Comparative Study of Nutritional Composition, Physiological Indicators, and Genetic Diversity in Litopenaeus vannamei from Different Aquaculture Populations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Measurement Methods
2.3. Biochemical Composition Analysis
2.4. Nutritional Quality Assessment
2.5. Antioxidant Enzyme Activity Assays
2.6. Genetic Diversity
2.7. Data Analysis
3. Results
3.1. Analysis of Phenotypic Traits
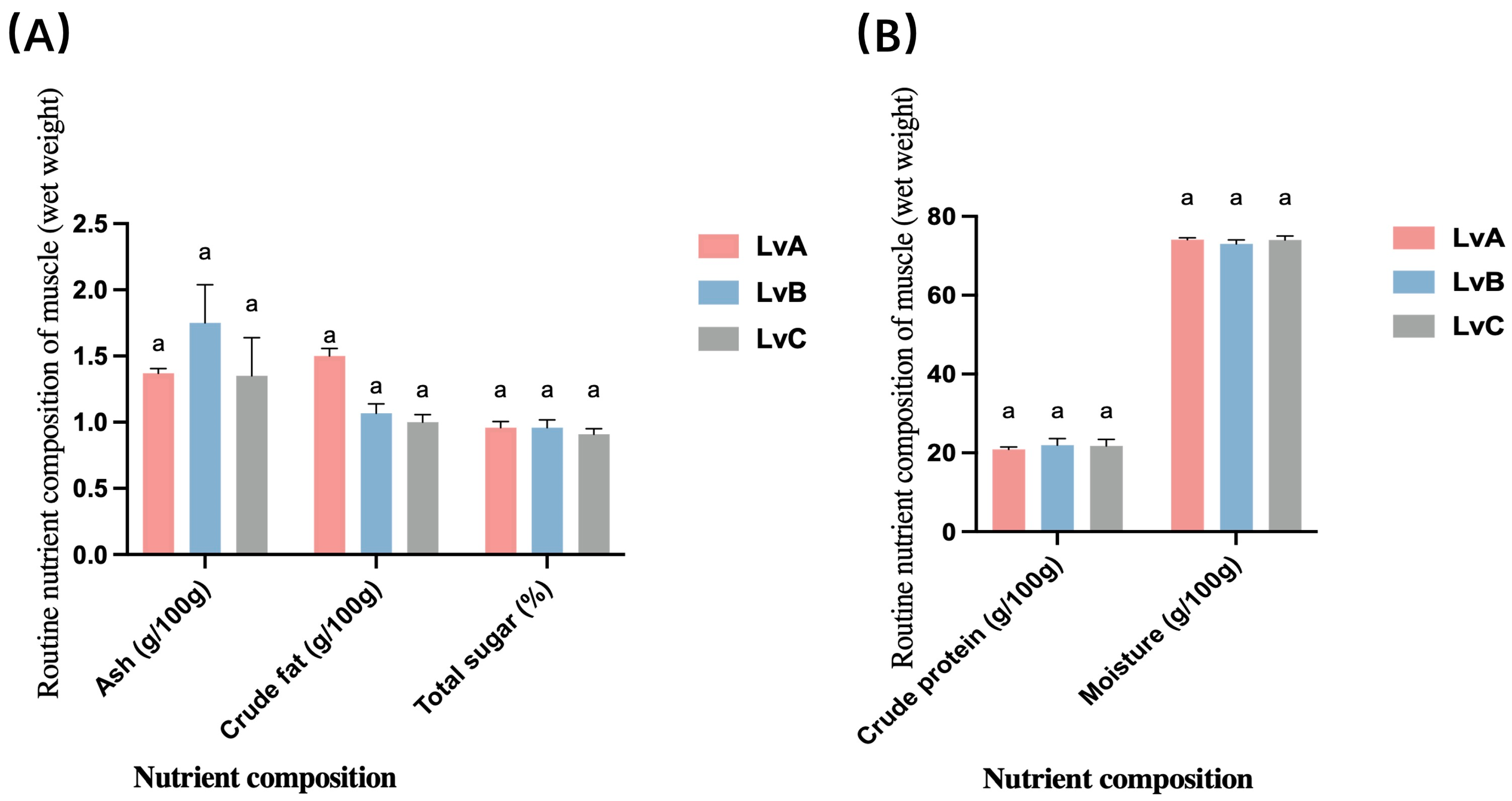
3.2. Conventional Nutrients
3.3. Amino Acid Composition and Content
3.4. Fatty Acid Composition and Content
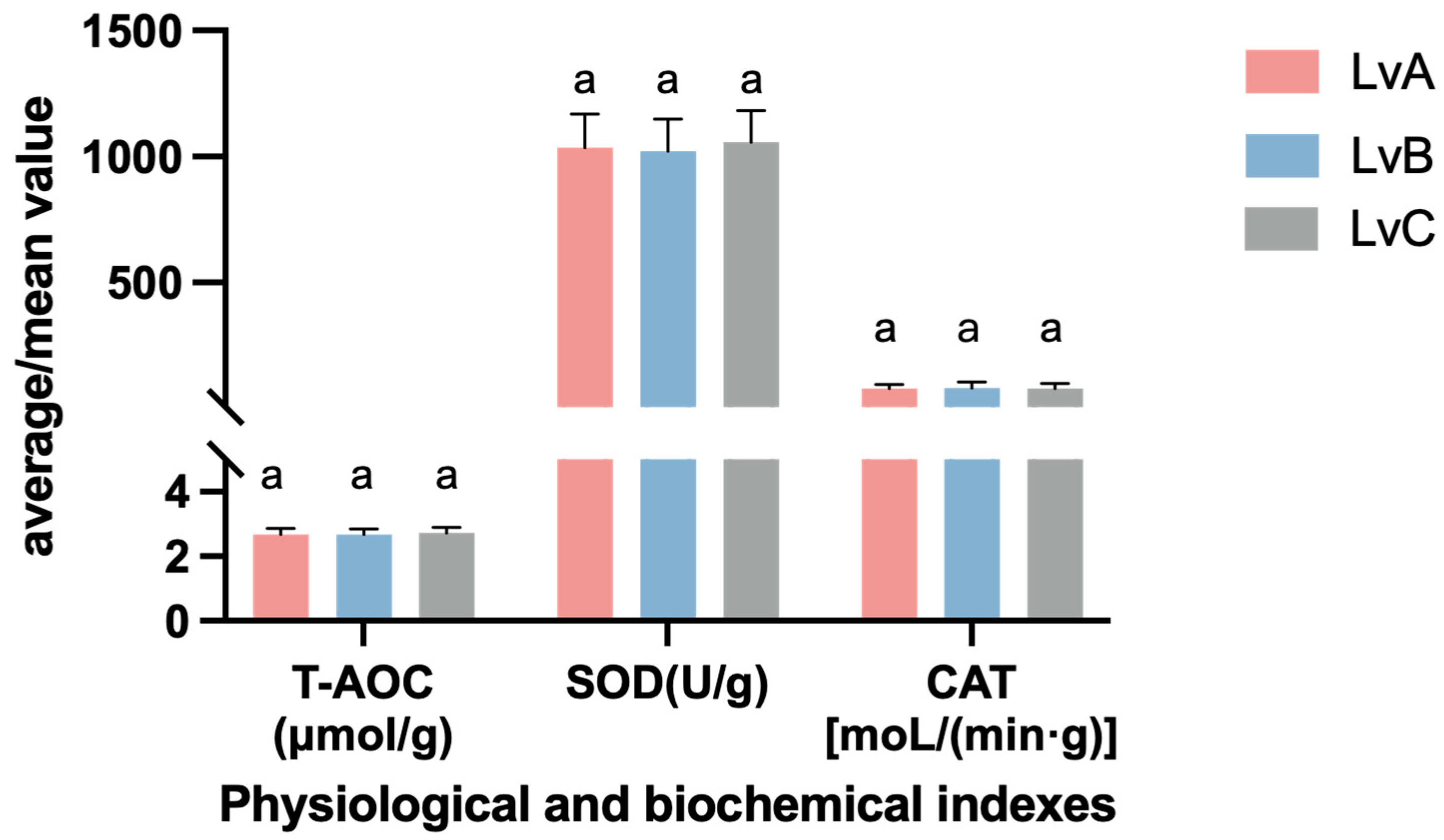
3.5. Analysis of Physiological Indicators
3.6. Genetic Diversity and Population Structure Analysis
Statistical Analysis of Genetic Diversity Parameters
4. Discussion
4.1. Analysis of Differences in Phenotypic Traits of Different Populations of Litopenaeus vannamei
4.2. Analysis of Differences in the Routine Nutrient Composition of the Muscles of L. vannamei from Different Stocks
4.3. Analysis of Differences in Muscle Amino Acid Composition of Different Stocks of Litopenaeus vannamei
4.4. Analysis of Differences in Muscle Fatty Acid Composition of Different Populations of Litopenaeus vannamei
4.5. Analysis of Differences in Physiological Indexes of Different Stocks of Litopenaeus vannamei
4.6. Analysis of Differences in Genetic Diversity of Different Stocks of Litopenaeus vannamei
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.M. Analyzation of Single Nucleotide Polymorphisms in CAT Gene and SOD Gene and Associated with Resistant Hypoxia Traits in Litopenaeus Vannamei. Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2017. [Google Scholar]
- Li, M.; Wen, H.; Huang, F.; Wu, M.; Yu, L.; Jiang, M.; Lu, X.; Tian, J. Role of arginine supplementation on muscular metabolism and flesh quality of Pacific white shrimp (Litopenaeus vannamei) reared in freshwater. Front. Nutr. 2022, 9, 980188. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.A.; Andrade, S.C.S.; Freitas, P.D. Identification of SNPs potentially related to immune responses and growth performance in Litopenaeus vannamei by RNA-seq analyses. PeerJ 2018, 6, e5154. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yu, Y.; Zhang, Q.; Bao, Z.; Xiang, J.; Li, F. Comparative Transcriptome Analysis Reveals the Adaptation Mechanism to High Salinity in Litopenaeus vannamei. Front. Mar. Sci. 2022, 9, 864338. [Google Scholar] [CrossRef]
- Lind, C.E. Population Genetics, Phylogeography and the Effects of Aquaculture on Genetic Diversity of the Silver-Lipped Pearl Oyster, Pinctada Maxima (Jameson). Ph.D. Thesis, James Cook University, Douglas, Australia, 2009. [Google Scholar]
- Zhao, Y.; Zhu, X.; Li, Z.; Xu, W.; Dong, J.; Wei, H.; Li, Y.; Li, X. Genetic diversity and structure of Chinese grass shrimp, Palaemonetes sinensis, inferred from transcriptome-derived microsatellite markers. BMC Genet. 2019, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Teletchea, F. Fish domestication in aquaculture: 10 unanswered questions. Anim. Front. 2021, 11, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.-H.; Gwo, J.-C. Genetic diversity and stock identification of small abalone (Haliotis diversicolor) in Taiwan and Japan. PLoS ONE 2017, 12, e0179818. [Google Scholar] [CrossRef]
- Yang, M.; Ding, F.J. Correlation Analysis between Phenotypic Traits and Body Weight of Macrobrachium rosenbergii at Different Month Ages. J. Jiangsu Ocean. Univ. Nat. Sci. Ed. 2021, 30, 1–9. [Google Scholar]
- GB5009.3-2016; Determination of Moisture in Food. National Standards of the People’s Republic of China: Beijing, China, 2016.
- GB/T 6438-92; Determination of Crude Ash in Feedstuffs. National Standards of the People’s Republic of China: Beijing, China, 1992.
- GB/T 6432-94; Determination of Crude Protein by Kjeldahl Method. National Standards of the People’s Republic of China: Beijing, China, 1994.
- GB 5009.6-2016; National Food Safety Standard-Determination of Crude Fat in Food. National Health and Family Planning Commission: Beijing, China, 2016.
- GB/T 9695.31-2008; Determination of Total Sugar in Food by Ultraviolet-Visible Spectrophotometry. National Standards of the People’s Republic of China: Beijing, China, 2008.
- GB/T 5009.124-2016; National Food Safety Standard-Determination of Amino Acids in Foods by Hydrochloric Acid Hydrolysis. National Health and Family Planning Commission: Beijing, China, 2016.
- GB/T 5009.168-2016; National Food Safety Standard-Determination of Fatty Acids in Foods by Gas Chromatography. National Health and Family Planning Commission: Beijing, China, 2016.
- Zhou, F.; Xiao, J.; He, Z.; Zhang, H.; He, F.; Wu, J. The Muscular Proximate Composition and Activities of Digestive and Immune Enzymes in Different Sizes of Mozambican Tiger Shrimp Penaeus monodon. Fish. Sci. 2013, 32, 653–656. [Google Scholar]
- Chen, S.; Huang, T.; Zhou, Y.; Han, Y.; Xu, M.; Gu, J. AfterQC: Automatic filtering, trimming, error removing and quality control for fastq data. BMC Bioinform. 2017, 18, 80. [Google Scholar] [CrossRef]
- Stuart, K.C.; Edwards, R.J.; Sherwin, W.B.; Rollins, L.A. Contrasting Patterns of Single Nucleotide Polymorphisms and Structural Variation Across Multiple Invasions. Mol. Biol. Evol. 2023, 40, msad046. [Google Scholar] [CrossRef]
- Xiong, X.; Samollow, P.B.; Cao, W.; Metz, R.; Zhang, C.; Leandro, A.C.; VandeBerg, J.L.; Wang, X. Genetic and genomic architecture in eight strains of the laboratory opossum Monodelphis domestica. G3 Genes Genomes Genet. 2022, 12, jkab389. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, L.; Ding, X.; Ma, Y. Genome-Wide Association Analysis of Reproductive Traits in Chinese Holstein Cattle. Genes 2023, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Park, N.; Kim, H.; Oh, J.; Kim, J.; Heo, C.; Kim, J. PAPipe: A Pipeline for Comprehensive Population Genetic Analysis. Mol. Biol. Evol. 2024, 41, msae040. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, T.; Gao, T.; Song, N. Whole-genome resequencing reveals genetic diversity and selection signals in warm temperate and subtropical Sillago sinica populations. BMC Genom. 2023, 24, 547. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Zhao, L.; Wu, X.; Ying, Z.; Ling, Y.; Cai, Z.; Ye, J.; Li, Q.; Tong, W.; Weng, X.; et al. Effects of Different Feeding Strategies on Growth, Digestion and Antioxidant capacity of Chinese Hook Snout Carp Opsariicjthys bidens. J. Fish. Sci. 2024, 1–7. [Google Scholar]
- Lu, C.; Yang, W.; Feng, Y.; Liu, Y.; Li, T.; Li, L.; Cao, Y.; Xu, C. Effect of Heating Temperature on Volatile Components of Sweet Potato Caramel Flavor. Flavour Fragr. Cosmet. 2024, 2, 133–141. [Google Scholar]
- Yu, H.; Liu, F.; Zhu, G.; Ren, X.; Liu, X.; Ling, W.; Su, S. Analysis of Genetic Diversity of P. clarkii Culture Population in Chuzhou Based on Mitochondrial COI Gene. J. Anhui Agric. Sci. 2022, 50, 111–113+125. [Google Scholar]
- Zhou, Z.; Zhang, L.; Huo, Y.; Li, H.; He, X.; Zhang, Y. Morphological differences and genetic diversity of Paratapes undulatus from an area of southern Fujian and Beihai sea, Guangxi. J. Appl. Oceanogr. 2023, 42, 581–590. [Google Scholar]
- Wang, J.; Wang, Q.; Qin, Z.; Chen, Y.; Li, J.; Li, J. Development of SSR markers from genomic data for Litopenaeus vannamei and analysis of genetic diversity in different cultured populations. J. Fish. 2023, 47, 64–74. [Google Scholar]
- Luo, X.; Qin, W.; Liang, H.; Sha, H.; Zou, G. Genetic monitoring of Changfeng silver carp based on microsatellite. J. Hydrobiol. 2022, 46, 725–734. [Google Scholar]
- Yang, X.; Xia, M.; Jiang, J.; Wang, J.; Chen, N.; Liao, M.; Lian, Y. Genetic diversity of Pseudorasbora elongata based on mitochondrial Cyt b gene and D-loop region sequences. J. Fish. Sci. 2023, 30, 1031–1041. [Google Scholar]
- Zhao, M.; Li, Q.; Qin, Q.; Cai, Y.X.; Xu, Z.Q.; Wang, M.H.; Chen, M.H.; Chen, X.H.; Ge, J.C.; Bian, W.J. Genetic diversity based on microsatellite markers in five channel catfish (Ictalurus punctatus) populations. Fish. Sci. 2011, 32, 24–30. [Google Scholar]
- Li, X.; Cui, Q.; Yuan, C. Morphological Differences and Discriminant Analysis of Six Populations of Litopenaeus vannamei. J. Hydroecology 2010, 31, 87–91. [Google Scholar]
- Yao, G.; Li, X. Determination of Nutritional Components of Macrobrachium rosenbergii. Fish. Sci. Technol. Inf. 1981, 4, 23+32. [Google Scholar]
- Xu, X.; Liu, X.; Yan, B.; Xu, J.; Xu, G.; Shao, Y.; Wu, J. Nutritional Component Analysis and Quality Evaluation of Penaeus japonicus. Food Sci. 2011, 32, 297–301. [Google Scholar]
- Zhang, T.; Lin, H.; Ge, J.; Lu, Q.; Peng, G.; Li, X. Analysis of Muscle Nutritional Quality of Wild Populations of Macrobrachium nipponense and Exopalaemon modestus. Feed. Res. 2008, 1, 59–63. [Google Scholar]
- Chen, X.; Chen, Q.; Xie, D. Evaluation of Meat Yield and Muscle Nutritional Value of Litopenaeus vannamei. Fish. Sci. Technol. Inf. 2001, 4, 165–168. [Google Scholar]
- Li, L. Analysis of Muscle Nutritional Components of Macrobrachium nipponense and Procambarus clarkii in Nanwan Reservoir. Water Conserv. Fish. 2005, 3, 28–29. [Google Scholar]
- Huang, Y.; Yang, X.; Wang, Y.; Li, Y.; Zhou, K.; Sun, Z.; Me, Z.; Lu, J.; Lai, Q. Analysis and quality evaluation of nutrient components in muscle of Litopenaeus vannamei of two saline—Alkali aquaculture and mariculture. Fish. Inf. Strategy 2023, 38, 60–66. [Google Scholar]
- Mai, K.; Wei, Y.; Wang, J.; Zhang, W. Main nutrient compositions of Antarctic krill and its application in aquafeeds. J. Ocean. Univ. China Nat. Sci. Ed. 2016, 46, 1–15. [Google Scholar]
- Anzani, C.; BoukidM, F.; Drummond, L.; Mullen, A.M.; Álvarez, C. Optimising the use of proteins from rich meat co-products and non-meat alternatives: Nutritional, technological and allergenicity challenges. Food Res. Int. 2020, 137, 109575. [Google Scholar] [CrossRef] [PubMed]
- Shan, F.; Liu, B.; Sun, C.; Zhou, Q.; Song, C.; Sun, H.; Yang, Z. Effect of Feed Vitamin E Content on Growth Performance, Serum Biochemical Indicators and Muscle Quality of Macrobrachium rosenbergii. J. Anim. Nutr. 2019, 31, 214–225. [Google Scholar]
- Tang, J.; Qin, B.; Ye, J.; Dai, Y. Effects of stocking modes on growth traits and muscle nutritional composition of Macrobrachium nipponense. J. Zhejiang Agric. 2024, 36, 254–263. [Google Scholar]
- FAO/WHO. Protein Quality Evaluation: Report of the Joint FAO/WHO Expert Consultation, Bethesda, MD, USA, 4–8 December 1989; Food and Agriculture Organization of the United Nations: Rome, Italy, 1991. [Google Scholar]
- Fauconneau, B.; Alami-Durante, H.; Laroche, M.; Marcel, J.; Vallot, D. Growth and meat quality relations in carp. Aquaculture 1995, 129, 265–297. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.Z.; Leng, X.J.; Gao, J.Z.; Sun, P.Y.; Qu, H.C.; Zhang, X.X.; Song, X.L. Comparison of muscle composition of wild and cultured discus fishes Symphysodon spp. J. Shanghai Ocean. Univ. 2016, 25, 719–725. [Google Scholar]
- Liu, Z.; Liu, Q.; Zhang, D.; Wei, S.; Sun, Q.; Xia, Q.; Shi, W.; Ji, H.; Liu, S. Comparison of the Proximate Composition and Nutritional Profile of Byproducts and Edible Parts of Five Species of Shrimp. Foods 2021, 10, 2603. [Google Scholar] [CrossRef]
- Lin, J.W.; Zhang, C.X.; Sun, Y.Z.; Zhai, S.W.; Song, K.; Ye, J. Complete replacement of fish meal by poultry by-product meal in practical diets supplemented with coated amino acids for Pacific white shrimp Litopenaeus vannamei. Chin. J. Anim. Nutr. 2015, 27, 1709–1721. [Google Scholar]
- Shi, R.H. Genetic and Molecular Analysis of Fatty Acid Quality Traits of the Pacific Oyster. Ph.D. Thesis, University of Chinese Academy of Sciences (Institute of Oceanology, Chinese Academy of Sciences), Beijing, China, 2022. [Google Scholar]
- Huang, B.X.; Wang, D.W.; Wang, J.F. Progress in the research on polyunsaturated fatty acids. Agric. Eng. Technol. Process. Agric. Prod. Prog. Biotechnol. 2009, 8, 26–30. [Google Scholar]
- Aydın Yaz, Y.; Yıldırım, N.; Yaz, Y.; Tekin, N.; İnal, M.; Şahin, F.M. Role of Oxidative Stress in Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma. Turk. J. Ophthalmol. 2019, 49, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Attia, H.G.; El-Morshedy, S.M.; Nagy, A.M.; Ibrahim, A.M.; Aleraky, M.; Abdelrahman, S.S.; Osman, S.M.; Alasmari, S.M.; El Raey, M.A.; Abdelhameed, M.F. Citrus clementine Peel Essential Oil Ameliorates Potassium Dichromate-Induced Lung Injury, Insights into the PI3K/AKT Pathway. Metabolites 2024, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Fan, Y.; Zhang, Z.; Chen, C.; Chen, C.; Wang, X.; Deng, J.; Peng, G.; Hu, Y.; Cao, S.; et al. Sodium selenite inhibits deoxynivalenol-induced injury in GPX1-knockdown porcine splenic lymphocytes in culture. Sci. Rep. 2018, 8, 17676. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.; Wright, J.M. Microsatellite DNA in Fishes. Rev. Fish Biol. Fish. 1997, 7, 331–363. [Google Scholar] [CrossRef]
- Li, J.L.; Li, H.X.; Tang, Y.K.; Yu, J.H.; Yu, F. Genetic difference analysis of two genetically improved farmed tilapia populations by using microsatellite marker. J. South. Agric. 2015, 46, 138–143. [Google Scholar]
- Ren, S.; Mather, P.B.; Tang, B.; Hurwood, D.A. Levels of genetic diversity and inferred origins of Penaeus vannamei culture resources in China: Implications for the production of a broad synthetic base population for genetic improvement. Aquaculture 2018, 491, 221–231. [Google Scholar] [CrossRef]
- Crawford, A.M.; Littlejohn, R.P. The use of DNA markers in deciding conservation priorities in sheep and other livestock. Anim. Genet. Resour. Inf. 1998, 23, 21–26. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; Xia, J. Population Structure, Genetic Diversity, and Conservation Strategies of a Commercially Important Sleeper Fish, Odontobutis potamophilus (Gobiiformes: Odontobutidae) Based on Gene-Capture Data. Front. Genet. 2022, 13, 843848. [Google Scholar] [CrossRef]
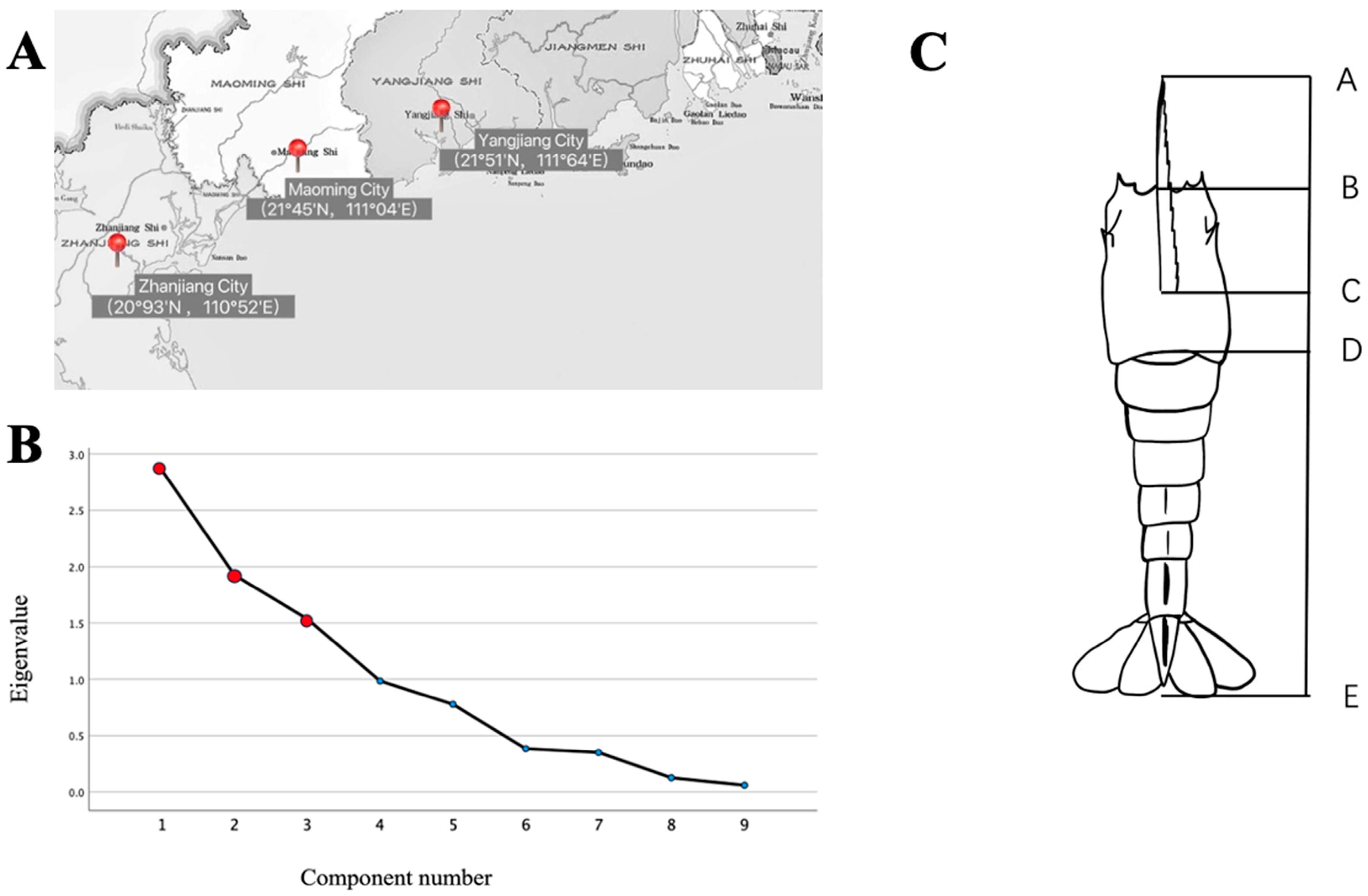
| Survey Point | Sample Name | Quantities | Average Weight (g) | Average Armor Length (mm) | Month | Water Temperature (°C) | Salinity (ppt) | Cultivation Environment |
|---|---|---|---|---|---|---|---|---|
| Maoming City | LvA | 30 | 59.28 ± 6.39 | 143.47 ± 3.12 | 3 to 4 | 27.5 | 30 | Natural bottom ponds |
| Zhanjiang City | LvB | 30 | 60.01 ± 6.63 | 142.87 ± 2.50 | 3 to 4 | 27.4 | 29 | Concrete bottoms |
| Yangjiang City | LvC | 30 | 60.32 ± 8.94 | 145.17 ± 4.32 | 3 to 4 | 27.4 | 30 | Canvas bottoms |
| Variant | LvA | LvB | LvC |
|---|---|---|---|
| Body length/second step foot length | 6.0533 ± 0.3866 ab | 6.1767 ± 0.32129 a | 5.9167 ± 0.35631 b |
| Body length/basal distance of fifth footsteps | 11.9667 ± 1.0083 a | 11.9533 ± 0.6622 a | 12.0300 ± 0.84431 a |
| Body length/cephalothoracic armor length | 3.7500 ± 0.2418 ab | 3.8867 ± 0.1717 a | 3.6000 ± 0.2084 b |
| Body length/frontal horn length | 8.5767 ± 0.76954 ab | 8.9167 ± 0.6097 a | 8.5033 ± 0.7573 b |
| Frontal horn length/cephalothoracic armor length | 0.4367 ± 0.4901 a | 0.4367 ± 0.4901 a | 0.4267 ± 0.4498 a |
| First whip length/cephalothoracic armor length | 1.5733 ± 0.2586 a | 1.6467 ± 0.2713 a | 1.5000 ± 0.31948 a |
| Second whip length/cephalothoracic armor length | 0.2900 ± 0.10939 a | 0.4267 ± 0.06400 b | 0.3967 ± 0.0556 b |
| First whip length/body length | 0.4167 ± 0.6989 a | 0.4200 ± 0.0610 a | 0.4067 ± 0.0785 a |
| Second whip length/body length | 0.0800 ± 0.4068 a | 0.100 ± 0.0000 b | 0.1000 ± 0.0000 b |
| Morphological Proportionality Traits | Principal Component | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Body length/second step foot length | 0.339 | 0.012 | −0.118 |
| Body length/basal distance of fifth footsteps | 0.214 | −0.049 | −0.060 |
| Body length/cephalothoracic armor length | 0.286 | 0.088 | −0.053 |
| Body length/frontal horn length | 0.301 | −0.057 | 0.118 |
| Frontal horn length/cephalothoracic armor length | −0.113 | 0.176 | −0.248 |
| First whip length/cephalothoracic armor length | 0.035 | 0.485 | 0.009 |
| Second whip length/cephalothoracic armor length | 0.010 | 0.047 | 0.463 |
| First whip length/body length | −0.048 | 0.482 | 0.003 |
| Second whip length/body length | −0.163 | 0.010 | 0.555 |
| Contribution of each principal component (%) | 31.756 | 21.383 | 17.065 |
| Cumulative contribution (%) | 31.756 | 53.139 | 70.204 |
| Amino Acids | LvA | LvB | LvC |
|---|---|---|---|
| Aspartic acid Asp @ | 1.54 ± 0.09 a | 1.53 ± 0.04 a | 1.50 ± 0.05 a |
| Threonine Thr * | 0.60 ± 0.03 a | 0.59 ± 0.01 a | 0.57 ± 0.02 a |
| Serine Ser | 0.53 ± 0.03 a | 0.53 ± 0.01 a | 0.50 ± 0.02 a |
| Glutamic acid Glu @ | 2.43 ± 0.15 a | 2.45 ± 0.06 a | 2.45 ± 0.06 a |
| Glycine Gly @ | 1.04 ± 0.06 a | 1.08 ± 0.03 a | 1.04 ± 0.03 a |
| Alanine Ala @ | 0.96 ± 0.04 a | 0.97 ± 0.02 a | 0.96 ± 0.03 a |
| Cystine Cys | 0.22 ± 0.02 a | 0.17 ± 0.02 b | 0.16 ± 0.01 b |
| Val * | 0.73 ± 0.04 a | 0.73 ± 0.02 a | 0.70 ± 0.02 a |
| Methionine Met * | 0.41 ± 0.02 a | 0.38 ± 0.01 b | 0.35 ± 0.01 b |
| Isoleucine IIe * | 0.66 ± 0.03 a | 0.65 ± 0.01 a | 0.63 ± 0.02 a |
| Leucine Leu * | 1.25 ± 0.07 a | 1.22 ± 0.03 a | 1.18 ± 0.04 a |
| Tyr tyrosine | 0.48 ± 0.02 a | 0.45 ± 0.01 a | 0.41 ± 0.01 b |
| Phenylalanine Phe * | 0.67 ± 0.03 a | 0.60 ± 0.01 b | 0.61 ± 0.02 b |
| Lys * | 1.21 ± 0.06 a | 1.20 ± 0.03 a | 1.20 ± 0.03 a |
| Histidine His & | 0.33 ± 0.02 a | 0.32 ± 0.01 a | 0.31 ± 0.01 a |
| Arginine Arg & | 0.95 ± 0.05 a | 0.95 ± 0.03 a | 0.91 ± 0.03 b |
| Proline Pro | 1.27 ± 0.08 a | 1.30 ± 0.06 a | 1.25 ± 0.06 a |
| EAA | 5.86 ± 0.31 a | 5.70 ± 0.13 b | 5.55 ± 0.16 c |
| SEAA | 1.28 ± 0.07 a | 1.27 ± 0.04 a | 1.22 ± 0.03 a |
| NEAA | 8.13 ± 0.47 a | 8.17 ± 0.21 a | 7.97 ± 0.28 b |
| DAA | 5.97 ± 0.34 a | 6.04 ± 0.15 a | 5.95 ± 0.17 a |
| TAA | 15.27 ± 0.84 a | 15.13 ± 0.38 a | 14.73 ± 0.47 a |
| EAA/TAA | 0.38 ± 0.00 a | 0.37 ± 0.00 a | 0.37 ± 0.00 a |
| EAA/NEAA | 0.72 ± 0.00 a | 0.70 ± 0.00 a | 0.70 ± 0.00 a |
| Fatty Acids | LvA | LvB | LvC |
|---|---|---|---|
| Palmitic acid C16:0 | 168.30 ± 2.69 a | 138.53 ± 15.23 b | 148.87 ± 9.56 ab |
| Palmitoleic acid C16:1 | 5.77 ± 0.12 a | 4.10 ± 0.60 b | 5.13 ± 0.42 c |
| C17:0 heptadecanoic acid | 10.67 ± 0.12 a | 9.03 ± 1.03 b | 9.90 ± 0.56 ab |
| Stearic acid C18:0 | 126.43 ± 2.27 a | 109.67 ± 13.27 b | 114.70 ± 6.42 b |
| Oleic acid C18:1 n9c | 123.07 ± 2.04 a | 106.27 ± 13.55 b | 104.53 ± 3.40 b |
| Linoleic acid C18:2n6c | 160.57 ± 2.57 a | 138.77 ± 17.04 b | 140.23 ± 8.30 b |
| Arachidic acid C20:0 | 7.80 ± 0.10 a | 5.13 ± 4.46 b | 7.40 ± 0.40 a |
| Linolenic acid C18:3n3 | 9.33 ± 0.15 a | 7.57 ± 0.97 b | 7.70 ± 0.60 b |
| Eicosatetraenoic acid C20:1 | 6.60 ± 0.10 a | 6.07 ± 0.87 a | 6.07 ± 0.55 a |
| Eicosadienoic acid C20:2 | 15.33 ± 0.40 a | 14.03 ± 1.68 a | 14.40 ± 0.87 a |
| Docosanoic acid C22:0 | 9.53 ± 0.15 a | 8.60 ± 1.61 a | 8.73 ± 0.49 a |
| Arachidonic acid ARAC20:4n6 | 31.57 ± 0.45 a | 27.17 ± 3.63 b | 28.97 ± 1.46 b |
| Erucic acid C22:1n9 | 3.90 ± 0.00 a | 4.37 ± 0.42 b | 6.47 ± 0.45 b |
| XXIII carbonic acid C23:0 | 1.12 ± 1.92 a | 1.13 ± 1.96 a | 1.13 ± 1.96 a |
| Eicosapentaenoic acid C20:5n3(EPA) | 54.80 ± 0.92 a | 47.37 ± 4.97 b | 51.10 ± 2.97 ab |
| C22:6n3(DHA) | 60.30 ± 0.95 a | 54.93 ± 5.51 b | 60.30 ± 3.06 a |
| Total fatty acid content TOTAL | 793.97 ± 12.94 a | 682.73 ± 85.68 b | 715.63 ± 42.04 c |
| Total saturated fatty acids ΣSFA | 317.83 ± 5.32 a | 267.17 ± 36.44 b | 285.97 ± 19.03 b |
| Monounsaturated fatty acids ΣMUFA | 10.50 + 0.10 b | 10.43 + 1.25 b | 12.53 + 0.76 a |
| Polyunsaturated fatty acids ΣPUFA | 277.10 ± 4.49 a | 242.42 ± 28.63 b | 251.60 ± 13.75 b |
| DHA ± EPA | 115.10 ± 1.87 a | 103.30 ± 10.47 b | 111.40 ± 5.86 a |
| n-3 series polyunsaturated fatty acidsn-3ΣPUFA | 124.43 ± 2.02 a | 109.87 ± 11.43 b | 119.10 ± 6.46 b |
| n-6 series polyunsaturated fatty acidsn-6ΣPUFA | 192.13 ± 3.01 a | 165.93 ± 20.67 b | 169.20 ± 9.42 b |
| Population | SNP Number | SNP Density (SNP/Kb) | Nucleotide Diversity (π) | Polymorphism Information Content (PIC) | Observed Heterozygosity (Ho) | Inbreeding Coefficient (FIS) |
|---|---|---|---|---|---|---|
| LvA | 29047925 | 17.461 | 3.11 × 10−5 | 0.303 ± 0.125 | 0.048 ± 0.001 | 0.835 |
| LvB | 25491738 | 15.323 | 2.98 × 10−5 | 0.317 ± 0.124 | 0.048 ± 0.001 | 0.834 |
| LvC | 26677995 | 16.036 | 15.84 × 10−5 | 0.300 ± 0.130 | 0.033 ± 0.014 | 0.887 |
| Population 1 | Population 2 | Population Differentiation Coefficient (FST) | Genetic Distance (DR) | Gene Flow (Nm) |
|---|---|---|---|---|
| LvA | LvB | 0.056 | 0.057 | 4.214 |
| LvA | LvC | 0.084 | 0.088 | 2.726 |
| LvB | LvC | 0.106 | 0.112 | 2.108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cao, S.; Jiang, S.; Huang, J.; Yang, Q.; Jiang, S.; Yang, L.; Zhou, F. Comparative Study of Nutritional Composition, Physiological Indicators, and Genetic Diversity in Litopenaeus vannamei from Different Aquaculture Populations. Biology 2024, 13, 722. https://doi.org/10.3390/biology13090722
Li Y, Cao S, Jiang S, Huang J, Yang Q, Jiang S, Yang L, Zhou F. Comparative Study of Nutritional Composition, Physiological Indicators, and Genetic Diversity in Litopenaeus vannamei from Different Aquaculture Populations. Biology. 2024; 13(9):722. https://doi.org/10.3390/biology13090722
Chicago/Turabian StyleLi, Yundong, Siyao Cao, Shigui Jiang, Jianhua Huang, Qibin Yang, Song Jiang, Lishi Yang, and Falin Zhou. 2024. "Comparative Study of Nutritional Composition, Physiological Indicators, and Genetic Diversity in Litopenaeus vannamei from Different Aquaculture Populations" Biology 13, no. 9: 722. https://doi.org/10.3390/biology13090722






