. Exp. Mar. Biol. Ecol. 1998, 221, 209–220. [Google Scholar] [CrossRef]
- Wang, Q.H. Effects of Diel Fluctuating Environmental Factors on Germination, Growth of Macroalage and Their Ecophysiological Mechanisms; Ocean University of China: Qingdao, China, 2008. [Google Scholar]
- Wu, Y.; Gao, K.; Riebesell, U. CO2-induced seawater acidification affects physiological performance of the marine diatom Phaeodactylum tricornutum. Biogeosciences 2010, 7, 2915–2923. [Google Scholar] [CrossRef]
- James, I.L.M.; Michael, D.M. Plant Growth and Climate Change; Blackwell Publishing: Oxford, UK, 2006; pp. 17–47. ISBN 79780470994184. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Tao, X.; Zhang, S.; Zhao, X. Effects of Ocean Acidification and Temperature Coupling on Photosynthetic Activity and Physiological Properties of Ulva fasciata and Sargassum horneri. Biology 2024, 13, 640. https://doi.org/10.3390/biology13080640
Wang K, Tao X, Zhang S, Zhao X. Effects of Ocean Acidification and Temperature Coupling on Photosynthetic Activity and Physiological Properties of Ulva fasciata and Sargassum horneri. Biology. 2024; 13(8):640. https://doi.org/10.3390/biology13080640
Chicago/Turabian StyleWang, Kai, Xiang Tao, Shouyu Zhang, and Xu Zhao. 2024. "Effects of Ocean Acidification and Temperature Coupling on Photosynthetic Activity and Physiological Properties of Ulva fasciata and Sargassum horneri" Biology 13, no. 8: 640. https://doi.org/10.3390/biology13080640
APA StyleWang, K., Tao, X., Zhang, S., & Zhao, X. (2024). Effects of Ocean Acidification and Temperature Coupling on Photosynthetic Activity and Physiological Properties of Ulva fasciata and Sargassum horneri. Biology, 13(8), 640. https://doi.org/10.3390/biology13080640




