Exploring Extremotolerant and Extremophilic Microalgae: New Frontiers in Sustainable Biotechnological Applications
Simple Summary
Abstract
1. Introduction
2. Food Industry Applications: Extreme Microalgae Biomolecules
2.1. Protein and Amino Acid Composition
2.2. Lipids and Fatty Acids
2.3. Natural Pigments
2.3.1. Carotenes and Xanthophylls
β-Carotene
Lutein
Astaxanthin
Canthaxanthin
2.3.2. Phycobiliproteins (PBs)
3. Textile and Cosmetic Industries
3.1. Sunscreen Products
3.2. Moisturization
3.3. Anti-Aging Creams
3.4. Colorants (Makeup)
3.5. Textile Pigments
4. Usage for Bioremediation
4.1. Bioremediation of Organic Pollutants
4.1.1. Petroleum Hydrocarbons (PHs) and Polycyclic Aromatic Hydrocarbons (PAHs)
4.1.2. Pesticides
4.2. Bioremediation of Inorganic Pollutants
4.2.1. Heavy Metals
4.2.2. Radioactive Compounds
4.3. Bioremediation of Emerging Contaminants (ECs)
Pharmaceutical’s Products (PhACs)
5. Renewable Energy Sources
5.1. Biodiesel
5.2. Biogas (“Biomethane”)
5.3. Bioethanol
5.4. Biohydrogen
6. Pharmaceutic and Therapeutic Industries
6.1. Ocular Health
6.2. Anti-Cancer Activity
6.3. Antimicrobial and Antioxidant Activity
7. Future Directions, Challenges, and Conclusions
| Biotechnological Industry | Microalgae Specie | Isolation Place | Extreme Characteristics | Biotechnological Application |
|---|---|---|---|---|
| Food industry | Arthrospira platensis | Alkaline and hypersaline lakes [58] | Alkali- and halophilic [58] | High protein content for food supplements [58] |
| Arthrospira maxima (LIMS-PS-1691) | Alkaline lakes [121] | Alkaliphilic [122] | Biomass and nutritional compounds production [122] | |
| Chlamydomonas malina RCC2488 | Beaufort Sea of Artic Ocean [74] | Psychrophilic [74] | Source of lipids and poly-unsaturated fatty acids (PUFAs) [74] | |
| Chlamydomonas nivalis | Liquid water in snow and glaciers of alpine and polar regions [101] | Psychrophilic [101] | Source of astaxanthin, β-carotene, tocopherol and lutein [101,140] | |
| Chlorella vulgaris CA1 | Dairy wastewater [59] | Ammonia-tolerant [59] | High protein content [59] | |
| Chlorella zofingiensis | Fresh water [104] | Halotolerant [104] | Source of astaxanthin and lipids for nutritional supplements [103] | |
| Coccomyxa acidophila | Acidic waters of Tinto River, Spain [92] | Acidophilic [92] | Source of lutein [92] | |
| Coccomyxa melkoniani SCCA 048 | Polluted mine waters of Rio Irvi River, Italy [70] | Resistant to heavy metal contamination [70] | Source of lipids with high nutritional value [70] | |
| Coccomyxa onubensis | Acidic waters of Tinto River, Spain [95] | Acidophilic [95] | Accumulation of lutein for food supplements [95,308] | |
| Cyanidioschyzon merolae 10D | Phelgrean fields, Italy [118] | Acido-, halo- and thermophilic [118,153] | Source of thermostable phycocyanin [118,153] | |
| Cyanidium caldariumm | Acid thermal area of Yellowstone National Park [117] | Acidophilic [117] | Source of phycocyanin [117] | |
| Dactylococcus dissociatus MT1 | Sahara Dessert of Algeria [106] | Resistant to solar ration and extreme daily and seasonal temperature variations [106] | Source of lutein and β-carotene, and lipids with antioxidant properties [106,309] | |
| Dunaliella salina | Shambar Salt Lakes, India [310] | Halophilic [310] | Source of β-carotene [310] | |
| Dunaliella tertiolecta DCCBC26 | Salt Lake of Urmia, Iran [90] | Halophilic [90] | Production of antioxidants and lipids [89] | |
| Galdieria phlegrea ACUF 009 | Cryptoendolithic environments of the Phlegrean Fields, Italy [120] | Acido- and thermophilic [120] | Source of thermostable C-phycocyanin for food colorant and preservative [119] | |
| Galdieria sulphuraria CCMEE 5587.1 | Unknown, obtained from Culture Collection of Microorganisms from Extreme Environments (Pacific Northwest National Laboratory, Richland, USA) [62] | Acidophilic and thermotolerant [62] | High protein content for food supplements [62] | |
| Graesiella sp. | “AinEchfa” hot spring, Tunisia [75] | Thermophilic [75] | Source of lipids with high nutrional value [75] | |
| Mesataenium berggrenii | Tiefenbach Glacier, Austrian Alps [96] | Psychrophilic [96] | Source of lutein and β-carotene [96] | |
| Microchloropsis gaditana CCMP526 | Gippsland Lakes, Australia [65] | Halotolerant [65] | Protein fortification in food [64] | |
| Synechococcus sp. PCC 6715 | Hot springs Yellowstone, USA [115] | Thermophilic [115] | Source of thermostable phycocyanin [115] | |
| Synechococcus vulcanus | Hot springs in Yellowstone National Park, USA [311] | Thermophilic [123] | Source of phycocyanin [123] | |
| Textile and cosmetics industries | Aphanothece halophytica | Isolated from Solar Lake, Sinai [146] | Halotolerant [146] | Source of mycosporine-2-glycine [146] |
| Chlamydomonas nivalis | Liquid water in snow and glaciers of alpine and polar regions [101] | Psychrophilic [101] | Source of astaxanthin, β-carotene, tocopherol and lutein [101,140] | |
| Chlorella vulgaris BUACC25 | Sonapur Sea Beach, Ganjam, Odisha [145] | Halotolerant [145] | Source of antioxidants (phenols and flavonoids) [145] | |
| Chlorella vulgaris M-207A7 | Beverage Technology Research Laboratory’s culture collection [144] | Halotolerant [144] | High chlorophyll content due to induced mutation [144] | |
| Chroococcidiopsis sp. B13 | Solar panels [136] | Resistant to desication, ionizing radiation and UV light [136] | Source of antioxidants [137] | |
| Cyanidioschyzon merolae 10D | Phelgrean fields, Italy [118] | Acido-, halo- and thermophilic [118,153] | Source of thermostable phycocyanin [118,153] | |
| Cyanidium caldariumm | Acid thermal area of Yellowstone National Park [117] | Acidophilic [117] | Source of phycocyanin [117] | |
| Coccomyxa melkonianni SCCA048 | Polluted mine waters of Rio Irvi River, Italy [70] | Resistant to heavy metal contamination [70] | Source of lutein and high lipid content [139] | |
| Dunaliella salina | Shambar Salt Lakes, India [310] | Halotolerant [310] | Source of yellow pigment β-carotene [310] | |
| Dunaliella tertiolecta DCCBC26 | Salt Lake of Urmia, Iran [90] | Halophilic [90] | Source of antioxidants and lipids [89] | |
| Galdieria phlegrea ACUF 009 | Cryptoendolithic environments of the Phlegrean Fields, Italy [120] | Acido- and thermophilic [120] | Source of thermostable C-phycocyanin [119] | |
| Mesataenium berggrenii | Tiefenbach Glacier, Austrian Alps [96] | Psychrophilic [96] | Source of β-carotene [96] | |
| Synechococcus lividus | Thermal alkaline hot springs of Yellowstone National Park [151] | Thermophile [151] | Fount of C-phycocyanin [151] | |
| Bioremediation | Chlamydomonas acidophila RT46 | Acidic waters of Tinto River, Spain [196] | Acidophilic and resistant to heavy metal contamination [196] | Removal of cadmiun [196] |
| Coccomyxa actinobiotis | Storage pool of element in research nuclear reactor, France [209] | Resistant to ionizing radiations and metallotolerant [209] | Bioremediation of radioactive and silver-polluted waters [209,210] | |
| Coccomyxa melkoniani SCCA 048 | Polluted mine waters of Rio Irvi River, Italy (43) | Resistant to heavy metal contamination (43) | Phycoremediation of heavy metals [69] | |
| Coccomyxa subellipsoidea C-169 | Marbel Point, Antarctica [312] | Psychrotolerant [312] | Degradation of organophosphates [312] | |
| Cyanidium caldarium | Littoral zone of Lake Caviahue, Argentina [185] | Acidophilic [185] | Bioindicator of Polycyclic aromatic hydrocarbons pollution [185] | |
| Desmodesmus sp. MAS1 | Local soil and lake water sample [232] | Acid-tolerant [232] | Bioremediation of acid soils [313], heavy metal removal [202] | |
| Dunaliella bardawil | Sambar Lake, India [314] | Halotolerant [314] | Bioremediation of aluminum polluted environments [203] | |
| Euglena gracilis | Acid and heavy metal polluted waters [198] | Acidophilic and metallotolerant [198] | Bioremediation by heavy metal remotion [198] | |
| Euglena mutabilis | Acid mine drainage near Reigous creek, France [201] | Acidophilic and metallotolerant [201] | Bioindicator for arsenic and other heavy metal contamination [200] | |
| Galdieria phlegrea ACUF 784.3 | Geothermal volcanic soils [197] | Acido- and thermophilic [197] | Municipal wastewater treatment [197] | |
| Galdieria sulphuraria 074 W | Sulfuric and acidic hot springs from Mt. Lawu, Indonesia [315] | Acido- and thermophilic [315] | Removal of cesium (Cs) [174] | |
| Nannochloropsis oculate | Unknown, obtained from the Culture Collection of Algae at the University of Texas Austin, USA [184] | Halophilic [184] | Removal of polyhydroxyalkanoates (PHAs) [184] | |
| Nannochloropsis sp. | Unknown, obtained from Varicon Aqua Solution, UK [220] | Halotolerant [220] | Removal of pharmaceuticals compounds [220] | |
| Pinnularia aljustrelica | Acidic waters of Aljustrel mining area, Portugal [316] | Acidophilic and metallotolerant [316] | Bioindicator of acid mine drainage [178] | |
| Pinnularia braunii | Streams near agricultural used lands Manyame, Zimbabwe [179] | Acidophilic and metallotolerant [179] | Bioindicator of water quality [179] | |
| Renewable energy industry | Acutodesmus obliquus MR | Freshwater samples of South Korea [317] | Psychrotolerant [247] | High lipid cell content for biodiesel production [247] |
| Asterarcys quadricellulare | Water bodies near a JSW steel plant, India [251] | Thermophilic and high CO2 tolerance [251] | High carbohydrate cell content for bioethanol and biohydrogen production [251] | |
| Chlorella sorokiniana | Water bodies near a JSW steel plant, India [251] | Thermophilic and high CO2 tolerance [251] | High lipid cell content for biogas and biodiesel production [251] | |
| Desertifilum tharense | Thermal water of Turkey [255] | Thermo- and alkalitolerant [255] | High biochemical methane potential for biogas production [255] | |
| Dunaliella terticola CCAP 19/30 | Saline marine environments [235] | Alkali- and halophilic [235] | High lipid cell content for biodiesel production [235] | |
| Dunaliella viridis Teod. | Maharlu Salt Lake, Iran [248] | Halophilic [248] | High lipid cell content for biodiesel production [248] | |
| Gladieria sulphuraria ACUF 64 | Sulfuric mine Ciavolotta, Italy [236] | Acidophilic [236] | High biomass productivity for biofuel feedstock [236] | |
| Gladieria sulphuraria CCMEE 5587.1 | Unknown, obtained from Culture Collection of Microorganisms from Extreme Environments (Pacific Northwest National Laboratory, Richland, U.S.A.) [266] | Acidophilic [266] | High heating value for biohydrogen production [266] | |
| Phormidium animale | Thermal water of Turkey [255] | Thermo- and alkalitolerant [255] | High biochemical methane potential for biogas production [255] | |
| Picochlorum renovo | Brackish and marine waters [260] | Halophile and thermotolerant [260] | High biomass and carbohydrates productivity for bioethanol and biohydrogen production [260] | |
| Pseudochlorella sp. YKT1 | Sulfuric mine drainage in Nagano Prefecture, Japan [250] | Acidophilic [250] | High lipid cell content biodiesel and biogas production [250] | |
| Pharmaceutics and therapeutics industries | Chlamydomonas acidophila RT46 | Acidic waters of Tinto River, Spain (73) | Acidophilic and resistant to heavy metal contamination (73) | Source of lutein and β-carotene [318] |
| Dactylococcus dissociatus MT1 | Sahara Dessert of Algeria [106] | Resistant to solar ration and extreme daily and seasonal temperature variations [106] | Efficient producer of canthaxanthin [106] | |
| Dunaliella salina | Shambar Salt Lakes, India [310] | Halotolerant [310] | Source of phytosterols [89] | |
| Dunaliella tertiolecta | Salt Lake of Urmia, Iran [90] | Halophilic [90] | Source of phytosterols [89] | |
| Haematococcus pluvialis | Mountainous and valley areas of the Black Sea, Caucasus and Crimea [293] | Can support high salinity [293] | Accumulates large quantities of astaxanthin and produces short chain fatty acids with antimicrobial properties [293] | |
| Chloromonas polyptera | Snow, Antarctica [294] | Psychrophilic [294] | Abundant accumulation of astaxanthin [294] | |
| Chlorella vulgaris KNUA007 | Meltwater stream, King George Island, Antarctica [319] | Cold-tolerant [319] | Rich in nutritional fatty acids (cardiovascular health) [319] | |
| Chlamydomonas sp. JSC4 | Ocean of southern Taiwan [320] | Halotolerant [320] | Source of lutein [281] | |
| Dunaliella sp. ST10 | Hyperaline pond in the “Saline di Tarquinia” on Tyrrhenian Coast, Central Italy [282] | Halotolerant [282] | Fount of lutein [282] | |
| Chlorella zofingiensis | Fresh water [104] | Halotolerant [104] | High astaxanthin and canthaxanthin content [104,288] | |
| Nostoc linckia | Soil on both sides of the water stream of the Helwan hot springs, Egypt [299] | Thermophilic [299] | Source of phenols with antimicrobial properties [299] | |
| Tetraselmis sp. KCTC 12236 BP | Young Heung Island, Incheon, Korea [302] | Halotolerant [302] | Accumulates polysaccharides with antimicrobial activity [303] | |
| Arthrospira platensis | River Krishna, Tungabhadra, India [305] | Halotolerant [304] | Concentration of polysaccharides with antimicrobial activity [304] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ikram, S.F.; Kumar, D.; Singh, V.; Tripathi, B.N.; Kim, B.H. Microalgal and Cyanobacterial Diversity of Two Selected Hot Springs of Garhwal Himalaya, Uttarakhand, India. Fundam. Appl. Limnol. 2021, 195, 111–127. [Google Scholar] [CrossRef]
- Chekanov, K.; Fedorenko, T.; Kublanovskaya, A.; Litvinov, D.; Lobakova, E. Diversity of Carotenogenic Microalgae in the White Sea Polar Region. FEMS Microbiol. Ecol. 2020, 96, 183. [Google Scholar] [CrossRef]
- Arsad, S.; Mulasari, Y.W.; Sari1, N.Y.; Lusiana, E.D.; Risjani, Y.; Musa, M.; Mahmudi, M.; Prasetiya, F.S.; Sari, L.A. Global Journal of Environmental Science and Management Microalgae Diversity in Several Different Sub-Habitats. Glob. J. Environ. Sci. Manag. 2022, 8, 32. [Google Scholar] [CrossRef]
- Sánchez-Baracaldo, P.; Cardona, T. On the Origin of Oxygenic Photosynthesis and Cyanobacteria. New Phytol. 2020, 225, 1440–1446. [Google Scholar] [CrossRef]
- Leão, P.N.; Engene, N.; Antunes, A.; Gerwick, W.H.; Vasconcelos, V. The Chemical Ecology of Cyanobacteria. Nat. Prod. 2012, 29, 372–391. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F.; Acien-Fernandez, F.G.; Molina-Grima, E. Microalgae Research Worldwide. Algal Res. 2018, 35, 50–60. [Google Scholar] [CrossRef]
- Sánchez, M.; Bernal-Castillo, J.; Rozo, C.; Rodríguez, I. Spirulina (arthrospira): An Edible Microorganism: A Review. Univ. Sci. 2003, 8, 7–24. [Google Scholar]
- Wan, D.; Wu, Q.; Kuča, K. Spirulina. Nutraceuticals: Efficacy, Safety and Toxicity; Springer: Berlin/Heidelberg, Germany, 2021; pp. 959–974. [Google Scholar] [CrossRef]
- Graziani, G.; Schiavo, S.; Nicolai, M.A.; Buono, S.; Fogliano, V.; Pinto, G.; Pollio, A. Microalgae as Human Food: Chemical and Nutritional Characteristics of the Thermo-Acidophilic Microalga Galdieria sulphuraria. Food Funct. 2012, 4, 144–152. [Google Scholar] [CrossRef]
- Tertychnaya, T.N.; Manzhesov, V.I.; Andrianov, E.A.; Yakovleva, S.F. New Aspects of Application of Microalgae Dunaliella salina in the Formula of Enriched Bread. IOP Conf. Ser. Earth Environ. Sci. 2020, 422, 012021. [Google Scholar] [CrossRef]
- Canelli, G.; Tarnutzer, C.; Carpine, R.; Neutsch, L.; Bolten, C.J.; Dionisi, F.; Mathys, A. Biochemical and Nutritional Evaluation of Chlorella and Auxenochlorella Biomasses Relevant for Food Application. Front. Nutr. 2020, 7, 565996. [Google Scholar] [CrossRef]
- Widyaningrum, D.; Prianto, A.D. Chlorella as a Source of Functional Food Ingredients: Short Review. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Babylon, Iraq, 2 August 2021; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 794. [Google Scholar]
- Darwish, R.; Gedi, M.A.; Akepach, P.; Assaye, H.; Zaky, A.S.; Gray, D.A. Chlamydomonas reinhardtii Is a Potential Food Supplement with the Capacity to Outperform Chlorella and Spirulina. Appl. Sci. 2020, 10, 6736. [Google Scholar] [CrossRef]
- Masi, A.; Leonelli, F.; Scognamiglio, V.; Gasperuzzo, G.; Antonacci, A.; Terzidis, M.A. Chlamydomonas reinhardtii: A Factory of Nutraceutical and Food Supplements for Human Health. Molecules 2023, 28, 1185. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, N.; Sarma, A.K. Assessment of a Novel Algal Strain Chlamydomonas debaryana NIREMACC03 for Mass Cultivation, Biofuels Production and Kinetic Studies. Appl. Biochem. Biotechnol. 2015, 176, 2253–2266. [Google Scholar] [CrossRef]
- Tripathi, S.; Choudhary, S.; Poluri, K.M. Insights into Lipid Accumulation Features of Coccomyxa sp. IITRSTKM4 under Nutrient Limitation Regimes. Environ. Technol. Innov. 2021, 24, 101786. [Google Scholar] [CrossRef]
- Bortolini, D.G.; Maciel, G.M.; Fernandes, I.d.A.A.; Pedro, A.C.; Rubio, F.T.V.; Branco, I.G.; Haminiuk, C.W.I. Functional Properties of Bioactive Compounds from Spirulina Spp.: Current Status and Future Trends. Food Chem. Mol. Sci. 2022, 5, 100134. [Google Scholar] [CrossRef]
- Čížková, M.; Vítová, M.; Zachleder, V.; Čížková, M.; Vítová, M.; Zachleder, V. The Red Microalga Galdieria as a Promising Organism for Applications in Biotechnology. In Microalgae—From Physiology to Application; InTech Open: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Hosseinzadeh Gharajeh, N.; Valizadeh, M.; Dorani, E.; Hejazi, M.A. Biochemical Profiling of Three Indigenous Dunaliella Isolates with Main Focus on Fatty Acid Composition towards Potential Biotechnological Application. Biotechnol. Rep. 2020, 26, e00479. [Google Scholar] [CrossRef]
- Hyrslova, I.; Krausova, G.; Mrvikova, I.; Stankova, B.; Branyik, T.; Malinska, H.; Huttl, M.; Kana, A.; Doskocil, I. Functional Properties of Dunaliella salina and Its Positive Effect on Probiotics. Mar. Drugs 2022, 20, 781. [Google Scholar] [CrossRef]
- Cezare-Gomes, E.A.; Mejia-da-Silva, L.d.C.; Pérez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; de Carvalho, J.C.M. Potential of Microalgae Carotenoids for Industrial Application. Appl. Biochem. Biotechnol. 2019, 188, 602–634. [Google Scholar] [CrossRef]
- Song, X.; Bo, Y.; Feng, Y.; Tan, Y.; Zhou, C.; Yan, X.; Ruan, R.; Xu, Q.; Cheng, P. Potential Applications for Multifunctional Microalgae in Soil Improvement. Front. Environ. Sci. 2022, 10, 1035332. [Google Scholar] [CrossRef]
- Patras, D.; Moraru, C.V.; Socaciu, C. Bioactive Ingredients from Microalgae: Food and Feed Applications. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Food Sci. Technol. 2019, 76, 1. [Google Scholar] [CrossRef]
- Khavari, F.; Saidijam, M.; Taheri, M.; Nouri, F. Microalgae: Therapeutic Potentials and Applications. Mol. Biol. Rep. 2021, 48, 4757–4765. [Google Scholar] [CrossRef]
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from Microalgae for Cosmetic Applications. Cosmetics 2021, 8, 52. [Google Scholar] [CrossRef]
- Navarro, F.; Forján, E.; Vázquez, M.; Toimil, A.; Montero, Z.; Ruiz-Domínguez, M.d.C.; Garbayo, I.; Castaño, M.; Vílchez, C.; Vega, J.M. Antimicrobial Activity of the Acidophilic Eukaryotic Microalga Coccomyxa onubensis. Phycol. Res. 2017, 65, 38–43. [Google Scholar] [CrossRef]
- Ampofo, J. Abbey, Lord Microalgae: Bioactive Composition, Health Benefits, Safety and Prospects as Potential High-Value Ingredients for the Functional Food Industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef]
- Mohammad Nabavi, S.; Sanches Silva, A. Nonvitamin and Nonmineral Nutritional Supplements; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Barbosa, M.; Inácio, L.G.; Afonso, C.; Maranhão, P. The Microalga Dunaliella and Its Applications: A Review. Appl. Phycol. 2023, 4, 99–120. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Sydney, E.B.; Schafranski, K.; Barretti, B.R.V.; Sydney, A.C.N.; Zimmerman, J.F.D.A.; Cerri, M.L.; Mottin Demiate, I. Biomolecules from Extremophile Microalgae: From Genetics to Bioprocessing of a New Candidate for Large-Scale Production. Process Biochem. 2019, 87, 37–44. [Google Scholar] [CrossRef]
- Lafarga, T.; Sánchez-Zurano, A.; Morillas-España, A.; Acién-Fernández, F.G. Extremophile Microalgae as Feedstock for High-Value Carotenoids: A Review. Int. J. Food Sci. Technol. 2021, 56, 4934–4941. [Google Scholar] [CrossRef]
- Matos De Opitz, C.L.; Sass, P. Tackling Antimicrobial Resistance by Exploring New Mechanisms of Antibiotic Action. Future Microbiol. 2020, 15, 703–708. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, H.; Du, H.; Jesumani, V.; He, W.; Cheong, K.L.; Li, T.; Hong, T. Industry Chain and Challenges of Microalgal Food Industry-a Review. Crit. Rev. Food Sci. Nutr. 2022, 64, 4789–4816. [Google Scholar] [CrossRef]
- Chang, M.; Liu, K. Arthrospira platensis as Future Food: A Review on Functional Ingredients, Bioactivities and Application in the Food Industry. Int. J. Food Sci. Technol. 2024, 59, 1197–1212. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation into Innovative Food Products with Potential Health Benefits. Front. Nutr. 2018, 5, 00058. [Google Scholar] [CrossRef]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.Y.; Filaire, E. Microalgae N-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, Composition, Production, Processing and Applications of Chlorella vulgaris: A Review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae Biomass as an Alternative Ingredient in Cookies: Sensory, Physical and Chemical Properties, Antioxidant Activity and in Vitro Digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Lafarga, T.; Acién, G. Microalgae for the Food Industry: From Biomass Production to the Development of Functional Foods. Foods 2022, 11, 765. [Google Scholar] [CrossRef]
- Soru, S.; Malavasi, V.; Concas, A.; Caboni, P.; Cao, G. A Novel Investigation of the Growth and Lipid Production of the Extremophile Microalga Coccomyxa melkonianii SCCA 048 under the Effect of Different Cultivation Conditions: Experiments and Modeling. Chem. Eng. J. 2019, 377, 120589. [Google Scholar] [CrossRef]
- Chacón-Lee, T.L.; González-Mariño, G.E. Microalgae for “Healthy” Foods-Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Maroneze, M.M.; Deprá, M.C.; Sartori, R.B.; Dias, R.R.; Zepka, L.Q. Bioactive Food Compounds from Microalgae: An Innovative Framework on Industrial Biorefineries. Curr. Opin. Food Sci. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Raczyk, M.; Polanowska, K.; Kruszewski, B.; Grygier, A.; Michałowska, D. Effect of Spirulina (Arthrospira platensis) Supplementation on Physical and Chemical Properties of Semolina (Triticum durum) Based Fresh Pasta. Molecules 2022, 27, 355. [Google Scholar] [CrossRef] [PubMed]
- Hernández-López, I.; Alamprese, C.; Cappa, C.; Prieto-Santiago, V.; Abadias, M.; Aguiló-Aguayo, I. Effect of Spirulina in Bread Formulated with Wheat Flours of Different Alveograph Strength. Foods 2023, 12, 3724. [Google Scholar] [CrossRef] [PubMed]
- Kejžar, J.; Jagodic Hudobivnik, M.; Nečemer, M.; Ogrinc, N.; Masten Rutar, J.; Poklar Ulrih, N. Characterization of Algae Dietary Supplements Using Antioxidative Potential, Elemental Composition, and Stable Isotopes Approach. Front. Nutr. 2021, 7, 618503. [Google Scholar] [CrossRef] [PubMed]
- Dinçoğlu, A.H.; Akça, S.S.; Çalışkan, Z. Effect of Spirulina platensis on Probiotic, Nutritional, and Quality Properties of Yogurt. Int. Food Res. J. 2024, 31, 157–168. [Google Scholar] [CrossRef]
- Fantechi, T.; Contini, C.; Casini, L. Pasta Goes Green: Consumer Preferences for Spirulina-Enriched Pasta in Italy. Algal Res. 2023, 75, 103275. [Google Scholar] [CrossRef]
- Lucas, B.F.; Alberto Vieira Costa, J.; Brunner, T.A. Attitudes of Consumers toward Spirulina and Açaí and Their Use as a Food Ingredient. LWT 2023, 178, 114600. [Google Scholar] [CrossRef]
- Ferreira de Oliveira, A.P.; Bragotto, A.P.A. Microalgae-Based Products: Food and Public Health. Future Foods 2022, 6, 100157. [Google Scholar] [CrossRef]
- Mosibo, O.K.; Ferrentino, G.; Udenigwe, C.C. Microalgae Proteins as Sustainable Ingredients in Novel Foods: Recent Developments and Challenges. Foods 2024, 13, 733. [Google Scholar] [CrossRef]
- Boukid, F.; Castellari, M. Food and Beverages Containing Algae and Derived Ingredients Launched in the Market from 2015 to 2019: A Front-of-Pack Labeling Perspective with a Special Focus on Spain. Foods 2021, 10, 173. [Google Scholar] [CrossRef]
- Varshney, P.; Mikulic, P.; Vonshak, A.; Beardall, J.; Wangikar, P.P. Extremophilic Micro-Algae and Their Potential Contribution in Biotechnology. Bioresour. Technol. 2015, 184, 363–372. [Google Scholar] [CrossRef]
- Liu, J.; Chen, F. Biology and Industrial Applications of Chlorella: Advances and Prospects. Adv. Biochem. Eng. Biotechnol. 2014, 153, 1–35. [Google Scholar] [CrossRef]
- Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Gayen, K.; Bhowmick, T.K. Downstream Processing of Microalgae for Pigments, Protein and Carbohydrate in Industrial Application: A Review. Food Bioprod. Process. 2018, 110, 60–84. [Google Scholar] [CrossRef]
- Volkmann, H.; Imianovsky, U.; Oliveira, J.L.B.; Sant’Anna, E.S. Cultivation of Arthrospira (spirulina) Platensis in Desalinator Wastewater and Salinated Synthetic Medium: Protein Content and Amino-Acid Profile. Braz. J. Microbiol. 2008, 39, 98–101. [Google Scholar] [CrossRef]
- Pang, N.; Bergeron, A.D.; Gu, X.; Fu, X.; Dong, T.; Yao, Y.; Chen, S. Recycling of Nutrients from Dairy Wastewater by Extremophilic Microalgae with High Ammonia Tolerance. Environ. Sci. Technol. 2020, 54, 15366–15375. [Google Scholar] [CrossRef]
- Markou, G.; Arapoglou, D.; Eliopoulos, C.; Balafoutis, A.; Taddeo, R.; Panara, A.; Thomaidis, N. Cultivation and Safety Aspects of Arthrospira platensis (spirulina) Grown with Struvite Recovered from Anaerobic Digestion Plant as Phosphorus Source. Algal Res. 2019, 44, 101716. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a Future Food Source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Herrera, C.A.; Vera-López Portillo, F.; Hernández-Chávez, G.T.; Martinez, A. Single-Cell Protein Production Potential with the Extremophilic Red Microalgae Galdieria sulphuraria: Growth and Biochemical Characterization. J. Appl. Phycol. 2022, 34, 1341–1352. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Qazi, W.M.; Ballance, S.; Kousoulaki, K.; Uhlen, A.K.; Kleinegris, D.M.M.; Skjånes, K.; Rieder, A. Protein Enrichment of Wheat Bread with Microalgae: Microchloropsis gaditana, Tetraselmis chui and Chlorella vulgaris. Foods 2021, 10, 3078. [Google Scholar] [CrossRef]
- Karthikaichamy, A.; Deore, P.; Srivastava, S.; Coppel, R.; Bulach, D.; Beardall, J.; Noronha, S. Temporal Acclimation of Microchloropsis Gaditana CCMP526 in Response to Hypersalinity. Bioresour. Technol. 2018, 254, 23–30. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional Evaluation of Australian Microalgae as Potential Human Health Supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, E.; Silva, J.; Mendonça, S.H.; Abreu, M.H.; Domingues, M.R. Lipidomic Approaches towards Deciphering Glycolipids from Microalgae as a Reservoir of Bioactive Lipids. Mar. Drugs 2016, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Villarruel-López, A.; Ascencio, F.; Nunõ, K. Microalgae, a Potential Natural Functional Food Source—A Review. Pol. J. Food Nutr. Sci. 2017, 67, 251–263. [Google Scholar] [CrossRef]
- Fais, G.; Malavasi, V.; Scano, P.; Soru, S.; Caboni, P.; Cao, G. Metabolomics and Lipid Profile Analysis of Coccomyxa melkonianii SCCA 048. Extremophiles 2021, 25, 357–368. [Google Scholar] [CrossRef]
- Soru, S.; Malavasi, V.; Caboni, P.; Concas, A.; Cao, G. Behavior of the Extremophile Green Alga Coccomyxa melkonianii SCCA 048 in Terms of Lipids Production and Morphology at Different PH Values. Extremophiles 2019, 23, 79–89. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Barbosa, C.R.; Pereira, R.D.; Malcata, F.X. Fatty Acid Composition of Several Wild Microalgae and Cyanobacteria, with a Focus on Eicosapentaenoic, Docosahexaenoic and α-Linolenic Acids for Eventual Dietary Uses. Food Res. Int. 2011, 44, 2721–2729. [Google Scholar] [CrossRef]
- Barta, D.G.; Coman, V.; Vodnar, D.C. Microalgae as Sources of Omega-3 Polyunsaturated Fatty Acids: Biotechnological Aspects. Algal Res. 2021, 58, 102410. [Google Scholar] [CrossRef]
- Ramesh Kumar, B.; Deviram, G.; Mathimani, T.; Duc, P.A.; Pugazhendhi, A. Microalgae as Rich Source of Polyunsaturated Fatty Acids. Biocatal. Agric. Biotechnol. 2019, 17, 583–588. [Google Scholar] [CrossRef]
- Morales-Sánchez, D.; Schulze, P.S.C.; Kiron, V.; Wijffels, R.H. Production of Carbohydrates, Lipids and Polyunsaturated Fatty Acids (PUFA) by the Polar Marine Microalga Chlamydomonas malina RCC2488. Algal Res. 2020, 50, 102016. [Google Scholar] [CrossRef]
- Gara-Ali, M.; Zili, F.; Hosni, K.; Ben Ouada, H.; Ben-Mahrez, K. Lipophilic Extracts of the Thermophilic Cyanobacterium Leptolyngbya sp. and Chlorophyte graesiella sp. and Their Potential Use as Food and Anticancer Agents. Algal Res. 2021, 60, 102511. [Google Scholar] [CrossRef]
- Mendes-Silva, T.D.C.D.; da Silva Andrade, R.F.; Ootani, M.A.; Mendes, P.V.D.; da Silva, M.R.F.; Souza, K.S.; dos Santos Correia, M.T.; da Silva, M.V.; de Oliveira, M.B.M. Biotechnological Potential of Carotenoids Produced by Extremophilic Microorganisms and Application Prospects for the Cosmetics Industry. Adv. Microbiol. 2020, 10, 397–410. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-Derived Pigments for the Food Industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Bonos, E.; Florou-Paneri, P. Innovative Microalgae Pigments as Functional Ingredients in Nutrition. In Handbook of Marine Microalgae: Biotechnology Advances; Springer: Berlin/Heidelberg, Germany, 2015; pp. 233–243. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.J.; Chang, J.S. Recent Advances in Lutein Production from Microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795. [Google Scholar] [CrossRef]
- Ahmed, F.; Fanning, K.; Netzel, M.; Schenk, P.M. Induced Carotenoid Accumulation in Dunaliella salina and Tetraselmis Suecica by Plant Hormones and UV-C Radiation. Appl. Microbiol. Biotechnol. 2015, 99, 9407–9416. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Ryan, L.; O’Brien, N. Comparison of the Uptake and Secretion of Carotene and Xanthophyll Carotenoids by Caco-2 Intestinal Cells. Br. J. Nutr. 2007, 98, 38–44. [Google Scholar] [CrossRef]
- Gateau, H.; Solymosi, K.; Marchand, J.; Schoefs, B. Carotenoids of Microalgae Used in Food Industry and Medicine. Mini-Rev. Med. Chem. 2016, 17, 1140–1172. [Google Scholar] [CrossRef]
- Limón, P.; Malheiro, R.; Casal, S.; Acién-Fernández, F.G.; Fernández-Sevilla, J.M.; Rodrigues, N.; Cruz, R.; Bermejo, R.; Pereira, J.A. Improvement of Stability and Carotenoids Fraction of Virgin Olive Oils by Addition of Microalgae Scenedesmus almeriensis Extracts. Food Chem. 2015, 175, 203–211. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; López-Pozo, M.; Stewart, J.J.; Adams, W.W. Zeaxanthin and Lutein: Photoprotectors, Anti-Inflammatories, and Brain Food. Molecules 2020, 25, 3607. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; El-Baroty, G.S. The Potential Use of Microalgal Carotenoids as Dietary Supplements and Natural Preservative Ingredients. J. Aquat. Food Prod. Technol. 2013, 22, 392–406. [Google Scholar] [CrossRef]
- Hu, C.C.; Lin, J.T.; Lu, F.J.; Chou, F.P.; Yang, D.J. Determination of Carotenoids in Dunaliella salina Cultivated in Taiwan and Antioxidant Capacity of the Algal Carotenoid Extract. Food Chem. 2008, 109, 439–446. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Nascimento, T.C.; Pinheiro, P.N.; Vendruscolo, R.G.; Wagner, R.; de Rosso, V.V.; Jacob-Lopes, E.; Zepka, L.Q. Bioaccessibility of Microalgae-Based Carotenoids and Their Association with the Lipid Matrix. Food Res. Int. 2021, 148, 110596. [Google Scholar] [CrossRef] [PubMed]
- Pourkarimi, S.; Hallajisani, A.; Nouralishahi, A.; Alizadehdakhel, A.; Golzary, A. Factors Affecting Production of Beta-Carotene from Dunaliella salina Microalgae. Biocatal. Agric. Biotechnol. 2020, 29, 101771. [Google Scholar] [CrossRef]
- Tammam, A.; Fakhry, E.; El-Sheekh, M. Effect of Salt Stress on Antioxidant System and the Metabolism of the Reactive Oxygen Species in Dunaliella salina and Dunaliella tertiolecta. Afr. J. Biotechnol. 2011, 10, 3795–3808. [Google Scholar]
- Fazeli, M.R.; Tofighi, H.; Samadi, N.; Jamalifar, H. Effects of Salinity on β-Carotene Production by Dunaliella tertiolecta DCCBC26 Isolated from the Urmia Salt Lake, North of Iran. Bioresour. Technol. 2006, 97, 2453–2456. [Google Scholar] [CrossRef]
- Çakmak, Z.E.; Çakmak, T. Evaluation of an Indigenous Dunaliella strain for β-Caroten and Neutral Lipid Production as a Response to Cobalt Deprivation. Adv. Renew. Energy 2016, 3. [Google Scholar]
- Casal, C.; Cuaresma, M.; Vega, J.M.; Vilchez, C. Enhanced Productivity of a Lutein-Enriched Novel Acidophile Microalga Grown on Urea. Mar. Drugs 2011, 9, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Cuaresma, M.; Casal, C.; Forján, E.; Vílchez, C. Productivity and Selective Accumulation of Carotenoids of the Novel Extremophile Microalga Chlamydomonas acidophila Grown with Different Carbon Sources in Batch Systems. J. Ind. Microbiol. Biotechnol. 2011, 38, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sevilla, J.M.; Acién Fernández, F.G.; Molina Grima, E. Biotechnological Production of Lutein and Its Applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef]
- Vaquero, I.; Ruiz-Domínguez, M.C.; Márquez, M.; Vílchez, C. Cu-Mediated Biomass Productivity Enhancement and Lutein Enrichment of the Novel Microalga Coccomyxa onubensis. Process Biochem. 2012, 47, 694–700. [Google Scholar] [CrossRef]
- Remias, D.; Holzinger, A.; Lutz, C. Physiology, Ultrastructure and Habitat of the Ice Alga Mesotaenium berggrenii (Zygnemaphyceae, Chlorophyta) from Glaciers in the European Alps. Phycologia 2009, 48, 302–312. [Google Scholar] [CrossRef]
- Iwamoto, H.; Soccol, C.R.; Molina-Aulestia, D.T.; Cardoso, J.; de Melo Pereira, G.V.; de Souza Vandenberghe, L.P.; Manzoki, M.C.; Ambati, R.R.; Ravishankar, G.A.; de Carvalho, J.C. Lutein from Microalgae: An Industrial Perspective of Its Production, Downstream Processing, and Market. Fermentation 2024, 10, 106. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential Health-Promoting Effects of Astaxanthin: A High-Value Carotenoid Mostly from Microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Villaró, S.; Ciardi, M.; Morillas-españa, A.; Sánchez-zurano, A.; Acién-fernández, G.; Lafarga, T. Microalgae Derived Astaxanthin: Research and Consumer Trends and Industrial Use as Food. Foods 2021, 10, 2303. [Google Scholar] [CrossRef]
- Zheng, Y.; Xue, C.; Chen, H.; He, C.; Wang, Q. Corrigendum: Low-Temperature Adaptation of the Snow Alga Chlamydomonas nivalis Is Associated with the Photosynthetic System Regulatory Process. Front. Microbiol. 2021, 12, 698706. [Google Scholar] [CrossRef]
- Gorton, H.L.; Williams, W.E.; Vogelmann, T.C. The Light Environment and Cellular Optics of the Snow Alga Chlamydomonas nivalis (Bauer) Wille. Photochem. Photobiol. 2001, 73, 611–620. [Google Scholar] [CrossRef]
- Tambat, V.S.; Patel, A.K.; Singhania, R.R.; Vadrale, A.P.; Tiwari, A.; Chen, C.W.; Dong, C. Di Sustainable Mixotrophic Microalgae Refinery of Astaxanthin and Lipid from Chlorella zofingiensis. Bioresour. Technol. 2023, 387, 129635. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an Alternative Microalgal Producer of Astaxanthin: Biology and Industrial Potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef]
- Abe, K.; Hattori, H.; Hirano, M. Accumulation and Antioxidant Activity of Secondary Carotenoids in the Aerial Microalga Coelastrella striolata Var. Multistriata. Food Chem. 2007, 100, 656–661. [Google Scholar] [CrossRef]
- Grama, B.S.; Chader, S.; Khelifi, D.; Agathos, S.N.; Jeffryes, C. Induction of Canthaxanthin Production in a Dactylococcus Microalga Isolated from the Algerian Sahara. Bioresour. Technol. 2014, 151, 297–305. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Rimbach, G. Canthaxanthin: From Molecule to Function. Mol. Nutr. Food Res. 2017, 61, 1600469. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Razavi, S.H.; Mousavi, S.M. Microbial Canthaxanthin: Perspectives on Biochemistry and Biotechnological Production. Eng. Life Sci. 2013, 13, 408–417. [Google Scholar] [CrossRef]
- Corato, A.; Le, T.T.; Baurain, D.; Jacques, P.; Remacle, C.; Franck, F. A Fast-Growing Oleaginous Strain of Coelastrella Capable of Astaxanthin and Canthaxanthin Accumulation in Phototrophy and Heterotrophy. Life 2022, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Tounsi, L.; Ben Hlima, H.; Hentati, F.; Hentati, O.; Derbel, H.; Michaud, P.; Abdelkafi, S. Microalgae: A Promising Source of Bioactive Phycobiliproteins. Mar. Drugs 2023, 21, 440. [Google Scholar] [CrossRef] [PubMed]
- Kobylewski, S.; Jacobson, M.F. Toxicology of Food Dyes. Int. J. Occup. Environ. Health 2012, 18, 220–246. [Google Scholar] [CrossRef]
- Mysliwa-Kurdziel, B.; Solymosi, K. Phycobilins and Phycobiliproteins Used in Food Industry and Medicine. Mini-Rev. Med. Chem. 2016, 17, 1173–1193. [Google Scholar] [CrossRef] [PubMed]
- Martelli, G.; Folli, C.; Visai, L.; Daglia, M.; Ferrari, D. Thermal Stability Improvement of Blue Colorant C-Phycocyanin from Spirulina platensis for Food Industry Applications. Process Biochem. 2014, 49, 154–159. [Google Scholar] [CrossRef]
- García, A.B.; Longo, E.; Bermejo, R. The Application of a Phycocyanin Extract Obtained from Arthrospira platensis as a Blue Natural Colorant in Beverages. J. Appl. Phycol. 2021, 33, 3059–3070. [Google Scholar] [CrossRef]
- Klepacz-Smółka, A.; Pietrzyk, D.; Szeląg, R.; Głuszcz, P.; Daroch, M.; Tang, J.; Ledakowicz, S. Effect of Light Colour and Photoperiod on Biomass Growth and Phycocyanin Production by Synechococcus PCC 6715. Bioresour Technol. 2020, 313, 123700. [Google Scholar] [CrossRef]
- Liang, Y.; Kaczmarek, M.B.; Kasprzak, A.K.; Tang, J.; Shah, M.M.R.; Jin, P.; Klepacz-Smółka, A.; Cheng, J.J.; Ledakowicz, S.; Daroch, M. Thermosynechococcaceae as a Source of Thermostable C-Phycocyanins: Properties and Molecular Insights. Algal Res. 2018, 35, 223–235. [Google Scholar] [CrossRef]
- Eisele, L.E.; Bakhru, S.H.; Liu, X.; MacColl, R.; Edwards, M.R. Studies on C-Phycocyanin from Cyanidium Caldarium, a Eukaryote at the Extremes of Habitat. Biochim. Et. Biophys. Acta (BBA)-Bioenerg. 2000, 1456, 99–107. [Google Scholar] [CrossRef][Green Version]
- Villegas-Valencia, M.; González-Portela, R.E.; de Freitas, B.B.; Al Jahdali, A.; Romero-Villegas, G.I.; Malibari, R.; Kapoore, R.V.; Fuentes-Grünewald, C.; Lauersen, K.J. Cultivation of the Polyextremophile Cyanidioschyzon merolae 10D during Summer Conditions on the Coast of the Red Sea and Its Adaptation to Hypersaline Sea Water. Front. Microbiol. 2023, 14, 1157151. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.; Imbimbo, P.; Marseglia, A.; Illiano, A.; Fontanarosa, C.; Amoresano, A.; Olivieri, G.; Pollio, A.; Monti, D.M.; Merlino, A. A Thermophilic C-Phycocyanin with Unprecedented Biophysical and Biochemical Properties. Int. J. Biol. Macromol. 2020, 150, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Carfagna, S.; Landi, V.; Coraggio, F.; Salbitani, G.; Vona, V.; Pinto, G.; Pollio, A.; Ciniglia, C. Different Characteristics of C-Phycocyanin (C-PC) in Two Strains of the Extremophilic Galdieria phlegrea. Algal Res. 2018, 31, 406–412. [Google Scholar] [CrossRef]
- Ling, N.; Mao, Y.; Zhang, X.; Mo, Z.; Wang, G.; Liu, W. Sequence Analysis of Arthrospira maxima Based on Fosmid Library. J. Appl. Phycol. 2007, 19, 333–346. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; Dinh, T.B.; Choi, S.; De Saeger, J.; Depuydt, S.; Brown, M.T.; Han, T. Commercial Potential of the Cyanobacterium Arthrospira maxima: Physiological and Biochemical Traits and the Purification of Phycocyanin. Biology 2022, 11, 628. [Google Scholar] [CrossRef]
- Adir, N.; Dobrovetsky, Y.; Lerner, N. Structure of C-Phycocyanin from the Thermophilic Cyanobacterium Synechococcus Vulcanus at 2.5 Å: Structural Implications for Thermal Stability in Phycobilisome Assembly. J. Mol. Biol. 2001, 313, 71–81. [Google Scholar] [CrossRef]
- Danh, N.T. Research on the Popularity of Cosmetics and Its Future in Vietnam Market. Qual.-Access Success. 2022, 23, 19–22. [Google Scholar] [CrossRef]
- Ratajczak, P.; Landowska, W.; Kopciuch, D.; Paczkowska, A.; Zaprutko, T.; Kus, K. The Growing Market for Natural Cosmetics in Poland: Consumer Preferences and Industry Trends. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1877. [Google Scholar] [CrossRef]
- Rybczyńska-Tkaczyk, K.; Grenda, A.; Jakubczyk, A.; Kiersnowska, K.; Bik-Małodzińska, M. Natural Compounds with Antimicrobial Properties in Cosmetics. Pathogens 2023, 12, 320. [Google Scholar] [CrossRef]
- Salinas, T.; Piquette-Miller, M. Within Our Skin. Clin. Pharmacol. Ther. 2017, 102, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Adique, A.; Sarkar, P.; Shenai, V.; Sampath, M.; Lai, R.; Qi, J.; Wang, M.; Farage, M.A. The Impact of Routine Skin Care on the Quality of Life. Cosmetics 2020, 7, 59. [Google Scholar] [CrossRef]
- Yarkent, Ç.; Gürlek, C.; Oncel, S.S. Potential of Microalgal Compounds in Trending Natural Cosmetics: A Review. Sustain. Chem. Pharm. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Amberg, N.; Fogarassy, C. Green Consumer Behavior in the Cosmetics Market. Resources 2019, 8, 137. [Google Scholar] [CrossRef]
- Zhuang, D.; He, N.; Khoo, K.S.; Ng, E.P.; Chew, K.W.; Ling, T.C. Application Progress of Bioactive Compounds in Microalgae on Pharmaceutical and Cosmetics. Chemosphere 2022, 291, 132932. [Google Scholar] [CrossRef] [PubMed]
- Žmitek, K.; Žmitek, J.; Rogl Butina, M.; Hristov, H.; Pogačnik, T.; Pravst, I. Dietary Lutein Supplementation Protects against Ultraviolet-Radiation-Induced Erythema: Results of a Randomized Double-Blind Placebo-Controlled Study. J. Funct. Foods 2020, 75, 104265. [Google Scholar] [CrossRef]
- Dang, L.; Wang, Y.; Xue, Y.; He, L.; Li, Y.; Xiong, J. Low-Dose UVB Irradiation Prevents MMP2-Induced Skin Hyperplasia by Inhibiting Inflammation and ROS. Oncol. Rep. 2015, 34, 1478–1486. [Google Scholar] [CrossRef]
- Anbualakan, K.; Tajul Urus, N.Q.; Makpol, S.; Jamil, A.; Mohd Ramli, E.S.; Md Pauzi, S.H.; Muhammad, N. A Scoping Review on the Effects of Carotenoids and Flavonoids on Skin Damage Due to Ultraviolet Radiation. Nutrients 2022, 15, 92. [Google Scholar] [CrossRef]
- Tuj Johra, F.; Kumar Bepari, A.; Tabassum Bristy, A.; Mahmud Reza, H. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef]
- Baldanta, S.; Arnal, R.; Blanco-Rivero, A.; Guevara, G.; Navarro Llorens, J.M. First Characterization of Cultivable Extremophile Chroococcidiopsis Isolates from a Solar Panel. Front. Microbiol. 2023, 14, 982422. [Google Scholar] [CrossRef]
- Assunção, J.; Amaro, H.M.; Lopes, G.; Tavares, T.; Malcata, F.X.; Guedes, A.C. Exploration of Marine Genus Chroococcidiopsis sp.: A Valuable Source for Antioxidant Industry? J. Appl. Phycol. 2021, 33, 2169–2187. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Papadaki, S.; Krokida, M. Life Cycle Analysis of β-Carotene Extraction Techniques. J. Food Eng. 2015, 167, 51–58. [Google Scholar] [CrossRef]
- Marcella, P.; Tempesta, S.; Malavasi, V.; Barghini, P.; Fenice, M. Lutein Production by Coccomyxa sp. SCCA048 Isolated from a Heavy Metal-Polluted River in Sardinia (Italy). J. Environ. Prot. Ecol. 2015, 16, 1262–1272. [Google Scholar]
- Leya, T.; Rahn, A.; Lütz, C.; Remias, D. Response of Arctic Snow and Permafrost Algae to High Light and Nitrogen Stress by Changes in Pigment Composition and Applied Aspects for Biotechnology. FEMS Microbiol. Ecol. 2009, 67, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Stoyneva-Gärtner, M.; Uzunov, B.; Gärtner, G. Enigmatic Microalgae from Aeroterrestrial and Extreme Habitats in Cosmetics: The Potential of the Untapped Natural Sources. Cosmetics 2020, 7, 27. [Google Scholar] [CrossRef]
- Martínez-Ruiz, M.; Martínez-González, C.A.; Kim, D.H.; Santiesteban-Romero, B.; Reyes-Pardo, H.; Villaseñor-Zepeda, K.R.; Meléndez-Sánchez, E.R.; Ramírez-Gamboa, D.; Díaz-Zamorano, A.L.; Sosa-Hernández, J.E.; et al. Microalgae Bioactive Compounds to Topical Applications Products—A Review. Molecules 2022, 27, 3512. [Google Scholar] [CrossRef]
- Lodén, M. The Clinical Benefit of Moisturizers. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 672–688. [Google Scholar] [CrossRef]
- Nakanishi, K.; Deuchi, K. Culture of a High-Chlorophyll-Producing and Halotolerant Chlorella vulgaris. J. Biosci. Bioeng. 2014, 117, 617–619. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Dash, S.R.; Nayak, R.; Behera, C.; Jena, M. Evaluation of the Anti-Bacterial Activity of Methanolic Extract of Chlorella vulgaris Beyerinck [Beijerinck] with Special Reference to Antioxidant Modulation. Future J. Pharm. Sci. 2021, 7, 17. [Google Scholar] [CrossRef]
- Tarasuntisuk, S.; Palaga, T.; Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-2-Glycine Exerts Anti-Inflammatory and Antioxidant Effects in Lipopolysaccharide (LPS)-Stimulated RAW 264.7 Macrophages. Arch. Biochem. Biophys. 2019, 662, 33–39. [Google Scholar] [CrossRef]
- Zheng, W.; Li, H.; Go, Y.; Chan, X.H.; Huang, Q.; Wu, J. Research Advances on the Damage Mechanism of Skin Glycation and Related Inhibitors. Nutrients 2022, 14, 4588. [Google Scholar] [CrossRef] [PubMed]
- Waditee-Sirisattha, R.; Kageyama, H.; Fukaya, M.; Rai, V.; Takabe, T. Nitrate and Amino Acid Availability Affects Glycine Betaine and Mycosporine-2-Glycine in Response to Changes of Salinity in a Halotolerant Cyanobacterium Aphanothece halophytica. FEMS Microbiol. Lett. 2015, 362, fnv198. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, R.; Rai, A.K. Microcystin Congeners Contribute to Toxicity in the Halophilic Cyanobacterium Aphanothece halophytica. Arch. Biol. Sci. 2014, 66, 1441–1446. [Google Scholar] [CrossRef]
- Kim, S.-K.; Ravichandran, D.; Khan, S.; Kim, Y. Prospective of the Cosmeceuticals Derived from Marine Organisms. Biotechnol. Bioprocess. Eng. 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Edwards, M.R.; MacColl, R.; Eisele, L.E. Some Physical Properties of an Unusual C-Phycocyanin Isolated from a Photosynthetic Thermophile. Biochim. Biophys. Acta (BBA)-Bioenerg. 1996, 1276, 64–70. [Google Scholar] [CrossRef][Green Version]
- Carabantes, A.G.; Dufossé, L. Microalgal Phycobiliproteins for Food/Feed Applications. In Handbook of Food and Feed from Microalgae: Production, Application, Regulation, and Sustainability; Springer: Berlin/Heidelberg, Germany, 2023; pp. 161–169. [Google Scholar] [CrossRef]
- Yoshida, C.; Murakami, M.; Niwa, A.; Takeya, M.; Osanai, T. Efficient Extraction and Preservation of Thermotolerant Phycocyanins from Red Alga Cyanidioschyzon merolae. J. Biosci. Bioeng. 2021, 131, 161–167. [Google Scholar] [CrossRef]
- Ranasinghe, L.; Jayasooriya, V.M. Ecolabelling in Textile Industry: A Review. Resour. Environ. Sustain. 2021, 6, 100037. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Chan, H.L.; Choi, T.M.; Yue, X. Effects of Carbon Tariffs Trading Policy on Duopoly Market Entry Decisions and Price Competition: Insights from Textile Firms of Developing Countries. Int. J. Prod. Econ. 2016, 181, 470–484. [Google Scholar] [CrossRef]
- You, S.; Cheng, S.; Yan, H. The Impact of Textile Industry on China’s Environment. Int. J. Fash. Des. Technol. Educ. 2009, 2, 33–43. [Google Scholar] [CrossRef]
- Belpaire, C.; Reyns, T.; Geeraerts, C.; Van Loco, J. Toxic Textile Dyes Accumulate in Wild European Eel Anguilla anguilla. Chemosphere 2015, 138, 784–791. [Google Scholar] [CrossRef]
- Mutaf-Kılıc, T.; Demir, A.; Elibol, M.; Oncel, S.S. Microalgae Pigments as a Sustainable Approach to Textile Dyeing: A Critical Review. Algal Res. 2023, 76, 103291. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from Microalgae: A Review of Recent Developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Prieto, A.; Pedro Cañavate, J.; García-González, M. Assessment of Carotenoid Production by Dunaliella salina in Different Culture Systems and Operation Regimes. J. Biotechnol. 2011, 151, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Shariati, M. Dunaliella Biotechnology: Methods and Applications. J. Appl. Microbiol. 2009, 107, 14–35. [Google Scholar] [CrossRef]
- Geremia, E.; Ripa, M.; Catone, C.M.; Ulgiati, S. A Review about Microalgae Wastewater Treatment for Bioremediation and Biomass Production—A New Challenge for Europe. Environments 2021, 8, 136. [Google Scholar] [CrossRef]
- Giovanella, P.; Vieira, G.A.L.; Ramos Otero, I.V.; Pais Pellizzer, E.; de Jesus Fontes, B.; Sette, L.D. Metal and Organic Pollutants Bioremediation by Extremophile Microorganisms. J. Hazard. Mater. 2020, 382, 121024. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V.A. Polycyclic Aromatic Hydrocarbons: A Critical Review of Environmental Occurrence and Bioremediation. Environ. Manag. 2017, 60, 758–783. [Google Scholar] [CrossRef]
- Ahmad, I.; Abdullah, N.; Koji, I.; Yuzir, A.; Mohamad, S.E. Potential of Microalgae in Bioremediation of Wastewater. Bull. Chem. React. Eng. Catal. 2021, 16, 413–429. [Google Scholar] [CrossRef]
- Gupta, S.; Pawar, S.B.; Pandey, R.A. Current Practices and Challenges in Using Microalgae for Treatment of Nutrient Rich Wastewater from Agro-Based Industries. Sci. Total Environ. 2019, 687, 1107–1126. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.S. Bioremediation of Heavy Metals Using Microalgae: Recent Advances and Mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Verma, P.; Lavecchia, R.; Zuorro, A. Microalgae-Based Biorefineries for Sustainable Resource Recovery from Wastewater. J. Water Process Eng. 2021, 40, 101747. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Sahoo, D.; Pandey, A. Resource Recovery through Bioremediation of Wastewaters and Waste Carbon by Microalgae: A Circular Bioeconomy Approach. Environ. Sci. Pollut. Res. 2021, 28, 58837–58856. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Sun, Y.; Zhou, Y.; Kumar, M.; Usman, M.; Li, J.; Shao, J.; Wang, L.; Tsang, D.C.W. Bioremediation of Water Containing Pesticides by Microalgae: Mechanisms, Methods, and Prospects for Future Research. Sci. Total Environ. 2020, 707, 136080. [Google Scholar] [CrossRef] [PubMed]
- Bauenova, M.O.; Sadvakasova, A.K.; Mustapayeva, Z.O.; Kokociński, M.; Zayadan, B.K.; Wojciechowicz, M.K.; Balouch, H.; Akmukhanova, N.R.; Alwasel, S.; Allakhverdiev, S.I. Potential of Microalgae Parachlorella kessleri Bh-2 as Bioremediation Agent of Heavy Metals Cadmium and Chromium. Algal Res. 2021, 59, 102463. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.L.; Malcata, X.X. Use of the Microalga Scenedesmus obliquus to Remove Cadmium Cations from Aqueous Solutions. World J. Microbiol. Biotechnol. 2009, 25, 1573–1578. [Google Scholar] [CrossRef]
- Dubey, S.; Chen, C.W.; Haldar, D.; Tambat, V.S.; Kumar, P.; Tiwari, A.; Singhania, R.R.; Dong, C.D.; Patel, A.K. Advancement in Algal Bioremediation for Organic, Inorganic, and Emerging Pollutants. Environ. Pollut. 2023, 317, 120840. [Google Scholar] [CrossRef]
- Fukuda, S.Y.; Yamamoto, R.; Iwamoto, K.; Minoda, A. Cellular Accumulation of Cesium in the Unicellular Red Alga Galdieria Sulphuraria under Mixotrophic Conditions. J. Appl. Phycol. 2018, 30, 3057–3061. [Google Scholar] [CrossRef]
- Papazi, A.; Karamanli, M.; Kotzabasis, K. Comparative Biodegradation of All Chlorinated Phenols by the Microalga Scenedesmus obliquus—The Biodegradation Strategy of Microalgae. J. Biotechnol. 2019, 296, 61–68. [Google Scholar] [CrossRef]
- Wu, N.; Dong, X.; Liu, Y.; Wang, C.; Baattrup-Pedersen, A.; Riis, T. Using River Microalgae as Indicators for Freshwater Biomonitoring: Review of Published Research and Future Directions. Ecol. Indic. 2017, 81, 124–131. [Google Scholar] [CrossRef]
- Cavalletti, E.; Sardo, A.; Bianco, V.; Miccio, L.; Pirone, D.; Sirico, D.; Valentino, M.; Spinelli, M.; Ferraro, P. Microalgae as Potential Bioindicators for Heavy Metal Pollution. In Proceedings of the 2021 IEEE International Workshop on Metrology for the Sea: Learning to Measure Sea Health Parameters (MetroSea), Reggio Calabria, Italy, 4–6 October 2021; pp. 449–453. [Google Scholar] [CrossRef]
- Rivera, M.J.; Luís, A.T.; Grande, J.A.; Sarmiento, A.M.; Dávila, J.M.; Fortes, J.C.; Córdoba, F.; Diaz-Curiel, J.; Santisteban, M. Physico-Chemical Influence of Surface Water Contaminated by Acid Mine Drainage on the Populations of Diatoms in Dams (Iberian Pyrite Belt, SW Spain). Int. J. Environ. Res. Public Health 2019, 16, 4516. [Google Scholar] [CrossRef]
- Mangadze, T.; Bere, T.; Mwedzi, T. Epilithic Diatom Flora in Contrasting Land-Use Settings in Tropical Streams, Manyame Catchment, Zimbabwe. Hydrobiologia 2015, 753, 163–173. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Ecological Risk Estimation of Organophosphorus Pesticides in Riverine Ecosystems. Chemosphere 2017, 188, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Choi, Y.Y.; Sim, S.J. Emerging Prospects of Mixotrophic Microalgae: Way Forward to Sustainable Bioprocess for Environmental Remediation and Cost-Effective Biofuels. Bioresour. Technol. 2020, 300, 122741. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.N.V. Recovery of Resources from Biowaste for Pollution Prevention. In Environmental Materials and Waste: Resource Recovery and Pollution Prevention; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–19. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. A New Look on Factors Affecting Microbial Degradation of Petroleum Hydrocarbon Pollutants. Int. Biodeterior. Biodegrad. 2017, 120, 71–83. [Google Scholar] [CrossRef]
- Marques, I.M.; Oliveira, A.C.V.; de Oliveira, O.M.C.; Sales, E.A.; Moreira, Í.T.A. A Photobioreactor Using Nannochloropsis Oculata Marine Microalgae for Removal of Polycyclic Aromatic Hydrocarbons and Sorption of Metals in Produced Water. Chemosphere 2021, 281, 130775. [Google Scholar] [CrossRef]
- Diaz, M.; Mora, V.; Pedrozo, F.; Nichela, D.; Baffico, G. Evaluation of Native Acidophilic Algae Species as Potential Indicators of Polycyclic Aromatic Hydrocarbon (PAH) Soil Contamination. J. Appl. Phycol. 2015, 27, 321–325. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Monllor-Alcaraz, L.S.; Postigo, C.; Uggetti, E.; López de Alda, M.; Díez-Montero, R.; García, J. Microalgae-Based Bioremediation of Water Contaminated by Pesticides in Peri-Urban Agricultural Areas. Environ. Pollut. 2020, 265, 114579. [Google Scholar] [CrossRef]
- Uwamahoro, C.; Jo, J.-H.; Jang, S.-I.; Jung, E.-J.; Lee, W.-J.; Bae, J.-W.; Kwon, W.-S.; Uwamahoro, C.; Jo, J.-H.; Jang, S.-I.; et al. Assessing the Risks of Pesticide Exposure: Implications for Endocrine Disruption and Male Fertility. Int. J. Mol. Sci. 2024, 25, 6945. [Google Scholar] [CrossRef] [PubMed]
- Goh, P.S.; Lau, W.J.; Ismail, A.F.; Samawati, Z.; Liang, Y.Y.; Kanakaraju, D. Microalgae-Enabled Wastewater Treatment: A Sustainable Strategy for Bioremediation of Pesticides. Water 2023, 15, 70. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, F.; Ou, J.-J. Global Pesticide Consumption and Pollution: With China as a Focus. Int. Acad. Ecol. Environ. Sci. 2011, 1, 125. [Google Scholar]
- Nicodemus, T.J.; DiRusso, C.C.; Wilson, M.; Black, P.N. Reactive Oxygen Species (ROS) Mediated Degradation of Organophosphate Pesticides by the Green Microalgae Coccomyxa subellipsoidea. Bioresour. Technol. Rep. 2020, 11, 100461. [Google Scholar] [CrossRef]
- Dixit, R.; Wasiullah, X.; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of Heavy Metals from Soil and Aquatic Environment: An Overview of Principles and Criteria of Fundamental Processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Douay, F.; Pelfrêne, A.; Planque, J.; Fourrier, H.; Richard, A.; Roussel, H.; Girondelot, B. Assessment of Potential Health Risk for Inhabitants Living near a Former Lead Smelter. Part 1: Metal Concentrations in Soils, Agricultural Crops, and Homegrown Vegetables. Environ. Monit. Assess. 2013, 185, 3665–3680. [Google Scholar] [CrossRef] [PubMed]
- Goutam Mukherjee, A.; Ramesh Wanjari, U.; Renu, K.; Vellingiri, B.; Valsala Gopalakrishnan, A. Heavy Metal and Metalloid—Induced Reproductive Toxicity. Environ. Toxicol. Pharmacol. 2022, 92, 103859. [Google Scholar] [CrossRef] [PubMed]
- Samadani, M.; Perreault, F.; Oukarroum, A.; Dewez, D. Effect of Cadmium Accumulation on Green Algae Chlamydomonas reinhardtii and Acid-Tolerant Chlamydomonas CPCC 121. Chemosphere 2018, 191, 174–182. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Perera, I.A.; Megharaj, M. Acid-Tolerant Microalgae Can Withstand Higher Concentrations of Invasive Cadmium and Produce Sustainable Biomass and Biodiesel at PH 3.5. Bioresour. Technol. 2019, 281, 469–473. [Google Scholar] [CrossRef]
- Puente-Sánchez, F.; Díaz, S.; Penacho, V.; Aguilera, A.; Olsson, S. Basis of Genetic Adaptation to Heavy Metal Stress in the Acidophilic Green Alga Chlamydomonas acidophila. Aquat. Toxicol. 2018, 200, 62–72. [Google Scholar] [CrossRef]
- Di Cicco, M.R.; Palmieri, M.; Altieri, S.; Ciniglia, C.; Lubritto, C. Cultivation of the Acidophilic Microalgae Galdieria phlegrea with Wastewater: Process Yields. Int. J. Environ. Res. Public Health 2021, 18, 2291. [Google Scholar] [CrossRef]
- Santiago-Martínez, M.G.; Lira-Silva, E.; Encalada, R.; Pineda, E.; Gallardo-Pérez, J.C.; Zepeda-Rodriguez, A.; Moreno-Sánchez, R.; Saavedra, E.; Jasso-Chávez, R. Cadmium Removal by Euglena gracilis Is Enhanced under Anaerobic Growth Conditions. J. Hazard. Mater. 2015, 288, 104–112. [Google Scholar] [CrossRef]
- Jasso-Chávez, R.; Campos-García, M.L.; Vega-Segura, A.; Pichardo-Ramos, G.; Silva-Flores, M.; Santiago-Martínez, M.G.; Feregrino-Mondragón, R.D.; Sánchez-Thomas, R.; García-Contreras, R.; Torres-Márquez, M.E.; et al. Microaerophilia Enhances Heavy Metal Biosorption and Internal Binding by Polyphosphates in Photosynthetic Euglena gracilis. Algal Res. 2021, 58, 102384. [Google Scholar] [CrossRef]
- Halter, D.; Casiot, C.; Heipieper, H.J.; Plewniak, F.; Marchal, M.; Simon, S.; Arsène-Ploetze, F.; Bertin, P.N. Surface Properties and Intracellular Speciation Revealed an Original Adaptive Mechanism to Arsenic in the Acid Mine Drainage Bio-Indicator Euglena mutabilis. Appl. Microbiol. Biotechnol. 2012, 93, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Casiot, C.; Bruneel, O.; Personné, J.C.; Leblanc, M.; Elbaz-Poulichet, F. Arsenic Oxidation and Bioaccumulation by the Acidophilic Protozoan, Euglena mutabilis, in Acid Mine Drainage (Carnoulès, France). Sci. Total Environ. 2004, 320, 259–267. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Pannerselvan, L.; Venkateswarlu, K.; Megharaj, M. Potential of Acid-Tolerant Microalgae, Desmodesmus sp. MAS1 and Heterochlorella sp. MAS3, in Heavy Metal Removal and Biodiesel Production at Acidic PH. Bioresour. Technol. 2019, 278, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, N.; Shariati, M. Aluminum Remediation from Medium by Dunaliella. Ecol. Eng. 2014, 67, 76–79. [Google Scholar] [CrossRef]
- Fukuda, S.Y.; Iwamoto, K.; Atsumi, M.; Yokoyama, A.; Nakayama, T.; Ishida, K.I.; Inouye, I.; Shiraiwa, Y. Global Searches for Microalgae and Aquatic Plants That Can Eliminate Radioactive Cesium, Iodine and Strontium from the Radio-Polluted Aquatic Environment: A Bioremediation Strategy. J. Plant Res. 2014, 127, 79–89. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Zhang, D. Bioremediation of Strontium (Sr) Contaminated Aquifer Quartz Sand Based on Carbonate Precipitation Induced by Sr Resistant Halomonas sp. Chemosphere 2012, 89, 764–768. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Safonov, A.; Zelenina, D.; Ershova, Y.; Boldyrev, K. Evaluation of Biosorption and Bioaccumulation Capacity of Cyanobacteria Arthrospira (spirulina) Platensis for Radionuclides. Algal Res. 2020, 51, 102075. [Google Scholar] [CrossRef]
- Hosseini, A.; Brown, J.E.; Gwynn, J.P.; Dowdall, M. Review of Research on Impacts to Biota of Discharges of Naturally Occurring Radionuclides in Produced Water to the Marine Environment. Sci. Total Environ. 2012, 438, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, C.; Wang, J. Bioremediation of Cesium-Contaminated Soil by Sorghum Bicolor and Soil Microbial Community Analysis. Geomicrobiol. J. 2016, 33, 216–221. [Google Scholar] [CrossRef]
- Rivasseau, C.; Farhi, E.; Atteia, A.; Couté, A.; Gromova, M.; De Gouvion Saint Cyr, D.; Boisson, A.M.; Féret, A.S.; Compagnon, E.; Bligny, R. An Extremely Radioresistant Green Eukaryote for Radionuclide Bio-Decontamination in the Nuclear Industry. Energy Environ. Sci. 2013, 6, 1230–1239. [Google Scholar] [CrossRef]
- Leonardo, T.; Farhi, E.; Pouget, S.; Motellier, S.; Boisson, A.M.; Banerjee, D.; Rébeillé, F.; Den Auwer, C.; Rivasseau, C. Silver Accumulation in the Green Microalga Coccomyxa actinabiotis: Toxicity, in Situ Speciation, and Localization Investigated Using Synchrotron XAS, XRD, and TEM. Environ. Sci. Technol. 2016, 50, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Rempel, A.; Gutkoski, J.P.; Nazari, M.T.; Biolchi, G.N.; Cavanhi, V.A.F.; Treichel, H.; Colla, L.M. Current Advances in Microalgae-Based Bioremediation and Other Technologies for Emerging Contaminants Treatment. Sci. Total Environ. 2021, 772, 144918. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, A.; Blaney, L.; Kao, J.; Tyagi, R.D.; Zhang, T.C.; Surampalli, R.Y. Emerging Contaminants in Landfill Leachate and Their Sustainable Management. Environ. Earth Sci. 2015, 73, 1357–1368. [Google Scholar] [CrossRef]
- Meganathan, B.; Rathinavel, T.; Rangaraj, S. Trends in Microbial Degradation and Bioremediation of Emerging Contaminants. Phys. Sci. Rev. 2023, 8, 2261–2283. [Google Scholar] [CrossRef]
- Patel, N.; Khan, Z.A.; Shahane, S.; Rai, D.; Chauhan, D.; Kant, C.; Chaudhary, V.K. Emerging Pollutants in Aquatic Environment: Source, Effect, and Challenges in Biomonitoring and Bioremediation-a Review. Pollution 2020, 6, 99–113. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Ralph, P.J. Microalgal Bioremediation of Emerging Contaminants—Opportunities and Challenges. Water Res. 2019, 164, 114921. [Google Scholar] [CrossRef]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and Fate of Pharmaceutical Products and By-Products, from Resource to Drinking Water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef]
- Qi, X.; Xiong, J.Q.; Zhao, C.Y.; Ru, S. Unraveling the Key Driving Factors Involved in Cometabolism Enhanced Aerobic Degradation of Tetracycline in Wastewater. Water Res. 2022, 226, 119285. [Google Scholar] [CrossRef]
- de Jesus Oliveira Santos, M.; Oliveira de Souza, C.; Marcelino, H.R. Blue Technology for a Sustainable Pharmaceutical Industry: Microalgae for Bioremediation and Pharmaceutical Production. Algal Res. 2023, 69, 102931. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Li, X.; Wang, R.; Zhai, J. Carbamazepine Toxicity and Its Co-Metabolic Removal by the Cyanobacteria Spirulina Platensis. Sci. Total Environ. 2020, 706, 135686. [Google Scholar] [CrossRef]
- Encarnação, T.; Palito, C.; Pais, A.A.C.C.; Valente, A.J.M.; Burrows, H.D. Removal of Pharmaceuticals from Water by Free and Imobilised Microalgae. Molecules 2020, 25, 3639. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.P.; Almeida, C.M.R.; Salgado, M.A.; Carvalho, M.F.; Mucha, A.P. Pharmaceutical Compounds in Aquatic Environments— Occurrence, Fate and Bioremediation Prospective. Toxics 2021, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, G. 200 Years of Fossil Fuels and Climate Change (1900–2100). J. Geol. Soc. India 2023, 99, 1043–1062. [Google Scholar] [CrossRef]
- Yap, J.K.; Sankaran, R.; Chew, K.W.; Halimatul Munawaroh, H.S.; Ho, S.H.; Rajesh Banu, J.; Show, P.L. Advancement of Green Technologies: A Comprehensive Review on the Potential Application of Microalgae Biomass. Chemosphere 2021, 281, 130886. [Google Scholar] [CrossRef]
- Knápek, J.; Králík, T.; Vávrová, K.; Valentová, M.; Horák, M.; Outrata, D. Policy Implications of Competition between Conventional and Energy Crops. Renew. Sustain. Energy Rev. 2021, 151, 111618. [Google Scholar] [CrossRef]
- Lupp, G.; Steinhäußer, R.; Starick, A.; Gies, M.; Bastian, O.; Albrecht, J. Forcing Germany’s Renewable Energy Targets by Increased Energy Crop Production: A Challenge for Regulation to Secure Sustainable Land Use Practices. Land. Use Policy 2014, 36, 296–306. [Google Scholar] [CrossRef]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B.; Schenk, P.M.; Thomas-Hall, S.R.; et al. Second Generation Biofuels: High-Efficiency Microalgae for Biodiesel Production. Bioenergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Tan, C.H.; Show, P.L.; Chang, J.S.; Ling, T.C.; Lan, J.C.W. Novel Approaches of Producing Bioenergies from Microalgae: A Recent Review. Biotechnol. Adv. 2015, 33, 1219–1227. [Google Scholar] [CrossRef]
- Mathushika, J.; Gomes, C. Development of Microalgae-Based Biofuels as a Viable Green Energy Source: Challenges and Future Perspectives. Biointerface Res. Appl. Biochem. 2021, 12, 3849–3882. [Google Scholar] [CrossRef]
- da Silva, T.L.; Moniz, P.; Silva, C.; Reis, A. The Dark Side of Microalgae Biotechnology: A Heterotrophic Biorefinery Platform Directed to ω-3 Rich Lipid Production. Microorganisms 2019, 7, 670. [Google Scholar] [CrossRef]
- Nascimento, I.A.; Marques, S.S.I.; Cabanelas, I.T.D.; de Carvalho, G.C.; Nascimento, M.A.; de Souza, C.O.; Druzian, J.I.; Hussain, J.; Liao, W. Microalgae Versus Land Crops as Feedstock for Biodiesel: Productivity, Quality, and Standard Compliance. Bioenergy Res. 2014, 7, 1002–1013. [Google Scholar] [CrossRef]
- Wild, K.J.; Trautmann, A.; Katzenmeyer, M.; Steingaß, H.; Posten, C.; Rodehutscord, M. Chemical Composition and Nutritional Characteristics for Ruminants of the Microalgae Chlorella vulgaris Obtained Using Different Cultivation Conditions. Algal Res. 2019, 38, 101385. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Cole, N.; Dharmarajan, R.; Venkateswarlu, K.; Megharaj, M. Sustainable Production of Biomass and Biodiesel by Acclimation of Non-Acidophilic Microalgae to Acidic Conditions. Bioresour. Technol. 2019, 271, 316–324. [Google Scholar] [CrossRef]
- Haris, N.; Manan, H.; Jusoh, M.; Khatoon, H.; Katayama, T.; Kasan, N.A. Effect of Different Salinity on the Growth Performance and Proximate Composition of Isolated Indigenous Microalgae Species. Aquac. Rep. 2022, 22, 100925. [Google Scholar] [CrossRef]
- Abiusi, F.; Trompetter, E.; Pollio, A.; Wijffels, R.H.; Janssen, M. Acid Tolerant and Acidophilic Microalgae: An Underexplored World of Biotechnological Opportunities. Front. Microbiol. 2022, 13, 820907. [Google Scholar] [CrossRef]
- Roy, U.K.; Wagner, J.; Radu, T. Production of Metabolites in Microalgae Under Alkali Halophilic Growth Medium Using a Dissolved Inorganic Carbon Source. Waste Biomass Valorization 2023, 14, 3339–3354. [Google Scholar] [CrossRef]
- Abiusi, F.; Trompetter, E.; Hoenink, H.; Wijffels, R.H.; Janssen, M. Autotrophic and Mixotrophic Biomass Production of the Acidophilic Galdieria sulphuraria ACUF 64. Algal Res. 2021, 60, 102513. [Google Scholar] [CrossRef]
- Gupta, P.L.; Lee, S.M.; Choi, H.J. A Mini Review: Photobioreactors for Large Scale Algal Cultivation. World J. Microbiol. Biotechnol. 2015, 31, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Khoo, K.S.; Chew, K.W.; Yew, G.Y.; Leong, W.H.; Chai, Y.H.; Show, P.L.; Chen, W.H. Recent Advances in Downstream Processing of Microalgae Lipid Recovery for Biofuel Production. Bioresour. Technol. 2020, 304, 122996. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Awad, F.N.; Qi, X.; Sahu, J.N. Recent Advances in Biological Pretreatment of Microalgae and Lignocellulosic Biomass for Biofuel Production. Renew. Sustain. Energy Rev. 2019, 105, 105–128. [Google Scholar] [CrossRef]
- Rana, M.S.; Prajapati, S.K. Multifarious Applications of Nanoparticles in Microalgae for Bioenergy Generation: State-of-the-Art Review. J. Environ. Chem. Eng. 2023, 11, 109145. [Google Scholar] [CrossRef]
- Muscat, A.; de Olde, E.M.; de Boer, I.J.M.; Ripoll-Bosch, R. The Battle for Biomass: A Systematic Review of Food-Feed-Fuel Competition. Glob. Food Sec 2020, 25, 100330. [Google Scholar] [CrossRef]
- Mittal, V.; Talapatra, K.N.; Ghosh, U.K. A Comprehensive Review on Biodiesel Production from Microalgae through Nanocatalytic Transesterification Process: Lifecycle Assessment and Methodologies. Int. Nano Lett. 2022, 12, 351–378. [Google Scholar] [CrossRef]
- Neag, E.; Stupar, Z.; Maicaneanu, S.A.; Roman, C. Advances in Biodiesel Production from Microalgae. Energies 2023, 16, 1129. [Google Scholar] [CrossRef]
- Tien Thanh, N.; Mostapha, M.; Lam, M.K.; Ishak, S.; Kanna Dasan, Y.; Lim, J.W.; Tan, I.S.; Lau, S.Y.; Chin, B.L.F.; Hadibarata, T. Fundamental Understanding of In-Situ Transesterification of Microalgae Biomass to Biodiesel: A Critical Review. Energy Convers. Manag. 2022, 270, 116212. [Google Scholar] [CrossRef]
- Hossain, F.M.; Rainey, T.J.; Ristovski, Z.; Brown, R.J. Performance and Exhaust Emissions of Diesel Engines Using Microalgae FAME and the Prospects for Microalgae HTL Biocrude. Renew. Sustain. Energy Rev. 2018, 82, 4269–4278. [Google Scholar] [CrossRef]
- Lu, N.; Wei, D.; Chen, F.; Yang, S.T. Lipidomic Profiling and Discovery of Lipid Biomarkers in Snow Alga Chlamydomonas Nivalis under Salt Stress. Eur. J. Lipid Sci. Technol. 2012, 114, 253–265. [Google Scholar] [CrossRef]
- Lee, N.; Oh, H.S.; Oh, H.M.; Kim, H.S.; Ahn, C.Y. Enhanced Growth and Lipid Production in Psychrotolerant Acutodesmus by Controlling Temperature-Dependent Nitrogen Concentration. Biomass Bioenergy 2019, 127, 105267. [Google Scholar] [CrossRef]
- Kharati-Koupaei, M.; Moradshasi, A. Effects of Sodium Nitrate and Mixotrophic Culture on Biomass and Lipid Production in Hypersaline Microalgae Dunaliella viridis Teod. Braz. Arch. Biol. Technol. 2016, 59, e16150437. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic Digestion of Microalgae as a Necessary Step to Make Microalgal Biodiesel Sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef]
- Hirooka, S.; Higuchi, S.; Uzuka, A.; Nozaki, H.; Miyagishima, S.Y. Acidophilic Green Alga Pseudochlorella sp. YKT1 Accumulates High Amount of Lipid Droplets under a Nitrogen-Depleted Condition at a Low-PH. PLoS ONE 2014, 9, e107702. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.; Beardall, J.; Bhattacharya, S.; Wangikar, P.P. Isolation and Biochemical Characterisation of Two Thermophilic Green Algal Species-Asterarcys quadricellulare and Chlorella sorokiniana, Which Are Tolerant to High Levels of Carbon Dioxide and Nitric Oxide. Algal Res. 2018, 30, 28–37. [Google Scholar] [CrossRef]
- Khan, S.; Das, P.; Abdul Quadir, M.; Thaher, M.I.; Mahata, C.; Sayadi, S.; Al-Jabri, H. Microalgal Feedstock for Biofuel Production: Recent Advances, Challenges, and Future Perspective. Fermentation 2023, 9, 281. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from Microalgae: Technologies, Challenges and Opportunities. Renew. Sustain. Energy Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- Mendez, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C. Chlorella vulgaris vs. Cyanobacterial Biomasses: Comparison in Terms of Biomass Productivity and Biogas Yield. Energy Convers. Manag. 2015, 92, 137–142. [Google Scholar] [CrossRef]
- Perendeci, N.A.; Yılmaz, V.; Ertit Taştan, B.; Gökgöl, S.; Fardinpoor, M.; Namlı, A.; Steyer, J.P. Correlations between Biochemical Composition and Biogas Production during Anaerobic Digestion of Microalgae and Cyanobacteria Isolated from Different Sources of Turkey. Bioresour. Technol. 2019, 281, 209–216. [Google Scholar] [CrossRef]
- Kusmiyati, K.; Hadiyanto, H.; Fudholi, A. Treatment Updates of Microalgae Biomass for Bioethanol Production: A Comparative Study. J. Clean. Prod. 2023, 383, 135236. [Google Scholar] [CrossRef]
- Phwan, C.K.; Ong, H.C.; Chen, W.H.; Ling, T.C.; Ng, E.P.; Show, P.L. Overview: Comparison of Pretreatment Technologies and Fermentation Processes of Bioethanol from Microalgae. Energy Convers. Manag. 2018, 173, 81–94. [Google Scholar] [CrossRef]
- Gronchi, N.; De Bernardini, N.; Cripwell, R.A.; Treu, L.; Campanaro, S.; Basaglia, M.; Foulquié-Moreno, M.R.; Thevelein, J.M.; Van Zyl, W.H.; Favaro, L.; et al. Natural Saccharomyces cerevisiae Strain Reveals Peculiar Genomic Traits for Starch-to-Bioethanol Production: The Design of an Amylolytic Consolidated Bioprocessing Yeast. Front. Microbiol. 2022, 12, 768562. [Google Scholar] [CrossRef]
- Chetty, B.J.; Inokuma, K.; Hasunuma, T.; van Zyl, W.H.; den Haan, R. Improvement of Cell-Tethered Cellulase Activity in Recombinant Strains of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2022, 106, 6347–6361. [Google Scholar] [CrossRef]
- Dahlin, L.R.; Gerritsen, A.T.; Henard, C.A.; Van Wychen, S.; Linger, J.G.; Kunde, Y.; Hovde, B.T.; Starkenburg, S.R.; Posewitz, M.C.; Guarnieri, M.T. Development of a High-Productivity, Halophilic, Thermotolerant Microalga Picochlorum renovo. Commun. Biol. 2019, 2, 388. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Markham, J.; Kinchin, C.; Grundl, N.; Tan, E.C.D.; Humbird, D. Process Design and Economics for the Production of Algal Biomass: Algal Biomass Production in Open Pond Systems and Processing through Dewatering for Downstream Conversion; National Renewable Energy Laboratory: Golden, CO, USA, 2016. [Google Scholar]
- Li, S.; Li, F.; Zhu, X.; Liao, Q.; Chang, J.S.; Ho, S.H. Biohydrogen Production from Microalgae for Environmental Sustainability. Chemosphere 2022, 291, 132717. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.J.; Kondo, A.; Chang, J.S. Recent Insights into Biohydrogen Production by Microalgae—From Biophotolysis to Dark Fermentation. Bioresour. Technol. 2017, 227, 373–387. [Google Scholar] [CrossRef]
- Javed, M.A.; Zafar, A.M.; Aly Hassan, A.; Zaidi, A.A.; Farooq, M.; El Badawy, A.; Lundquist, T.; Mohamed, M.M.A.; Al-Zuhair, S. The Role of Oxygen Regulation and Algal Growth Parameters in Hydrogen Production via Biophotolysis. J. Environ. Chem. Eng. 2022, 10, 107003. [Google Scholar] [CrossRef]
- Kumar, G.; Zhen, G.; Kobayashi, T.; Sivagurunathan, P.; Kim, S.H.; Xu, K.Q. Impact of PH Control and Heat Pre-Treatment of Seed Inoculum in Dark H2 Fermentation: A Feasibility Report Using Mixed Microalgae Biomass as Feedstock. Int. J. Hydrogen Energy 2016, 41, 4382–4392. [Google Scholar] [CrossRef]
- Banihashemi, F.; Ibrahim, A.F.M.; Deng, S.; Lin, J.Y.S. Pyrolysis and Gasification Characteristics of Galdieria sulphuraria Microalgae. Bioenergy Res. 2023, 16, 611–621. [Google Scholar] [CrossRef]
- Scherer, F.M. Chapter 25 The Pharmaceutical Industry. Handb. Health Econ. 2000, 1, 1297–1336. [Google Scholar] [CrossRef]
- Xia, D.; Qiu, W.; Wang, X.; Liu, J. Recent Advancements and Future Perspectives of Microalgae-Derived Pharmaceuticals. Mar. Drugs 2021, 19, 703. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.; Baltz, R.H. Natural Product Discovery: Past, Present, and Future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Thuan, N.H.; An, T.T.; Shrestha, A.; Canh, N.X.; Sohng, J.K.; Dhakal, D. Recent Advances in Exploration and Biotechnological Production of Bioactive Compounds in Three Cyanobacterial Genera: Nostoc, Lyngbya, and Microcystis. Front. Chem. 2019, 7, 474915. [Google Scholar] [CrossRef]
- Bhattacharjee, M. Pharmaceutically Valuable Bioactive Compounds of Algae. Asian J. Pharm. Clin. Res. 2016, 9, 43–47. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural Products: A Continuing Source of Novel Drug Leads. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Gupta, E.; Singh, P.; Prasad, R. Application of Microalgae Metabolites in Food and Pharmaceutical Industry. In Preparation of Phytopharmaceuticals for the Management of Disorders: The Development of Nutraceuticals and Traditional Medicine; Springer: Berlin/Heidelberg, Germany, 2021; pp. 391–408. [Google Scholar] [CrossRef]
- Silva, M.; Kamberovic, F.; Uota, S.T.; Kovan, I.M.; Viegas, C.S.B.; Simes, D.C.; Gangadhar, K.N.; Varela, J.; Barreira, L. Microalgae as Potential Sources of Bioactive Compounds for Functional Foods and Pharmaceuticals. Appl. Sci. 2022, 12, 5877. [Google Scholar] [CrossRef]
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and Prevention Strategies for Non-Communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health 2020, 8, 574111. [Google Scholar] [CrossRef]
- Zimmer, J.P.; Billy, R.; Hammond, J. Possible Influences of Lutein and Zeaxanthin on the Developing Retina. Clin. Ophthalmol. 2007, 1, 25. [Google Scholar] [PubMed]
- Stringham, J.M.; Bovier, E.R.; Wong, J.C.; Hammond, B.R. The Influence of Dietary Lutein and Zeaxanthin on Visual Performance. J. Food Sci. 2010, 75, R24–R29. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary Sources of Lutein and Zeaxanthin Carotenoids and Their Role in Eye Health. Nutrients 2013, 5, 1169. [Google Scholar] [CrossRef]
- Saha, S.K.; Ermis, H.; Murray, P. Marine Microalgae for Potential Lutein Production. Appl. Sci. 2020, 10, 6457. [Google Scholar] [CrossRef]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein Production from Biomass: Marigold Flowers versus Microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef]
- Ma, R.; Zhao, X.; Xie, Y.; Ho, S.H.; Chen, J. Enhancing Lutein Productivity of Chlamydomonas sp. via High-Intensity Light Exposure with Corresponding Carotenogenic Genes Expression Profiles. Bioresour. Technol. 2019, 275, 416–420. [Google Scholar] [CrossRef]
- Barghini, P.; Giovannini, V.; Fenice, M.; Gorrasi, S. High Lutein Production by a Halo-Tolerant Strain of Dunaliella sp. (Chlorophyceae) Isolated from Solar Salterns in Central Italy. J. Environ. Prot. Ecol. 2018, 19, 704–712. [Google Scholar]
- Sathasivam, R.; Ki, J.S. A Review of the Biological Activities of Microalgal Carotenoids and Their Potential Use in Healthcare and Cosmetic Industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef]
- Palozza, P.; Maggiano, N.; Calviello, G.; Lanza, P.; Piccioni, E.; Ranelletti, F.O.; Bartoli, G.M. Canthaxanthin Induces Apoptosis in Human Cancer Cell Lines. Carcinogenesis 1998, 19, 373–376. [Google Scholar] [CrossRef]
- Abdel-Fatth, G.; Watzl, B.; Huang, D.; Watson, R.R. Beta-Carotene in Vitro Stimulates Tumor Necrosis Factor Alpha and Interleukin 1 Alpha Secretion by Human Peripheral Blood Mononuclear Cells. Nutr. Res. 1993, 13, 863–871. [Google Scholar] [CrossRef]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kaderi Kibria, K.M.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Sadeq, M.A.; Ashry, M.H.; Ghorab, R.M.F.; Afify, A.Y. Causes of Death among Patients with Cutaneous Melanoma: A US Population-Based Study. Sci. Rep. 2023, 13, 10257. [Google Scholar] [CrossRef]
- Pelah, D.; Sintov, A.; Cohen, E. The Effect of Salt Stress on the Production of Canthaxanthin and Astaxanthin by Chlorella zofingiensis Grown under Limited Light Intensity. World J. Microbiol. Biotechnol. 2004, 20, 483–486. [Google Scholar] [CrossRef]
- Kahlos, K.; Kangas, L.; Hiltunen, R. Ergosterol Peroxide, an Active Compound from Inonotus Radiatus. Planta Med. 1989, 55, 389–390. [Google Scholar] [CrossRef]
- Yasukawa, K.; Akihisa, T.; Kanno, H.; Kaminaga, T.; Izumida, M.; Sakoh, T.; Tamura, T.; Takido, M. Inhibitory Effects of Sterols Isolated from Chlorella vulgaris on 12-0-Tetradecanoylphorbol-13-Acetate-Induced Inflammation and Tumor Promotion in Mouse Skin. Biol. Pharm. Bull. 1996, 19, 573–576. [Google Scholar] [CrossRef]
- Chang, M.X.; Xiong, F.; Pak, S.; Liang Ooi, S. Astaxanthin and Its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Terao, J. Revisiting Carotenoids as Dietary Antioxidants for Human Health and Disease Prevention. Food Funct. 2023, 14, 7799–7824. [Google Scholar] [CrossRef] [PubMed]
- Chelebieva, E.S.; Dantsyuk, N.V.; Chekanov, K.A.; Chubchikova, I.N.; Drobetskaya, I.V.; Minyuk, G.S.; Lobakova, E.S.; Solovchenko, A.E. Identification and Morphological-Physiological Characterization of Astaxanthin Producer Strains of Haematococcus pluvialis from the Black Sea Region. Appl. Biochem. Microbiol. 2018, 54, 639–648. [Google Scholar] [CrossRef]
- Remias, D.; Wastian, H.; Lütz, C.; Leya, T. Insights into the Biology and Phylogeny of Chloromonas polyptera (Chlorophyta), an Alga Causing Orange Snow in Maritime Antarctica. Antarct. Sci. 2013, 25, 648–656. [Google Scholar] [CrossRef]
- Santoyo, S.; Rodríguez-Meizoso, I.; Cifuentes, A.; Jaime, L.; García-Blairsy Reina, G.; Señorans, F.J.; Ibáñez, E. Green Processes Based on the Extraction with Pressurized Fluids to Obtain Potent Antimicrobials from Haematococcus pluvialis Microalgae. LWT-Food Sci. Technol. 2009, 42, 1213–1218. [Google Scholar] [CrossRef]
- Hsiao, C.P.; Siebert, K.J. Modeling the Inhibitory Effects of Organic Acids on Bacteria. Int. J. Food Microbiol. 1999, 47, 189–201. [Google Scholar] [CrossRef]
- Kadry, A.A.; El-Antrawy, M.A.; El-Ganiny, A.M. Impact of Short Chain Fatty Acids (SCFAs) on Antimicrobial Activity of New β-Lactam/β-Lactamase Inhibitor Combinations and on Virulence of Escherichia coli Isolates. J. Antibiot. 2023, 76, 225–235. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef]
- El-fayoumy, E.A.; Shanab, S.M.; Hassan, O.M.A.; Shalaby, E.A. Enhancement of Active Ingredients and Biological Activities of Nostoc linckia Biomass Cultivated under Modified BG-110 Medium Composition. Biomass Convers. Biorefin 2023, 13, 6049–6066. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus Aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida Albicans Pathogenicity Mechanisms. Virulence 2013, 4, 119. [Google Scholar] [CrossRef]
- Shin, D.-W.; Bae, J.-H.; Cho, Y.; Ryu, Y.-J.; Kim, Z.-H.; Lim, S.-M.; Lee, C.-G. Isolation of New Microalga, Tetraselmis sp. KCTC12236BP, and Biodiesel Production Using Its Biomass. J. Mar. Biosci. Biotechnol. 2016, 8, 39–44. [Google Scholar] [CrossRef]
- Amna Kashif, S.; Hwang, Y.J.; Park, J.K. Potent Biomedical Applications of Isolated Polysaccharides from Marine Microalgae Tetraselmis Species. Bioprocess. Biosyst. Eng. 2018, 41, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, E.A.; Shanab, S.M.M.; Singh, V. Salt Stress Enhancement of Antioxidant and Antiviral Efficiency of Spirulina Platensis. J. Med. Plants Res. 2010, 4, 2622–2632. [Google Scholar] [CrossRef]
- Cm, N. Isolation and Acclimatization of Arthrospira platensis from Tungabhadra Reservoir for Mass Culture in Semi-Arid Region At Ballari, Karnataka. Electron. J. Bio. 2021, 10, 2021–2277. [Google Scholar] [CrossRef]
- Núñez-Montero, K.; Rojas-Villalta, D.; Barrientos, L. Antarctic Sphingomonas sp. So64.6b Showed Evolutive Divergence within Its Genus, Including New Biosynthetic Gene Clusters. Front. Microbiol. 2022, 13, 1007225. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Guerra, I.; Costa, M.; Silva, J.; Gouveia, L. Future Perspectives of Microalgae in the Food Industry. In Cultured Microalgae for the Food Industry: Current and Potential Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 387–433. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Robles, M.; Martín, L.; Beltrán, Á.; Gava, R.; Cuaresma, M.; Navarro, F.; Vílchez, C. Ultrasound-Based Recovery of Anti-Inflammatory and Antimicrobial Extracts of the Acidophilic Microalga Coccomyxa onubensis. Mar. Drugs 2023, 21, 471. [Google Scholar] [CrossRef]
- Bouzidi, N.E.; Grama, S.B.; Khelef, A.E.; Yang, D.; Li, J. Inhibition of Antioxidant Enzyme Activities Enhances Carotenogenesis in Microalga Dactylococcus dissociatus MT1. Front. Bioeng. Biotechnol. 2022, 10, 1014604. [Google Scholar] [CrossRef]
- Singh, P.; Baranwal, M.; Reddy, S.M. Antioxidant and Cytotoxic Activity of Carotenes Produced by Dunaliella salina under Stress. Pharm. Biol. 2016, 54, 2269–2275. [Google Scholar] [CrossRef] [PubMed]
- Copeland, J.J. Yellowstone Thermal Myxophyceae. Ann. N. Y. Acad. Sci. 1936, 36, 4–223. [Google Scholar] [CrossRef]
- Allen, J.W.; DiRusso, C.C.; Black, P.N. Triacylglycerol Synthesis during Nitrogen Stress Involves the Prokaryotic Lipid Synthesis Pathway and Acyl Chain Remodeling in the Microalgae Coccomyxa subellipsoidea. Algal Res. 2015, 10, 110–120. [Google Scholar] [CrossRef]
- Shanthakumar, S.; Abinandan, S.; Venkateswarlu, K.; Subashchandrabose, S.R.; Megharaj, M. Algalization of Acid Soils with Acid-Tolerant Strains: Improvement in PH, Carbon Content, Exopolysaccharides, Indole Acetic Acid and Dehydrogenase Activity. Land. Degrad. Dev. 2021, 32, 3157–3166. [Google Scholar] [CrossRef]
- Vanitha, A.; Narayan, M.S.; Murthy, K.N.C.; Ravishankar, G.A. Comparative Study of Lipid Composition of Two Halotolerant Alga, Dunaliella bardawil and Dunaliella salina. Int. J. Food Sci. Nutr. 2007, 58, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Gross, W.; Schnarrenberger, C. Heterotrophic Growth of Two Strains of the Acido-Thermophilic Red Alga Galdieria sulphuraria. Plant Cell Physiol. 1995, 36, 633–638. [Google Scholar] [CrossRef]
- Luís, A.T.; Novais, M.H.; van de Vijver, B.; Almeida, S.F.P.; da Silva, E.A.F.; Hoffmann, L.; Ector, L. Pinnularia aljustrelica sp. Nov. (Bacillariophyceae), a New Diatom Species Found in Acidic Waters in the Aljustrel Mining Area (Portugal) and Further Observations on the Taxonomy and Ecology of P. Acidophila Hofmann et Krammer and P. Acoricola Hustedt. Fottea 2012, 12, 27–40. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, L.; Zhou, A.; Yang, F.; Yue, Z.; Wang, J. Metabolomic and Physiological Changes of Acid-Tolerant Graesiella sp. MA1 during Long-Term Acid Stress. Environ. Sci. Pollut. Res. 2023, 30, 97209–97218. [Google Scholar] [CrossRef]
- Garbayo, I.; Cuaresma, M.; Vílchez, C.; Vega, J.M. Effect of Abiotic Stress on the Production of Lutein and β-Carotene by Chlamydomonas acidophila. Process Biochem. 2008, 43, 1158–1161. [Google Scholar] [CrossRef]
- Jo, S.W.; Do, J.M.; Kang, N.S.; Park, J.M.; Lee, J.H.; Kim, H.S.; Hong, J.W.; Yoon, H.S. Isolation, Identification, and Biochemical Characteristics of a Cold-Tolerant Chlorella vulgaris KNUA007 Isolated from King George Island, Antarctica. J. Mar. Sci. Eng. 2020, 8, 935. [Google Scholar] [CrossRef]
- Nakanishi, A.; Aikawa, S.; Ho, S.H.; Chen, C.Y.; Chang, J.S.; Hasunuma, T.; Kondo, A. Development of Lipid Productivities under Different CO2 Conditions of Marine Microalgae Chlamydomonas sp. JSC4. Bioresour. Technol. 2014, 152, 247–252. [Google Scholar] [CrossRef]
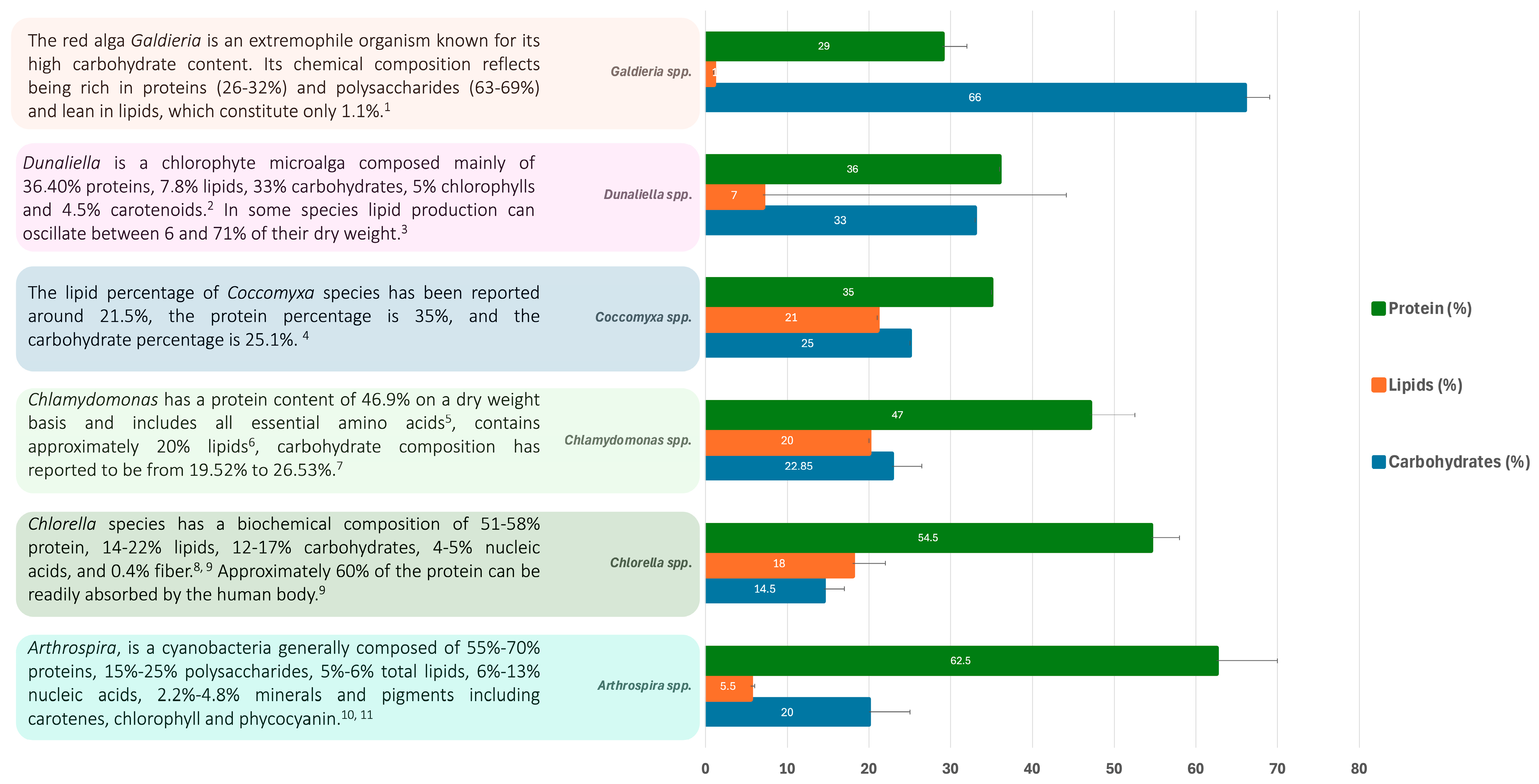
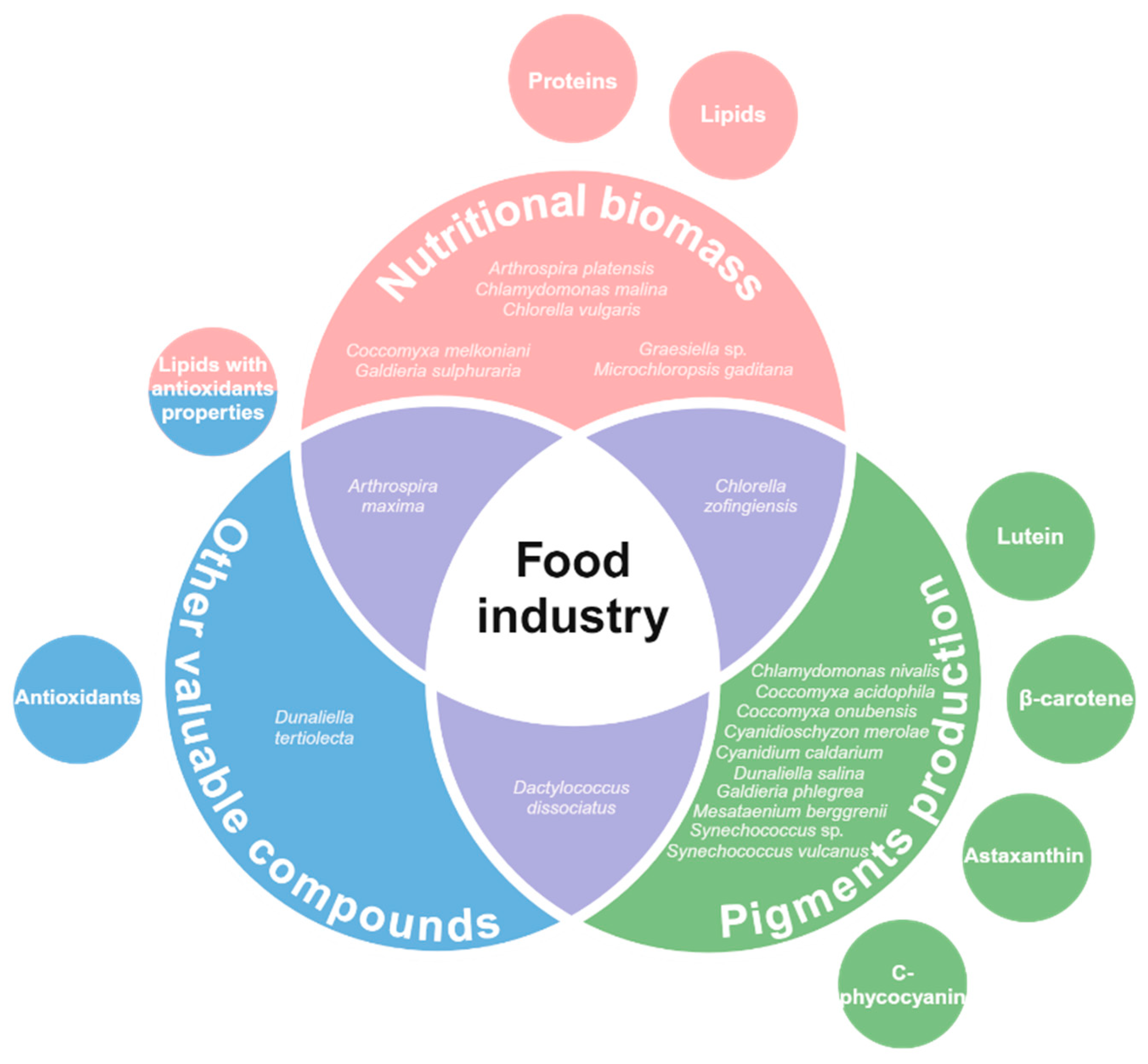
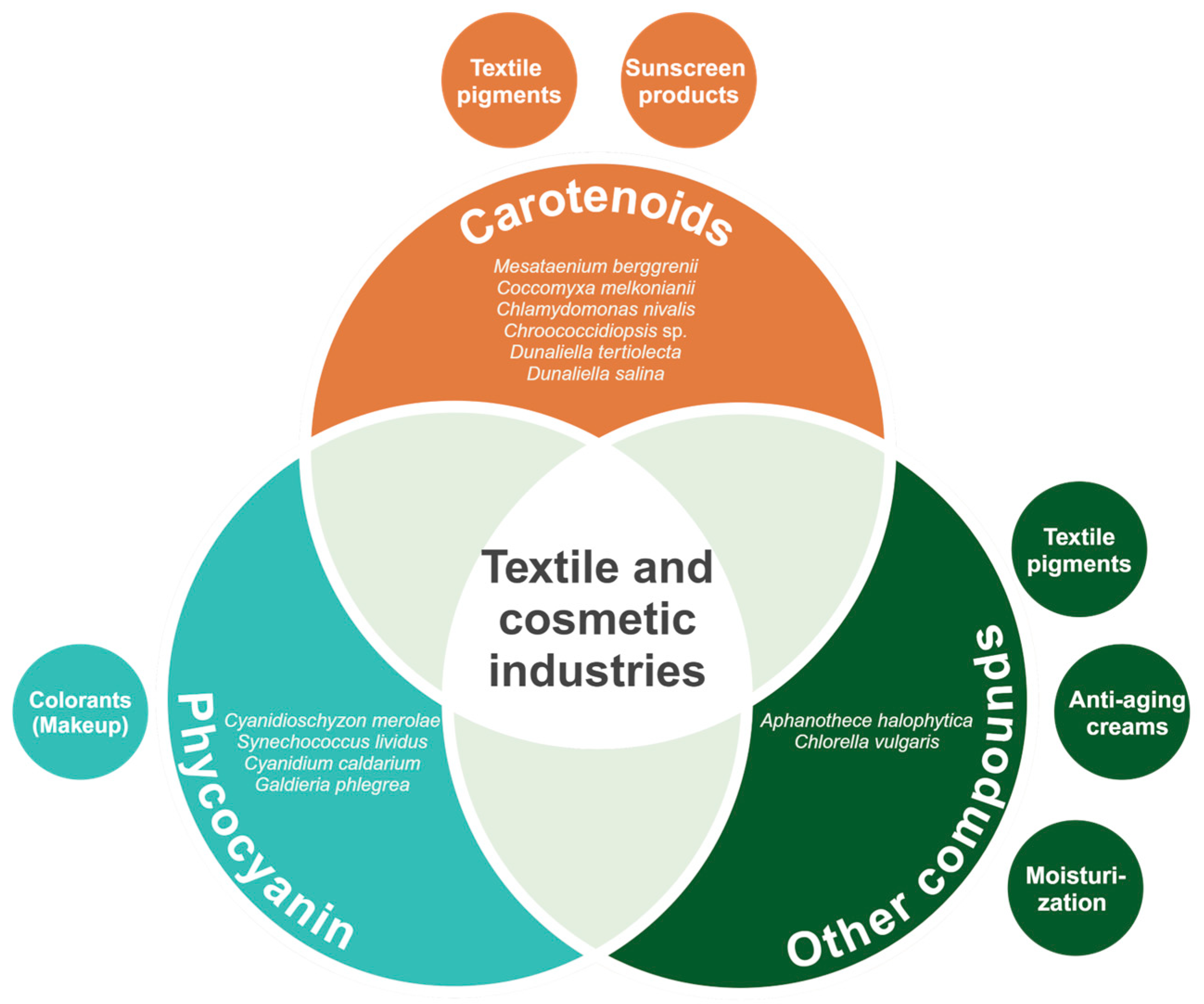
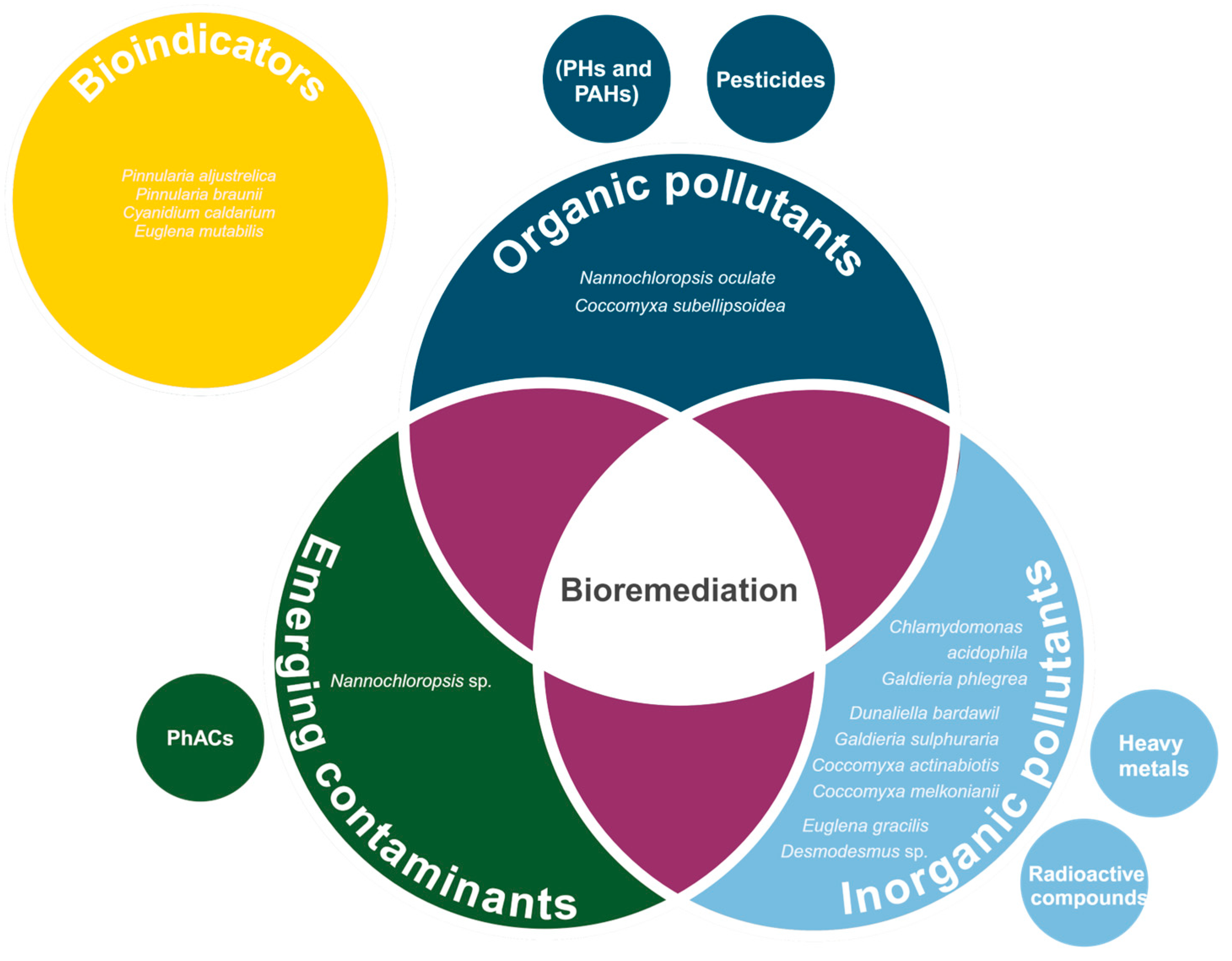
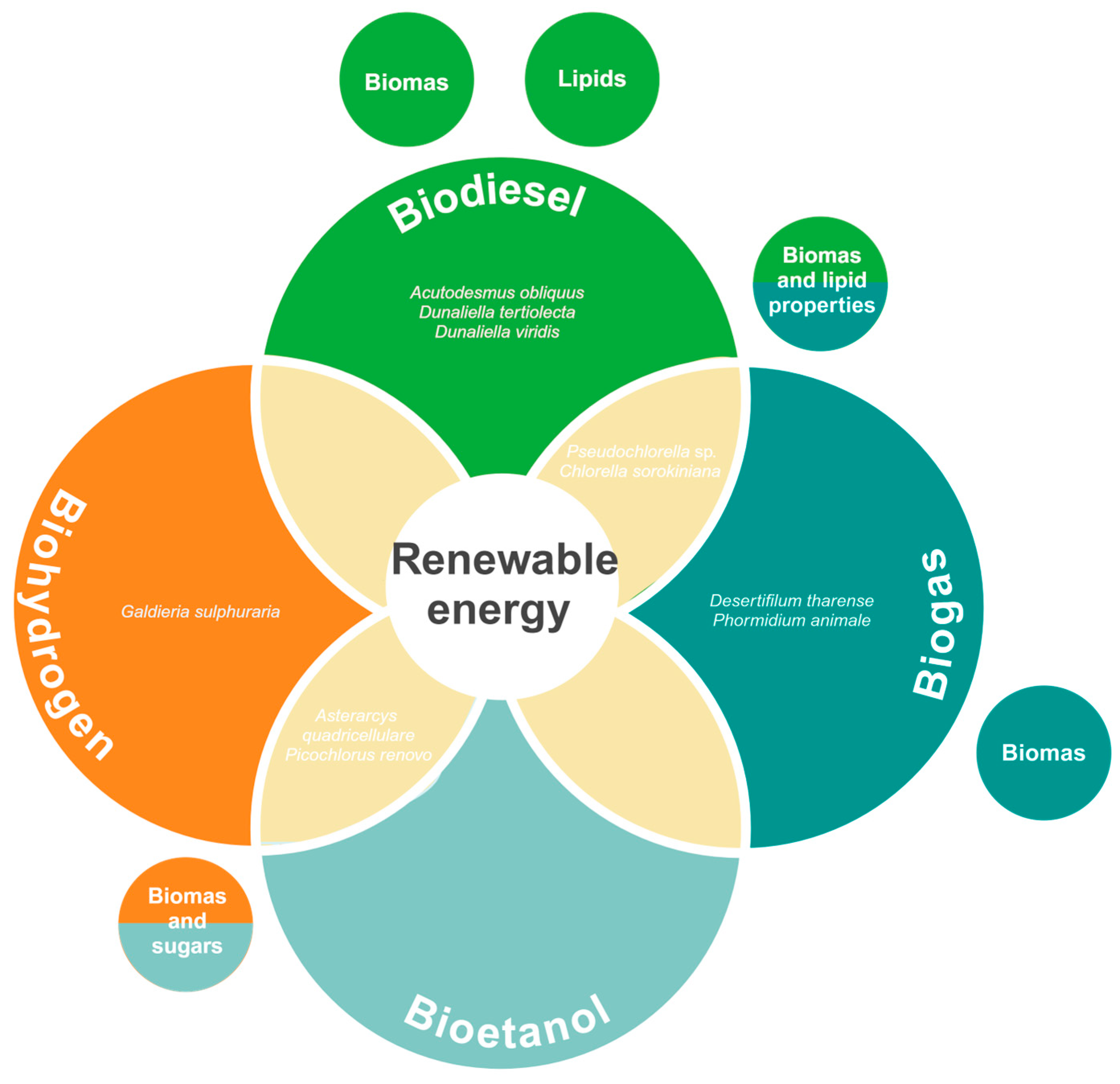
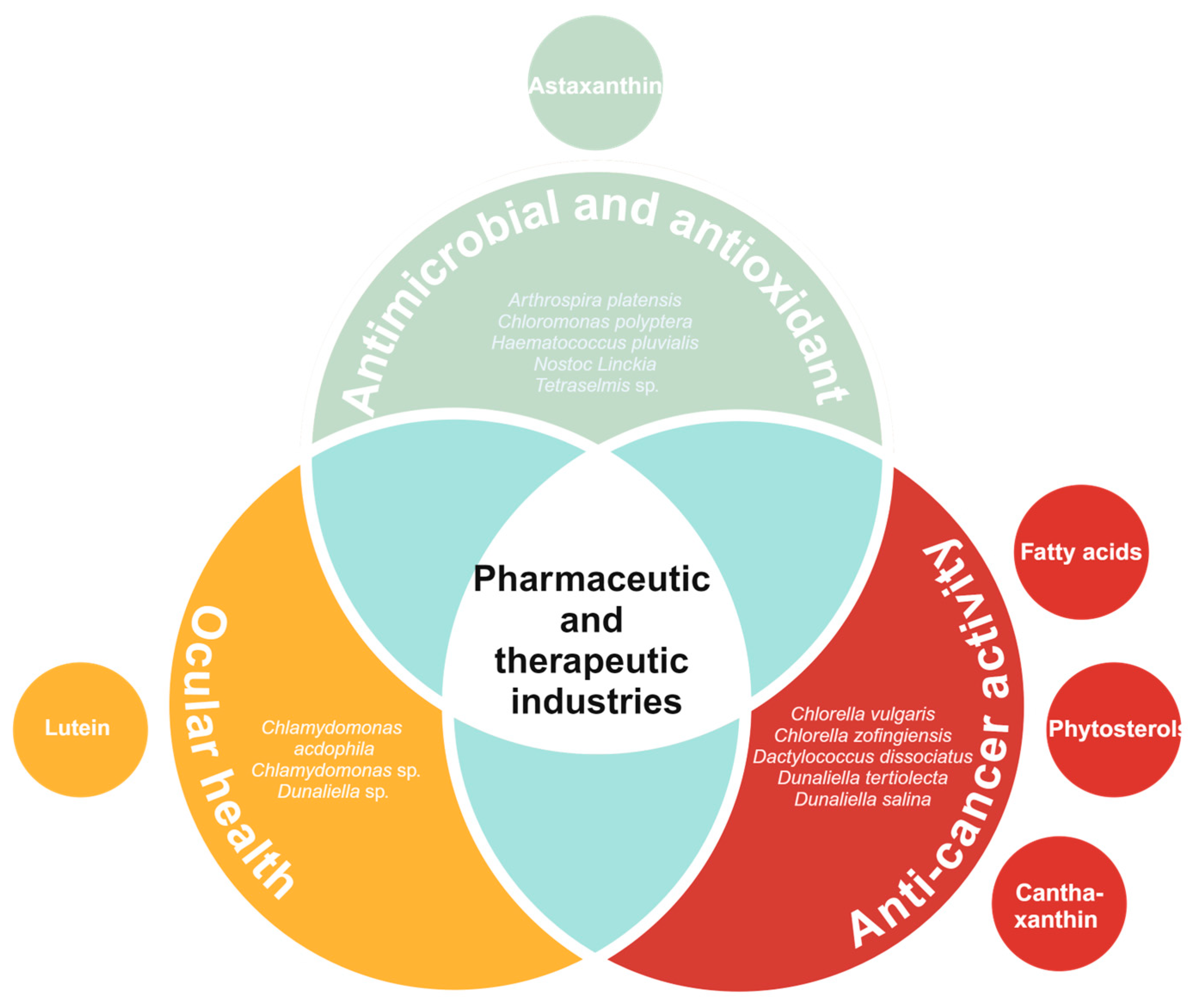
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Villalta, D.; Rojas-Rodríguez, D.; Villanueva-Ilama, M.; Guillén-Watson, R.; Murillo-Vega, F.; Gómez-Espinoza, O.; Núñez-Montero, K. Exploring Extremotolerant and Extremophilic Microalgae: New Frontiers in Sustainable Biotechnological Applications. Biology 2024, 13, 712. https://doi.org/10.3390/biology13090712
Rojas-Villalta D, Rojas-Rodríguez D, Villanueva-Ilama M, Guillén-Watson R, Murillo-Vega F, Gómez-Espinoza O, Núñez-Montero K. Exploring Extremotolerant and Extremophilic Microalgae: New Frontiers in Sustainable Biotechnological Applications. Biology. 2024; 13(9):712. https://doi.org/10.3390/biology13090712
Chicago/Turabian StyleRojas-Villalta, Dorian, David Rojas-Rodríguez, Melany Villanueva-Ilama, Rossy Guillén-Watson, Francinie Murillo-Vega, Olman Gómez-Espinoza, and Kattia Núñez-Montero. 2024. "Exploring Extremotolerant and Extremophilic Microalgae: New Frontiers in Sustainable Biotechnological Applications" Biology 13, no. 9: 712. https://doi.org/10.3390/biology13090712
APA StyleRojas-Villalta, D., Rojas-Rodríguez, D., Villanueva-Ilama, M., Guillén-Watson, R., Murillo-Vega, F., Gómez-Espinoza, O., & Núñez-Montero, K. (2024). Exploring Extremotolerant and Extremophilic Microalgae: New Frontiers in Sustainable Biotechnological Applications. Biology, 13(9), 712. https://doi.org/10.3390/biology13090712







