Protective Effect of Polysaccharides Isolated from Sargassum horneri against H2O2-Induced Oxidative Stress Both In Vitro, in Vero Cells, and In Vivo in Zebrafish
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Regents
2.2. Plant Material and Sample Preparation
2.3. The Cell Cultures
2.4. The Assay for Cytotoxicity
2.5. Measurement of Intracellular ROS Levels
2.6. The Effect of SHP on Intracellular MDA Content and SOD Activity
2.7. Maintenance of Zebrafish
2.8. The Survival Rate of Zebrafish Embryos
2.9. Measurement of Oxidative Stress-Related Indicators (SOD, CAT, GSH-PX, and MDA) in Zebrafish
2.10. Determination of ROS in Zebrafish
2.11. Statistical Analysis
3. Results
3.1. FTIR Spectrum of S. horneri Polysaccharide (SHP)
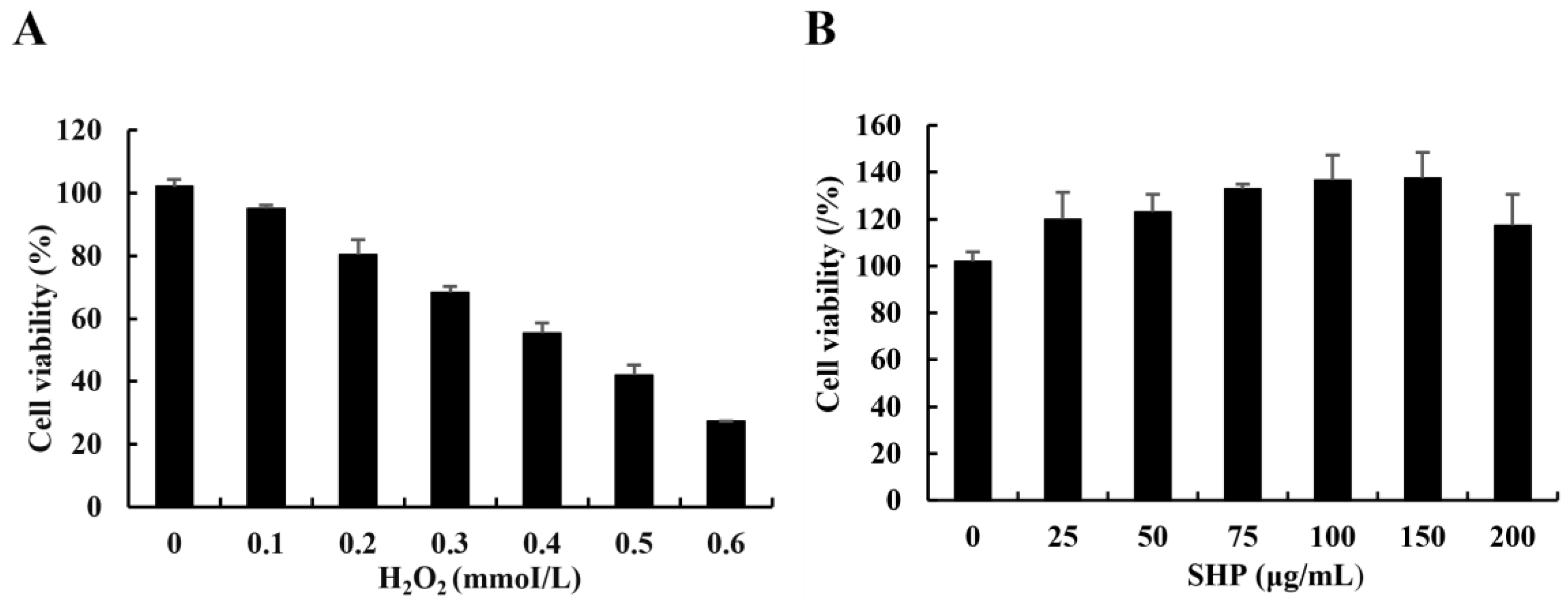
3.2. Determination of the H2O2 Damage Model
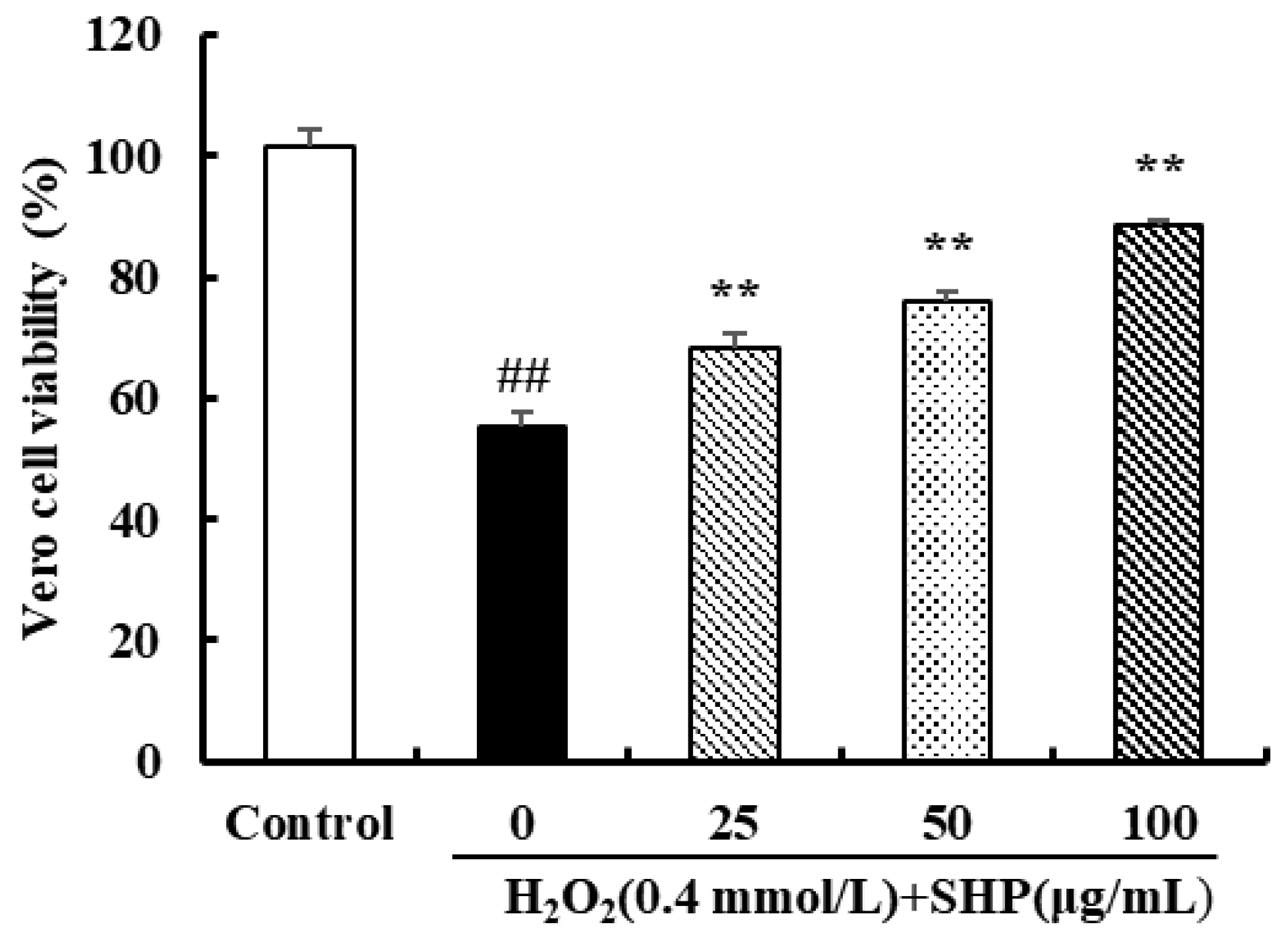
3.3. The Effect of SHP against Oxidative Stress Induced by H2O2 in Vero Cells
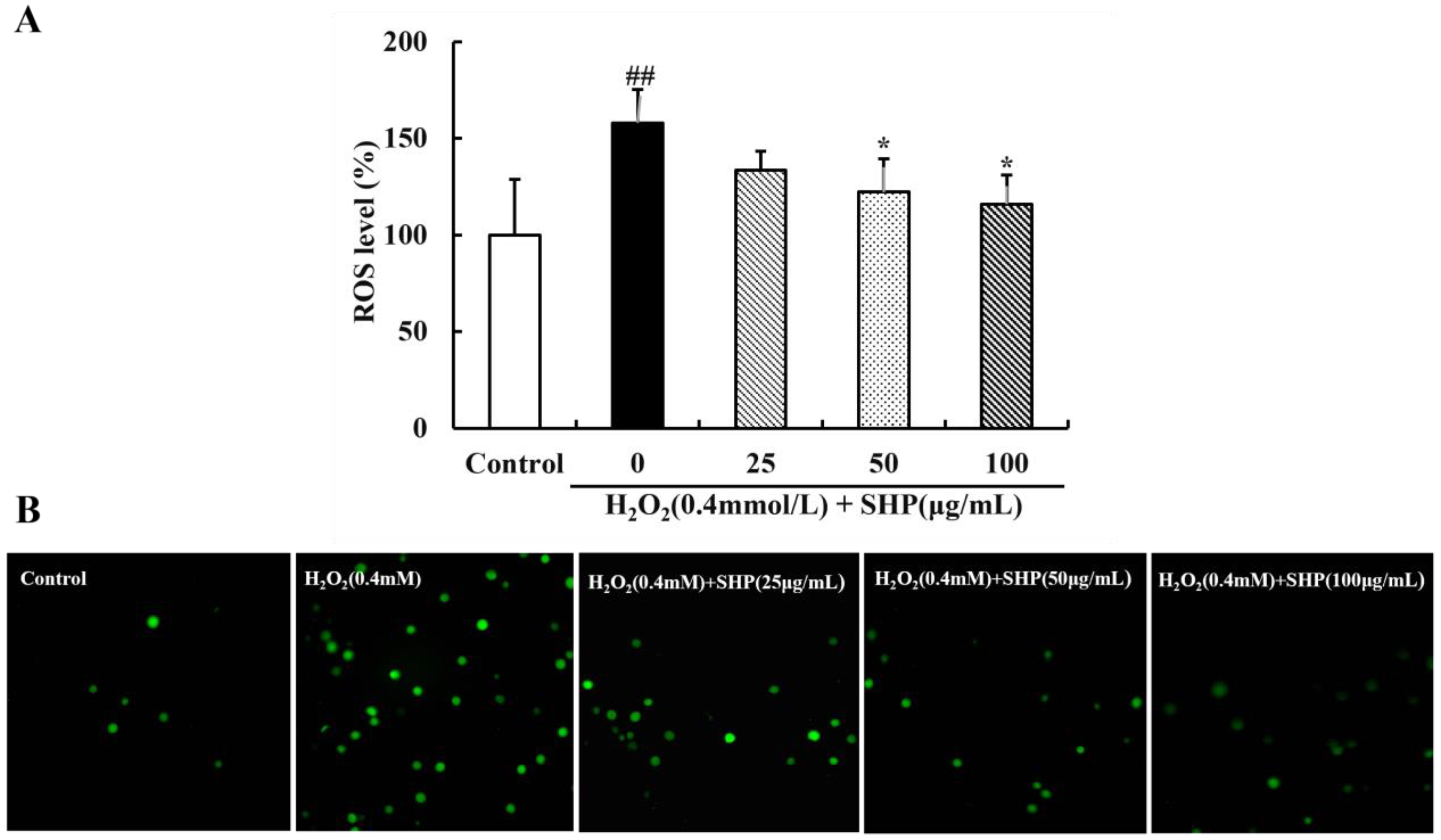
3.4. The Scavenging Effect of SHP on ROS in Vero Cells
3.5. Effects of SHP on Intracellular MDA Content and SOD Activity
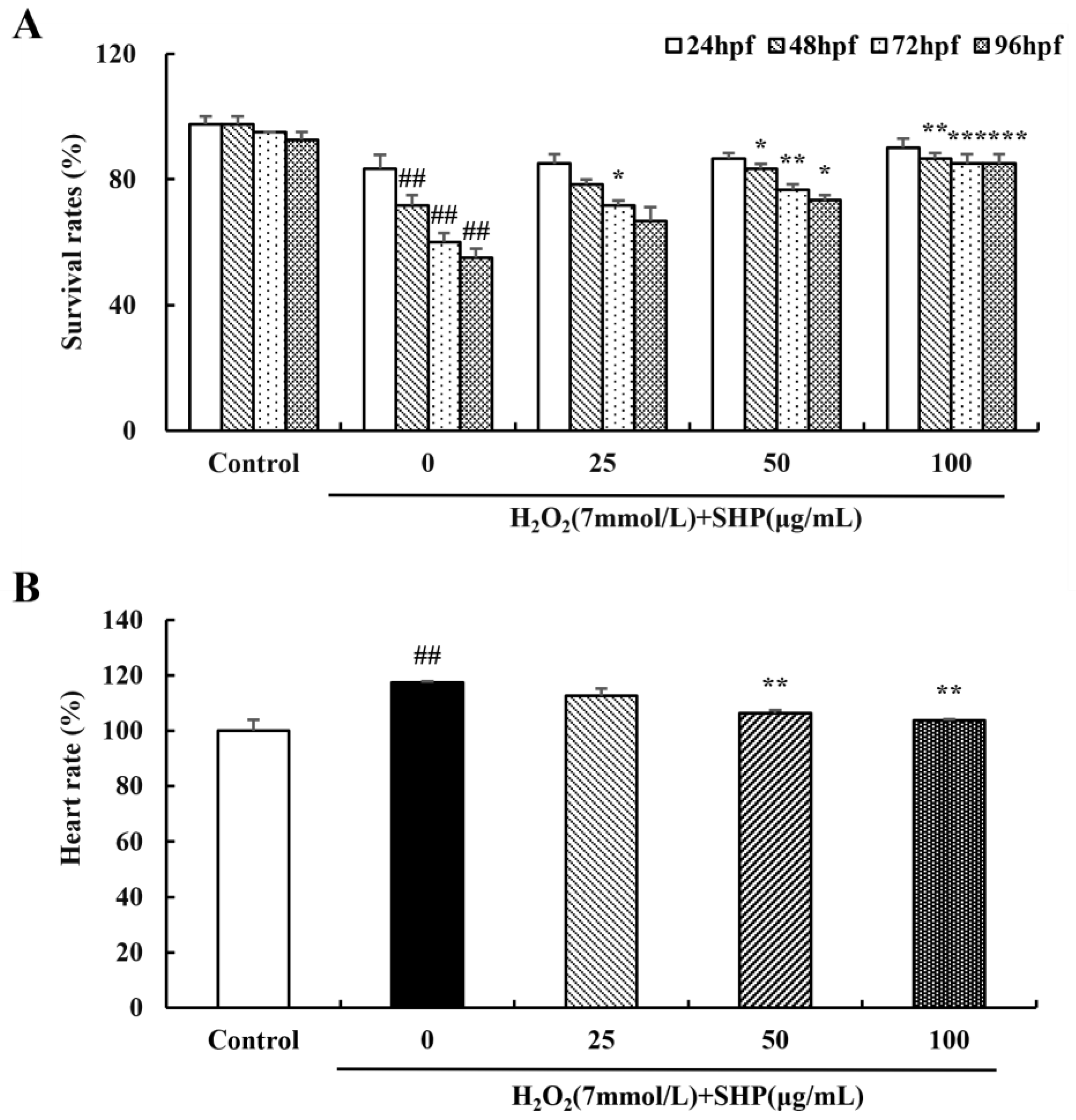
3.6. Effects of H2O2 on Survival Rate and Hatching Rate of Zebrafish
3.7. Effects of H2O2 on the Growth and Development of Zebrafish
3.8. Effects of SHP on H2O2-Induced Zebrafish
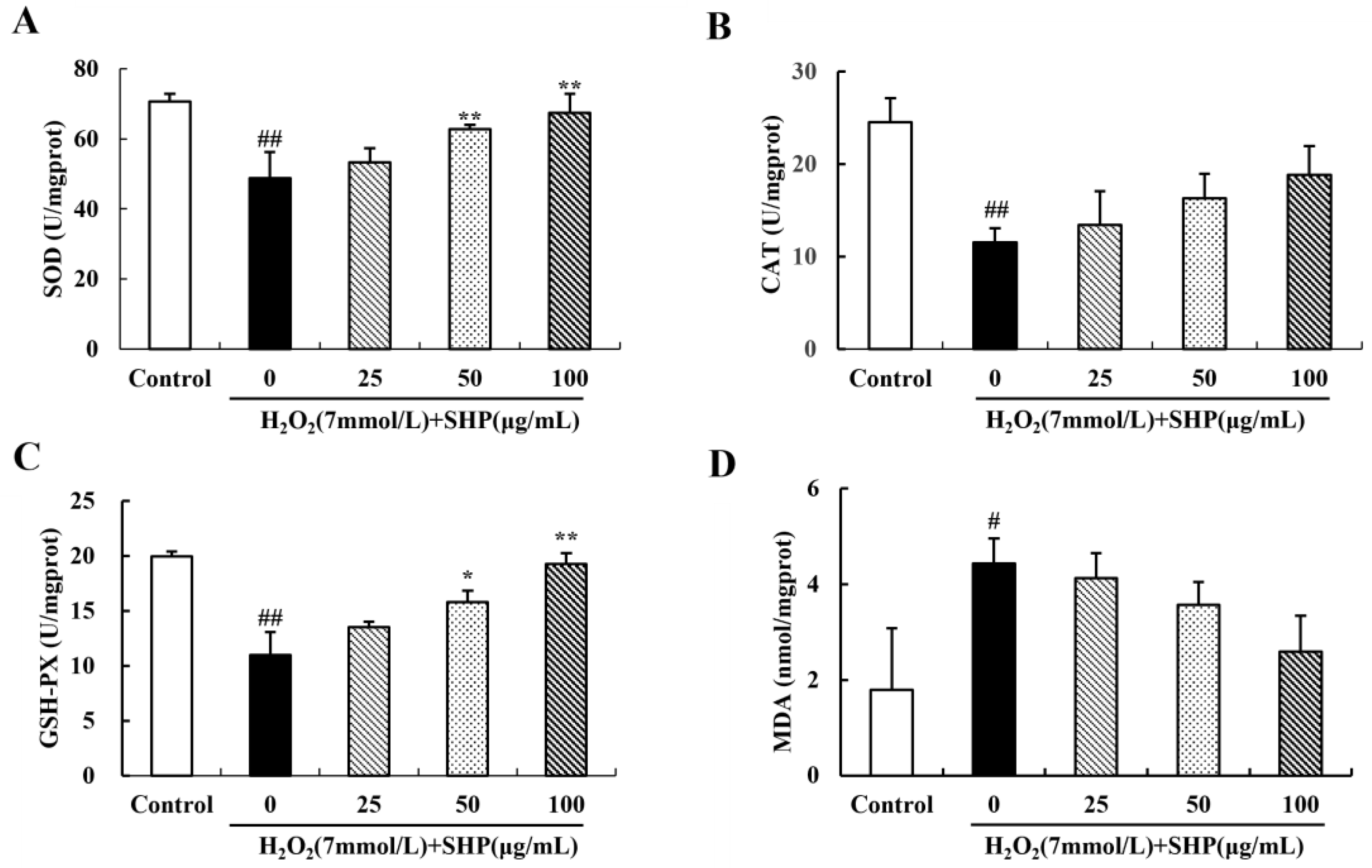
3.9. Effects of SHP on the Activities of SOD and CAT and on the Levels of GSH-PX and MDA in Zebrafish
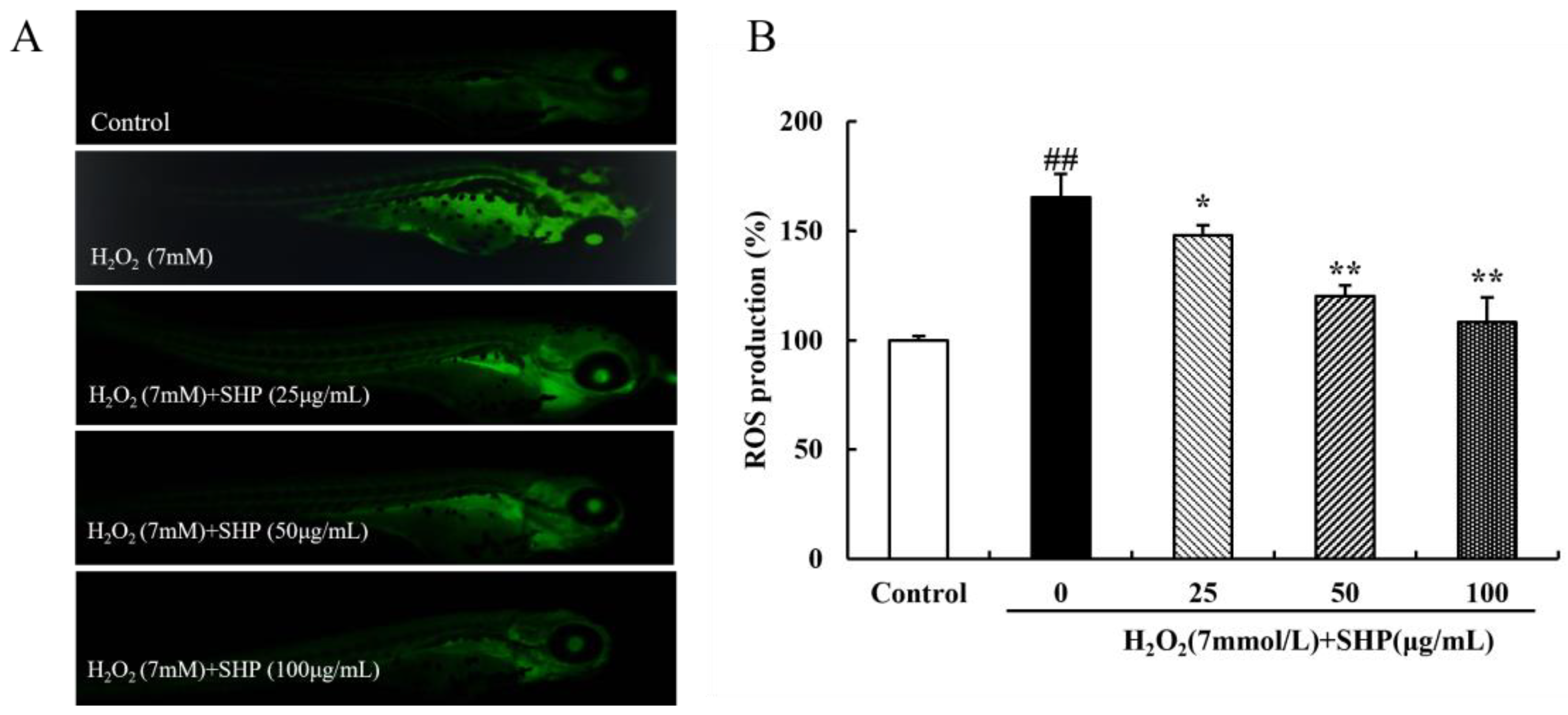
3.10. The Role of SHP on H2O2-Induced ROS in Zebrafish
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernando, I.P.S.; Kim, M.; Son, K.T.; Jeong, Y.; Jeon, Y.J. Antioxidant activity of marine algal polyphenolic compounds: A mechanistic approach. J. Med. Food 2016, 19, 615–628. [Google Scholar] [CrossRef]
- Wang, L.; Jo, M.J.; Katagiri, R.; Harata, K.; Ohta, M.; Ogawa, A.; Kamegai, M.; Ishida, Y.; Tanoue, S.; Kimura, S.; et al. Antioxidant effects of citrus pomace extracts processed by super-heated steam. LWT Food Sci. Technol. 2018, 90, 331–338. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hwang, J.W.; Sung, S.H.; Jeon, Y.J.; Jeong, J.H.; Jeon, B.T.; Moon, S.H.; Park, P.J. Antioxidant activity and protective effect of extract of Celosia cristata L. flower on tert-butyl hydroperoxide-induced oxidative hepatotoxicity. Food Chem. 2015, 168, 572–579. [Google Scholar] [CrossRef]
- Begum, R.; Howlader, S.; Mamun-Or-Rashid, A.N.M.; Rafiquzzaman, S.M.; Ashraf, G.M.; Albadrani, G.M.; Sayed, A.A.; Peluso, I.; Abdel-Daim, M.M.; Uddin, M.S. Antioxidant and signal-modulating effects of brown seaweed-derived compounds against oxidative stress-associated pathology. Oxidative Med. Cell. Longev. 2021, 2021, 9974890. [Google Scholar] [CrossRef]
- Sarniak, A.; Lipińska, J.; Tytman, K.; Lipińska, S. Endogenous mechanisms of reactive oxygen species (ROS) generation. Adv. Hyg. Exp. Med. 2016, 70, 1150–1165. [Google Scholar] [CrossRef]
- Wojtovich, A.P.; Berry, B.J.; Galkin, A. Redox signaling through compartmentalization of reactive oxygen species: Implications for health and disease. Antioxid. Redox Signal. 2019, 31, 591–593. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Je, J.G.; Jayawardena, T.U.; Kim, Y.S.; Ko, J.Y.; Fu, X.T.; Jeon, Y.J. Protective effects of sulfated polysaccharides isolated from the enzymatic digest of Codium fragile against hydrogen peroxide-induced oxidative stress in in vitro and in vivo models. Algal Res. 2020, 48, 101891. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Yang, H.W.; Kim, H.S.; Jeon, Y.J. Protective effect of sulfated polysaccharides from a Celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-induced photoaging in vitro in human keratinocytes and in vivo in zebrafish. Mar. Life Sci. Technol. 2019, 1, 104–111. [Google Scholar] [CrossRef]
- Dimitrova-Shumkovska, J.; Krstanoski, L.; Veenman, L. Potential beneficial actions of fucoidan in brain and liver injury, disease, and intoxication-potential implication of Sirtuins. Mar. Drugs 2020, 18, 242. [Google Scholar] [CrossRef]
- Saraswati; Giriwono, P.E.; Iskandriati, D.; Tan, C.P.; Andarwulan, N. Sargassum seaweed as a source of anti-inflammatory substances and the potential insight of the tropical species: A review. Mar. Drugs 2019, 17, 590. [Google Scholar] [CrossRef]
- Riccio, G.; Lauritano, C. Microalgae with immunomodulatory activities. Mar. Drugs 2020, 18, 2. [Google Scholar] [CrossRef]
- Marques, M.L.M.; Presa, F.B.; Viana, R.L.S.; Costa, M.; Amorim, M.O.R.; Bellan, D.L.; Alves, M.; Costa, L.S.; Trindade, E.S.; Rocha, H.A.O. Anti-thrombin, anti-adhesive, anti-migratory, and anti-proliferative activities of sulfated galactans from the tropical green seaweed, Udotea flabellum. Mar. Drugs 2019, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Anticoagulant activity of sulfated Ulvan isolated from the green macroalga Ulva rigida. Mar. Drugs 2019, 17, 291. [Google Scholar] [CrossRef]
- Jun, J.Y.; Jung, M.J.; Jeong, I.H.; Yamazaki, K.; Kawai, Y.; Kim, B.M. Antimicrobial and antibiofilm activities of sulfated polysaccharides from marine algae against dental plaque bacteria. Mar. Drugs 2018, 16, 301. [Google Scholar] [CrossRef]
- Rasmussen, R.S.; Morrissey, M.T. Marine biotechnology for production of food ingredients. Adv. Food Nutr. Res. 2007, 52, 237–292. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Kim, H.S.; Lee, W.; Cui, Y.R.; Lee, H.G.; Kim, Y.T.; Ko, J.Y.; Jeon, Y.J. Protective effect of polysaccharides from Celluclast-assisted extract of Hizikia fusiforme against hydrogen peroxide-induced oxidative stress in vitro in Vero cells and in vivo in zebrafish. Int. J. Biol. Macromol. 2018, 112, 483–489. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Kim, E.A.; Lee, S.H.; Ko, C.I.; Cha, S.H.; Kang, M.C.; Kang, S.M.; Ko, S.C.; Lee, W.W.; Ko, J.Y.; Lee, J.H.; et al. Protective effect of fucoidan against AAPH-induced oxidative stress in zebrafish model. Carbohydr. Polym. 2014, 102, 185–191. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Wang, L.; Sanjeewa, K.K.A.; Kang, S.I.; Lee, J.S.; Jeon, Y.J. Antioxidant potential of sulfated polysaccharides from Padina boryana: Protective effect against oxidative stress in an in vitro and in vivo zebrafish model. Mar. Drugs 2020, 18, 212. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. Improvement of antioxidant and moisture-preserving activities of Sargassum horneri polysaccharide enzymatic hydrolyzates. Int. J. Biol. Macromol. 2015, 74, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Chen, X.; Sun, P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr. Polym. 2014, 105, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.S.; Liu, L.J.; OuYang, X.K.; Qu, Y.L.; Chen, Y.; Ding, G.F. Protective effect of polysaccharides from Sargassum horneri against oxidative stress in RAW264.7 cells. Int. J. Biol. Macromol. 2014, 68, 98–106. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Pei, P.; Zheng, C.; Cheong, K.L.; Huang, N. Unravelling property of polysaccharides from Sargassum sp. as an anti-wrinkle and skin whitening property. Int. J. Biol. Macromol. 2019, 140, 216–224. [Google Scholar] [CrossRef]
- Wen, Z.S.; Xiang, X.W.; Jin, H.X.; Guo, X.Y.; Liu, L.J.; Huang, Y.N.; OuYang, X.K.; Qu, Y.L. Composition and anti-inflammatory effect of polysaccharides from Sargassum horneri in RAW264.7 macrophages. Int. J. Biol. Macromol. 2016, 88, 403–413. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, S.Y.; Kim, H.S.; Ahn, G.; Jee, Y.; Jeon, Y.J. In vitro and in vivo anti-inflammatory activities of high molecular weight sulfated polysaccharide; containing fucose separated from Sargassum horneri: Short communication. Int. J. Biol. Macromol. 2018, 107, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, S.Y.; Kim, H.S.; Ahn, G.; Kim, J.; Jeon, Y.J. Fucoidan isolated from invasive Sargassum horneri inhibit LPS-induced inflammation via blocking NF-κB and MAPK pathways. Algal Res. 2019, 41, 101561. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, S.Y.; Lee, H.G.; Je, J.G.; Jee, Y.; Jeon, Y.J. Sargassum horneri (Turner) inhibit urban particulate matter-induced inflammation in MH-S lung macrophages via blocking TLRs mediated NF-kappa B and MAPK activation. J. Ethnopharmacol. 2020, 249, 112363. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Liu, J.; Chen, X.; Fang, Z.; Sun, P. Structural features and antitumor activity of a purified polysaccharide extracted from Sargassum horneri. Int. J. Biol. Macromol. 2015, 73, 124–130. [Google Scholar] [CrossRef]
- Lee, P.T.; Quan Tran, H.T.; Huang, H.T.; Nan, F.H.; Lee, M.C. Sargassum horneri extracts stimulate innate immunity, enhance growth performance, and upregulate immune genes in the white shrimp Litopenaeus vannamei. Fish Shellfish. Immunol. 2020, 102, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, H.S.; Jeon, Y.J.; Jee, Y.; Kim, K.N.; Lee, K.; Fernando, I.P.S.; Ahn, G. Sargassum horneri (Turner) C. Agardh ethanol extract attenuates fine dust-induced inflammatory responses and impaired skin barrier functions in HaCaT keratinocytes. J. Ethnopharmacol. 2021, 273, 114003. [Google Scholar] [CrossRef]
- Cruz-Rivera, E.; Flores-Diaz, M.; Hawkins, A. A fish kill coincident with dense Sargassum accumulation in a tropical bay. Bull. Mar. Sci. 2015, 91, 455–456. [Google Scholar] [CrossRef]
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef]
- Schell, J.M.; Goodwin, D.S.; Siuda, A.N.S. Recent Sargassum inundation events in the Caribbean shipboard observations reveal dominance of a previously rare form. Oceanography 2015, 28, 8–10. [Google Scholar] [CrossRef]
- Sfriso, A.; Facca, C. Annual growth and environmental relationships of the invasive species Sargassum muticum and Undaria pinnatifida in the lagoon of Venice. Estuar. Coast. Shelf Sci. 2013, 129, 162–172. [Google Scholar] [CrossRef]
- Wei, S.Y.; Cai, C.N.; He, P.M.; Jia, R. Protective Effect of Polysaccharide from Sargassum horneri on H2O2-induced Oxidative Stress in HaCaT Cells. J. Trop. Subtrop. Bot. 2023, 31, 232–240. [Google Scholar] [CrossRef]
- Wang, L.; Kim, H.S.; Oh, J.Y.; Je, J.G.; Jeon, Y.J.; Ryu, B. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against UVB-induced damage in vitro in human dermal fibroblasts and in vivo in zebrafish. Food Chem. Toxicol. 2020, 136, 110963. [Google Scholar] [CrossRef]
- Pallavi, S.; Bhushan, J.A.; Shanker, D.R.; Mohammad, P. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Yu, J.P.; Liu, Y.F.; Teng, X.J.; Ming, M.; Lv, P.; An, P.; Liu, S.Q.; Yu, H.G. Effects of Ginkgo biloba extract on inflammatory mediators (SOD, MDA, TNF-alpha, NF-kappa Bp65, IL-6) in TNBS-induced colitis in rats. Mediat. Inflamm. 2006, 2006, 92642. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, E.A.; Kim, Y.S.; Yu, S.K.; Choi, C.; Lee, J.S.; Kim, Y.T.; Nah, J.W.; Jeon, Y.J. Protective effects of polysaccharides from Psidium guajava leaves against oxidative stresses. Int. J. Biol. Macromol. 2016, 91, 804–811. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Zeng, F.S.; Yao, Y.F.; Wang, L.F.; Li, W.J. Polysaccharides as antioxidants and prooxidants in managing the double-edged sword of reactive oxygen species. Biomed. Pharmacother. 2023, 159, 114221. [Google Scholar] [CrossRef]
- Kang, S.M.; Heo, S.J.; Kim, K.N.; Lee, S.H.; Jeon, Y.J. Isolation and identification of new compound, 2,7″-phloroglucinol-6,6′-bieckol from brown algae, Ecklonia cava and its antioxidant effect. J. Funct. Foods 2012, 4, 158–166. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative stress in human pathology and aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Stara, A.; Kristan, J.; Zuskova, E.; Velisek, J. Effect of chronic exposure to prometryne on oxidative stress and antioxidant response in common carp (Cyprinus carpio L.). Pestic. Biochem. Physiol. 2013, 105, 18–23. [Google Scholar] [CrossRef]
- Vijay Sankar, N.P.; Jagtap, A.S.; Baghel, R.S.; Imchen, T.; Manohar, C.S. Chapter 28—Elucidation of the antioxidant potential of marine macroalgal biomolecules for healthcare applications: Current status and future prospects. In Marine Antioxidants; Kim, S.-K., Shin, K.-H., Venkatesan, J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 365–377. [Google Scholar] [CrossRef]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef]
- Imen, M.Y.B. Free radical metabolism in human erythrocytes. Clin. Chim. Acta 2008, 390, 1–11. [Google Scholar] [CrossRef]
- Yokozawa, T.; Liu, Z.W.; Chen, C.P. Protective effects of Glycyrrhizae radix extract and its compounds in a renal hypoxia (ischemia)-reoxygenation (reperfusion) model. Phytomedicine 2000, 6, 439–445. [Google Scholar] [CrossRef]
- Lee, J.H.; Ko, J.Y.; Oh, J.Y.; Kim, E.A.; Kim, C.Y.; Jeon, Y.J. Evaluation of phlorofucofuroeckol-A isolated from Ecklonia cava (Phaeophyta) on anti-lipid peroxidation in vitro and in vivo. Algae 2015, 30, 313–323. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saikia, S.K. Use of zebrafish as a model organism to study oxidative stress: A review. Zebrafish 2022, 19, 165–176. [Google Scholar] [CrossRef]
- Gyimah, E.; Zhu, X.; Zhang, Z.; Guo, M.; Xu, H.; Mensah, J.K.; Dong, X.; Zhang, Z.; Gyimah, G.N.W. Oxidative stress and apoptosis in bisphenol AF-induced neurotoxicity in zebrafish embryos. Environ. Toxicol. Chem. 2022, 41, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.E.; Timme-Laragy, A.R.; Karchner, S.I.; Stegeman, J.J. Nrf2 and Nrf2-related proteins in development and developmental toxicity: Insights from studies in zebrafish (Danio rerio). Free Radic. Biol. Med. 2015, 88, 275–289. [Google Scholar] [CrossRef] [PubMed]


| Sample | (μg/mL) | Vero | |
|---|---|---|---|
| SOD (U/mgprot) | MDA (nmol/mgprot) | ||
| Control | - | 52.43 ± 1.31 | 7.17 ± 1.29 |
| H2O2 | - | 22.9 ± 4.46 ## | 19.04 ± 2.53 ## |
| SHP | 25 | 28.28 ± 5.77 | 14.09 ± 0.51 ** |
| 50 | 30.80 ± 6.04 | 11.09 ± 2.10 ** | |
| 100 | 40.70 ± 8.95 ** | 9.39 ± 0.92 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.; Wang, L.; Yang, J.; Xu, R.; Jia, R.; He, P. Protective Effect of Polysaccharides Isolated from Sargassum horneri against H2O2-Induced Oxidative Stress Both In Vitro, in Vero Cells, and In Vivo in Zebrafish. Biology 2024, 13, 651. https://doi.org/10.3390/biology13090651
Wei S, Wang L, Yang J, Xu R, Jia R, He P. Protective Effect of Polysaccharides Isolated from Sargassum horneri against H2O2-Induced Oxidative Stress Both In Vitro, in Vero Cells, and In Vivo in Zebrafish. Biology. 2024; 13(9):651. https://doi.org/10.3390/biology13090651
Chicago/Turabian StyleWei, Shuangyan, Li Wang, Jia Yang, Ruihang Xu, Rui Jia, and Peimin He. 2024. "Protective Effect of Polysaccharides Isolated from Sargassum horneri against H2O2-Induced Oxidative Stress Both In Vitro, in Vero Cells, and In Vivo in Zebrafish" Biology 13, no. 9: 651. https://doi.org/10.3390/biology13090651
APA StyleWei, S., Wang, L., Yang, J., Xu, R., Jia, R., & He, P. (2024). Protective Effect of Polysaccharides Isolated from Sargassum horneri against H2O2-Induced Oxidative Stress Both In Vitro, in Vero Cells, and In Vivo in Zebrafish. Biology, 13(9), 651. https://doi.org/10.3390/biology13090651






